| Citation: | Urban OLSSON, Per ALSTRÖM. 2013: Molecular evidence suggests that the enigmatic Sulawesi endemic Geomalia heinrichi belongs in the genus Zoothera (Turdidae, Aves). Avian Research, 4(2): 155-160. DOI: 10.5122/cbirds.2013.0015 |
The taxonomic status of the Sulawesi endemic Geomalia heinrichi has long been debated, and it has variously been treated as a babbler (Timaliidae) or a turdid (Turdidae). We estimated the phylogeny of 43 taxa in the family Turdidae based on the mitochondrial cytochrome b gene and the nuclear myoglobin intron 2 and ornithine decarboxylase introns 6-7. Geomalia heinrichi was shown to be part of the Zoothera clade with high support. We propose that Geomalia is transferred to Zoothera under the name Zoothera heinrichi.
The Sulawesi endemic Geomalia (Geomalia heinrichi) is a medium-sized terrestrial bird living in mossy forest at 1700–3500 m a.s.l., mainly 1800–2200 m (Collar, 2005). In some respects it resembles both thrushes (Turdidae) and babblers (Timaliidae/Pellorneidae/Leiotrichidae), and no consensus as to its taxonomic position has been reached to date. The species was discovered by Gerd Heinrich in 1930 in the Latimodjong mountains in central Sulawesi. He made his first observation already on his first day in the field, and initially mistook it for an Island Thrush (Turdus poliocephalus). Throughout the description of his first encounters with the species, he referred to it as a thrush (quoted in Stresemann, 1940). Stresemann (1931) provided a detailed description, pointing out morphological features of which some seemed to indicate that Geomalia was closer to thrushes, and others suggesting that it belonged to the babblers. The early attempts to classify Geomalia by Stresemann(1931, 1940) followed a tradition in which specific characters judged to be more important than others were relied on to determine affinity, rather than synapomorphies. His initial conclusion (Stresemann, 1931) was that he thought that Geomalia was related to the shortwings, particularly Heinrichia calligyna, alternatively best placed in its own monotypic subfamily, Geomaliinae. Later, Stresemann (1940) reconsidered this and placed Geomalia in Timaliinae.
Following Stresemann's initial descriptions and taxonomic conclusions (Stresemann, 1931, 1940), very little primary data have been added. The taxonomic position of this monotypic genus has remained controversial, with opinion swinging between babbler or thrush affinity. Ripley (1952) was the first to disagree with Stresemann (1940), by placing Geomalia in Turdidae, immediately before Zoothera in a linear taxonomy, although without providing any reasons. Subsequent authors have followed either of these two positions. Most major world lists or handbooks have placed Geomalia before Zoothera/Geokichla in Turdidae (Sibley and Monroe, 1990; Dickinson, 2003; Clement, 2000; Clements et al., 2012; Gill and Donsker, 2013). Collar (2005) emphasized the uncertain phylogenetic position of Geomalia by placing it among the first species in Turdinae. Collar(2004, 2005) noted that Geomalia has a distinct juvenile plumage, with spotted underparts, unlike any babbler.
White and Bruce (1986) placed Geomalia in Timaliidae, but mixed up their reference to Stresemann by stating that he placed Geomalia in Timaliidae in 1931, which in fact he did in 1940. They further stated that Stresemann (1931) did not mention a thrush-like juvenile plumage, which is correct in the sense that Stresemann (1931) did not refer to the juvenile plumage as "thrush-like". However, his description, although somewhat vague, stated that the feathers of the breast are edged grey-black in juveniles, not uniformly colored as in the adult (Stresemann, 1931). Holmes and Philipps (1996) and Coates and Bishop (1997) followed White and Bruce (1986) in placing Geomalia in Timaliidae, in the latter case reiterating the incorrect reference to Stresemann (1931).
No cladistic analysis of morphological characters including Geomalia has been undertaken. We here present a hypothesis of the phylogenetic position of Geomalia heinrichi based on DNA sequences of mitochondrial and nuclear sequences obtained from one of Heinrich's specimens. To the best of our knowledge, this is the first molecular study of the phylogenetic position of Geomalia heinrichi.
DNA was extracted from feathers and a claw from a juvenile Geomalia heinrichi heinrichi housed in the Museum für Naturkunde der Humboldt-Universität, Berlin, collected in the Latimodjong Mountains, southern Sulawesi on 16 July 1939 by Gerd Heinrich (ZMB 34.160; field number 1018), and from tissue samples of a few additional taxa (Supplementary Table S1).
DNA was extracted using QIA Quick DNEasy Kit (Qiagen, Inc) according to the manufacturer's instructions, but with 30 µL DTT added to the initial incubation step for the extraction from feathers, toepads and claws. For the amplification and sequencing of the fresh samples, we sequenced the mitochondrial cytochrome b (cytb) gene and the entire nuclear myoglobin intron 2 (myo) following the protocols described in Olsson et al. (2005), and the nuclear ornithine decarboxylase exon 6 (partial), intron 6, exon 7, intron 7 and exon 8 (partial) (ODC) following the protocols described in Friesen et al. (1999) and Allen and Omland (2003).
We sequenced a few additional taxa, and downloaded some more sequences from GenBank (Supplementary Table S1). Both the cytb gene and the nuclear introns from the museum toepad samples were sequenced in small fragments, using a range of specific primer combinations as described by Irestedt et al. (2006), Dario Zuccon (personal communication) and some primers designed specifically for this study (Supplementary Table S2). All sequences have been submitted to GenBank (Supplementary Table S1).
Sequences were aligned using MegAlign 4.03 in the DNAstar package (DNAstar Inc.).
Phylogenies were estimated by Bayesian inference (BI) using MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003). All analyses were run under the best-fit models according to the Bayesian Information Criterion (BIC), calculated in jModeltest 0.1.1 (Posada, 2008a, 2008b). The preferred model for the cytb data was the general time-reversible (GTR) model (Lanave et al., 1984; Tavaré, 1986; Rodríguez et al., 1990), assuming rate variation across sites according to a discrete gamma distribution with four rate categories (Γ; Yang, 1994), and an estimated proportion of invariant sites (I; Gu et al., 1995) (GTR + Γ + I). For ODC the preferred model was GTR + I, and for myo the HKY (Hasegawa et al., 1985) model + Γ.
The three loci were analysed both separately and concatenated. In the combined BI analysis, the data were divided into locus-specific partitions. In each analysis, four Metropolis-coupled MCMC chains were run for 30 million generations and sampled every 1000 generations. The posterior distributions were summarized as a majority-rule consensus tree. The first 7.5 million generations of each run, well after the chains reached stationarity, were discarded as burn-in.
The concatenated data were also analysed by maximum likelihood bootstrapping (MLBS) and parsimony bootstrapping (MPBS). MLBS (1000 replicates) was conducted with RAxML-HPC2 version 7.3.2 (Stamatakis, 2006; Stamatakis et al., 2008) on the Cipres portal (Miller et al., 2010). The data were partitioned by locus, and as per default GTRCAT was used for the bootstrapping phase, and GTRGAMMA for the final tree inference. The MPBS was performed in PAUP* (Swofford, 2001): heuristic search strategy, 10000 replicates, starting trees obtained by stepwise addition (random addition sequence, 10 replicates), TBR branch swapping. Myophonus caeruleus, which is part of Muscicapidae, the sister group of Turdidae (Sangster et al., 2010), was used as outgroup in all analyses.
The aligned cytb of 44 taxa comprised 1038 characters, of which 374 (36%) were parsimony informative. For 41 sequences the complete target stretch was available, while 3 included missing data and varied between 976 and 1011 bp in length. We found no unexpected stop codons, indels or distinct double peaks in the chromatograms that would indicate the presence of nuclear pseudogenes (e.g. Sorenson and Quinn, 1998).
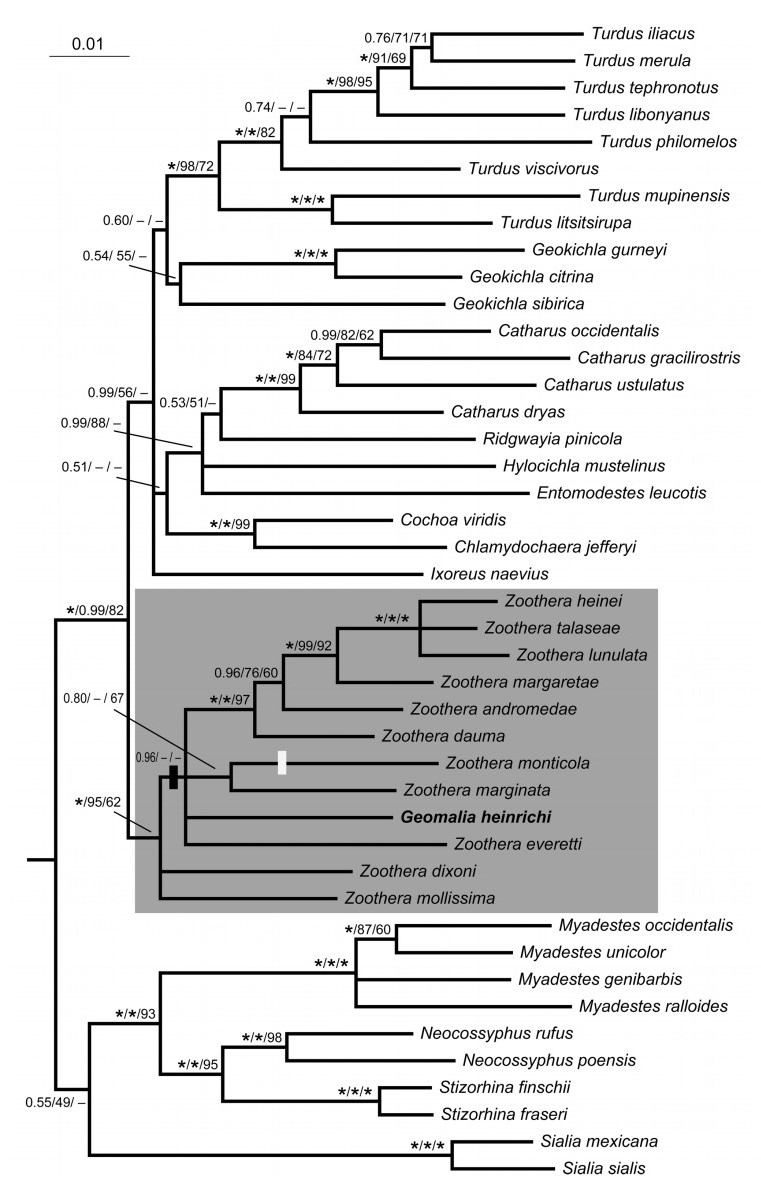
The length of the nuclear introns varied depending on multiple indels. The aligned myo sequences of 43 taxa comprised 758 characters, of which 94 (12%) were parsimony informative. For 36 sequences the complete target stretch of 695–713 bp in length was obtained. Nine sequences included missing data. Eight of these varied between 674 and 705 bp in length, one sequence was only 181 bp, and for Ridgwayia pinicola no sequence from this locus was available. Seven indels were shared by two or more taxa, and one of these, a 2 bp deletion, supported the position of Geomalia in the phylogeny (see below; Fig. 1).
The aligned ODC of 43 taxa comprised 733 characters, of which 104 (14%) were parsimony informative. For 29 sequences the complete target stretch of 690–706 bp in length was available. Fourteen sequences included missing data, the majority varying between 499 and 691 bp in length. The Geomalia sequence was 191 bp, and Ridgwayia pinicola 156 bp. For Zoothera everetti sequence data from this locus was missing.
The trees based on single-locus analyses (hereafter SLAs) varied in resolution. All three SLAs recovered Geomalia as part of the Zoothera clade, with good support (PP 0.92 in the cytb tree; PP 0.97 in the ODC tree and PP 0.99 in the myo tree) (Supplementary Figs. S1–S3).
The tree based on the concatenated sequences (Fig. 1) was overall congruent with various trees presented by Cibois and Cracraft (2004), Klicka et al. (2005), Nylander et al. (2008), Voelker et al. (2007) and Voelker and Klicka (2008). In agreement with previous studies, the exact positions of some problematic taxa, such as Geokichla sibirica, Ixoreus naevius, Ridgwayia pinicola, Hylocichla mustelinus and Entomodestes leucotis were uncertain to some degree. Geomalia was part of the Zoothera clade with PP 1.00, 95% MLBS and 62% MPBS, although its exact position within the clade was unresolved.
The phylogeny is overall relatively well supported and in agreement with previous publications (e.g. Cibois and Cracraft, 2004; Klicka et al., 2005; Nylander et al., 2008; Voelker et al., 2007; Voelker and Klicka, 2008) regarding reference taxa. We are not concerned here with minor differences within clades; all major clades found in earlier works are recovered here, and the position of Geomalia as part of the Zoothera clade is corroborated both by the concatenated data and by all of the SLAs. Within the Zoothera clade, the position of Geomalia is unclear. The present data indicate that it stems from a basal node, suggesting that it may have become isolated on Sulawesi early in the Zoothera radiation. The juvenile plumage of Geomalia shows a dark vertical bar on the rear ear-coverts and a dark malar stripe, and the breast feathers have dark scallops (photos available on www.orientalbirdimages.org), all traits recalling several Zoothera species. It is worth noting that the Borneo endemic Z. everetti resembles Geomalia in having warm orange underparts (albeit with whitish belly), lacking the dark spotting or scalloping typical of the genus Zoothera, and in showing a facial pattern reminiscent of juvenile Geomalia. Juvenile Z. everetti has more heavily scalloped underparts than juvenile Geomalia. It thus appears that the typical Zoothera pattern is retained in juveniles, whereas it has become lost in adults in both these species. The characters that have suggested to some earlier workers that Geomalia is a babbler may be adaptions to its apparently obligate terrestrial life-style. As Geomalia is firmly placed in Zoothera, we propose that it should be assigned to that genus, under the name Zoothera heinrichi.
We are grateful to Pascal Eckhoff and Sylke Frahnert at the Museum für Naturkunde der HumboldtUniversität, Berlin for generously granting permission to sample a specimen of Geomalia heinrichi, and Jon Fjeldså for help with the sampling of this specimen and for providing a description of the juvenile plumage and some other comments; and to Jornvall Foundation and the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (No. 2011T2S04) for other support (both to P.A.).
Available on Chinese Birds website, links at http://www.chinesebirds.net, including
Tables S1–S2
Figures S1–S3
|
Clement P. 2000. Thrushes. Princeton University Press, Princeton, N.J.
|
|
Coates BJ, Bishop KD. 1997. A Guide to the Birds of Wallacea. Dove Publ., Alderley, Australia.
|
|
Collar NJ. 2004. Four odd "thrushes". BirdingAsia, 1: 18–21.
|
|
Collar NJ. 2005. Family Turdidae (Thrushes), Geomalia. In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the Birds of the World (Vol. 10). Lynx Edicions, Barcelona, Spain, p 633.
|
|
Dickinson E (ed. ). 2003. The Howard and Moore Complete Checklist of the Birds of the World. 3rd ed. Helm, London.
|
|
Gu X, Fu Y-X, Li W-H. 1995. Maximum likelihood estimation of the heterogeneity of substitution rate among nucleotide sites. Mol Biol Evol, 12: 546–557.
|
|
Holmes DA, Philipps K. 1996. The Birds of Sulawesi. Oxford University Press, Kuala Lumpur.
|
|
Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA, pp 1–8.
|
|
Ripley SD. 1952. The Thrushes: a Taxonomic Study. Postilla 13.
|
|
Sibley CG, Ahlquist JE. 1990. Phylogeny and Classification of Birds: a Study of Molecular Evolution. Yale University Press, New Haven, CT.
|
|
Stresemann E. 1931. Zur ornithologie des Latimodjong-Gebirges in südlichen Central-Celebes. Orn Monatsb, 39: 7–14.
|
|
Swofford DL. 2001. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods). Version 4.08b. Sinauer Associates, Sunderland, MA.
|
|
Tavaré S. 1986. Some probabilistic and statistical problems on the analysis of DNA sequences. Lec Math Life Sci, 17: 57–86.
|
|
White CMN, Bruce MD. 1986. The Birds of Wallacea. B.O.U. Check-list No. 7. B.O.U., London.
|