| Citation: | Fenliang Kuang, Wei Wu, David Li, Chris J. Hassell, Grace Maglio, Kar-Sin K. Leung, Jonathan T. Coleman, Chuyu Cheng, Pavel S. Tomkovich, Zhijun Ma. 2022: Detecting the non-breeding region and migration route of Whimbrels (Numenius phaeopus rogachevae) in the East Asian–Australasian Flyway. Avian Research, 13(1): 100011. DOI: 10.1016/j.avrs.2022.100011 |
Determining the migration routes and connections of migratory birds at the population level helps clarify intraspecific differences in migration. Five subspecies have been recognized in the Whimbrel (Numenius phaeopus) in Eurasia. Ssp. rogachevae is the most recently described subspecies. It breeds in Central Siberia, while its non-breeding region and migration routes are still unclear. We tracked the migration of Eurasian Whimbrels captured at three non-breeding sites (Moreton Bay in east coast of Australia, Roebuck Bay in Northwest Australia and Sungei Buloh Wetland in Singapore) and two migration stopover sites (Chongming Dongtan and Mai Po Wetland in China). We determined the breeding sites and inferred the subspecies of the tagged birds in the East Asian – Australasian Flyway (EAAF) based on the known breeding distribution of each subspecies. Of the 30 tagged birds, 6 and 21 birds bred in the breeding range of ssp. rogachevae and variegatus, respectively; one bred in the presumed transition area between the breeding range of ssp. phaeopus and rogachevae, and two bred in the region between the breeding range of ssp. rogachevae and variegatus. The birds that bred in the ssp. rogachevae breeding range spent their non-breeding season in the northern Sumatra, Singapore, East Java and Northwest Australia and mainly stopped over along China's coasts during migration. None of our birds bred in the exclusive breeding range of the phaeopus subspecies. Previous studies have predicted that rogachevae whimbrels migrate along the Central Asian Flyway and spend the non-breeding season in West India and East Africa. We found that at least some rogachevae whimbrels migrate along the EAAF and spend the non-breeding season in Southeast Asia and Australia. The ssp. phaeopus is at best sparsely distributed in the EAAF in the west region, or possibly does not occur at all.
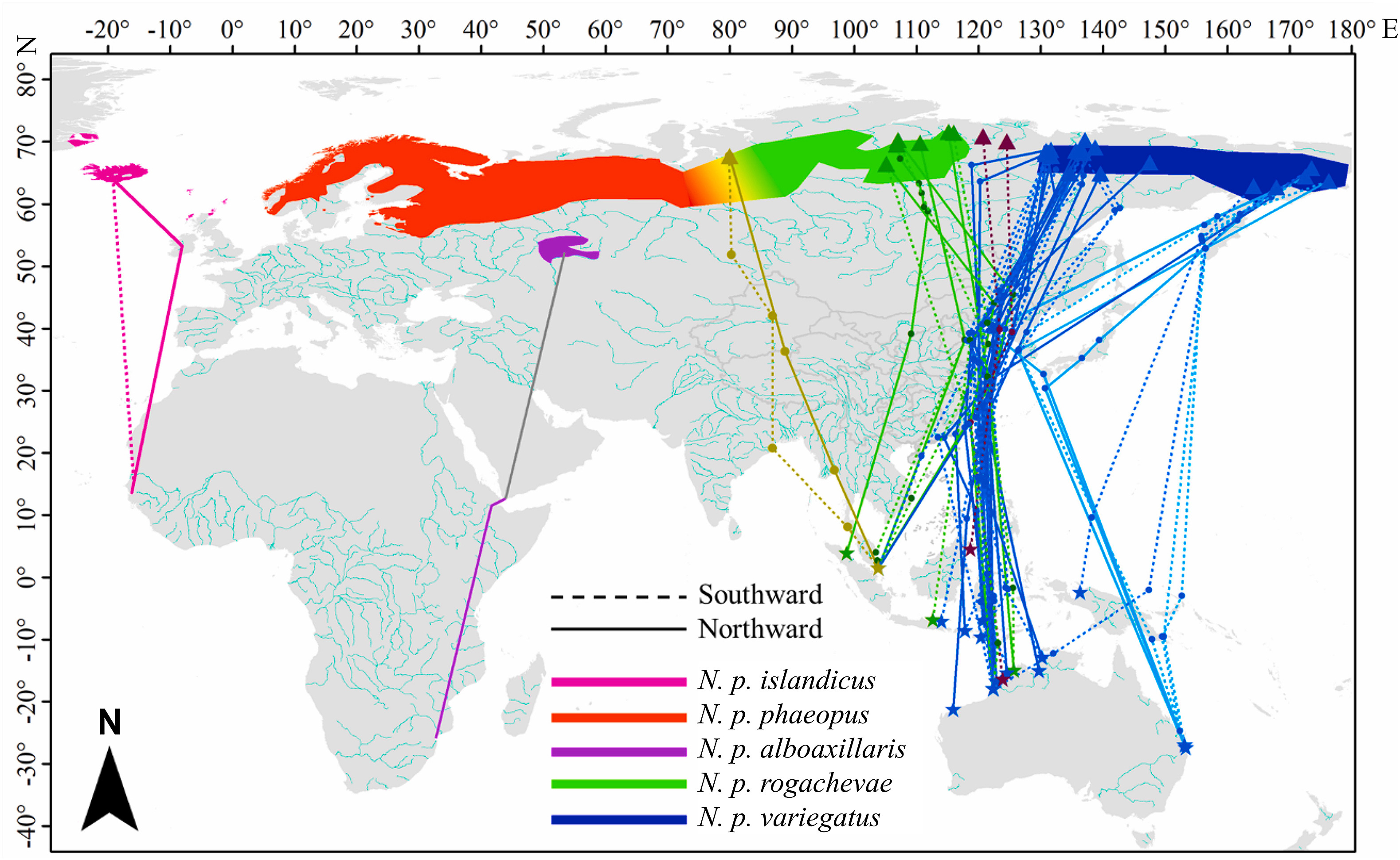
Seven subspecies of Whimbrel (Numenius phaeopus) have been described in the world (del Hoyo and Collar, 2014). According to the latest IOC World Bird list, the Whimbrels are divided into Hudsonian Whimbrel (Numenius hudsonicus) and Eurasian Whimbrel (N. phaeopus) (Gill et al., 2021), which is supported by genomic analysis (Tan et al., 2019). Subspecies rufiventris of Hudsonian Whimbrel breed in Alaska and Northwest Canada and spends non-breeding season from western USA to South America (Gill et al., 2021). Subspecies hudsonicus of Hudsonian Whimbrel breed in Hudson Bay area to Northeast Canada and the bulk of the population spends the non-breeding season in the Caribbean and South America (Gill et al., 2021) , and small numbers have been reported from New Zealand (Gill et al., 2010). Five subspecies of Eurasian Whimbrel (N. phaeopus) are recognized (Gill et al., 2021). The breeding and non-breeding distribution of four of these subspecies are known (Brown et al., 2014; Birdlife International and NatureServe, 2015; Carneiro et al., 2019; see Fig. 1). N. p. islandicus breeds in Iceland and the British Isles (also in Greenland and Faroe Islands) and spends non-breeding season in West Africa; N. p. phaeopus breeds in the region from North Europe to Western Siberia and spends non-breeding season in Africa; N. p. alboaxillaris breeds in the region from Western Kazakhstan to Southwestern Siberia and spends non-breeding season on the islands and coasts of the West Indian Ocean; N. p. variegatus breeds in the range from central to Eastern Siberia and spends non-breeding season on the coasts of South Asia, Southeast Asia and Australia. The most recently described subspecies, N. p. rogachevae (Tomkovich, 2008), breeds in Central Siberia. The non-breeding sites are still unclear (Fig. 1; Tomkovich, 2008; Lappo et al., 2012; Brown et al., 2014). It has been speculated that the rogachevae whimbrels migrate along the Central Asian Flyway (Tomkovich, 2008) and spend the non-breeding season in Western India and Eastern Africa (Skeel and Mallory, 2020; Gill et al., 2021), but there is still a lack of evidence.
In the East Asian – Australasian Flyway (EAAF), both ssp. phaeopus and variegatus have been widely reported and that their main non-breeding grounds are located in the western and eastern regions respectively. N. p. phaeopus mainly distributed from the coast of Bangladesh (Ripley, 1982) and Sri Lanka (Phillips, 1975). The non-breeding variegatus Whimbrel widely distributed along the coast from Bangladesh (Ripley, 1982) (some birds occasionally visited to Sri Lanka; Phillips, 1975) to Mariana Islands (Stinson et al., 1997) and Micronesia (Baker, 1951) in the Central Pacific. A few variegatus whimbrels spend non-breeding season in the Malay Peninsula, Wallacea region and Sunda Islands, while most variegatus whimbrels spend non-breeding season in eastern Indonesia, the Guadalcanal Islands and the Florida Island of the Solomon archipelago in the South Pacific, New Guinea, and Australia (Bishop, 2006; Crossland et al., 2016).
Some studies have considered that N. p. variegatus is the only subspecies of the Eurasian Whimbrel in Southeast Asia and Australia (Brown et al., 2014; del Hoyo and Collar, 2014; Gill et al., 2021). However, there are plenty of records of specimens collected in the early 1900s indicated that a few individuals of the Whimbrels were referred to as “phaeopus” subspecies in Thailand (Deignan, 1946, 1963) and Peninsula Malaya (Robinson and Chasen, 1936). Moreover, an increasing number of recent studies and field guides have indicated that Eurasian Whimbrels with morphological features in lower back and rump similar to those of ssp. phaeopus occur in Southeast Asia, the Malay Peninsula, the Wallacea region, and the Greater Sunda Islands (Lekagul and Round, 1991, Wells, 1999; Grantham, 2000; Robson, 2007; Jeyarajasingam and Pearson, 2012; Arlott, 2018). Wells (1999) indicated that some Eurasian Whimbrels with a white lower back and rump (considered phaeopus) are distributed along the west coast of the Malay Peninsula, while both the considered phaeopus and variegatus whimbrels are distributed along the east coast. Until ssp. rogacheva was described most (all) observers looked at lower back/rump patters to distinguish ssp. phaeopus from variegatus. The potential importance of underwing/axillary patterning has only been recognised quite recently (Hayman et al., 1986; Tomkovich, 2008). Grantham (2000) also found that more than 95% of Eurasian Whimbrels that passed through Alas Purwo National Park in the far southeast corner of Java during migration exhibit intermediate plumage characteristics between variegatus and phaeopus: white lower back and rump with no obvious spots, and the underwing coverts with some dark spots. The descriptions of the morphological features are consistent with the ssp. rogachevae: the black spotting on the lower back and rump of ssp. rogachevae are less intense dark than that of ssp. variegatus, and the dark bars on the axillary feathers of ssp. rogachevae are wider than those of the ssp. phaeopus (Tomkovich, 2008). Some individuals exhibited the intermediate plumage characteristics between ssp. variegatus and phaeopus were also accounted in Indonesia (Cramp, 1983), Malay Peninsula (Robinson and Chasen, 1936), Thailand (Jorgensen, 1949) and Borneo (MacKinnon and Phillipps, 1993). In addition, field survey found that non-breeding whimbrels in Northwest Australia showed a variation in the spots colour of lower back and rump from less intense dark to strong intense dark, and they passed through Java during migration (Kuang et al., 2000). The results from Grantham (2000) may thus suggest that the Eurasian Whimbrels passing through Java and exhibiting the intermediate plumage characteristics were most likely from Northwest Australia.
Both breeding range and non-breeding range of variegatus Whimbrel distributed in the east, and of phaeopus Whimbrel in the west, of the EAAF, suggesting that the subspecies of Eurasian Whimbrel exhibits parallel migration (Newton, 2008). Because the breeding range of the ssp. rogachevae is located between the breeding ranges of ssp. phaeopus and variegatus, it can be predicted that some whimbrels that spend non-breeding season in Southeast Asia and Northwest Australia and match the plumage features of ssp. rogachevae are ssp. rogachevae rather than the ssp. phaeopus. However, direct empirical evidence is still sparse. Some recent studies based on individual tracking in EAAF reported the migration route and breeding ground distribution of Eurasian Whimbrel (Kuang et al., 2020; Li et al., 2020), but did not elaborate the distribution of ssp. rogachevae. In this study, we integrated and analyzed the migration tracking data of Eurasian Whimbrels from stopover sites and non-breeding sites in the EAAF, to identify some of the breeding sites and judge the subspecies of satellite-tagged birds based on the known breeding distribution of each subspecies. We focused on whether the tagged Eurasian Whimbrels bred in the breeding range of ssp. rogachevae and then identified the migration route and non-breeding sites of the presumed rogachevae Whimbrels. We further discussed the subspecies distribution of Eurasian Whimbrels in the EAAF.
From 2017 to 2019, Eurasian Whimbrels were captured at three non-breeding sites, Moreton Bay (MB) on east coast of Australia, Roebuck Bay (RB) in Northwest Australia, and Sungei Buloh Wetlandin Singapore, and two migratory stopover sites, Chongming Dongtan (CMDT) and Mai Po Wetland (MPW) in China, and deployed tags on birds using the leg-looped method (refer to Kuang et al., 2020; Li et al., 2020). Two types of tags, 5-g solar Platform Terminal Transmitters (PTTs, Microwave Telemetry, Inc., Columbia, Maryland, US) and a 7-g solar-powered Global Positioning System-Global System for Mobile Communication (GPS-GSM, Hunan Global Messenger Technology Co. Ltd., Hunan, China) were used. All the tagged birds were identified as adults based on plumage characters (Prater et al., 1977). The weight of each tag was less than 3% of the body mass of the whimbrels (see details in Kuang et al., 2020; Li et al., 2020). Photos were taken of the lower back and rump for some birds, which can be referred to for subspecies identification (Tomkovich, 2008).
As mentioned above, underwing/axillary patterns are the main features to distinguish spp. rogachevae from phaeopus, and lower back and rump are the main features to distinguish spp. rogachevae from variegatus. However, we did not take the underwing photos of the tagged birds. Of the tagged birds in this study, the subspecies therefore were inferred by matching the breeding sites of the tagged birds with the known breeding range of the subspecies. We collated published literature to determine the breeding ranges of each subspecies of Eurasian Whimbrels (Fig. 1; Lappo et al., 2012; BirdLife International and NatureServe, 2015; Tan et al., 2019). The breeding, non-breeding, and migration stopover sites of the tagged birds were determined using the methods in previous studies (Kuang et al., 2020). Briefly, the locations in Siberia where the tagged birds stayed for the longest periods and when two successive fixes were less than 50 km apart were identified as the breeding sites. The locations of fixes at the southernmost sites for each individual where the tagged birds stayed for the longest periods were identified as non-breeding sites. During migration, sites where the tagged birds stayed within 0.5 degrees of latitude for more than 48 h and where two successive fixes were less than 50 km apart were identified as stopover sites (Kuang et al., 2020). The geographical location of the non-breeding, stopover, and breeding sites for each tagged bird was the arithmetic mean of all the geographical coordinates at each site. For 12 birds tracked for more than 2 years, only the data from the first year were used in the analysis because the geographical locations of both breeding and non-breeding sites of the same birds were similar in different years.
Breeding sites were identified for 30 tagged birds (Fig. 1; Appendix Table S1), including 14 tagged at CMDT (Appendix Tables S1) and 7 at RB in Northwest Australia, 4 in Singapore, 3 at MB on east coast of Australia, and 2 at MPW (Appendix Table S1). The non-breeding sites of the 14 birds tagged at CMDT were located in Northwest Australia and on the coasts of the Malay Archipelago, Indonesia, Malaysia and the Philippines. The two birds tagged at MPW spent the non-breeding season in Karratha, West Australia and North Sumatra, Indonesia (Appendix Table S1).
Breeding sites of all 30 tagged birds ranged in northern Siberia, from the Lower Taz River in Western Siberia (67.65° N, 79.90° E) to south-eastern Chukotka (176.27° E, 63.69° N; Fig. 1; Appendix Table S1). One bird bred at the presumed transition area of the breeding range of ssp. phaeopus and rogachevae, 6 in the breeding range of ssp. rogachevae, 21 at the breeding range of ssp. variegatus, and 2 came from non-breeding sites in Malaysia (birds were tagged at CMDT) and Northwest Australia bred in the region between the known breeding ranges of ssp. rogachevae and variegatus (114° E to 132° E; Fig. 1; Appendix Table S2).
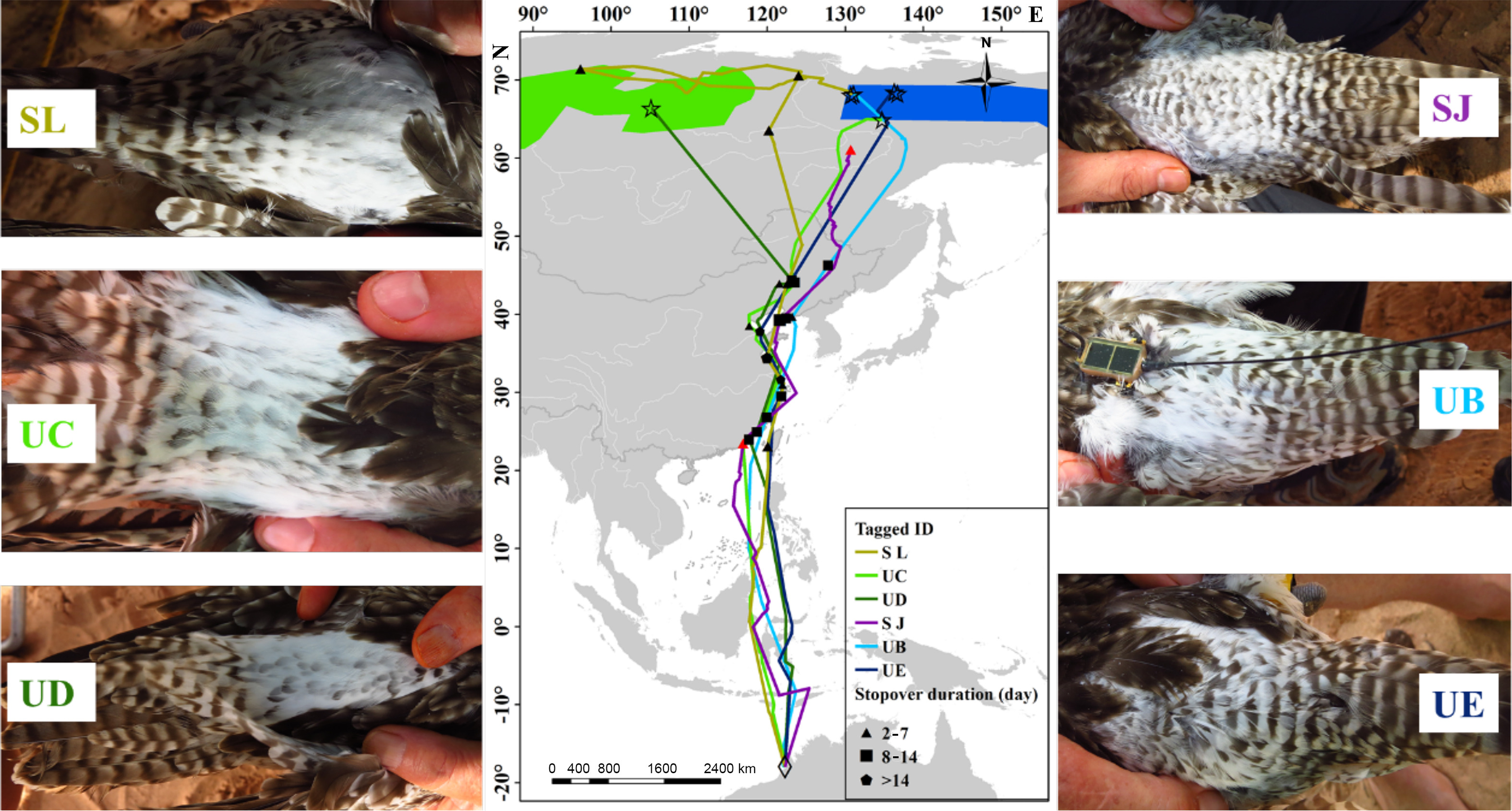
Of the six tagged whimbrels that bred in the breeding range of ssp. rogachevae, four birds were tracked for both northward and southward migration, one for complete northward migration, and one (tagged at CMDT) for incomplete northward and complete southward migration (Fig. 1; Appendix Table S2; Fig. S2). During northward migration, four birds departing from Singapore (two birds) and Northwest Australia (two birds) stopped over on the coasts of China (mainly in the Yellow Sea, including Bohai Bay) and then flew to breeding sites in Siberia (Fig. 1; Appendix Table S2; Fig. S2). Another bird was tagged at CMDT during southward migration departed from the non-breeding site in Sumatra, flew across inland China and stopped over for 23 days in Inner Mongolia, China before flying to its breeding site (Appendix Table S2). These 5 tracked whimbrels stopped over 1–6 times during northward migration with an average stopover duration of 27 ± 15 days (range: 10–47 days; Appendix Table S2). During southward migration, the five tracked whimbrels that bred in the breeding range of the ssp. rogachevae stopped over on the coasts of China, Vietnam, Peninsular Malaysia and Indonesia (Fig. 2; Appendix Table S2). These five birds stopped over 2–3 times during southward migration with an average stopover duration of 48 ± 15 days (range: 34–73 days; Appendix Table S2). Of the five birds, two birds spent the non-breeding season in Singapore, one in the East Java and two in Northwest Australia (Fig. 1; Appendix Table S2; Fig. S2).
Of the six birds tagged at Roebuck Bay (RB) in Northwest Australia, three birds exhibited intense dark spotting on the lower back and rump. Two of them stayed in the breeding range of the ssp. variegatus (Fig. 2), and another was shot in the Sakha (Yakutia) Republic (red triangle in Fig. 2, 130.71° E, 61.09° N, close to the breeding range of the ssp. variegatus) during its northward migration. The other three birds exhibited less intense dark spotting (a typical feature of ssp. rogachevae), one of them stayed in the breeding range of ssp. rogachevae, and two of them stayed in the western part of the breeding range of the ssp. variegatus (Fig. 2).
This study provided new information on the breeding sites of Eurasian Whimbrels in the EAAF according to migration tracking of birds from two stopover sites and three non-breeding sites. Our results suggest that both rogachevae and variegatus occur in the EAAF. We found that two tagged birds bred in the region between the known breeding ranges of ssp. rogachevae and variegatus (114° E to 132° E; Fig. 1; Appendix Table S2). This region (between the valleys of the Anabar and Yana Rivers) has been suggested as the gap of breeding whimbrels due to a lack of breeding records in this poorly surveyed area (Lappo et al., 2012).The two birds stayed in the region for 63 and 65 days respectively, suggesting that birds likely breed there rather than using the area as a stopover site or temporary stay before/after breeding activities. Due to the lack of detailed information in photos and DNA, we cannot determine the subspecies of these two tagged birds. In the future, systematic analysis of DNA together with standardised photos of lower back/rump and underwing/axillary pattern will be helpful to determine the subspecies at the gaps.
The six tagged birds that bred in the breeding range of the ssp. rogachevae came from Southeast Asia and Northwest Australia, including one from Sumatra Island, two from Singapore, one from the East Java and two from Northwest Australia (Fig. 1; Appendix Table S2; Fig. S2). No birds came from Moreton Bay (MB) on the east coast of Australia. Of the six tagged birds, there was a single migration for the two birds that spent non-breeding period in Sumatra Island and East Java, while other 4 birds returned to their non-breeding sites during southward migration. This suggests that the Whimbrels are sitefaithful to their non-breeding sites. As mentioned above, Eurasian Whimbrels with plumage features of the ssp. phaeopus have been largely reported in southeast Asia (Grantham, 2000; Robson, 2005; Iqbal and Ridwan, 2009; Jeyarajasingam and Pearson, 2012; Arlott, 2018), but those birds are probably the ssp. rogachevae (see discussions below). All these results suggest that rogachevae whimbrels may be common in Southeast Asia and Northwest Australia.
Although the collected material of ssp. rogacheva was limited, genetic study on the Whimbrels has identified that there is a barrier between the ssp. rogachevae from Central Siberia and phaeopus in sub-Arctic Europe in Eurasia. However, the conclusion is based on two single specimens and therefore should be taken with cautions. The results also indicate that ssp. rogachevae and variegatus can be united into a cluster distinct from ssp. phaeopus (Tan et al., 2019). According to the plumage features of birds at non-breeding sites, many previous studies have reported that some individuals similar with ssp. phaeopus occurs in Southeast Asian (Lekagul and Round, 1991, Wells, 1999; Grantham, 2000; Robson, 2007; Iqbal and Ridwan, 2009; Jeyarajasingam and Pearson, 2012; Arlott, 2018). However, of the total 30 tagged birds in this study (from two stopover sites and three non-breeding sites in the EAAF), none bred in the exclusive breeding range of the ssp. phaeopus. Actually, some of these previous studies were published before ssp. rogachevae was described (Tomkovich, 2008), and others may not have been aware that the subspecies had been described. In these circumstances, the authors would have probably assumed that Whimbrels with extensive white rumps were ssp. phaeopus. Our tracking results indicate that the ssp. phaeopus might be sparsely distributed in the EAAF in the west region, or probably does not occur at all. The birds similar with ssp. phaeopus in plumage traits that was previously reported in Southeast Asian might be the newly described ssp. rogachevae, which has a close relationship with the ssp. variegatus (Tan et al., 2019).
One of the major differences in plumage features between the ssp. rogachevae and variegatus is that the former has relatively less intense dark spotting on the lower back and rump (Tomkovich, 2008). Our study found that two of the six birds tagged at RB in Northwest Australia and exhibited intense dark spotting on the lower back and rump stayed in the breeding range of the ssp. variegatus (Fig. 2). For the one bird shot in the Sakha (Yakutia) Republic, its breeding site was also probably in the breeding region of ssp. variegatusbecause according to its light direction We thus believe that it has not yet arrived its breeding site, inferring from their migration route northward migration. The other three birds exhibited less intense dark spotting (a typical feature of rogachevae), one of them stayed in the breeding ranges of ssp. rogachevae, and two of them stayed in the western part of the breeding range of the ssp. variegatus (Fig. 2). It is likely that the westernmost breeding range of ssp. variegatus can be the transition area of breeding rogachevae and variegatus Whimbrel, or the Whimbrels nesting in the region have plumage attributes intermediate between ssp. rogachevae and variegatus. It may also be related to that the current knowledge of breeding distributions is still quite patchy (Lappo et al., 2012; Tan et al., 2019). All these imply that there is more to be understood about the breeding distribution and the division of the subspecific taxa. Ssp. rogachevae and variegatus may form a more allied population than shown, and ssp. rogachevae clustered more closely to ssp. variegatus (Tan et al., 2019).
Egg-laying and incubation of Eurasian Whimbrel last for approximately 3 and 25 days respectively (Skeel and Mallory, 2020). It takes at least 5 days to finish pair formation and nest building before the start of egg laying (PST personal observation). Thus, offspring hatch occurred at least 33 days after the adult Whimbrels arrived at breeding site. The tagged birds stayed at least 33 days at their breeding sites (median = 63 days, range from 33 to 76 days, n = 30; Appendix Table S3), suggesting that they underwent breeding activities. The average arrival date of tagged birds at the breeding sites was June 1 (from May 28 to June 4 for 95% confidence interval; Appendix Table S3). The peak period of food availability, as predicted by the Normalized Difference Vegetation Index (Sweet et al., 2015), occurs in early July in Eastern Siberia, such as at North Yakutia (Varlamova and Solovyev, 2015). Consequently, whimbrels that complete egg laying in early June might match the maximum food availability for their offspring. The one tagged bird (ID E116), arriving at breeding site too late (in late June) and staying there too short (33 days) (Appendix Table S3), was unlikely to breed successfully.
This study identified the breeding sites of non-breeding Eurasian Whimbrel in Southeast Asia, and northwest and east coast of Australia, as well as the non-breeding area and migration activities of the presumed rogachevae Whimbrel in the EAAF. Our results indicate that birds showing characteristics of rogachevae Whimbrels are not uncommon in Southeast Asia and Northwest Australia. They mainly stopover along China's coasts during both northward and southward migration and stay on coast of North Sumatra and East Java in Indonesia, in Singapore, and Broome and Wyndham in Northwest Australia in the non-breeding season. However, phaeopus Whimbrels might be sparsely distributed in the EAAF but mainly in the western region, or possibly does not occur at all. We highlight that migration tracking helps distinguish the distribution and migration connections of different populations in the same species, especially those that are difficult to distinguish in the field.
ZM and FK conceived the idea. WW, CJH, GM, KSKL, JTC and CC conducted the fieldwork and deployed tags on birds. FK and ZM analyzed the data and led manuscript writing. WW, DL, CJH, GM, KSKL, CC, and PST participated in writing and editing the manuscript. All authors read and approved the final manuscript.
All experimental protocols of bird capture and handling were strictly complied with the requirement of Chinese Wild Animal Protection Law and approved by Chongming Dongtan National Nature Reserve for Whimbrels captured and tagged at Chongming Dongtan and by the Agriculture, Fisheries and Conservation Department of the Government of the Hong Kong SAR, China for Whimbrels captured and tagged at Mai Po Nature Reserve. Tagging and catching Whimbrels in Australia were carried out under the license of the Australian Bird and Bat Banding Scheme (ABBBS). Catching Whimbrels on the shores of Roebuck Bay was conducted with the permission of the Yawuru People. The Whimbrels in Northwest Australia were tagged under the Regulation 17 Licence 08-00741-3 authorised to CJH from the Department of Biodiversity, Conservation and Attractions. The Whimbrels were tagged in Southeast Australia under the license CVL1337 issued to JTC by the Department of National Parks, Sport and Racing, Queensland Government and Department of Primary Industries Animal Ethics license CA 2015-03-845.
The authors declare that they have no competing interests.
We thank the Chongming Dongtan National Nature Reserve, WWF-Hong Kong, Australasian Wader Studies Group (AWSG), and the Northwest Australia Expedition 2018 team for their support of the fieldwork, particularly the late Dr Clive Minton. We appreciate Roz Jessop, Michael Dawkins, Prue Wright, Robert Bush, Brad Woodworth, Chi-Yeung Choi, Bingrun Zhu, Weipin Chen, Xuesong Feng, Xiaodan Wang, Mianjuan Ke and volunteers of the Hong Kong Waterbirds Ringing Group for their assistance with the fieldwork. We thank National Parks Board Singapore for providing tracking data and photos of tagged birds for this paper. AWSG acknowledges the Yawuru People via the offices of Nyamba Buru Yawuru Limited for permission to catch birds on the shores of Roebuck Bay, the traditional lands of the Yawuru people. CJH thanks his funders, WWF Netherlands, Spinoza Premium of Netherlands Organization Prize for Scientific Research to Theunis Piersma and MAVA (Foundation Pour La Nature). We thank Camilo Carneiro, Danny Rogers, Gary Allport and another two anonymous reviewers for their comments and suggestions on an earlier version of the manuscript. This study was financially supported by the National Natural Science Foundation of China (No. 31830089 and 31772467), Science and Technology Commission of Shanghai Municipality (21DZ1201902), World Wide Fund for Nature Beijing Office (10003881), Shanghai Landscaping and City Appearance Administrative Bureau (G201610), and Scientific Research Fund of Yunnan Provincial Education Department (2022J0847).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100011.
|
Allport, G.A., Atkinson, P.W., Carvalho, M., Clark, N.A., Green, R.E., 2018. Local site use and frst northbound migration track of non-breeding Steppe Whimbrel Numenius phaeopus alboaxillaris (Lowe 1921). Wader Study 125, 219–227
|
|
Arlott, N., 2018. Birds of the Philippines, Sumatra, Java, Bali, Borneo, Sulawesi, the Lesser Sundas and the Moluccas. William Collins, London.
|
|
Baker, R.H., 1951. The Avifauna of Micronesia, Its Origin, Evolution, and Distribution. University of Kansas, Lawrence.
|
|
BirdLife International, NatureServe, 2015. Bird Species Distribution Maps of the World. BirdLife International and Arlington: NatureServe, Cambridge.
|
|
Bishop KD. Shorebirds in New Guinea: their status, conservation and distribution. Stilt. 2006;50:103-134
|
|
Carneiro C, Gunnarsson TG, Alves JA. Faster migration in autumn than in spring: seasonal migration patterns and non-breeding distribution of Icelandic whimbrels Numenius phaeopus islandicus. J Avian Biol. 2019;e01938
|
|
Cramp SC. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic. Vol.3. Waders to gulls. Oxford: Oxford university press; 1983
|
|
Crossland AC, Butcher S, Crutchley P, Mugan ND, Kake J. A survey of waders on part of Guadalcanal and the Florida group, Solomon Islands. Stilt. 2016;69-70:48-56
|
|
Deignan HG. Checklist of the birds of Thailand. Washington, Smithsonian Institution;1963
|
|
del Hoyo J, Collar NJ. HBW and Birdlife International illustrated checklist of the birds of the world. Vol.1. Non-passerines. Barcelona: Lynx Edicions; 2014
|
|
Gill BJ, Bell BD, Chambers GK, Medway DG, Palma RL, Scofield RP, et al. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th ed. Wellington: Te Papa Press; 2010
|
|
Grantham MJ. A note on the passage of variegatus and phaeopus type whimbrels through Alas Purwo National Park, East Java. Kukila. 2000;11:133-135
|
|
Hayman, P., Marchant, J., Prater, T., 1986. Shorebirds: An Identification Guide to the Waders of the World. Croom Helm, London
|
|
Iqbal M, Ridwan A. Summering of whimbrel in southern Sumatra, Indonesia. Stilt. 2009;55:20-24
|
|
Jeyarajasingam A. A field guide to the birds of Peninsular Malaysia and Singapore. Oxford: Oxford University Press; 2012
|
|
Jorgensen AA. Siams Vadefugle. III. Charadriiae (Tringinae). Dansk Orn For Tidsskr. 1949;43:216-237
|
|
Lappo EG, Tomkovich PP, Syroechkovski EE. Atlas of breeding waders in the Russian Arctic. Moscow: UF Ofsetnaya Pechat; 2012
|
|
Lekagul, B., Round, P. D., 1991. A Guide to the Birds of Thailand. Saha Karn Bhaet, Bangkok
|
|
MacKinnon J, Phillipps K. A field guide to the birds of Borneo, Sumatra, Java, and Bali (The Greater Sunda Islands). Oxford: Oxford university press; 1993
|
|
Newton I. The migration ecology of birds. London: Academic Press; 2008
|
|
Phillips WWA. A 1975 annotated checklist of the birds of Ceylon (Sri Lanka). Colombo: Wildlife and Nature Protection Society of Ceylon; 1975
|
|
Prater AJ, Marchant JH, Vuorinen J. Guide to the identification and ageing of Holarctic waders. Tring: BTO; 1977
|
|
Ripley SD. A synopsis of the birds of India and Pakistan: together with those of Nepal, Bhutan, Bangladesh, and Sri Lanka. Bombay: Bombay natural history society; 1982
|
|
Robinson HT, Chasen FN. The birds of the Malay Peninsula, Vol. 3: sporting birds, birds of the shore and estuaries. London: Witherby; 1936
|
|
Robson C. New Holland field guide to birds of South-East Asia: Thailand, Peninsular Malaysia, Singapore, Vietnam, Cambodia, Laos, Myanmar. London: New Holland; 2005
|
|
Stinson DW, Wiles GJ, Reichel JD. Occurrence of migrant shorebirds in the Mariana Islands. J Field Ornitol. 1997;68:42-55
|
|
Tomkovich PS. A new subspecies of the whimbrel (Numenius phaeopus) from central Siberia. Zool Zurnal. 2008;87:1092-1099
|
|
Wells DR. The birds of the Thai-Malay Peninsula, Vol. 1: non-passerines. London: Academic Press; 1999
|