| Citation: | Jonas Rafael Rodrigues Rosoni, Carla Suertegaray Fontana, Caio José Carlos. 2023: Timing of breeding as a determinant of nest success of the vulnerable Chestnut Seedeater (Sporophila cinnamomea) in grasslands of southern South America. Avian Research, 14(1): 100082. DOI: 10.1016/j.avrs.2023.100082 |
The breeding traits of Sporophila seedeaters have been relatively well studied in recent years; nevertheless, a group of ten species in the genus, known as southern capuchinos, remain understudied. That is the case with Chestnut Seedeater (Sporophila cinnamomea), a species vulnerable to extinction, which breeds in the grasslands of southeast South America and, after reproduction, migrates towards the Cerrado region in central Brazil. Here, we investigated breeding ecology and calculated average clutch size, productivity, the sex ratio of nestlings, and estimated nest success. Then we tested (1) whether there is a relationship between the number of active nests and environmental variables, (2) whether the nestling sex ratio deviates from the 1:1 ratio, (3) whether clutch size varies between breeding seasons, and (4) whether the nest success is related to starting date, nest age, plant support, nest height from the ground, and clutch size. During two breeding seasons (October–March 2018–2020), we monitored 98 nests. We generated survival models with five interacting covariates to assess the survival of the nests. We recorded the entire breeding period for Chestnut Seedeater, which was estimated to be 4.6 months, similar to other migratory seedeaters. Clutch size did not differ between breeding seasons. The sex ratio of nestlings was not significantly different from the 1:1 ratio. Nest success was 31%, and predation was the leading cause of unsuccessful nests (83%). The daily survival rate was 0.95 ± 0.01. The main predictor of nest survival was the covariate starting date. These findings, added to other aspects of the species' natural history described here, may help illuminate the ecology and behavior of Chestnut Seedeater and other southern endangered capuchinos, and grassland-dependent species of South America.
Reproduction is perhaps the most critical part of a species' life cycle due to its high energetic investment and direct link to individual fitness (Stubbs, 1977; Dobson, 2012). In birds, for example, life-history traits such as the timing of breeding, clutch size, parental care, annual number of broods, and fertility are mutually related and have consequences on nest success (Böhning-Gaese et al., 2000; Martin et al., 2000b; Dunn, 2004; Duca and Marini, 2014; Boyce et al., 2015).
Factors such as nest type and location, latitudinal gradient, and migratory behavior can influence the clutch size, a widely studied trait (Lack, 1948; Slagsvold, 1982; Böhning-Gaese et al., 2000). Variations in clutch sizes can occur between and within species and tend to increase at high latitudes compared with the tropics (Farner, 1972). However, identifying patterns related to breeding season and nest success could still be better understood for Neotropical birds (Lany et al., 2016). Furthermore, variations in the breeding season may be associated with environmental changes (e.g., precipitation and temperature), which could result in changes in the egg laying timing (Lany et al., 2016). Understanding such patterns can help clarify issues about ecological and behavioral interactions during reproduction (Stutchbury and Morton, 2001; Dunn and Møller, 2014), in addition to answering how reproductive strategies evolve (Covas, 2012) and what is the importance of breeding phenology in birds (Dunn and Møller, 2014).
Approximately two-thirds of the world's bird diversity occur in the tropics (Dias et al., 2009). Tropical and some subtropical (non-temperate) birds tend to have longer breeding seasons, smaller clutch sizes, and lower nest success than temperate birds (Ricklefs and Bloom, 1977; Stutchbury and Morton, 2001; Chiarani and Fontana, 2015; Pretelli et al., 2015). Although the breeding biology of subtropical nesting birds has been generally well known (e.g., Di Giacomo et al., 2011; Browne et al., 2021), there are still essential gaps that require further investigation and testing, mainly with detailed data that can be used to increase knowledge about life history theory (Fierro-Calderón et al., 2021). For example, evaluation of how environmental factors, such as temperature variation and day length, can influence the variation in clutch sizes (Lack, 1948; Slagsvold, 1982; Hochachka, 1990), survival of young, and, consequently, overall nest success (Mayfield, 1975; Dinsmore and Dinsmore, 2007), will help to understand how these factors act directly on population dynamics and recruitment of juveniles (Fletcher et al., 2006).
The genus Sporophila currently includes 42 species of small, granivorous passerines that mainly inhabit non-forested areas in the Neotropics (Ridgely and Tudor, 1989; Di Giacomo and Kopuchian, 2016; Gill et al., 2022). Within this genus, a group of ten species with low genetic differentiation (Estalles et al., 2022), known as "southern capuchinos", are smaller than their congeners and occur in sympatry at breeding sites (Silva, 1999; Campagna et al., 2015) and across a large part of their range in the grasslands of southeastern South America (SESA, sensu Azpiroz et al., 2012b). The SESA grasslands are the most extensive and threatened grassland ecosystem in the Neotropics (Azpiroz et al., 2012b), being also the habitat for many birds reliant on native grassland to feed and nest (Fontana and Bencke, 2015).
Overall, the breeding traits of 14 Sporophila seedeaters have been well-documented in South America (Repenning and Fontana, 2019; Rosoni et al., 2020). However, only four of the ten species of southern capuchinos have been systematically researched. In tropical latitudes in the Cerrado biome (22° S), nests, eggs, and nestlings have been described for Pearly-bellied Seedeater (S. pileata) (Freitas et al., 2018). In the subtropical upland grasslands (28° S) of southern Brazil, studies provided information on nest success, parental care, reproductive phenology, clutch size, nests, eggs, and nestlings of Tawny-bellied Seedeater (S. hypoxantha) and Black-bellied Seedeater (S. melanogaster) (Rovedder, 2011; Franz and Fontana, 2013, 2021). While in the pampas grasslands of Argentina (28° S), research for Iberá Seedeater (S. iberaensis) provided information on nest success, nest description, clutch size, incubation period, and nestling development (Turbek et al., 2019; Browne et al., 2021). These species showed similar breeding traits; nevertheless, differences were also noticed. For example, the breeding season length varied from 83 to 132 days, with peaks ranging from November to January (Rovedder, 2011; Franz and Fontana, 2013; Freitas et al., 2018; Turbek et al., 2019). Although these efforts have considerably improved our knowledge of the natural history of southern capuchinos, the breeding biology of some species, especially the threatened ones, remains comparatively understudied. That is the case with Chestnut Seedeater (Sporophila cinnamomea).
In this study, we first report on the Chestnut Seedeater breeding phenology, clutch size, and brood parasitism. Next, we provide estimates of hatchability, fecundity, egg success, nestling survival, annual nestling production, nestling sex ratio, and nest survival and success. Then, we tested four interrelated predictions in two groups: the first group related to breeding biology and the second to nest survival.
We predicted that the number of active nests would be positively associated with precipitation, temperature, and photoperiod. These relationships were found in the congeners Pearly-bellied Seedeater, Black-bellied Seedeater, and Rusty-collared Seedeater (S. collaris) (Rovedder, 2011; Freitas, 2014; Rosoni et al., 2019). However, in the case of the Chestnut Seedeater, we expected a positive association between the number of active nests and photoperiod and precipitation since the temperature was less influential in other studies at the same latitude (Rosoni et al., 2019). Next, we predicted that the nestling sex ratio will not differ from 1:1. The 1:1 pattern is directly related to the reproductive strategy of the genus, as has been studied in Black-bellied Seedeater, Tawny-bellied Seedeater, and Rusty-collared Seedeater (Rovedder, 2011; Franz and Fontana, 2013; Rosoni et al., 2019). Deviations from 1:1 in the nestling sex ratio could negatively affect the species' reproductive productivity by reducing breeding pairs, thus leading to a more accentuated population decline. Finally, we tested whether the average clutch size would vary between breeding seasons. For example, in Tropeiro Seedeater (S. beltoni), the clutch size did not differ between breeding seasons (Repenning and Fontana, 2016).
We also evaluated the influence of two main covariates on nest survival: we tested the prediction that the nest survival time model follows a linear trend. Nest survival models with linear trends have been found for Black-bellied Seedeater, Iberá Seedeater, and Tawny-bellied Seedeater (Rovedder, 2011; Browne et al., 2021; Franz and Fontana, 2021). Furthermore, linear models often show negative trends in daily survival rate (DSR) in altricial species (Repenning, 2012). Therefore, this analysis can also identify what covariates influence nest success. In this way, this approach would help in the elaboration of mitigation measures for the conservation of Chestnut Seedeater. Lastly, the nest predation rate is also determined by predator accessibility, and nest height is a good indicator for assessing the accessibility for different taxa of nest predators (Colombelli-Négrel and Kleindorfer, 2009). Thus, we tested the "predation avoidance hypothesis" of nest site selection. This hypothesis predicts that birds should adjust their nest-site preferences, i.e., nest height, according to variation in predation, although the effect of the nest height on the nest success was not found for Iberá Seedeater, Black-bellied Seedeater, and Tawny-bellied Seedeater, which breed in the same latitudes (Rovedder, 2011; Guan et al. 2018; Browne et al., 2021; Franz and Fontana, 2021). Additionally, knowing the average height of successful nests will help in vegetation management strategies of the grasslands of southern South America, where many obligatory grassland species breed.
Chestnut Seedeater breeds in the grasslands of northeast Argentina, western and southeast Uruguay, southeast Paraguay, and south Brazil during austral spring and summer (Rosoni et al., 2020; BirdLife International, 2022). After breeding, it migrates towards the north, spending the non-reproductive period in the grasslands of the Cerrado biome (Silva, 1999; Rosoni, 2022). The species is globally Vulnerable owing to a rapid population reduction caused by habitat fragmentation and loss and trapping for the bird trade (BirdLife International, 2022). Regionally, it is listed as Endangered in Argentina and Paraguay (MAyDS y AA Ministerio de Ambiente y Desarrollo Sustentable de la Nación y Aves Argentinas, 2015; MADS Ministerio del Ambiente y Desarrollo Sostenible, 2019), Vulnerable in Uruguay (Azpiroz et al., 2012a), and Near Threatened in Brazil (ICMBio Instituto Chico Mendes de Conservação da Biodiversidade, 2018). Currently, the available information on the species is restricted to its nest architecture and support plants, egg shape and color, incubation period, and nestling development (Maurício et al., 2013; Rosoni et al., 2020).
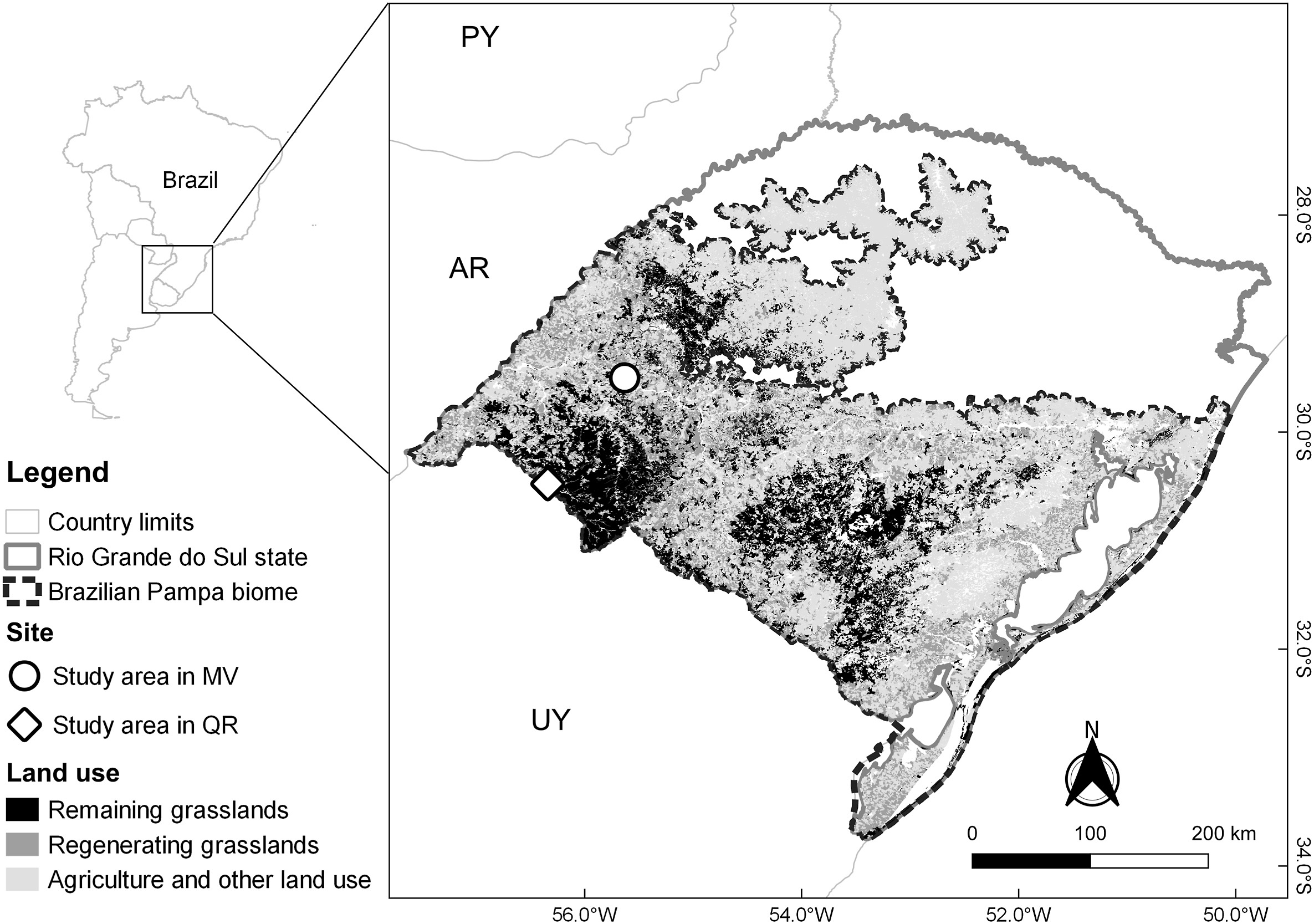
We searched for Chestnut Seedeaters in the Brazilian Pampa grasslands (Fig. 1), located in cooperative properties of the Santa Maria do Ibicuí Settlement in Manoel Viana (MV) (29.497736° S, 55.638431° W; 647 ha) and on private farms in Quaraí (QR), a municipality bordering Uruguay (30.485264° S, 56.340922° W; 679 ha). Both areas are grassland mosaics with dry soils and wetlands (Fig. 1). Vegetation is characterized by Poaceae, Apiaceae, and Onagraceae species (Rosoni et al., 2020). The climate in the region is classified as Humid Mesothermal (Cfa in the Köppen's classification) and characterized by hot summers with an average temperature above 22 ℃ and rain distributed throughout the year, with an average annual rainfall of 1600–1900 mm (Alvares et al., 2013).
We conducted fieldwork from October to March 2018–2020, during two breeding seasons with a daily sampling effort of 8 h (n = 2240 h). First, we identified breeding territories by the presence of males singing and showing territorial behavior. We searched for nests by systematically walking in the previously identified territories and observing the behavior of adults carrying nest materials or food for the nestlings (Winter et al., 2003).
We estimated the start of the breeding season from the date of the first nest found in the construction phase and established the end of the breeding season based on the date when the last nestling fledged (Larre et al., 2022). We checked nests every 3–4 days or daily when nests were close to starting or ending laying, hatching, or fledging. We determined clutch sizes when the number of eggs remained constant for at least 4–5 days after the initial encounter (Marini et al., 2014). We considered a nest successful when at least one young fledged. We defined a nest as predated when all the eggs or nestlings disappeared between two consecutive checks or when it was destroyed or wholly uprooted from the support plants (Di Giacomo et al., 2011) and as abandoned when there were no signs of activity and eggs failed to hatch after more than 12 days (Marini et al., 2014).
We captured and banded adults using mist nets and 7–8 days old nestlings from their nests by hand. The specimens were individualized with numbered metal and colored plastic rings. In addition, we collected blood samples of nestlings for sexing (Owen, 2011). Sex was determined by PCR through gene amplification of specific segments of the Z/W sex chromosomes (Dubiec and Zagalska-Neubauer, 2006).
We collected meteorológical data from stations located 15 km from the study site in QR (Quaraí–A831) and 22 km from the study site in MV (Alegrete–A826) (INMET Instituto Naciónal de Meteorologia, 2020). In addition, we took photoperiod data (length of the day in minutes) from Kohl-Moreira (2020).
We calculated the hatchability only for nests with eggs that survived from laying to hatching (Mayfield, 1975). The fecundity of females was the ratio of the total number of nestlings of all broods to the number of females breeding in one breeding season (Aguilar et al., 1999). We considered the number of breeding females equal to the number of nests found (Marini et al., 2009). The egg success was the ratio of hatchlings that developed and left the nest to the number of eggs from the encountered broods (Skutch, 1966).
We estimated the nestling survival as the ratio between the number of fledglings and the total number of hatches, considering only nests found during the construction and/or laying phase (Di Giacomo et al., 2011). The annual nestling production was the ratio of fledglings to the total number of broods (Ricklefs and Bloom, 1977).
We estimated nest success using three different methods: apparent nest success was the ratio of successful nests to the total number of nests monitored, resulting in a simple percentage (Marini et al., 2010). Second, we calculated DSR (±SD) based on the Mayfield protocol (Mayfield, 1975) for each nest period (egg: incubation period until hatching = 12 days; nestling: hatchling until fledgling = 10 days). Then, we modeled nest survival in the program MARK (DSR ± SE) (White and Burnham, 1999; Dinsmore and Dinsmore, 2007). Third, we calculated the cumulative probability based on the DSR generated in program MARK and the length of the nesting cycle through the calculation of DSRt (t = length of the nest cycle = 22 days, see "Results" section) (Chiarani and Fontana, 2015). Finally, we carried out nest success estimates with equal sample sizes between the methods, using the selected nests for the program MARK as a filter. We chose to use these three methods to facilitate a comparison between results from studies already published with congeners or even other grassland species when only one of the methods was presented.
We generated models in program MARK (White and Burnham, 1999; Dinsmore and Dinsmore, 2007) for all nests that had their fate (success/failure) known and followed the five recommended assumptions for using the model (Dinsmore and Dinsmore, 2007). In addition, we continuously numbered all the dates of the nests found on the first day, 6 November, corresponding to the day we found the first nest with an egg, until 15 March, the date of the last active nest (Appendix A).
To assess the effect on nest survival, we generated constant, linear, and quadratic models using the following covariates: (1) starting date, i.e., days passed since the first active nest was found; (2) nest age, i.e., days passed since the laying of the first egg in the nest on day zero. The use of this covariate can identify the increase in the probability of predation as nest age advances due to greater parental activity, odour, and noise; (3) support plant, i.e., plant species that the bird used as a nest support plant; (4) nest height, i.e., the height of the nest considering its edge to the ground. This covariate aims to assess whether different heights account for different predation rates; and (5) clutch size, for which we included a factor with two levels: two or three eggs/nestlings. In addition, we tested all possible combinations of covariates within the models, including models with a time trend with the logit-link function and the null model of constant survival with the sin-link function (Dinsmore and Dinsmore, 2007). We conducted the analyses in three steps: (1) we ran only the models with temporal trends, then (2) we added one more unique covariate specific to the nests in each temporal model, and finally, (3) we added to the best model selected in the second phase all other covariates measured to test for the effect of the interaction among them on nest survival.
In the MARK program (Rotella, 2021), we evaluated the strength of the models based on the Akaike criterion, with adjusted values for small samples (AICc) (Burnham et al., 2011). We consider best-fit models those combining the following quantitative measures: lower ΔAICc, higher Akaike weights (wi), and fewer parameters (Burnham et al., 2011). Additionally, we evaluated the relative importance of each covariate in the final set of models through the sum of the Akaike weights (wi) in models that included the covariate (Burnham and Anderson, 2002). Finally, we assumed independence between successive renesting attempts made by the same female throughout the breeding season since, at each attempt, the bird built a new nest, and nest survival was not associated with previous attempts (χ52 = 1.90, P = 0.85) (Di Giacomo et al., 2011; Browne et al., 2021).
We performed all statistical analyses using the software R 4.0.0 (R Core Team, 2020), considering a significance level of P < 0.05 (Fowler and Cohen, 1995). We performed a multiple linear regression with package 'stats' to evaluate the relationship of independent environmental variables to the dependent variable (the total number of active nests), with data grouped by a fortnight. For the precipitation variable only, we used values referring to the fortnight that precede each record of the active focal nests. The delayed ecological effect of precipitation on bird reproduction has already been identified in some studies with birds (Cavalcanti et al., 2016). We used the Binomial test with package 'stats' to assess the sex ratio of nestlings (Fowler and Cohen, 1995). Next, we applied the Wilcoxon-Mann-Whitney U-test using the 'stats' package with independent samples (Fowler and Cohen, 1995) to evaluate whether there was a difference in clutch sizes between breeding seasons. Finally, we used the Z-test (Hensler and Nichols, 1981) to assess the differences in DSRs and survival probabilities calculated during the egg and nestling phase estimated through the Mayfield protocol. We present values as mean ± SD.
We monitored 98 nests (57 from construction, seven from laying, 27 from incubation, and seven from the time they were found with nestlings). We recorded the construction of the first nest on 30 October (mid-austral spring), seven days after the first adult individuals arrived at the breeding sites. The first nest we found was also the first we recorded with eggs on 6 November. The last active nest was dated 15 March, when the fledglings left the nest (late summer).
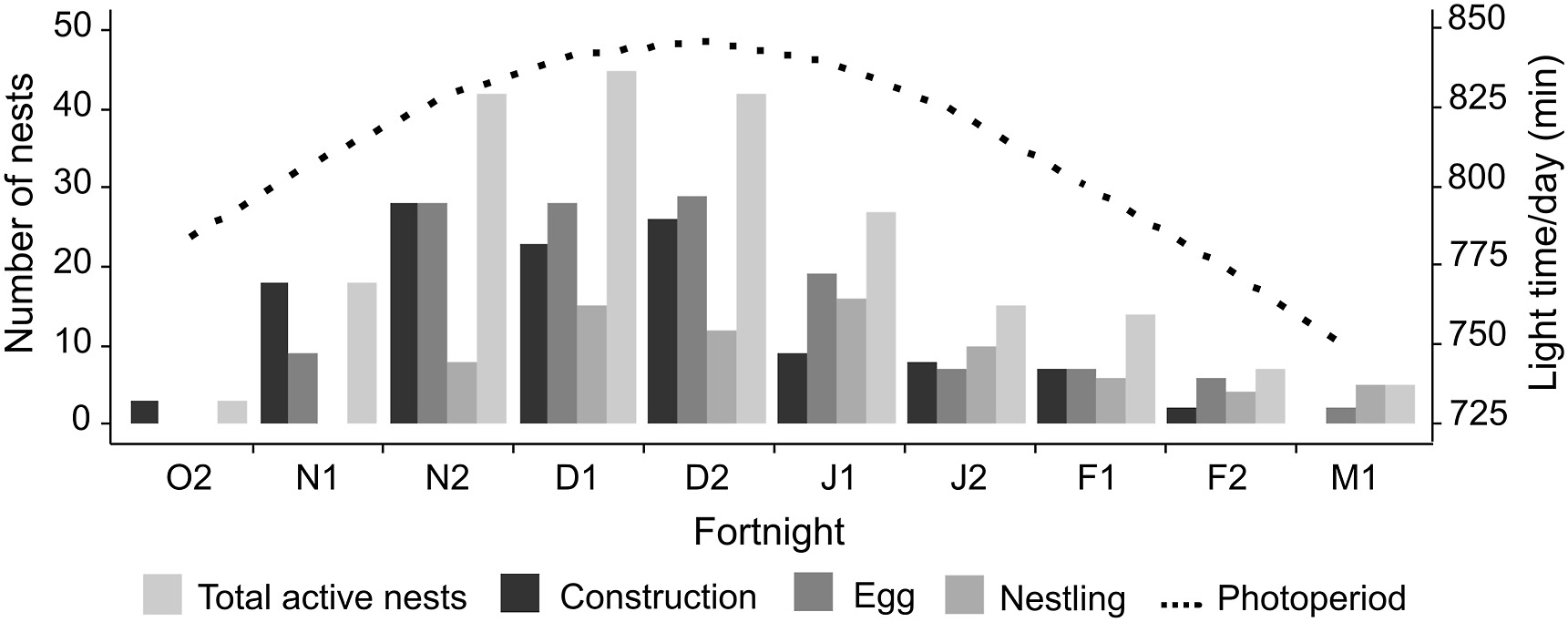
The breeding period was 139 days (October–March), or 4.6 months. The peak of reproductive activity was in the first half of December (n = 43 nests), coinciding with the peak of the longest day – an average of 14 h of sun exposure (Fig. 2). We observed the peak of nests under construction in the second half of November, with 28 nests being built. Nests with eggs or females in incubation occurred during the second half of December with 29 nests; consequently, the first half of January showed higher nesting activity (n = 16 nests) with nestlings.
The average monthly temperature during the breeding season was 23.3 ℃ (range: 7.2–37.4 ℃). The monthly average precipitation was 153.3 mm. Among the environmental variables, only the photoperiod showed a significant positive relationship with the total number of active nests (Table 1). The number of active nests increased as the days were longer (t = 4.17, P = 0.01, Fig. 2).
| Variables | Estimate | Standard error | t value | P value |
| Intercept | ‒217.30 | 52.2 | ‒4.16 | 0.01* |
| Average temperature | ‒0.01 | 4.94 | 0.00 | 1.00 |
| Maximum temperature | ‒4.00 | 4.42 | ‒0.90 | 0.41 |
| Minimum temperature | 1.96 | 2.16 | 0.90 | 0.41 |
| Precipitation | ‒0.32 | 0.32 | ‒0.99 | 0.37 |
| Photoperiod | 21.09 | 5.05 | 4.17 | 0.01* |
| Signif. codes: *P < 0.05. | ||||
About 77% of the nests contained two eggs (n = 63 nests), 22% had three eggs (n = 18 nests), and 1% had one egg. We could not define the clutch size of 16 nests; seven were discovered when they had nestlings, and nine were depredated before complete egg-laying. The mean clutch size was 2.10 ± 0.37 eggs in 2018–2019 and 2.33 ± 0.47 eggs in 2019–2020, and this difference was not significant (U = 143, P = 0.13). The hatchability was 0.88 ± 0.18 (n = 107 eggs, Table 2). The fecundity was 1.12 nestlings/female (n = 90 nests, Table 2). The egg success was 28% (n = 193 eggs, Table 2). The nestling survival was 50% (n = 49 nests), and the average annual production of nestlings was 0.96 ± 0.17 nestlings/brood (Table 2). The sex ratio of nestlings was 33 males to 27 females, leading to an equal ratio for both sexes (P = 0.51).
| Breeding parameters | Breeding season | ||
| 2018–2019 | 2019–2020 | Both seasons | |
| Nest found | 49 nests | 49 nests | 98 nests |
| Clutch size | 2.10 ± 0.37 (n = 42 nests) | 2.33 ± 0.47 (n = 40 nests) | 82 nests |
| Hatchability | 0.86 ± 0.23 (n = 25 nests, n = 52 eggs) | 0.90 ± 0.18 (n = 24 nests, n = 55 eggs) | 49 nests |
| Fecundity | 1.13 nestlings/female (n = 46 nests) | 1.11 nestlings/female (n = 44 nests) | 90 nests |
| Egg success | 37% (n = 43 nests, n = 91 eggs) | 20% (n = 44 nests, n = 102 eggs) | 87 nests |
| Nestling survival | 60% (n = 25 nests) | 41% (n = 24 nests) | 49 nests |
| Annual nestling production | 1.08 nestlings/brood (n = 25 nests) | 0.83 nestlings/brood (n = 24 nests) | 49 nests |
The apparent nest success of the nests was 31% (n = 27 nests) (Table 3). Predation was responsible for 83% (n = 49 nests) of unsuccessful nests. Birds abandoned four nests, cattle trampled three nests, Camponotus ants (Formicidae, Hymenoptera; Fig. 3D) attacked two, and one was lost due to bad weather.
| Breeding season | Number of nests | Apparent reproductive success (%) | Mayfield protocol | MARK | ||||||||
| % | Egg DSR | Z-test | P | Nestling DSR | Z-test | P | % | DSR | ||||
| 2018–2019 | 43 | 39 | 32 | 0.95 ± 0.01 | 0.87 | 0.37 | 0.95 ± 0.02 | 1.65 | 0.09 | 36 | 0.95 ± 0.01 | |
| 2019–2020 | 43 | 23 | 15 | 0.93 ± 0.01 | 0.89 ± 0.03 | 21 | 0.93 ± 0.01 | |||||
| All seasons | 86 | 31 | 23 | 0.94 ± 0.01 | – | – | 0.93 ± 0.01 | – | – | 29 | 0.95 ± 0.01 | |
Fifty-five unsuccessful nests were found intact (not deformed) after the disappearance of eggs or nestlings. Fifteen nests suffered partial damage; some were detached, and others destroyed. On one occasion, a female was preyed upon while incubating the eggs, which were left intact (Fig. 3A). In addition, we recorded larvae of Philornis flies (Diptera, Muscidae; Fig. 3B) parasitizing 12 nestlings; of these, four with low infestations fledged while the others suffered predation.
We recorded the first case of parasitism by the Shiny Cowbird (Molothrus bonariensis, Icteridae) in a Chestnut Seedeaters' nest with an egg of 24.86 mm × 19.86 mm on 12 December 2019 (Fig. 3C). There was partial predation of this nest during incubation and abandonment by the female afterwards.
The nest success calculated using the Mayfield protocol was 23% (Table 3). We found no difference in DSRs between breeding seasons for nests with eggs and nestlings (Table 3). Likewise, the probability of survival did not differ for nests with eggs and nestlings between breeding seasons (Table 4). The estimated cumulative probability of a nest surviving, from laying the first egg to the departure of the last chick, was 29% (22 days cycle) (Table 3). The DSR was 0.95 ± 0.01 (n = 86 nests).
| Breeding season | Probability of survival | |||||||||
| Egg | Nest days | Lost nests | Z-test | P | Nestlings | Nest days | Lost nests | Z-test | P | |
| 2018–2019 | 0.54 ± 0.08 | 323 | 16 | 0.93 | 0.35 | 0.59 ± 0.09 | 199 | 10 | 1.88 | 0.06 |
| 2019–2020 | 0.43 ± 0.08 | 284 | 19 | 0.33 ± 0.09 | 136 | 14 | ||||
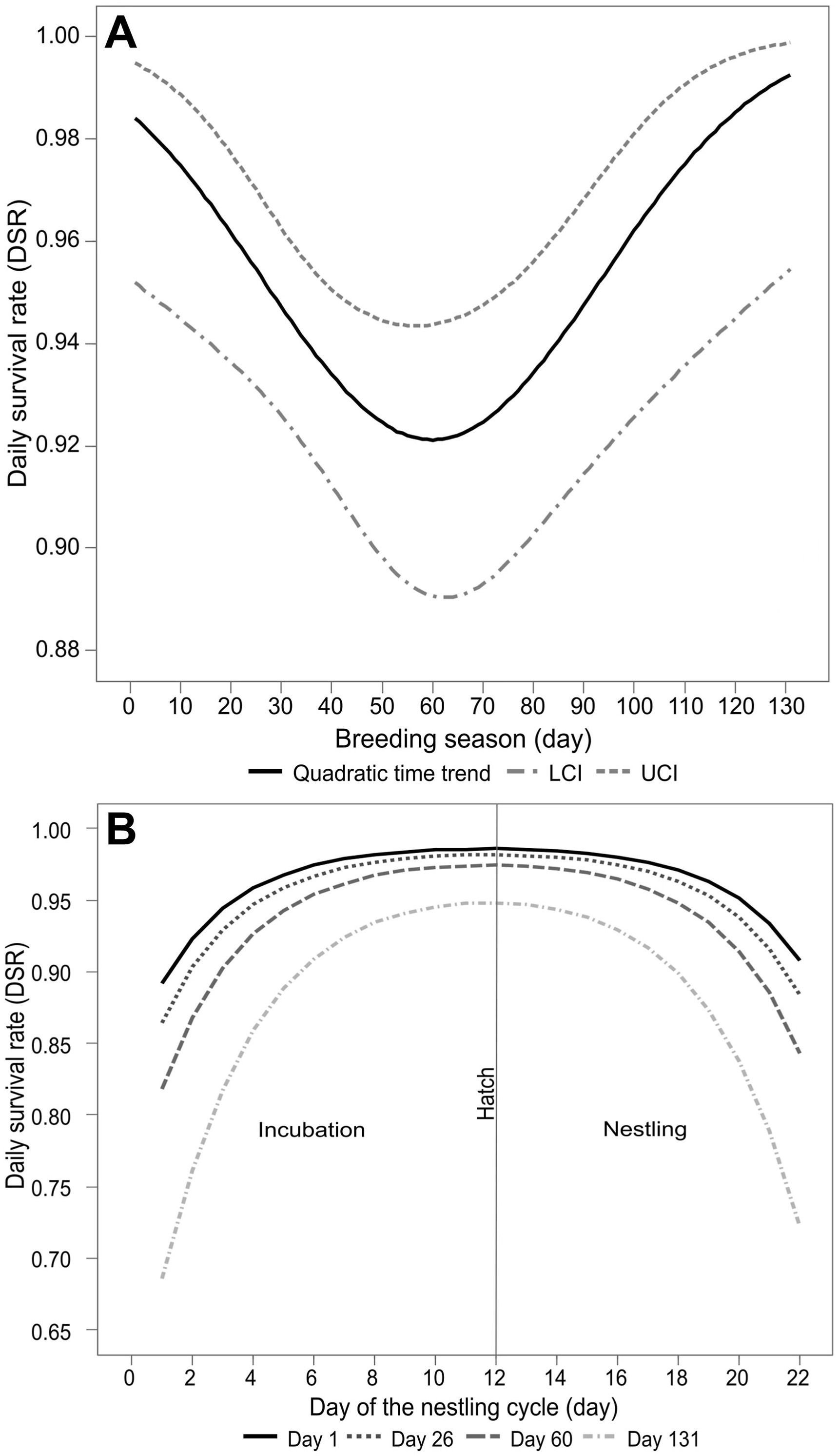
We considered 19 models in the nest survival analysis (Table 5). Among those that included time trends, the quadratic trend showed a relative importance of 0.89 (Table 5) and positively influenced the DSRs with increased rates from the middle to the end of the breeding season (Fig. 4A, Table 6). In the second step, the starting date was the covariate that best fitted the survival models with a relative importance of 0.98 (Table 5), but it had a negative coefficient (beta) value, with a later starting date having a lower DSR (Table 6).
| Model | AICc | wi | k | Deviance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt) | 0a | 0.89 | 3 | 340.98 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 4.89 | 0.08 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(t) | 6.83 | 0.03 | 2 | 349.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Second step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date) | 0b | 0.98 | 4 | 329.92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest) | 10.20 | 0.01 | 4 | 340.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Clutch) | 10.30 | 0.01 | 4 | 340.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Nest_age) | 10.54 | 0.01 | 4 | 340.46 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Sp_plant) | 11.01 | 0.00 | 4 | 340.93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 13.93 | 0.00 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Third step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest) | 0c | 0.22 | 5 | 329.33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Clutch) | 0.04 | 0.21 | 5 | 329.37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Sp_plant) | 0.43 | 0.17 | 5 | 329.76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Nest_age) | 0.49 | 0.17 | 5 | 329.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Clutch) | 1.43 | 0.11 | 6 | 328.74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Nest_age) | 1.98 | 0.08 | 6 | 329.29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Clutch+Sp_plant) | 3.41 | 0.04 | 7 | 328.69 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest+Clutch) | 9.95 | 0.00 | 5 | 339.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest+Sp_plant) | 10.76 | 0.00 | 5 | 340.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 12.50 | 0.00 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a AICc = 347.00. b AICc = 337.96. c AICc = 339.39. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Explanatory covariate | β | SE | 95% IC | |
| Lower | Upper | |||
| Nest height | 1.17 | 1.29 | ‒1.35 | 3.69 |
| Quadratic time trend | 0.01 | 0.01 | 0.01 | 0.01 |
| Nest support plant | 0.01 | 0.02 | ‒0.03 | 0.03 |
| Nest age | ‒0.03 | 0.04 | ‒0.11 | 0.05 |
| Starting date | ‒0.08 | 0.03 | ‒0.13 | ‒0.03 |
| Clutch | ‒0.28 | 0.32 | ‒0.90 | 0.34 |
The nest height showed a higher positive coefficient (Table 6), suggesting that nests placed at a greater height experience higher survival (successful nests = 48.26 ± 13.63 cm, n = 27 nests; unsuccessful nests = 41.78 ± 8.81 cm, n = 59 nests). On the other hand, the clutch size and nest support plants had a weakly negative coefficient (Table 6). Finally, the nest age negatively influenced the DSRs (Table 6). Although these covariates showed positive and negative coefficients for DSR, the 95% CI includes zero (0), suggesting that these covariates did not strongly influence DSRs (Table 6).
In the third step, six models were the best fit with the interaction of covariates (ΔAICc < 2) among candidate models (Table 5). Again, the starting date was the covariate that showed the highest relative importance in nest success with 1.0, leaving nest height at 0.44, clutch and nest age at 0.25 and nest support plant at 0.21 (Table 5). However, the 95% CI again included zero (Table 6).
The length of the breeding period of the Chestnut Seedeater (4.6 months) is similar to other migratory Sporophila species that breed in southern Brazil (Rovedder, 2011; Franz and Fontana, 2013; Repenning and Fontana, 2016). Furthermore, there seems to be a reproductive synchronism among congeners, since a reproductive peak activity from late November to mid-December has also been recorded for Tawny-bellied Seedeater (Franz and Fontana, 2013), Black-bellied Seedeater (Rovedder, 2011), and Tropeiro Seedeater (Repenning and Fontana, 2016).
As expected, precipitation was not a good predictor of the breeding peak of Chestnut Seedeater. The rainfall in the study region is well distributed throughout the year, with little effect on the development of grasses (Moreira et al., 2019), the main food of seedeaters. Because Chestnut Seedeater reproduces in a region with a subtropical climate, our results differ from studies with tropical species (insectivores) since their peaks of reproductive activity precede peaks of higher volumes of precipitation (Cavalcanti et al., 2016; França et al., 2020). Nonetheless, we found a significantly positive relationship between the peak of active nests and the photoperiod. The associations of photoperiod with environmental variables, such as temperature, precipitation, availability of food resources, and nest sites, trigger the beginning and determine the duration of the breeding season in birds (Stutchbury and Morton, 2001; Coppack and Pulido, 2004; Dunn and Møller, 2014). Examples of these associations are the positive correlations between photoperiod, precipitation, and seed production with the peak of active nests found for Tropeiro Seedeaters and the photoperiod with the peak of active nests for Rusty-collared Seedeater. These species breed in the southern Brazil grassland (Repenning and Fontana, 2016; Rosoni et al., 2019). According to the hypothesis of the availability of daylight, adults of migratory species that breed at higher latitudes tend to have a higher total rate of food delivery, resulting in the accelerated growth of the young in response to increased photoperiod (Sockman and Hurlbert, 2020). Thus, offspring that hatch during the period with the longest day length acquire a better body condition for migration (Coppack and Pulido, 2004), increasing the probability of being recruited into the population. Although we did not provide data regarding recruitment, our findings support this theory since we recorded a more significant number of Chestnut Seedeater couples reproducing during the longest days of the breeding period.
Clutch size (two eggs on average) was similar to other seedeaters that breed further south in South America (Di Giacomo, 2005; Vizentin-Bugoni et al., 2013; Turbek et al., 2019) and for other seedeaters that breed near the equator (Di Giacomo, 2005; Ferreira and Lopes, 2017). All this information confirms our prediction that the Chestnut Seedeater clutch size does not vary between seasons and appears to be a phylogenetically inert in the genus Sporophila. Tropical species usually have smaller clutch sizes (~2 eggs), generally regulated by ecological pressures (Stutchbury and Morton, 2001). Such pressures are related to higher rates of predation and the high energy cost of producing more eggs, reducing the rate of food delivery and the low probability of recruiting juveniles (Stutchbury and Morton, 2001). Furthermore, in seedeaters, the clutch size tends not to vary between latitudes (Repenning and Fontana, 2016). Here we hypothesize that clutch size is an inherited trait in the southern capuchino group since the group has experienced rapid radiation, presenting low genetic differentiation among the ten species (Campagna et al., 2015; Estalles et al., 2022). We highlight the need for further studies involving clutch size in phylogenetic analyses to help understand how reproductive strategies have evolved in the group and the genus.
The fecundity of 1.12 nestlings/female of Chestnut Seedeater was higher than that found for Rusty-collared Seedeater (0.90 nestlings/female, Rosoni et al., 2019). The fecundity of Chestnut Seedeater was also higher than those of other grasslands passerines: Straight-billed Reedhaunter (Limnoctites rectirostris, Furnariidae) from southern Brazil, with 0.60 nestlings/female (Larre et al., 2022) and Shrike-like Tanager (Neothraupis fasciata, Thraupidae) from the Cerrado, with 0.60 (Duca and Marini, 2014). In addition, the Chestnut Seedeater produced more yearly offspring than Tropeiro Seedeaters (0.57) and Rusty-collared Seedeaters (0.75). However, the egg success was equivalent to Rusty-collared Seedeater (20%) and lower than Tropeiro Seedeater (36%) (Repenning, 2012; Rosoni et al., 2019). We can hypothesize that the differences observed in the productivity of the Chestnut Seedeater in relation to its congeners can be attributed to factors such as (1) ecology, such as the availability of food resources, or (2) behavior, related to the maturity and experience of adults in reproduction. We stress that further studies with other Chestnut Seedeater populations are necessary to assess the species' population stability. According to Dunn and Møller (2014), high productivity and punctual arrivals in spring are key parameters to be assessed.
The sex ratio of Chestnut Seedeater nestlings was similar to that found for Black-bellied Seedeater (Rovedder, 2011), Tawny-bellied Seedeater (Franz and Fontana, 2013), Tropeiro Seedeater (Repenning and Fontana, 2016), and Rusty-collared Seedeater (Rosoni et al., 2019). When comparing the proportions among the five seedeater species, only the Tawny-bellied Seedeater had more females born. In the Black-bellied Seedeater territories monitored by Rovedder (2011), some adult males were observed without females throughout the breeding season, raising the hypothesis that this characteristic could result from the slight difference observed in the sexual proportions of the offspring. Our observation confirms this hypothesis, which predicts that more male births occur in populations. Alternatively, we also propose that the survival of female Chestnut Seedeaters may be lower than that of males, which can explain the observation of males alone in the breeding territory throughout the breeding season (JRRR, unpbl. data). Nevertheless, other factors may explain these proportions, e.g., site fidelity, female mortality, and differences in conspicuousness between males and females. These factors have been raised in a study with Iberá Seedeater in Argentina, in which a low rate of female return was detected in subsequent years of monitoring (Browne et al., 2021).
Several studies on breeding biology have highlighted that nest predation is the main cause of reproductive failure in birds (e.g., Chiarani and Fontana, 2015; Pretelli et al., 2015; Beier and Fontana, 2019; Larre et al., 2022). Studies with Tropeiro Seedeater (Repenning and Fontana, 2016) and Lined Seedeater (S. lineola, Ferreira and Lopes, 2017) recorded similar predation events on females in the nests during the incubation phase. The predation of nests caused by ants seems to occur regularly in nests of Passeriformes (Skutch, 1985), including Lined Seedeater and Black-bellied Seedeater (Rovedder, 2011; Ferreira and Lopes, 2017). It is worth noting that we recorded only a single nestling predation event, by the snake Patagonia Green Racer (Pseudablabes patagoniensis) (Rosoni et al. submitted). Nevertheless, we observed other potential reptile predators in the study area, e.g., the Argentine Black and White Tegu (Salvator merianae). Furthermore, birds and mammals in the region may depredate Chestnut Seedeater nests. For example, Guira Cuckoo (Guira guira), Great Kiskadee (Pitangus sulphuratus), Long-winged Harrier (Circus buffoni), Cinereous Harrier (Circus cinereus), and Pampas Fox (Lycalopex gymnocercus, Canidae) have been reported to prey on nests of other bird species (Repenning et al., 2009; Rovedder, 2011; Repenning, 2012; Browne et al., 2021; Colombo et al., 2021).
Nest success seems to depend on the degree of infestation by the larvae of Philornis flies (Rovedder, 2011; Franz and Fontana, 2021). For example, in a study with Blue-black Grassquit (Volatinia jacarina, Thraupidae) in central Brazil, Biagolini-Jr and Macedo (2021) found that nestlings with low infestation rates by larvae did not compromise the success of the nests. Although we did not test this effect of infestation by larvae on nestlings, we cannot rule out that this interaction affects the nest success of Chestnut Seedeaters (Beier and Fontana, 2019).
Our study is the first to present a case of brood parasitism in a southern capuchino seedeater. Records of brood parasitism in seedeaters by Shiny Cowbird have previously been reported for Lined Seedeater, Double-collared Seedeater (S. caerulescens), Ruddy-breasted Seedeater (S. minuta), Chestnut-bellied Seed Finch (S. angolensis) and Large-billed Seed Finch (S. crassirostris) (Lowther, 2019). However, because it is the first case recorded between latitudes 28° S and 30° S, it may be a worrying factor in the future. Furthermore, due to the current Chestnut Seedeater population decline, monitoring other populations of this species becomes necessary, as well as other southern capuchino seedeaters that may have their reproductive populations compromised due to brood parasitism in addition to other environmental factors (e.g., habitat loss or illegal trade of songbirds males; Repenning, 2012; BirdLife International, 2022) in the grasslands in southeast South America.
Considering the grassland seedeaters that nest in cycles of ~22 days, the estimated probability of nest survival (29%) was like that found for Black-bellied Seedeater (23%, Rovedder, 2011), Rusty-collared Seedeater (29%, Rosoni et al., 2019), Tropeiro Seedeater (20%, Repenning and Fontana, 2016), while almost the double compared to Iberá Seedeater (16%, Browne et al., 2021). Different factors can cause variations in the cumulative probability of nest survival in species of grassland birds, such as nest height (Repenning and Fontana, 2016), nest age (Chiarani and Fontana, 2015), nest support plant (Rovedder, 2011), breeding season (Di Giacomo et al., 2011), the gradient of urbanization (Pretelli et al., 2015), or clutch size (Rosoni et al., 2019).
The most parsimonious and better-performing models incorporated time trends as a quadratic factor. Our findings are similar to those reported for Tropeiro Seedeater and other grassland passerines (Grant et al., 2005; Repenning and Fontana, 2016). This result led us to suppose that the DSR varies across the breeding season and that such variation is associated with the abundance of predators (Grant et al., 2005; Di Giacomo et al., 2011). Previous studies with five North American grassland passerines also reported that the variation in DSR may be related to the nest-site availability and vegetation cover (Davis, 2005). The peak of food availability (grass seed) can also be the main factor that acts on the variation in DSR (Repenning and Fontana, 2016). Nevertheless, we consider that the models we generated in this study are more likely related to the fluctuation in the abundance of predators since predation was the leading cause of nest failure. These fluctuations could be related to a greater food supply, young predators' post-reproductive movements, and increased summer temperatures, which favor the abundance of reptiles (Davis, 2005; Grant et al., 2005; Cox et al., 2013).
Our models with covariate starting date agree with the study by Caccamise (1978), which relates the increase in predation rate with the advancement of the breeding season and the increase in the abundance of potential predators. This time trend has also been observed in Argentina in two grassland birds: Strange-tailed Tyrant (Alectrurus risora, Tyrannidae) and Iberá Seedeater (Di Giacomo et al., 2011; Browne et al., 2021). This similarity in the decrease of DSRs among species may be related to nest morphology (Di Giacomo and Di Giacomo, 2004), support plant, or nest height above the ground (Di Giacomo et al., 2011), making it more susceptible to predation. In North America, a pattern in predation rates in relation to the place where birds build their nests has been identified in a study with 123 bird species. Open-nest species that built their nests in shrubs in grassland environments showed higher predation rates than species that built other nest types and used different environments (Martin, 1995).
The effect of the start date is especially noticeable after hatching when we visualize DSRs for a 22 days nesting cycle. DSRs tend to increase throughout the incubation period, and after hatching in the mid-breeding stage, they decrease again. The same pattern was observed for Tropeiro Seedeater (Repenning and Fontana, 2016). A similar but inverse pattern has been found in nests of Clay-colored Sparrows (Spizella pallida, Passerellidae) and Vesper Sparrows (Pooecetes gramineus, Passerellidae), in which DSRs tended to decrease during the last days of incubation and further reduced after hatching. However, DSR rises again until the chicks leave the nests (Grant et al., 2005). Our results are consistent with Skutch's hypothesis (Skutch, 1949) that older nests are more vulnerable to predation when associated with intense parental care. Nevertheless, many recent studies on bird breeding biology in South America have contradicted Skutch's hypothesis (Berkunsky et al., 2016; Meireles et al., 2021; Larre et al., 2022). Visual, auditory, and olfactory signals increase the risk of the nest being found by predators through observing clues left by adults or nestlings (Grant et al., 2005). In addition, the vegetation phenology surrounding the nests is a factor that influences predation rates (Borgmann et al., 2013) and the location where the nest was built (Martin et al. 2000a).
Finally, we emphasize the great need and the importance of creating and adequately managing protected areas that link grasslands of different physiognomies (e.g., dry grasslands, wetlands, and marshes). These measures will improve the quality of habitats and breeding territories and consequently will act on the nesting success of the species. The information presented herein fills in one of the main knowledge gaps requested to be investigated in the Brazilian National Action Plan for the Conservation of Grassland Birds (ICMBio Instituto Chico Mendes de Conservação da Biodiversidade, 2021). Information on the natural history of this species and other grassland passerines can be an essential tool for the effective development of new conservation measures (Franz and Fontana, 2013). Furthermore, it will help develop further questions related to biology, the evolution of reproductive traits, distribution, and conservation of southern capuchino seedeaters, a group presenting a steady population decline.
This study was carried out under the permits granted by the Instituto Chico Mendes de Conservação da Natureza – ICMBio (permit SISBIO number 63556-1), and all procedures with the birds complied with local and national rules regarding the capture, marking, and handling of wild birds and was approved by the Centro de Pesquisa e Conservação de Aves Silvestres - CEMAVE (permit number 4332/1).
JRRR, CSF, and CJC conceptualized the study. JRRR data cured. JRRR performed the analysis. JRRR, CSF, and CJC performed funding acquisition. JRRR performed investigation. JRRR, CSF, and CJC elaborate the methodology. JRRR, CSF, and CJC managed the project. CSF and CJC supervised the project. JRRR wrote the first original draft paper. JRRR, CSF, and CJC revised the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank J. Paiva and V. Schwendler for their hospitality during fieldwork; we thank the owners I. Wagner, C.S. Fontana, A. Quinteiro, B. Pujol, M. Brasil (F. Outeiro, in memoriam) and H. Guterres for allowing us to access their private properties; C. Paiva, V. Silva, J. Just and J. Lopes for the company and help during the fieldwork; P. Filho (UFRGS) who kindly helped to identify the plants used in the nests; B.B. Rosa (UFPR) for helping to identify ants. Lastly, we would like to thank Y.S. Esmaeili for the English translation of the manuscript. This research is part of the project "Neotropical Grasslands Connection: Ecology, Migration, and Conservation of the Threatened Chestnut Seedeater Sporophila cinnamomea", supported by the Rufford Foundation, United Kingdom (ID 27044-1). In addition, this study was supported by grants to JRRR and CJC, who received doctoral and postdoctoral fellowships, respectively, from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (88882.439380/2019-01 and 88882.316294/2019-01), and to CSF, who received a research grant from the Conselho Naciónal de Desenvolvimento Científico e Tecnológico – CNPq (310608/2019-8).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100082.
|
Aguilar, T.M., Leite, L.O., Marini, M. Â., 1999. Biologia da nidificação de Lathrotriccus euleri (Cabanis, 1968) (Tyrannidae) em fragmentos de mata de Minas Gerais. Ararajuba 7, 125-133.
|
|
Azpiroz, A.B., Alfaro, M., Jimenez, S., 2012a. Lista Roja de las Aves del Uruguay: Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la IUCN. Dirección Naciónal de Medio Ambiente, Montevideo.
|
|
Biagolini-Jr, C., Macedo, R.H., 2021. Philornis infection in blue-black grassquits: impact on nestlings and risk factors involved. J. Avian Biol. 52, 1-20.
|
|
Böhning-Gaese, K., Halbe, B., Lemoine, N., Oberrth, R., 2000. Factors influencing the clutch size, number of broods and annual fecundity of North American and European land birds. Evol. Ecol. Res. 2, 823-839.
|
|
Browne, M., Turbek, S.P., Pasian, C., Di Giacomo, A.S., 2021. Low reproductive success of the endangered Iberá Seedeater in its only known breeding site, the Iberá Wetlands, Argentina. Ornithol. Appl. 123, duab008.
|
|
Burnham, K.P., Anderson, D.R., 2002. In: Model Selection and Multimodel Inference: A
Practical Information-Theoretic Approach, second ed. Springer-Verlag, New York.
|
|
Cavalcanti, L.M.P., Paiva, L.V., França, L.F., 2016. Effects of rainfall on bird reproduction in a semi-arid Neotropical region. Zoologia 33, e20160018.
|
|
Coppack, T., Pulido, F., 2004. Photoperiodic response and the adaptability of avian life cycles to environmental change. Adv. Ecol. Res. 35, 131-150.
|
|
Di Giacomo, A.G., 2005. Aves de la Reserva El Bagual. In: Di Giacomo, A.G.,
Krapovickas, S.F. (Eds.), Temas de Naturaleza y Conservación. Monografía de Aves Argentinas, Buenos Aires, pp. 201–465.
|
|
Di Giacomo, A.S., Di Giacomo, A.G., 2004. Extincion, historia natural y conservación de las poblaciones del Yetapa de Collar (Alectrurus risora) en la Argentina. Ornitol. Neotrop. 15, 145-157.
|
|
Di Giacomo, A.S., Kopuchian, C., 2016. Una nueva especie de Capuchino (Sporophila: Thraupidae) de los Esteros del Iberá, Corrientes, Argentina. Nuestras Aves 61, 3-5.
|
|
Dias, A.F.S., Maia, R., Dias, R.I., 2009. Breeding strategies of Tropical birds. In: Del
Claro, K., Oliveira, P.S., Rico-Gray, V. (Eds.), Tropical Biology and Conservation
Management – Vol. Ⅷ. Encyclopedia of Life Support Systems (EOLSS), Oxford, UK,
pp. 1–30.
|
|
Dinsmore, S.J., Dinsmore, J.J., 2007. Modeling avian nest survival in program MARK. Stud. Avian Biol. 34, 73-83.
|
|
Dubiec, A., Zagalska-Neubauer, M., 2006. Molecular techniques for sex identification in birds. Biol. Lett. 43, 3-12.
|
|
Duca, C., Marini, M. Â., 2014. High survival and low fecundity of a Neotropical savanna tanager. Emu 114, 121-128.
|
|
Dunn, P.O., 2004. Breeding dates and reproductive performance. Adv. Ecol. Res. 35, 69-87.
|
|
Farner, D.D., 1972. Breeding Biology of Birds: Proceedings of a Symposium on Breeding Behavior and Reproductive Physiology in Birds. National Academy of Sciences, Washington, D. C.
|
|
Fierro-Calderón, K., Loaiza-Muñoz, M., Sánchez-Martínez, M.A., Ocampo, D., David, S., Greeney, H.F., et al., 2021. Methods for collecting data about the breeding biology of Neotropical birds. J. Field Ornithol. 92, 315-341.
|
|
Fontana, C.S., Bencke, G.A., 2015. Biodiversidade de aves. In: Pillar, V.P., Lange, O. (Eds.), Os Campos Do Sul. Rede de Campos Sulinos - UFRGS, Porto Alegre, pp. 93-99.
|
|
Fowler, J., Cohen, L., 1995. Statistics for Ornithologists, BTO Guide. British Trust for Ornithology, Tring, UK.
|
|
França, L.F., Figueiredo-Paixão, V.H., Duarte-Silva, T.A., Santos, K.B., 2020. The effects of rainfall and arthropod abundance on breeding season of insectivorous birds, in a semi-arid neotropical environment. Zoologia 37, e37716.
|
|
Freitas, M.S., 2014. Biologia reprodutiva, seleção de sítios de nidificação e sucesso reprodutivo em aves campestres de cerrado na Estação Ecológica de Itirapina, SP. Master's thesis. Universidade de São Paulo.
|
|
Guan, H., Wen, Y., Wang, P., Lv, L., Xu, J., Li, J., 2018. Seasonal increase of nest height of the Silver-throated Tit (Aegithalos glaucogularis): can it reduce predation risk? Avian Res. 9, 1-8.
|
|
Hensler, G.L., Nichols, J.D., 1981. The Mayfield method of estimating nesting succes: a model, estimators and simulation results. Wilson Bull. 93, 42-53.
|
|
ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade], 2018. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, I. ICMBio/MMA, Brasília.
|
|
ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade], 2021. Plano de Ação Naciónal para Conservação das Aves dos Campos Sulinos, Portaria do PAN - Portaria n° 856, de 26 de dezembro de 2017. Brasília.
|
|
Lack, D., 1948. The significance of clutch-size. Part Ⅲ: Some interspecific comparisons. Ibis. 90, 25-45.
|
|
MADS [Ministerio del Ambiente y Desarrollo Sostenible], 2019. Resolución N° 254, de 09 de maio de 2019. Lista de Aves Protegidas de la Vida Silvestre (Paraguay).
|
|
Marini, M. Â., Duca, C., Maníca, L.T., 2010. Técnicas de pesquisa em biologia reprodutiva de aves. In: Von Matter, S., Straube, F.C., Accordi, I., Piacentini, V. de Q., Cândido-Jr., J.F. (Eds.), Ornitologia e Conservação: Ciência Aplicada, Técnicas de Pesquisa e Levantamento. Technical Books Editora, Rio de Janeiro, pp. 297-312.
|
|
Marini, M. Â., Sousa, N.O., Borges, F.J.A., Silveira, M.B., 2009. Biologia reprodutiva de Elaenia cristata (Aves: Tyrannidae) em cerrado do Brasil central. Neotrop. Biol. Conserv. 4, 3-12.
|
|
Marini, M.A., Vasconcellos, M.M., Lobo, Y., 2014. Reproductive biology and territoriality of the Wedge-tailed Grass-finch (Emberizoides herbicola) (Aves: Passeriformes). Biosci. J. 30, 853-862.
|
|
Martin, T.E., Scott, J., Menge, C., 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. B 267, 2287-2293.
|
|
Martin, T.E., Martin, P.R., Olson, C.R., Heidinger, B.J., Fontaine, J.J., 2000. Parental care and clutch sizes in North and South American birds. Science. 287, 1482-1485.
|
|
Maurício, G.N., Bencke, G.A., Repenning, M., Machado, D.B., Dias, R.A., Bugoni, L., 2013. Review of the breeding status of birds in Rio Grande do Sul, Brazil. Iheringia. Série Zool. 103, 163-184.
|
|
MAyDS y AA [Ministerio de Ambiente y Desarrollo Sustentable de la Nación y Aves Argentinas], 2015. Categorización de las Aves de la Argentina. Buenos Aires, Argentina.
|
|
Mayfield, H.F., 1975. Suggestions for calculating nest success. Wilson Bull. 87, 456-466.
|
|
Meireles, R.C., Lopes, L.E., Pichorim, M., Machoado, T.L.S.S., Duca, C., Solar, R., 2021. Nest survival of the threatened Campo Miner Geositta poeciloptera: a tropical cavity-nesting grassland bird. Austral Ecol. 46, 1236-1245.
|
|
R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
|
|
Repenning, M., 2012. História natural, com ênfase na biologia reprodutiva, de uma população migratória de Sporophila aff. plumbea (Aves; Emberizidae) do sul do Brasil. Master's thesis. Pontifícia Universidade Católica do Rio Grande do Sul, Brazil.
|
|
Repenning, M., Fontana, C.S., 2016. Breeding biology of the Tropeiro Seedeater (Sporophila beltoni). Auk 133, 484-496.
|
|
Ricklefs, R.E., Bloom, G., 1977. Components of avian breeding productivity. Auk 94, 86-96.
|
|
Ridgely, R.S., Tudor, G., 1989. The Birds of South America. University of Texas Press, Austin.
|
|
Rosoni, J.R.R., Fontana, C.S., Carlos, C.J., 2020. Nests, eggs, clutch size, and nestlings of the Chestnut Seedeater (Sporophila cinnamomea), a vulnerable species of South America. Wilson J. Ornithol. 132, 998-1007.
|
|
Rosoni, J.R.R., 2022. Caboclinho-de-chapéu-cinzento Sporophila cinnamomea (Aves: Passeriformes) o que faz no Pampa e para onde vai no Cerrado? História natural com enfâse em biologia reprodutiva, distribuição e migração. Doctor's thesis. Universidade Federal do Rio Grande do Sul, Brazil.
|
|
Rotella, J., 2021. Nest survival models. In: Cooch, E.G., White, G.C. (Eds.), Program MARK - A 'Gentle Introduction'. Ithaca, New York, pp. 1-19.
|
|
Rovedder, C.E., 2011. História natural de Sporophila melanogaster (Pelzen 1870) (Aves: Emberizidae) com ênfase em sua biologia reprodutiva. Master's thesis. Pontifícia Universidade Católica do Rio Grande do Sul, Brazil.
|
|
Silva, J.M.C., 1999. Seasonal movements and conservation of seedeaters of the genus Sporophila in South America. Stud. Avian Biol. 19, 272-280.
|
|
Skutch, A.F., 1949. Do tropical birds rear as many young as they can nourish? Ibis 91, 430-455.
|
|
Skutch, A.F., 1966. A breeding bird census and nesting success in Central America. Ibis. 108, 1-16.
|
|
Slagsvold, T., 1982. Clutch size variation in passerine birds: The nest predation hypothesis. Oecologia 54, 159-169.
|
|
Stutchbury, B.J.M., Morton, E.S., 2001. Behavioral Ecology of Tropical Birds. Academic Press, London.
|
|
Vizentin-Bugoni, J., Areta, J.I., Di Giacomo, A.G., Di Giacomo, A.S., Jacobs, F., Coimbra, M.A.A., Dias, R.A., 2013. Breeding biology and conservation of the Marsh Seedeater Sporophila palustris. Bird Conserv. Int. 23, 147-158.
|
|
White, G.C., Burnham, K.P., 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46, 120-139.
|
|
Winter, M., Hawks, S.E., Shaffer, J.A., Johnson, D.H., 2003. Guidelines for finding nests of passerine birds in tallgrass prairie. Prairie Nat. 35, 197-211.
|
| Variables | Estimate | Standard error | t value | P value |
| Intercept | ‒217.30 | 52.2 | ‒4.16 | 0.01* |
| Average temperature | ‒0.01 | 4.94 | 0.00 | 1.00 |
| Maximum temperature | ‒4.00 | 4.42 | ‒0.90 | 0.41 |
| Minimum temperature | 1.96 | 2.16 | 0.90 | 0.41 |
| Precipitation | ‒0.32 | 0.32 | ‒0.99 | 0.37 |
| Photoperiod | 21.09 | 5.05 | 4.17 | 0.01* |
| Signif. codes: *P < 0.05. | ||||
| Breeding parameters | Breeding season | ||
| 2018–2019 | 2019–2020 | Both seasons | |
| Nest found | 49 nests | 49 nests | 98 nests |
| Clutch size | 2.10 ± 0.37 (n = 42 nests) | 2.33 ± 0.47 (n = 40 nests) | 82 nests |
| Hatchability | 0.86 ± 0.23 (n = 25 nests, n = 52 eggs) | 0.90 ± 0.18 (n = 24 nests, n = 55 eggs) | 49 nests |
| Fecundity | 1.13 nestlings/female (n = 46 nests) | 1.11 nestlings/female (n = 44 nests) | 90 nests |
| Egg success | 37% (n = 43 nests, n = 91 eggs) | 20% (n = 44 nests, n = 102 eggs) | 87 nests |
| Nestling survival | 60% (n = 25 nests) | 41% (n = 24 nests) | 49 nests |
| Annual nestling production | 1.08 nestlings/brood (n = 25 nests) | 0.83 nestlings/brood (n = 24 nests) | 49 nests |
| Breeding season | Number of nests | Apparent reproductive success (%) | Mayfield protocol | MARK | ||||||||
| % | Egg DSR | Z-test | P | Nestling DSR | Z-test | P | % | DSR | ||||
| 2018–2019 | 43 | 39 | 32 | 0.95 ± 0.01 | 0.87 | 0.37 | 0.95 ± 0.02 | 1.65 | 0.09 | 36 | 0.95 ± 0.01 | |
| 2019–2020 | 43 | 23 | 15 | 0.93 ± 0.01 | 0.89 ± 0.03 | 21 | 0.93 ± 0.01 | |||||
| All seasons | 86 | 31 | 23 | 0.94 ± 0.01 | – | – | 0.93 ± 0.01 | – | – | 29 | 0.95 ± 0.01 | |
| Breeding season | Probability of survival | |||||||||
| Egg | Nest days | Lost nests | Z-test | P | Nestlings | Nest days | Lost nests | Z-test | P | |
| 2018–2019 | 0.54 ± 0.08 | 323 | 16 | 0.93 | 0.35 | 0.59 ± 0.09 | 199 | 10 | 1.88 | 0.06 |
| 2019–2020 | 0.43 ± 0.08 | 284 | 19 | 0.33 ± 0.09 | 136 | 14 | ||||
| Model | AICc | wi | k | Deviance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt) | 0a | 0.89 | 3 | 340.98 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 4.89 | 0.08 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(t) | 6.83 | 0.03 | 2 | 349.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Second step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date) | 0b | 0.98 | 4 | 329.92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest) | 10.20 | 0.01 | 4 | 340.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Clutch) | 10.30 | 0.01 | 4 | 340.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Nest_age) | 10.54 | 0.01 | 4 | 340.46 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Sp_plant) | 11.01 | 0.00 | 4 | 340.93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 13.93 | 0.00 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Third step | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest) | 0c | 0.22 | 5 | 329.33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Clutch) | 0.04 | 0.21 | 5 | 329.37 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Sp_plant) | 0.43 | 0.17 | 5 | 329.76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Nest_age) | 0.49 | 0.17 | 5 | 329.82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Clutch) | 1.43 | 0.11 | 6 | 328.74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Nest_age) | 1.98 | 0.08 | 6 | 329.29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Start_date+Height_nest+Clutch+Sp_plant) | 3.41 | 0.04 | 7 | 328.69 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest+Clutch) | 9.95 | 0.00 | 5 | 339.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(tt+Height_nest+Sp_plant) | 10.76 | 0.00 | 5 | 340.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| S(.) | 12.50 | 0.00 | 1 | 349.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a AICc = 347.00. b AICc = 337.96. c AICc = 339.39. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Explanatory covariate | β | SE | 95% IC | |
| Lower | Upper | |||
| Nest height | 1.17 | 1.29 | ‒1.35 | 3.69 |
| Quadratic time trend | 0.01 | 0.01 | 0.01 | 0.01 |
| Nest support plant | 0.01 | 0.02 | ‒0.03 | 0.03 |
| Nest age | ‒0.03 | 0.04 | ‒0.11 | 0.05 |
| Starting date | ‒0.08 | 0.03 | ‒0.13 | ‒0.03 |
| Clutch | ‒0.28 | 0.32 | ‒0.90 | 0.34 |