| Disturbance by photographers | Number of species | Incubation | Nestling | |||||
| Total nests | Predated | Predation rate (%) | Total nests | Predated | Predation rate (%) | |||
| Photographed | 29 | 83 | 2 | 2.4 | 81 | 9 | 11.1 | |
| Not photographed | 25 | 194 | 84 | 43.3 | 110 | 38 | 34.5 | |
| Citation: | Xiaocai Tan, Shilong Liu, Eben Goodale, Aiwu Jiang. 2022: Does bird photography affect nest predation and feeding frequency?. Avian Research, 13(1): 100036. DOI: 10.1016/j.avrs.2022.100036 |
Bird photography is a popular and growing form of ecotourism that contributes to the economic growth of local communities, but its disturbance to bird reproduction remains understudied. We worked in a tropical forest of southern China, which has experienced a sharp increase in the number of photographers in recent years. We compared nests that were photographed and those that were not, in their nest predation and parental feeding rates. Including nests of 42 species, the results demonstrate that the predation rate of nests that were not photographed (incubation stage: 43.3% of 194 nests; nestling stage: 34.5% of 110 nests) was significantly higher than that of photographed ones (incubation: 2.4% of 83 nests; nestling: 11.1% of 81 nests). Among different nest types, open cup nests in shrub and trees were most affected by photography, in both incubation and nestling stages. Of five factors investigated, including three natural factors (nest stage, structure and position), and two anthropogenic factors (photography and distance to forest edge), only photographic disturbance and nest structure had significant effects (open nests had higher predation). The feeding frequency at nests when photographers were present was not strongly different from when they were not present. Human activity therefore had no negative effects on the birds, but showed a positive effect on their nesting success, in terms of reducing nest predation rates. However, there needs to be further assessment of other aspects of nesting (e.g., clutch size, duration of nestlings in nests), and other kinds of stress responses (e.g., hormonal changes), before the total effect of bird photography can be understood.
Birding tourism has become a popular and growing subsector of ecotourism. Birding tourism is a way in which biodiversity can produce a considerable source of income to local communities (Sekercioglu, 2002; Basnet et al., 2021). Birders are an important group of stakeholders for conservation, as they are willing to pay more to see particular bird species at the destinations where they go in search of birds (Kolstoe and Cameron, 2017). Their tourism boosts the economic income of residents at those destinations, and they may also participate in wildlife conservation projects or competitions that contribute towards conservation (Sekercioglu, 2002; Shipley et al., 2019). But birding tourism has made wild birds' exposure to humans more widespread and frequent (Goodfellow, 2017), which may have negative effects on bird behavior and habitat selection (Sekercioglu, 2002). Further, recently there has become a new extension to birding tourism, in which the traditional way of watching birds with binoculars or spotting scopes has been replaced by the use of photographic equipment (Mancuso and Battiato, 2001; Wee and Tsang, 2008). Bird photographers are especially interested in taking pictures of bird nests, because they know the birds will return there and it also provides interesting insight into birds' lives (i.e. reproduction, growth and development). Yet because nesting is a very delicate life stage for birds, bird photography may also bring negative fitness outcomes to birds (Veríssimo et al., 2013; Slater et al., 2019).
One way in which photography could be disturbing to birds is if the birds consider the photographers as potential predators. Birds under natural conditions will flush when humans approach (Blumstein et al., 2005). Further, some characteristics of photography, such as following birds repeatedly, crouching, or using artificial lighting (i.e. flashes), have been shown to stimulate birds to escape more than a walking person (Slater et al., 2019). If birds feel threatened this could affect their nest success. For example, increasing birds' perception of predation risk has been shown to reduce adult feeding trips to nests and ultimately nestling survival (Zanette et al., 2011). Therefore, it is necessary to study whether bird photography changes aspects of nesting behavior such as feeding rates.
Another way in which photography could change nest success is indirectly through affecting nest predation. Nest predation risk is one of the main determinants of avian reproductive success (Ricklefs, 1969; Martin, 1993; Caro, 2005). Disturbance from humans was originally thought to have a negative impact by allowing nest predators to find nests (e.g., Götmark, 1992). However, meta-analyses on the effects of human activity near nests have found that human observers checking nests (Ibáñez-Álamo et al., 2012), or the installation of camera traps (Richardson et al., 2009) can actually decrease nest predation, presumably by scaring away predators. At the same time, there is a great degree of variance in nest predation among studies and species, due to other factors, both natural, such as predator type (Cox et al., 2012; Degregorio et al., 2016), and the habitat and vegetation around nests (Martin, 1993; Seibold et al., 2013), as well as anthropogenic effects such as fragmentation and urbanization (Stephens et al., 2004; Vetter et al., 2013; Vincze et al., 2017). Such variation requires further studies of the effects of ecotourists on nests, especially in the tropics (Sekercioglu, 2002).
Therefore, this study explored whether bird photography would affect the reproduction of birds by comparing nests affected by photography with those that were not, as well as comparing behavior at photographed nests when photographers were present and when they were absent. We worked at a site in southern China where the increase in bird photography has been rapid over the past decade, as it has elsewhere in China (Gao et al., 2009; Feng et al., 2018; Basnet et al., 2021). Our main objectives were to understand how: 1) photography, and other ecological factors, both natural and anthropogenic, influenced nest predation, and 2) whether photographer presence affected the frequency of feeding at photographed nests. We aim to provide data useful for the development of the bird tourism industry, as well as the species management of birds in this region.
This study was conducted in and nearby Nonggang National Nature Reserve (NNNR) (22°28′N, 106°57′E, 100–650 m a.s.l.), southwestern Guangxi Zhuang Autonomous Region, southern China. Nonggang has a tropical monsoon climate, with a clear distinction between the dry and wet season. Based on the data of Longzhou Meteorological Station from 1980 to 2010, annual rainfall averages 1260.2 ± 246.2 (standard deviation, SD) mm. The annual average temperature is 22.4 ± 0.5 ℃ (Jiang et al., 2017a). Inside the reserve, the forest is relatively mildly disturbed and forest coverage is over 90%. Sugarcane (Saccharum officinarum) and maize (Zea mays) are mainly cultivated in the farmland surrounding the protected area. NNNR is rich in bird resources, including seven globally threatened birds (Jiang et al., 2014), and it was the location of the discovery of the Nonggang Babbler (Stachyris nonggangensis; Zhou and Jiang, 2008). A high rate of nest predation is an important life history characteristic of most birds in this area, with a variety of nest predators being recorded including birds, mammals, and reptiles (Jiang et al., 2013, 2015; 2017b; Liu et al., in review).
The area has become a hot spot for birdwatching tourism, and attracts an increasing number of bird photographers. These photographers observe nests in groups, waiting at the nest for several hours to get good opportunities for photographs. Nests that are photographed are visited by between 3 and 10 people during the day. Local guides have set up blinds 5–10 m from the photographed nests made of dark-colored material that the observers stay within, with holes for the camera lenses. Photography occurs everyday and throughout the day (6:00–18:00 h), unless there is strong wind or rain.
Nests were found by local villagers through systematic searching, and observing the behavior and movements of parent birds to find nests. We monitored each nest every 2–3 days until the chicks left, or the nests were predated or abandoned by the parents. For each nest, we recorded various kinds of data. General information about the nest included: 1) bird species, 2) photographic disturbance (photographed or not; a nest was considered photographed if it was so at any time during the whole nest cycle, including both the incubation and the nestling stages), 3) location using a GPS. Temporal data included: 4) the date the nest was found, 5) the date the first egg was laid, 6) the date the first nestling hatched, 7) the date the first nestling left the nest. Information about the eggs/nestlings and their fate included: 8) clutch size, 9) brood size, 10) the number of individuals leaving the nest, and the fate of the nest, rated as 0 if predated and 1 if at least one nestling fledged. Information about the nests: included 11) nest type (a burrow in the ground or rock; a cavity in a tree; an open cup nest on the ground; an open cup nest in a shrub or tree; and a domed nest, in which the bird makes its own form of protection over the top of the nest), 12) height of the nest from the ground, 13) distance from the forest edge (ranging from 0 to 1500 m). Given our definition of predation (predation was only counted if all hatchlings or all nestlings were destroyed), if we found nests during the nestling stage, we automatically considered them as successful for incubation.
We observed the nests in ways to minimize our own disturbance. We observed nests with binoculars (BOSMA 8×42) at the furthest distance from which we could see the interior of the nest. For high nests, we used a cellphone camera mounted on a stick to observe its status. When taking data, we waited for the parents to leave the nest on a foraging trip, and wore gloves.
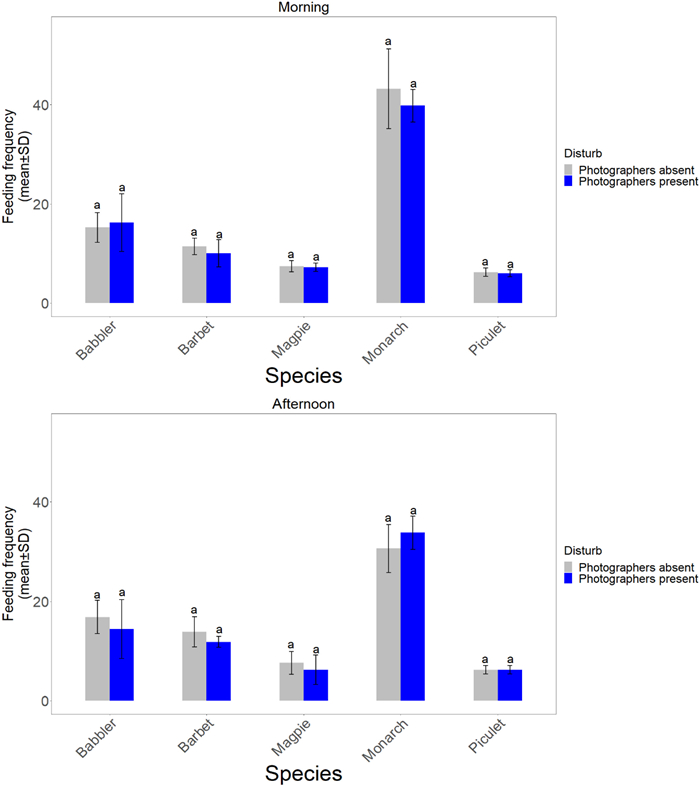
We observed and documented the feeding of five species in photographed nests, including three open-cup nesting species, Black-naped Monarch (Hypothymis azurea), Yellow-eyed Babbler (Chrysomma sinense) and Indochinese Green Magpie (Cissa hypoleuca), and two cavity nesting species, Great Barbet (Psilopogon virens) and White-browed Piculet (Sasia ochracea). These five species were chosen because they are the main subjects photographed by photographers. We recorded the feeding frequency of parent birds at the same nests under two different conditions: photographers present (throughout the observation) and photographers absent. We recorded the number of times parent birds returned with food during a 2-h period, between 8:00 to 10:00 h and between 16:00 and 18:00 h. These two periods of time were chosen as they were the peak periods of bird activity, and also favorite times for photography. For each species, we conducted five observations in each of four conditions (with and without photography, in morning and afternoon). Because the number of actively photographed nests was different for the five species, we collected this data at unequal numbers of nests: three nests for monarchs, three nests for babblers, two nests for magpies, one nest for barbets, and three nests for piculets. Each nest was observed in a balanced manner (visited equally when photographers were present and when they were absent, visited equally in morning and afternoon).
All statistical analyses were conducted in R version 3.6.0 (R Core Team, Geneva, Switzerland), and we reported mean values ± standard deviations. The nest predation rate was defined as the percentage of nests predated (Jiang et al., 2017b), i.e. predation rate = (the number of predated nests/total number of nests) × 100%. We used Fisher Exact Tests to compare the frequency of predation in the two conditions (photographed nests and not photographed nests) for all nests, and then separately for the five types of nests.
To understand the factors influencing nest predation, we constructed a generalized linear mixed model with the R package 'lme4' (Bates et al., 2015), using a binomial distribution to analyze the effects of different factors on bird breeding fate (0 = predated or 1 = successful). As fixed independent variables, we used environmental factors, including nest stage (incubation, nestling), nest position (ground, shrub, tree), nest structure (either enclosed — including burrows, cavities, and domes — or open), and anthropogenic factors (distance to the edge of the forest and photography status). Village and bird species were used as random variables. We checked for spatial autocorrelation with a Moran's I Test and found none, and the model was not overdispersed. However, no variance was explained by the random variables and we therefore simplified to a generalized linear model (GLM).
We then used model averaging to understand the relative importance of the different factors in the full model. We used the package "MuMIn" (Bartón, 2013) to find all models with delta corrected Akaike Information Criterion (ΔAICc) < 4 from the best model, and found the average coefficients using the 'zero' method of calculation. We consider variables that have 95% confidence intervals that do not cross zero to be influential, and we also report the "importance factor", which shows how consistently a variable is included in models with ΔAICc < 4.
For the feeding rate analysis, the data was balanced (between photographers present and photographers not present, between morning and evening), even though the total number of nests observed differed between the species. We therefore used independent sample t-tests for each species to determine whether the number of feeding trips within a 2-h period depended on whether photographers were present. However, we note that because some of the nests were observed more than others, the data breaks assumptions of independence and results therefore must be considered preliminary.
A total of 277 nests of 42 bird species, including 83 photographed nests and 194 nests that were not photographed, were observed in this study (see Appendix Table S1). Among these nests, 48.0% (133/277) were predated (Table 1). In the incubation stage, the predation rate for photographed nests (2/83) was less than that for not photographed ones (84/194; Fisher Exact Test, P < 0.001). In the nestling stage, the predation rate for photographed nests (9/81) was again less than for not photographed ones (38/110; Fisher Exact Test, P = 0.0039). A substantial number of species did not have nests in both treatments for the incubation stage (30 species), or for the nestling stage (29 species). However, if such species are taken out of the analysis, photographed nests still had lower predation than photographed nests for the incubation stage (P < 0.001), and this trend was marginally significant for the nestling stage (P = 0.054; Appendix Table S2).
| Disturbance by photographers | Number of species | Incubation | Nestling | |||||
| Total nests | Predated | Predation rate (%) | Total nests | Predated | Predation rate (%) | |||
| Photographed | 29 | 83 | 2 | 2.4 | 81 | 9 | 11.1 | |
| Not photographed | 25 | 194 | 84 | 43.3 | 110 | 38 | 34.5 | |
When dividing the dataset by the type of nest and nest stage, photographed nests on average had lower predation than not photographed nests, with one exception (open ground nests in the nestling stage, Table 2). Of five tests in the incubation stage, a significant difference between photographed and not photographed nests was seen for open shrub/tree nests (P < 0.001), dome nests (P = 0.021), and there was a marginally significant difference for cavity nests (P = 0.051). For the same five tests in the nestling stage, only open shrub/tree nests had a significant difference between photographed and not photographed nests (P = 0.0068).
| Disturb by photographers | Nest types | Number of species | Incubation | Nestling | |||||
| Total nests | Predated | Predation rate (%) | Total nests | Predated | Predation rate (%) | ||||
| Photographed | Burrow | 2 | 3 | 0 | 0.0 | 3 | 0 | 0.0 | |
| Cavity | 8 | 22 | 0 | 0.0 | 22 | 1 | 4.5 | ||
| Dome | 3 | 15 | 0 | 0.0 | 15 | 4 | 26.7 | ||
| Open ground | 2 | 4 | 0 | 0.0 | 4 | 1 | 25.0 | ||
| Open shrub/tree | 14 | 39 | 2 | 5.1 | 37 | 3 | 8.1 | ||
| Not photographed | Burrow | 1 | 2 | 1 | 50.0 | 1 | 0 | 0.0 | |
| Cavity | 5 | 11 | 3 | 27.3 | 8 | 2 | 25.0 | ||
| Dome | 6 | 82 | 31 | 37.8 | 51 | 16 | 31.4 | ||
| Open ground | 5 | 9 | 4 | 44.4 | 5 | 0 | 0.0 | ||
| Open shrub/tree | 8 | 90 | 45 | 50.0 | 45 | 20 | 44.4 | ||
The GLM analyses showed that among all the factors considered, disturbance (photographed vs. not photographed nest) was the strongest factor that influenced predation rates (Table 3), with photographed nests having lower predation. Nest structure also had a coefficient with a confidence interval that did not cross zero, with enclosed nests having lower predation. The other factors had little importance.
| Variable | Coefficient | 2.5% CI | 97.5% CI | Importance factor |
| Disturbance: not photographed vs. photographed | 2.39 | 1.69 | 3.09 | 1.00 |
| Nest stage: incubation vs. nestling | 0.03 | −0.37 | 0.61 | 0.21 |
| Nest structure: enclosed vs. open | −0.43 | −1.02 | −0.01 | 0.83 |
| Distance to edge | −0.01 | −0.53 | 0.43 | 0.20 |
| Nest position: ground vs. shrub | −0.07 | −1.63 | 0.71 | 0.15 |
| Nest position: ground vs. tree | −0.09 | −1.68 | 0.49 | (Same as above) |
None of the t-tests were significant, suggesting that the feeding rates in photographed nests did not differ depending on whether the photographers were present or not (Fig. 1).
The presence of humans close to animals tends to have a direct negative impact on wildlife (Blumstein et al., 2005). However, sometimes humans have a negative impact on predators, which leads to an indirect positive impact on their prey. For example, when human activity substantially decreased mongoose populations, nest predation on turtle eggs was reduced (Leighton et al., 2010), in which the author termed a "scarecrow effect". Similarly, the presence of industrial machinery and noise pollution lowered the abundance of an avian nest predator, changing nest predation patterns (Francis et al., 2009). Our results are in line with these kinds of indirect effects being more important than direct effects: feeding rates at nests were not strongly affected by the presence of photographers, but photographed nests had dramatically less nest predation. Below, we discuss the generally high rate of predation at this study site, the mechanism by which photography may affect nest success, and the feeding rate data, after first discussing some limitations of the study.
A major limitation of this study was that we do not have data on how much nests were visited by humans. Some colorful or rare species are particular targets for photographers, and their nest visitation, in terms of the numbers of days per week, and the number of hours per day, would presumably be higher than for more plain and common species. Unfortunately, because we were focusing on collecting the nest predation data, we were not able to collect this kind of detailed information, and so divided nests simply into those that were photographed and those that were not. We hope future studies can look at the effects of human visitation rate more carefully. Another limitation is that our feeding rate data was constrained by the number of active nests we could find during the nestling stage, and some individual nests were observed repeatedly. Thus, we view the feeding rate data as preliminary, although we believe that if there had been strong differences in feeding rate between photographed and not photographed nests, we would have detected that.
The high rates of nest predation in this study are typical of our study site in subtropical China. For example, rates of nest predation were previously measured as 75% for the Nonggang Babbler (Stachyris nonggangensis), a ground nesting species (Jiang et al., 2013), and 64% for Red-whiskered Bulbul (Pycnonotus jocosus), which makes open cup nests in trees (Jiang et al., 2015). Experiments on artificial nests in limestone outcrops showed nest predation at about 66%, with nest predation increasing later in the spring, caused most probably by greater activity by snakes with increasing temperatures (Jiang et al., 2017c). This current study was conducted during May–July and thus could also be the result of high snake activity. Cameratrap data from an artificial nest study in Nonggang shows that a wide variety of predators are present, including birds such as magpies and coucals (39 separate detected attacks on 160 artificial nests), small mammals such as mice and squirrels (18 attacks), as well as snakes (7 attacks, Liu et al., in review). In this forest, nest predation has even been observed by giant millipedes (Jiang et al., 2013). Our result that open cup nests were more predated than enclosed ones is consistent with other studies that have shown these kinds of nests are particularly vulnerable (e.g., Martin and Li, 1992).
In this high predation environment, the effect of photography was clear: the predation rate of photographed nests was much lower than that of not photographed nests, a result found for both nesting stages and for a variety of nest types in the incubation stage. It was surprising to us that the difference between photographed and not photographed nests tended to be greater during the incubation stage than during the nestling stage, because photographers during incubation stage have more limited opportunities to work, given that the adults are more stationary and may not be visible for enclosed nests. We view the reduction in predation for photographed nests as evidence that the activity of the villagers, who found the nests and prepared them for photographers visiting (i.e., construction of the hide), was a major factor affecting nest predation.
Our results show that bird photography does not always have a negative impact on bird reproduction, but rather can decrease nest predation rates, as part of a scarecrow effect. The result concurs with meta-analyses of human observer effects on nests (Ibáñez-Álamo et al., 2012), and of the installation of survellience cameras at nests (Richardson et al., 2009): in both these studies there were indications that nest predation could decrease with greater human activity. The cameratrap study that was occurring in Nonggang simultaneously with this one found that playback of traffic noise at nests strongly reduced predation by small mammals (Liu et al., in review). Mammals may be particularly sensitive to human presence or to equipment that they install (Richardson et al., 2009). For example, humans decreased the activity of mammalian nest predators in the studies of Miller and Hobbs (2000) and Ibáñez-Álamo and Soler (2010). It is also possible that birds could choose to select nest sites in areas in which photographers are active, similar to how some bird species nest near aggressive birds or other predators for protection (e.g., Quinn and Ueta, 2008).
We found that the presence of bird photographers did not strongly reduce the feeding frequency at the nests of the five species observed, although this part of our study should be extended in the future with greater sampling. Recent studies have shown that feeding trips to nests attract predator attention (Martin et al., 2000), and birds at higher risk, or perceived risk, of nest predation lower their feeding trips to the nest, which can lead to reduced nestling feeding (Eggers et al., 2004; Zanette et al., 2011). However, our results did not show any indication that the birds altered their behavior based on the presence of the photographers. Perhaps this was because the villagers' construction of blinds was successful in reducing the birds' detections of the photographers, or perhaps this was because the birds have habituated to these conditions. We compared photographed nests at times when photographers were present and not; future studies should also investigate feeding at nests that are not photographed. We believe it is likely that the amount of food given per trip (which is difficult to assess with binoculars for some species), and the total food intake of the nestlings, also did not depend on whether photographers were present, since the timing of foraging trips was similar, and hence the effort per trip was similar. However, this could also be checked in future studies; in this study, we did not want to give further disturbance to the nestlings by weighing them.
Although our study shows generally positive effects of photography on the aspects of bird fitness that we studied, we need to be cautious, for it is possible that other aspects of nesting behavior could be affected. Birds respond to changes in nest predation by adjusting clutch size (Slagsvol, 1982; Chalfoun and Martin, 2010), and the duration the nestlings stay in the nest (Hua et al., 2014), as well as feeding frequency. Therefore, it would be useful to look for impacts of photography on clutch size or the duration of nesting. Bird response to stress can include hormonal changes or decreases in immunity (Ellenberg et al., 2007) that should be explored. Knowing what we know presently, however, we can make one conclusion: although it seems that most bird photographers feel that their effects on the birds they photograph are quite trivial (Slater et al., 2019), here we show this perception can be misleading in some situations.
XT, AJ and EG developed the idea and wrote the first draft. SL helped design and conduct this project. All authors read and approved the final manuscript.
This research was approved by the Nonggang National Nature Reserve, the Guangxi University Animal Ethics Committee (GXU2018-043) and followed the laws of the country in which it was conducted (People's Republic of China). Precautions were taken to minimize the disturbance of the research on the bird nests (short observation times every 2–3 days when adults were away, all measurements made while wearing gloves).
Data on the nests' characteristics and fates are included in the Supplementary data section, as Appendix Table S1.
The authors declare that they have no competing interests.
We thank the field assistants from Nonggang for help in finding nests and the department of the Nonggang National Nature Reserve for support and approval of the research. Thanks to Jianli Bi, Qihong Li, and Liuyan Lu for help in the field, and to anonymous reviewers for insightful comments that improved the manuscript. This work was supported by the National Natural Science Foundation of China (Grant No. 31870370) and the Graduate Education Innovation Program of Guangxi (No. YCSW2020023).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100036.
|
Bates, D., Maechler, M., Bolker, B., Walker, S., 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48
|
|
Caro, T., 2005. Antipredator Defenses in Birds and Mammals. University of Chicago Press, Chicago, Illinois
|
|
Eggers, S., Griesser, M., Ekman, J., 2004. Predator-induced plasticity in nest visitation rates in the Siberian jay (Perisoreus infaustus). Behav. Ecol. 16, 309-315
|
|
Goodfellow, D.K., 2017. Couples and Avitourism: A mixed methods study of north american birdwatchers. Ph.D thesis. Southern Cross University, Lismore, NSW
|
|
Gotmark, F., 1992. The effects of investigator disturbance on nesting birds. In: Power, D.M. (Eds.), Current Ornithology, vol 9. Springer, Boston, MA
|
|
Jiang, A.W., Zhou, F., Liu, N.F., 2014. Significant recent ornithological records from the limestone area of south-west Guangxi, south China, 2004-2012. Forktail. 30, 122-129
|
|
Jiang, D.M., Nong, Z.Q., Jiang, A.W., Luo, X., 2015. Breeding ecology and nest site selection of Red-whiskered Bulbul (Pycnonotus jocosus) in limestone area, northern tropical region of China. Chinese J. Zool. 50, 359-365. (in Chinese)
|
|
Mancuso, M., Battiato, S., 2001. An introduction to the digital still camera technology. J. Syst. Res. 2, 1-8
|
|
Verissimo, D., Kanagavel, A., Seshadri, K.S., Raghavan, R., 2013. The tragedy of the nature photography commons. Asian J. Conserv. Biol. 2, 1-2
|
|
Wee, Y.C., Tsang, K.C., 2008. The changing face of birding in Singapore. Nat. Singapore. 1, 97-102
|
| Disturbance by photographers | Number of species | Incubation | Nestling | |||||
| Total nests | Predated | Predation rate (%) | Total nests | Predated | Predation rate (%) | |||
| Photographed | 29 | 83 | 2 | 2.4 | 81 | 9 | 11.1 | |
| Not photographed | 25 | 194 | 84 | 43.3 | 110 | 38 | 34.5 | |
| Disturb by photographers | Nest types | Number of species | Incubation | Nestling | |||||
| Total nests | Predated | Predation rate (%) | Total nests | Predated | Predation rate (%) | ||||
| Photographed | Burrow | 2 | 3 | 0 | 0.0 | 3 | 0 | 0.0 | |
| Cavity | 8 | 22 | 0 | 0.0 | 22 | 1 | 4.5 | ||
| Dome | 3 | 15 | 0 | 0.0 | 15 | 4 | 26.7 | ||
| Open ground | 2 | 4 | 0 | 0.0 | 4 | 1 | 25.0 | ||
| Open shrub/tree | 14 | 39 | 2 | 5.1 | 37 | 3 | 8.1 | ||
| Not photographed | Burrow | 1 | 2 | 1 | 50.0 | 1 | 0 | 0.0 | |
| Cavity | 5 | 11 | 3 | 27.3 | 8 | 2 | 25.0 | ||
| Dome | 6 | 82 | 31 | 37.8 | 51 | 16 | 31.4 | ||
| Open ground | 5 | 9 | 4 | 44.4 | 5 | 0 | 0.0 | ||
| Open shrub/tree | 8 | 90 | 45 | 50.0 | 45 | 20 | 44.4 | ||
| Variable | Coefficient | 2.5% CI | 97.5% CI | Importance factor |
| Disturbance: not photographed vs. photographed | 2.39 | 1.69 | 3.09 | 1.00 |
| Nest stage: incubation vs. nestling | 0.03 | −0.37 | 0.61 | 0.21 |
| Nest structure: enclosed vs. open | −0.43 | −1.02 | −0.01 | 0.83 |
| Distance to edge | −0.01 | −0.53 | 0.43 | 0.20 |
| Nest position: ground vs. shrub | −0.07 | −1.63 | 0.71 | 0.15 |
| Nest position: ground vs. tree | −0.09 | −1.68 | 0.49 | (Same as above) |