| Citation: | Yunzhu Liu, Lan Wu, Jia Guo, Shengwu Jiao, Sicheng Ren, Cai Lu, Yuyu Wang, Yifei Jia, Guangchun Lei, Li Wen, Liying Su. 2022: Habitat selection and food choice of White-naped Cranes (Grus vipio) at stopover sites based on satellite tracking and stable isotope analysis. Avian Research, 13(1): 100060. DOI: 10.1016/j.avrs.2022.100060 |
By combining satellite tracking, land-cover extracted from Landsite 8 images, and the traditional stable isotope analysis, we studied the habitat selection and food preference of a vulnerable migratory waterbird, the White-naped Crane (Grus vipio), in one of its key stagging sites, the Shandian River Basin in the semi-arid northern China, to provide knowledge that is critical for its conservation in the Anthropocene. Our results showed that the White-naped Cranes used both uplands and natural wetlands in the stopover site. While the cranes used farmland and natural land cover equally as night-time roosting grounds, they spent most daytime foraging at farmlands. Despite the extensive usage of croplands as their foraging ground, the Bayesian mixing models based on stable isotopic analysis revealed that crop residues after harvesting, such as Maize (Zea mays) and Naked Oat (Avena chinensis), were only a small fraction of the White-naped Cranes' diet (~ 19%), and their diet composited mainly natural plants, such as Allium ledebourianum, Potentilla anserina, and P. tanacetifoli. Moreover, more than 20% of the total wetlands in the region were modelled as home range of the cranes. On contrast, less than 10% of croplands and about 1% of the unused uplands were identified as home range. In addition, the entire core habitats were located in natural wetlands. Our findings demonstrated the importance of natural wetlands for the survival of the threatened crane. However, the satellite-derived land cover data showed that croplands increased rapidly in the last decade in this area, at the expense of natural wetlands. With the sharp decrease of White-naped Crane population in China, the conservation of stopover sites becomes imperative. Based on our analysis, we recommend the following management actions: conserving adequate natural wetland area, regulating anthropogenic pressures such as the use of herbicides, expanding the duration and extent of current conservation regulations, establishing a comprehensive monitoring program, and initiating basin-scale ecological restoration, for effective conservation of this threatened species. These integrated conservation strategies for migratory waterbirds are necessary, considering the rapid land-cover changes and agricultural expansion that have been occurring in East Asian-Australasian Flyway, especially in the semi-arid temperate zone.
Nearly 20% of the world's bird species are migratory, including most of the 870 waterbird species (Wetland International, 2012; del Hoyo et al., 2018). Stopover sites, critical areas used by migratory birds for refueling and re-energizing during their annual journeys often receive less attention than breeding and wintering grounds in conservation actions (Elphick and Oring, 2003; Kirby et al., 2008; Yang et al., 2011). During the Anthropocene, wetland loss, degradation and fragmentation all over the world have been driving rapid loss of biodiversity and increasing risk of species extinction (Gibbs, 2000). These processes also threaten the survival of waterbirds, forcing these birds to change their habitat use, feeding behaviors, and even their migration routes (Jia et al., 2021).
The East Asian-Australasian Flyway (EAAF) is considered the most threatened of the world's nine major flyways, and it has a higher proportion of endangered species compared to other flyways (IUCN, 2012). In China, human population pressure and modern farming have resulted in large-scale land reclamation, converting a significant number of wetlands into farmlands (Liu et al., 2004). Farmlands may offer supplementary foraging opportunities for some migratory waterbirds (Wu et al., 2020), but this also creates potential conflicts with humans (Fox et al., 2017). Wintering grounds for migratory waterbirds such as cranes have been relatively effectively protected under the national wildlife conservation legislation. For example, many important habitat areas for wintering cranes in China, such as East Dongting Lake, West Dongting Lake, large part of Poyang Lake, and Shenjinghu Lake, were gazetted as nature reserves (Wang et al., 2021a, Wang et al., 2021b; Xiang et al., 2021). However, although many studies have demonstrated the vital function of stopover sites as refueling stations for migratory birds (Xia et al., 2017), most are not sufficiently protected by law and their capacity to support migratory birds is continuing to degrade (Lei et al., 2019) with the continuation of loss and fragmentation of wetlands and the expansion of farmlands (Jia et al., 2021). The main reason for this lack of protection is the lack of understanding of their importance for migratory waterbirds and when and how these areas are used. Previous studies have shown that waterbirds can adapt rapidly to dramatic habitat changes by broadening their diet, increasing foraging time and expanding foraging areas, and this flexibility allows them to cope with the impacts of the environmental changes and human disturbance (Hamer and Furness, 1993; Karanth and Sunquist, 1995, 2000; Fedriani et al., 1999). In this respect, expansion of croplands may offer some short-term benefits for some species. However, the long-term effects on their evolutionary trajectory are not known.
Habitat selection is one of the fundamental questions in animal ecology (Jones, 2001; Cagnacci et al., 2010), especially regarding migrating waterbirds. Animal tracking technologies are rapidly developing and widely used in scientific research and conservation practice (Bridge et al., 2011). Utilization distribution of wildlife is a basic research field in conservation biology and animal movement ecology (Kranstauber et al., 2012) as it is focused on understanding the habitat requirements and adaptation of animals and is significant in estimating habitat selection. The recent use of satellite tracking technologies in research on migratory dynamics and resource utilization of birds has resulted in enormous progress in this study field (Bridge et al., 2011; Chen et al. 2021). Selection of habitats by birds, especially of feeding sites and roosting sites, can be detected, delineated and visualized by exploring the tracking data. Cranes are typical migrants and excellent navigators with high habitat fidelity. Due to their large body size, cranes could easily carry the equipment in satellite-tracking studies (Jia et al., 2021).
Paralleling to the recent developments in animal movement ecology, advances in stable isotope ecology have provided powerful tools to identify the food sources and to quantify dietary composition using carbon and nitrogen stable isotopes from different types of samples (Xu et al., 2022). As diet selection and feeding behavior are partially determined by food availability, the investigation of food preferences can aid in the identification of conservation sites for threatened species (Hirzel et al., 2004). Cranes are omnivorous animals, but they are mainly herbivorous at stopover and wintering sites and highly selective in their food preferences. They discriminate among plant species, individuals of the same species, and different parts of the same plant. The use of stable isotopes in foraging studies is based on the fact that different dietary items often have different isotopic signatures that can be detected through blood, feather or feces samples (DeNiro and Epstein, 1978; Hahn et al., 2012).
In this study, we combined isotopic diet composition resource analysis and telemetric data modeling to investigate food choice and habitat selection of the White-naped Crane (Grus vipio, hereinafter referred to as "WNC"), a threatened migratory species, in an area that is rapidly changing in terms of land cover, notably the reduction in the extent of wetlands. With the land cover maps derived from satellite images, we further quantified their usage of cropland and natural wetland. WNC has been listed as Vulnerable in the IUCN Red List since 1994 (BirdLife International, 2018), with an estimated population of less than 7000 individuals (Wetland International, 2012). WNCs are omnivorous but mainly plant-based. This species' wintering population in China has sharply decreased in the past 15 years, from 4000 individuals in 2002 to the current population size of 100–1500 (Wetland International, 2012). Habitat loss and agricultural expansion over the past 15 years are thought to be key threats behind this dramatic decrease (BirdLife International, 2018). Although most of the breeding sites (eastern steppe of Mongolia) and wintering sites (the mid-Yangtze River Basin) of WNC, have been protected, currently comprehensive knowledge regarding their stopover sites in China is lacking (Batbayar et al., 2021; Jia et al., 2021). Moreover, we cannot understand the true causes of the rapid population decrease nor implement effective conservation strategies without identifying stopovers sites and how these are used. By using stable isotope analysis and satellite tracking technologies, we studied the main food resources and the habitat utilization distribution (UD) of WNCs in one of their main stopover sites, the Shandian River Basin, in the semi-arid temperate zone of China. Our hypotheses are: 1) WNCs may use both wetlands and croplands as foraging sites; 2) the birds feeding on croplands use more food resources than those feeding in wetlands; 3) as wetlands are safer than croplands for these birds, WNCs use wetlands as night-time roosting sites.
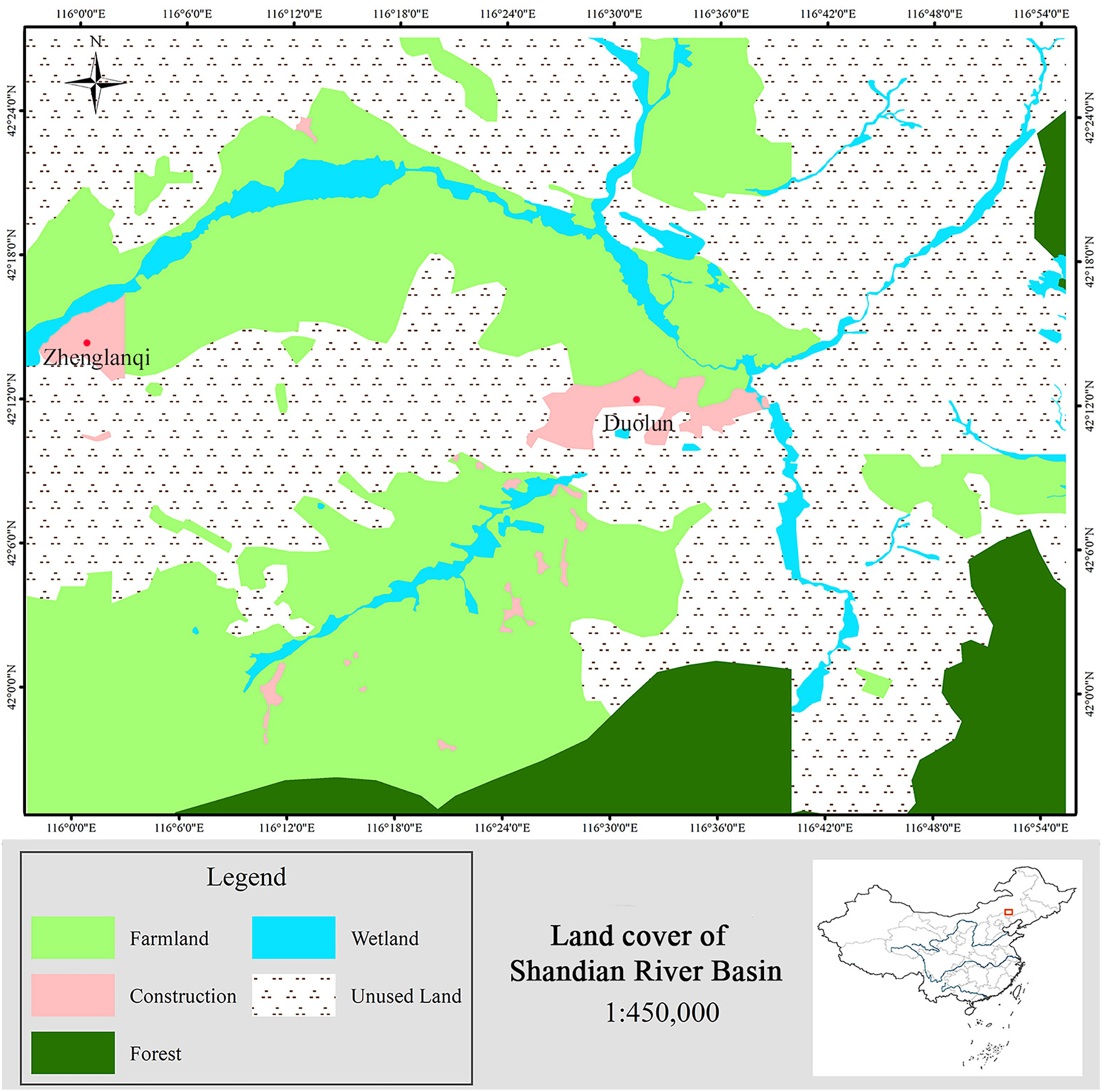
The White-naped Crane uses the Shandian River Basin (42.40°–41.91° N, 115.83°–116.81° E; Fig. 1) as its principal stopover site on its western flyway, almost 90% WNC use this area for staging site (Jia et al., 2021). Shandian River Basin is located in Duolun County and Zhenglanqi County in the central part of the Inner Mongolia Autonomous Region, and Guyuan County in Hebei Province, China. This area has a cold semi-arid climate and comprises an agro-pastoral ecotone located between the Hunshandake Desert and the Yanshan Mountains without a great diversity and high endemism of plants. The main land cover types are wetlands, grasslands, and croplands, all of which can be used by WNC during stopover. Because of the area's low temperatures and short frostless periods, corn, oats, buckwheat, potato, and other products are extensively cultivated in this region (Wu et al., 2011).
From August 2013–2016, the International Crane Foundation (ICF), the Mongolian Academy of Sciences (MAS), and the Wildlife Science and Conservation Center of Mongolia (WSCC) conducted the tracking of birds. Catching and handling cranes was permitted under a scientific research license No.04/2550 issued by the Ministry of Environment and Tourism in Mongolia. The Khurkh (48°22′28.2″ N, 11°21′32.5″ E) and Khuiten Valleys (48°17′15.9″ N, 110°5′17.9″ E) in Mongolia are the main breeding sites for the targeted subpopulation of WNC. Nine cranes caught in the two catchments were banded and GPS transmitters were attached to their legs. Three cranes were fitted with GPS-Argos tags (Model: 30 GPS; North Star Science and Technologies, USA) and the other six were equipped with GPS-GSM tags (Model: CTT-1060a; Cellular Tracking Technologies, USA; hereafter referred to as CTT data). The maximum weight of the transmitters is 60 g, and is about 1.2% of the average weight of the captured cranes; therefore, the fixation has minimal adverse effects on the birds. A GPS-GSM system was used to record the position of tagged cranes at a time interval of 30 min (hereinafter referred to as PTT data). All PTTs and CTTs were fixed on the crane during the period of wing molting, i.e., from July to August, when the WNC is flightless. The PTT data was downloaded daily via the Argos Message Retriever (PTT Tracker Ver1.0.0.3.7, GeoTrak, Inc. http://www.geotrakinc.com/ptt-tracker/, latest access: January 13, 2018). To increase the positioning accuracy and the number of useable fixes, the tags were programmed to record the Argos and GPS locations simultaneously (Hirzel et al., 2004). Each Argos location was assigned to a Location Class (LC) as follows: 3, 2, 1, 0, A, B, or Z, corresponding to an estimated error of: from < 150 m to > 1000 m (LC 3, 2, 1, and 0), no accuracy estimation (LC A and B), and invalid location (LC Z). The CTT data was downloaded directly from Cellular Tracking Technologies (http://account.celltracktech.com/, most recent access: January 13, 2018). The estimated GPS location accuracy (HDOP, fix ≥3) was from < 25 m to 76–100 m.
GPS records of fix (i.e., the time and coordinates of the relocations) were used to distinguish roosting and foraging behaviors and to calculate the daily utilization distribution of WNCs during the autumn migration season. Duplicate and low precision records were first screened and removed from the raw telemetry data to ensure the unique fix. The cleared telemetric data were then used to create 11 trajectories (one for each tagged bird per year). AdehabitatLT, a R package by (Calenge, 2011) was used to create, manipulate, and store these trajectories. Although the PTT and CTT are programmed to return a regular relocation, slightly delays are common in tracking devices, resulting in an irregular sequence. In addition, missing location points are expected as the GPS unit sometimes fails to receive signal (Calenge, 2011). More importantly, the sampling intervals of the two satellite tracking campaigns (CTT and PTT) were different. However, to ensure that we had enough location points to model utilization distribution (UD) and to delineate the home range core habitat of WNCs, both PTT and CTT data were used. Daytime relocations were used to model foraging habitats and nighttime fixes were used to define roosting habitats. Based on the sunrise and sunset times in the area, daytime and nighttime were considered to be at 6:30–17:30 and 17:30–6:30, respectively.
As temporal autocorrelation is an intrinsic property of animal tracking sequential data (Kuhn et al., 2009; Tomkiewicz et al., 2010), the biased random bridge (BRB) approach was used to estimate the UD of WNCs. BRB is in principle similar to the random Brownian bridge approach (Horne et al., 2007), but with several improvements for more accurate estimation of UD (Benhamou, 2011), especially for sequences with large intervals. The Brownian bridge approach estimates the probability density of a trajectory passing through any point of the study area, which is modelled as a conditional random walk between consecutive pairs of relocations. The modelled probability density is dependent on the time interval between locations, the Euclidean distance between locations, and the Brownian motion variance, which is a tuning parameter related to the animal's mobility (Horne et al., 2007). The Brownian bridge approach assumes that the animal motion between two consecutive position is random and purely diffusive: the animal moves randomly from the starting point and reaches the next position. The BRB approach improves the modelling by adding an advection element (i.e., a "drift") to the purely diffusive movement in the Brownian bridge; BRB assumes that the animal movement is controlled by a drift component (i.e., a general tendency to move towards the next position) and a diffusion component (tendency to move in directions other than the direction of the drift) (Benhamou and Cornélis, 2010). The addition of the drift is therefore more realistic representing animal movements (Calenge, 2011). A detailed description of the BRB approach can be found in the BRB approach is briefly outlined as follows, and the technical details of this approach is provided by Benhamou (2011).
Considering that one step in an animal trajectory includes two successive relocations r1 = (x1, y1) and r2 = (x2, y2) collected at time t1 and t2, the BRB approach estimates the probability density function (PDF) of the animal located at any place ri = (xi, yi) at time ti (with t1 < ti < t2) using a bivariate normal distribution:
| f(ri,ti|r1,r2)=t2−t14πD(t2−t1)exp[rmDrm4pi(t2−t1)] | (1) |
| rm=xi+pi(x2−x1)yi+pi(y2−y1) | (2) |
| pi=ti−t1t2−t1 | (3) |
where rm is the mean location, pi is the proportion of time from starting relocation to rm, and D is the diffusion matrix. From this BRB model, the home range and core areas were defined as the areas encompassed within 95% and 50% UD isopleths, respectively (Monsarrat et al., 2013).
Annual survey of WNC population during autumn of 2013–2016 was also carried out throughout the entire Shandian River Basin, using a point count method with a telescope (Swarovski ATS 80 HD 20-60 × 80). The survey data and the accompanying field observations provide valuable information in interpreting the outputs of UD modelling.
The 30 m resolution Landsat 8 Collection 1 Level-1 Precision and Terrain Correction product (L1TP) images from 24 August 2014 (Scene ID: LC08_L1TP_124031_20140826_20200911_02_T1, courtesy of the U.S. Geological Survey, obtained from https://earthexplorer.usgs.gov/) were used to interpret land cover in the Shandian River Basin. Based on our field observation from 2013 to 2016, the change in land cover at our study site during this time frame is neglectable; therefore, we select a year that lies in our survey timespan, which should not affect the following analysis. Images acquired in August were selected to fit the season of our survey, while images from later in the year would be subject to contamination from frost and/or snow.
Based on the Chinese Land Cover Classification System (Ogden et al., 2004), we simplified the land cover types in Shandian River Basin into five categories: unused land (including mainly barren land, desert steppe, and undeveloped land), wetland, farmland, construction (residence, roads, mining area, and industrial area), and forest. We used an object-oriented automated algorithm, combined with ground survey and radar data to classify the land cover in ERDAS IMAGINE 2016 and ArcGIS 10.0 designed by ESRI Company. The contribution of land cover types to staging habitats was then calculated by overlaying with the modelled UDs. Two land cover types are assumed to be suitable staging habitats: wetlands and farmlands.
Based on the GPS data, WNCs mainly used the Shandian River Basin as a stopover site in Duolun, Inner Mongolia. Therefore, we collected 30 fecal samples of WNC from the positions extracted from the GPS tracking to investigate the potential food resources. The visible dopants were removed from the fecal samples for further analysis. Based on previous studies, field observation of WNC's foraging, and knowledge on the natural and artificial habitat characteristics of the Shandian River Basin, three natural plant species Allium ledebourianum, Potentilla anserina, P. tanacetifolia and two crop plants Maize (Zea mays) and Naked Oat (Avena chinensis) were sampled.
Fecal samples were frozen at −18℃, lyophilized in vacuum until it reached a certain mass, and crushed into a find and homogeneous powder in a mortar and pestle. The samples were then placed in tin capsules for stable isotope analysis. Three samples of each plant were random selected then washed with distilled water in the lab, dried in an oven at 60℃, and then ground into a uniform powder for isotope analysis (Ogden et al., 2004).
Stable isotopic analysis was performed using a Finnegan MAT 253 (Thermo Scientific, USA) continuous-flow isotope ratio mass spectrometer equipped with the Flash Elemental Analyzer 1112 system. The stable isotope ratio was calculated (expressed as δ13C and δ15N [‰]) using the following equation:
| X‰=RsampleRstandard−1 | (4) |
where X is δ13C and δ15N, and R is the 13C/12C or 15N/14N. The reference standards for δ13C and δ15N were Pee Dee Belemnite limestone and atmospheric nitrogen, respectively. Analytical precisions of δ13C and δ15N in the laboratory were ±0.1% and ±0.3%, respectively.
To determine the contribution of each plant, R package "siar" was used (Parnell and Jackson, 2013). The Bayesian mixing models used to infer the intraindividual contribution were run with 500, 000 iterations, 50, 000 burn-in, and flat priors with the 'siarmcmcdirichletv4' function. This function used the Markov chain Monte Carlo (MCMC) to determine WNC diet composition (Parnell and Jackson, 2013). Enrichment factors for feces were 3.54 ± 0.74‰ (δ15N) and 1.63 ± 0.63‰ (δ13C) in all models. All statistical analyses were performed using the R 3.3.3 software (R Core Team, 2017). Results were presented as mean ± SD.
Our results showed that unused lands (mainly bare ground and desert) and farmlands (1663.7 km2) occupied most of the Shandian River Basin (Fig. 1). Overall, unused land (2394 km2) was the largest type of land cover in this area followed by farmland (1663.7 km2), forest (439.2 km2), wetland (207.5 km2), and construction land (91.3 km2). Within the Shandian River Basin, a total of 1871.2 km2 was classified as potentially suitable crane habitat (wetland and farmland), covering 39% of total mapped area.
During the autumn migration periods of 2013–2016, there were 647, 663, 883, and 714 cranes recorded in the Shandian River Basin, respectively, representing more than 60% of the western population of the species. From the tagged cranes, a total of 14 completed southward migratory trajectories were obtained. Note that some birds had multiyear routes, therefore more than one trajectory (Table 1). WNCs stayed at the Shandian River Basin for 16.9 ± 5.9 days (n = 14) during their autumn migration, which indicates that this area is the most important WNC stopover site. These cranes were observed feeding on wetlands and croplands, almost always at the same locations, and they rarely flew for long distances during staging at this area.
| IDs | Autumn season | |||
| Arrive | Leave | Days | Data bulks | |
| 130948 | 2013/10/11 14:00 | 2013/11/6 4:35 | 25.6 | 556 |
| 130950 | 2013/10/8 13:55 | 2013/10/29 4:53 | 20.6 | 70 |
| 139082 | 2014/10/12 19:32 | 2014/10/26 5:34 | 13.4 | 45 |
| 139082 | 2015/10/9 23:05 | 2015/10/28 6:18 | 18.3 | 66 |
| 139083 | 2014/10/13 0:30 | 2014/10/26 7:46 | 13.3 | 47 |
| 150758 | 2014/10/13 12:49 | 2014/10/26 5:34 | 12.7 | 15 |
| CGNE01 | 2016/10/8 15:02 | 2016/10/31 6:01 | 22.6 | 156 |
| Chukh | 2014/10/12 16:53 | 2014/10/26 8:15 | 13.6 | 645 |
| Chukh | 2015/10/15 13:38 | 2015/10/29 10:20 | 13.9 | 639 |
| Duaria | 2015/9/27 17:56 | 2015/10/28 10:19 | 30.7 | 1350 |
| Tenger | 2013/10/14 16:24 | 2013/10/29 9:50 | 14.7 | 690 |
| Tenger | 2014/10/17 11:37 | 2014/10/26 8:16 | 8.9 | 185 |
| Xiwang | 2015/9/27 17:57 | 2015/10/8 8:25 | 10.6 | 498 |
| Xiwang | 2016/10/10 13:34 | 2016/10/28 8:48 | 17.8 | 906 |
| Average | 16.9 | 5868 | ||
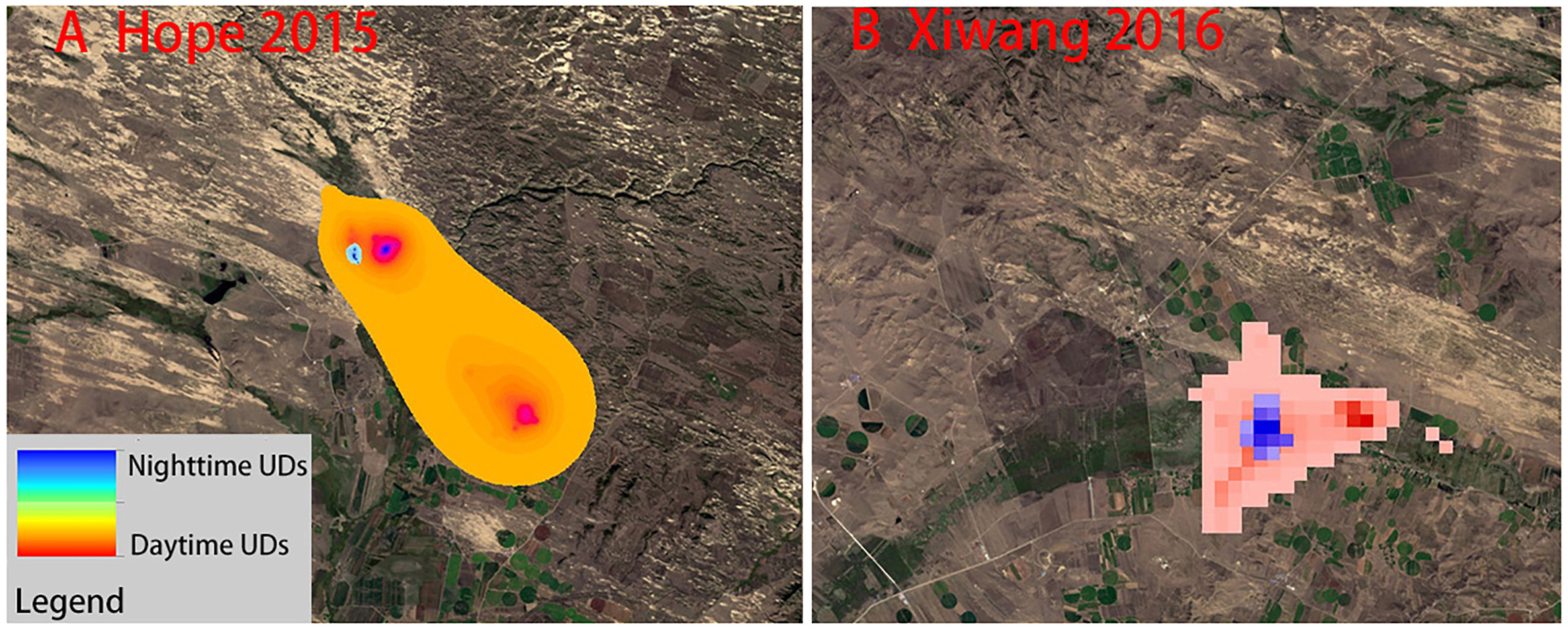
Using the outputs of the fitted BRB models, we calculated the UD of each trajectory. The 14 UDs were then pooled to map and delineate the home range and core habitats in our study area, and the results were presented in Fig. 2. We identified three separated home ranges in Shandian River Basin, where WNCs typically congregated.
By overlaying the summed UD with the land cover map, how the WNC used the landscape was revealed. Table 2 summarized the proportion of land cover types utilized by the birds. The WNC habitats as the same with daytime UDs were farmlands (153.7 km2), wetlands (46 km2), and unused lands (25.2 km2) in Shandian River Basin. These results are consistent with our ground observations. During the day, WNC used areas twice as extensive as those used at night-time (4.7% of the total area of 224.9 km2 vs. 2.2% of the total area of 106.3 km2; Table 2). Farmlands, wetlands, and unused lands accounted for 3.2%, 14.3%, and 1% of land use during the night and 9.2%, 22.2%, and 1.1% of land use during the day. Farmlands were the land type most used for staging sites, followed by wetlands and unused lands (50.3%, 27.9%, and 21.7% at night and 68.2%, 20.4%, and 11.2% during the day, respectively; Table 2). Dramatic differences in habitat use between daytime and night-time were found.
| Farmland | Wetland | Unused land | Total | ||
| Nighttime | Area (km2) | 53.5 | 29.7 | 23.1 | 106.3 |
| A | 3.2% | 14.3% | 1.0% | 2.2% | |
| B | 50.3% | 27.9% | 21.8% | ||
| Daytime | Area (km2) | 153.7 | 46.0 | 25.2 | 224.9 |
| A | 9.2% | 22.2% | 1.1% | 4.7% | |
| B | 68.3% | 20.5% | 11.2% | ||
| A, Land cover types (%) used within the Shandian River Basin; B, Land cover types (%) used within each type of habitat. | |||||
Large variations were found regarding the estimated WNC UD during daytime and night-time. During the daytime, WNCs were found to feed on corn and naked oat fields, whereas at night, they roosted on the wetlands (Fig. 3).
The stable isotope content of potential food sources was shown in Fig. 4. The results showed that maize had higher δ13C than four other potential food sources. Two Potentilla plants had higher δ15N than three other potential food sources.
Based on the Bayesian stable isotope mixing model, the three wetland plants, A. ledebourianum (27.89%), P. anserine (29.14%), and P. tanacetifolia (24.63%), accounted for 81.66% of the total WNC diet, while crop residuals Z. mays (2.73%) and Avena chinensis (15.61%) attributed to 18.34% of the bird's dietary intakes (Fig. 5). In addition, the diet model showed that the cranes had no preference regarding to native plants (the contribution was not significantly different). For crop residuals, the contribution of maize was significantly lower.
In this study, our results showed that the White-naped Cranes stayed an average of 16.9 (SD = 5.9) days in Shandian River Basin during their autumn migration season, suggesting the importance of this region as the refueling stopover site for the western flyway population of the species. The studied cranes used both farmlands and natural wetlands in the area, and they spent more daytime foraging in croplands but used natural wetlands as night-time roosting sites for considerable time. Despite the extensive usage of croplands as their feeding ground, the stable isotopic results showed that agricultural residues after crop harvesting, maize and naked oat in our study, were only a small fraction (18.34%) of the White-naped Cranes' diet, and the main food sources were from native wetland plants. This inconsistence in habitat usage and food intakes might be resulted from the elevated contribution of nocturnal foraging (Dugan, 1981), which is suggested as an adaptive strategy of wildlife to increased human activities (McMahon and Evans, 1992). The scarcity of crop residuals might be another reason for the marginal contribution to the crane diet (Alonso et al., 1994). Future study including food abundance survey could reveal more insights in habitat usage. There is an important caveat of using fecal isotopic signals to model potential diet composite. As foods that are easily digested, crop residual grains consisting of more starch and protein in this study, could be incorrectly interpreted. Using blood or plasma samples (that are extremely difficult to obtain) could provide more reliable estimates.
Many migratory waterbirds in EAAF, especially cranes and geese, heavily rely on wetlands as staging sites in semi-arid areas (Lei et al., 2019). During this period, the choice of foraging grounds was directly related to energy replenishment. The influence of food availability is obvious, especially in the food-poor seasons (Zou and Wu, 2006). Though the food selection is the most important part of habitat selection, and for cranes at stopover sites, supplementing food while ensuring safety is also a major factor in habitat selection, which is why the importance of natural wetlands is highlighted in the conclusions of this article. As a result of climate change, particularly the increase in temperature and change in precipitation patterns, and population increase, the northwards extension of farmlands has increased in the northern hemisphere (Wang et al., 2021a). Therefore, the landscape on which stopover sites are located have been experiencing rapid changes, including the reduction of wetland areas and increased habitat fragmentation. These changes largely threaten WNC, although some studies suggest that increase in croplands also presents them with new opportunities (Lambin et al., 2001; Pielke, 2005).
The Red-crowned Cranes (Grus japonensis) select farmland as the foraging ground based on the height, density, area of crop and the distances to open water and reed swamps (Liu et al., 2013). In the early and middle stages of wintering, farmland is the main foraging habitat, from which the Hooded Cranes (G. monacha) have the highest foraging frequency and efficiency (Zhang et al., 2015; Cai et al., 2019). The stable isotope result is consistent with the dietary habits of the White-naped Crane studied by the traditional fecal microscopic analysis (Zou et al., 2005, Zou et al., 2012). The research results of Zou and Wu (2006) also showed that WNC prefer to eat plants with high crude protein and low crude fiber as their main food. There was a significant correlation between the food composition ratio and plant nutrition of WNC, scilicet a highly significant negative correlation with crude fiber and a significant positive correlation with crude protein.
The measurement of naturally occurring stable isotope contents from bird samples provides quantifiable data on diet and habitat selection (Hobson et al., 1997, 2000). Moreover, the isotopic analysis of feces allows determination of which food resources are absorbed. The contents of all the natural-growing plants (i.e., A. ledebourianum, P. anserine, and P. tanacetifolia) were higher than the content of the crops (i.e., Z. mays and Avena chinensis) in the feces samples. This indicates that WNCs not only feed on crops but also in the natural wetland. As crops are more easily digested and absorbed than natural-growing plants, we expected the cellulose content from the natural-growing plants to be high and we found that the protein and starch contents of maize and naked oats were higher than the cellulose content. It is widely known that protein and starch can be easily digested and rapidly absorbed by WNCs; therefore, these resources may be targeted to obtain energy for migrations.
Recent changes in climate, mainly higher temperature and lower summer precipitation (Angerer et al., 2008), has resulted in rapid reclamation of wetlands into croplands and woodlands in the Shandian River Basin, especially in Duolun County, in the last 30 years (Liu and Tong, 2003; Wu et al., 2011; Nendel et al., 2018). These land use changes could be positive for WNCs as they may indicate the creation of more staging sites; however, they also indicate a reduction of these birds' natural habitats. Our results showed that the WNCs mainly foraged in wetlands and croplands during the day and that they spent more time roosting on wetlands during the night. This result differs from those of a study of WNCs from Bohai Bay (located further south than Duolun), which found that WNCs mainly used natural wetlands (Higuchi et al., 2004; Jia et al., 2021). More importantly, our results showed that key habitats were located entirely in wetlands, suggesting the irreplaceable functional role of wetlands in wildlife conservation (Keddy et al., 2009). Our analysis of utilization distribution indicated that foraging in croplands was more frequently-used than wetland. This area is in an arid and semi-arid region, and because of its latitude and location, the frost-free period is short, and the agricultural cultivation period is from May to October each year. The White-naped Cranes use this area from March to April and from October to November, which are non-agricultural periods, so the impact of human interference is low. However, presumably for safety from predators, such as dogs and cats, WNCs also used wetlands as roosting sites. The use of croplands may be an important adaptation strategy in the situation of rapid land cover change. However, a minimum area for roosting is still required. Thus, the ideal habitat would possibly be a wetland of an adequate size surrounded by croplands (Fig. 6).
Habitat selection and use can be influenced by many factors including food availability, predation risk, and social behaviors. Stopover sites connect breeding and wintering sites of waterbirds. Foraging in stopover sites is directly related to energy supply, migratory success rate, and population dynamics, especially during the season of food shortage. WNCs are omnivorous and their diet is variable according to available sources. Based on the analysis of potential food resources, we found that crops are a good food supplement. Moreover, compared with plants from wetlands, corn and naked oats may be more easily digestible. A. ledebourianum, P. anserine, and P. tanacetifolia have a high cellulose content, whereas corn and naked oats contain more protein and starch, which can be rapidly digested by WNCs. These findings are supported by the studies of WNC wintering and staging in Japan and Korea, where Common Cranes (Grus grus) and Hooded Cranes use croplands and are fed by local farmers. However, in Korea cranes still need wetlands to roost (Meine and Archibald, 1996; Harris and Mirande, 2013). Our study confirmed that the WNCs spent more than one third of their autumn migratory journey in Shandian River Basin, suggesting that it is one of the most important staging sites of WNC's western population (Jia et al., 2021). The study showed that the cranes used only a small proportion of the croplands but more than 20% of the natural wetlands were utilized as foraging or roosting habitats. Despite our results of potential food sources are inconclusive, the contribution of residual crops may be supplementary, and the diet of the cranes consists of considerable native wetland plants. WNCs seem to be highly adaptive birds that can quickly alter their diet. However, although crops contain many sources of protein that can provide herbivorous waterbirds with high-quality energy, foraging in croplands may expose waterbirds to threats such as poaching, predation, and disturbance.
Due to sharp decrease of White-naped Crane population in China, the conservation of stopover sites becomes imperative. We conclude that farmland plays an important role in the food supply for WNC now, but we must keep and management wetlands that allow the cranes to roost safely. Based on our analysis, we recommend the following management actions, including keeping monitor both WNC and the land use change of habitat, education and public awareness of local residents should be strengthened to reduce disturbance to migrating cranes, for effective conservation of this threatened species in the future. As the Shandian River Basin does not belong to a specific protected area, and because it provides important resources for WNCs, a new wetland reserve or wetland park should be established. This integrated conservation actions and strategies for migratory waterbirds are necessary, considering the rapid land-cover changes and agricultural expansion that have been occurring in the semi-arid temperate zone which is the important stopover site in East Asian-Australasian Flyway.
Conceptualization: YJ and GL; methodology: L. Wu and L. Wen; validation: YJ, GL and L. Wu; investigation: YL, JG, SJ, LS, and SR; writing-original draft preparation: YJ and YL; writing-review and editing: YJ, L. Wen and YW; supervision: YJ and CL; project administration: GL. All authors read and approved the final manuscript.
The scientific research license for catching and handling cranes was issued by the Ministry of Environment and Tourism in Mongolia (Permission No. 04/2550). Crane fecal samples were collected without any disturbance to the animals.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank the late James Harris and other staff from the International Crane Foundation, Wildlife Science and Conservation Center of Mongolia for the use of the tracking data. This research was funded by National Natural Science Foundation of China (No. 31971400) and the Fundamental Research Funds for the Central Universities (No. BLX202144). We wish to thank the first class General Financial Grant from the China Postdoctoral Science Foundation (No. 2017M620023), the Free Flying Wings Program, and the SEE Foundation.
|
Cai, T., Huettmann, F., Lee, K., Guo, Y., 2019. Analyzing stopover and wintering habitats of hooded cranes (Grus monacha): implications for conservation and species dispersion in the East Asia. Pakistan J. Zool. 51, 1323.
|
|
Calenge, C., 2011. Analysis of Animal Movements in R: the adehabitatLT Package. R Foundation for Statistical Computing, Vienna.
|
|
del Hoyo, J., Elliott, A., Christie, D., 2018. Handbook of the Birds of the World. Lynx Edicions, Barcelona.
|
|
Dugan, P.J., 1981. The importance of nocturnal foraging in shorebirds: a consequence of increased invertebrate prey activity. In: Jones, N.V., Wolff, W.J. (Eds. ), Feeding and Survival Strategies of Estuarine Organisms. Marine Science, vol. 15. Springer, Boston, pp. 251-260.
|
|
Liu, Q., Tong, Y., 2003. The effects of land use change on the ecoenvironmental evolution of farming-pastoral region in Northern China: with an emphasis on Duolun county in Inner Mongolia. Acta Ecol. Sin. 23, 1025-1030.
|
|
Meine, C.D., Archibald, G.W., 1996. The Cranes: status survey and conservation action plan. IUCN, Gland, Switzerland, and Cambridge.
|
|
Ogden, L.J.E., Hobson, K.A., Lank, D.B., 2004. Blood isotopic (δ13C and δ15N) turnover and diet-tissue fractionation factors in captive dunlin (Calidris alpina pacifica). Auk 121, 170-177.
|
|
Wetland International, 2012. Waterbird Population Estimates, fifth edition. Summary Report. Wetlands International, Wageningen.
|
|
Wu, H.Z., Ruhan, A., Guo, T.B., Sun, Z.Y., 2011. Impacts of land use change on ecosystem services value in Duolun County of Inner Mongolia based on RS and GIS. Sci. Geog. Phisica Sin. 31, 110-116.
|
|
Xia, S., Yu, X., Millington, S., Liu, Y., Jia, Y., Wang, L., et al., 2017. Identifying priority sites and gaps for the conservation of migratory waterbirds in China's coastal wetlands. Biol. Conserv. 210, 72-82.
|
|
Zou, H.F., Feng, X.D., Wu, Q.M., Wu, Y.N., Hao, M., Ma, J.Z., 2012. Diet component and preference of white-naped crane during courtship period in Zhalong Nature Reserve. J. Northeast For. Univ. 40, 69-76.
|
| IDs | Autumn season | |||
| Arrive | Leave | Days | Data bulks | |
| 130948 | 2013/10/11 14:00 | 2013/11/6 4:35 | 25.6 | 556 |
| 130950 | 2013/10/8 13:55 | 2013/10/29 4:53 | 20.6 | 70 |
| 139082 | 2014/10/12 19:32 | 2014/10/26 5:34 | 13.4 | 45 |
| 139082 | 2015/10/9 23:05 | 2015/10/28 6:18 | 18.3 | 66 |
| 139083 | 2014/10/13 0:30 | 2014/10/26 7:46 | 13.3 | 47 |
| 150758 | 2014/10/13 12:49 | 2014/10/26 5:34 | 12.7 | 15 |
| CGNE01 | 2016/10/8 15:02 | 2016/10/31 6:01 | 22.6 | 156 |
| Chukh | 2014/10/12 16:53 | 2014/10/26 8:15 | 13.6 | 645 |
| Chukh | 2015/10/15 13:38 | 2015/10/29 10:20 | 13.9 | 639 |
| Duaria | 2015/9/27 17:56 | 2015/10/28 10:19 | 30.7 | 1350 |
| Tenger | 2013/10/14 16:24 | 2013/10/29 9:50 | 14.7 | 690 |
| Tenger | 2014/10/17 11:37 | 2014/10/26 8:16 | 8.9 | 185 |
| Xiwang | 2015/9/27 17:57 | 2015/10/8 8:25 | 10.6 | 498 |
| Xiwang | 2016/10/10 13:34 | 2016/10/28 8:48 | 17.8 | 906 |
| Average | 16.9 | 5868 | ||
| Farmland | Wetland | Unused land | Total | ||
| Nighttime | Area (km2) | 53.5 | 29.7 | 23.1 | 106.3 |
| A | 3.2% | 14.3% | 1.0% | 2.2% | |
| B | 50.3% | 27.9% | 21.8% | ||
| Daytime | Area (km2) | 153.7 | 46.0 | 25.2 | 224.9 |
| A | 9.2% | 22.2% | 1.1% | 4.7% | |
| B | 68.3% | 20.5% | 11.2% | ||
| A, Land cover types (%) used within the Shandian River Basin; B, Land cover types (%) used within each type of habitat. | |||||