| Citation: | Per ALSTRÖM, Urban OLSSON, Fumin LEI. 2013: A review of the recent advances in the systematics of the avian superfamily Sylvioidea. Avian Research, 4(2): 99-131. DOI: 10.5122/cbirds.2013.0016 |
The systematics of the avian superfamily Sylvioidea are reviewed, focusing on studies of relationships among families and within genera, more superficially on taxonomic studies at the species level. For the families Bernieridae and Phylloscopidae, new analyses based on already published sequence data are presented. Our understanding of relationships has been vastly improved in recent years due to a large number of molecular studies. However, the relationships among the different families remain largely obscured, probably mainly as a result of rapid divergence of the different primary lineages (families). Also, species level taxonomy has been much improved in recent years due to a large number of studies applying molecular markers and/or vocalizations and other life-history data. It seems likely that the number of species will continue to increase, as new groups are being studied with modern integrative methods.
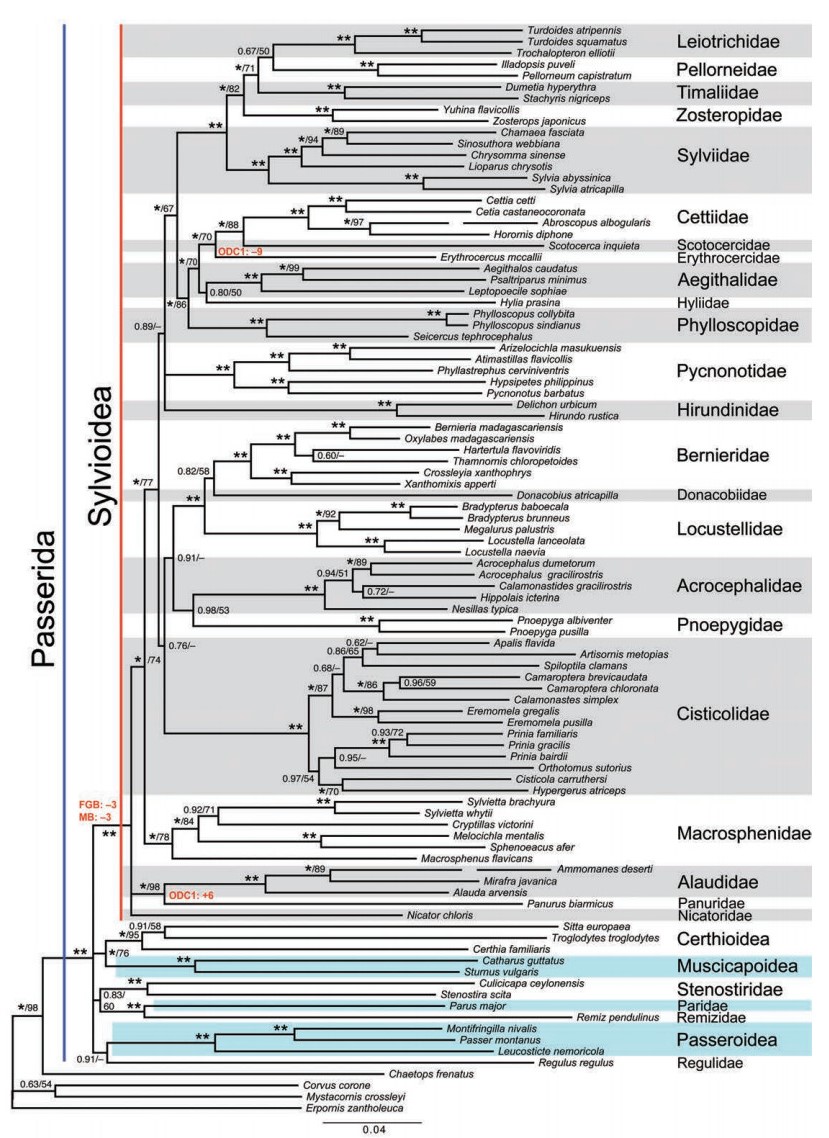
The Passerida was identified by Sibley and Ahlquist (1990) based on DNA-DNA hybridization studies as the largest radiation within oscine passerine birds (cf. Fig. 1). These authors recognized three superfamilies within Passerida: Muscicapoidea (e.g. waxwings, dippers, thrushes, Old World flycatchers, starlings and mockingbirds), Sylvioidea (e.g. nuthatches, treecreepers, tits, wrens, crests/kinglets, swallows, bulbuls, babblers and warblers), and Passeroidea (e.g. larks, pipits, wagtails, waxbills, weavers, finches, sparrows, cardinals, tanagers, wood-warblers, and icterids). Subsequent studies of DNA sequence data have indicated that both Muscicapoidea and Passeroidea, after minor taxonomic adjustments, can be recovered as monophyletic (Barker et al., 2002, 2004; Ericson and Johansson, 2003; Voelker and Spellman, 2003; Cibois and Cracraft, 2004; Beresford et al., 2005; Jønsson and Fjeldså, 2006; Johansson et al., 2008a; Spellman et al., 2008; Treplin et al., 2008). However, Sylvioidea sensu Sibley and Ahlquist (1990) and Sibley and Monroe (1990) has not been corroborated by later studies (e.g. Chikuni et al., 1996; Sheldon and Gill, 1996; Barker et al., 2002, 2004; Ericson and Johansson, 2003; Spicer and Dunipace, 2004; Beresford et al., 2005; Alström et al., 2006; Fregin et al., 2012). In addition to Muscicapoidea, Passeroidea and Sylvioidea, the superfamily Certhioidea (containing treecreepers, nuthatches, gnatcatchers and wrens) has been proposed by Cracraft et al. (2004) (cf. Fig. 1).
The superfamily Sylvioidea has received much attention in recent years, with three major studies of interfamilial relationships (Alström et al., 2006; Johansson et al., 2008a; Fregin et al., 2012) and multiple studies focusing on different families within it (e.g. Cibois et al., 1999, 2001; Helbig and Seibold, 1999; Sheldon et al., 2005; Moyle and Marks, 2006; Nguembock et al., 2007; Fregin et al., 2009; Gelang et al., 2009; Alström et al., 2011a, b, 2013; Moyle et al., 2012; Olsson et al., 2013a). The first comprehensive study of the whole superfamily (Alström et al., 2006) was based on one nuclear and one mitochondrial sequence. That study identified 10 well-supported major clades, which were proposed to be recognized at family level (Acrocephalidae, Aegithalidae, Alaudidae, Cettiidae, Cisticolidae, Hirundinidae, Megaluridae, Phylloscopidae, Pycnonotidae and Timaliidae), as well as three lineages comprising one to two species each that were not recognized nomenclaturally (Donacobius, Melocichla + Sylvietta and Panurus). Johansson et al. (2008a) recognized also Bernieridae, of which Alström et al (2006) had no representatives, and referred to the Melocichla + Sylvietta clade as the "Sphenoeacus group" (comprising Macrosphenus, Sphenoeacus, Bradypterus victorini and two species of Sylvietta). Both these groupings were previously identified by Beresford et al. (2005), the former as "Malagasy endemic 'warblers'" and the latter as the "Sphenoeacus group" (containing also Achaetops, in addition to the previously mentioned genera).
The finding that the type genus of Sylviidae, Sylvia, was nested within the large Timaliidae group (as previously discovered by Fjeldså et al., 1999; Cibois, 2003a, b) caused a taxonomic problem, as the older name Sylviidae has priority over Timaliidae (ICZN, 1999), and it would be inconvenient both to discard the name Timaliidae and to use the name Sylviidae in a completely new context. To solve this difficulty, it was suggested that Sylviidae be suppressed, and Timaliidae used for the group including mostly traditional Timaliidae species (Cibois, 2003a, b; Alström et al., 2006). However, Gelang et al. (2009), again based on DNA sequence data, resurrected Sylviidae, but restricted it to a clade containing mostly traditional Timaliidae species, and this arrangement has been followed by subsequent authors (Fregin et al., 2012; Moyle et al., 2012; Gill and Donsker, 2013).
The latest and most comprehensive analysis of Sylvioidea (Fregin et al., 2012) proposed new family names for the genera Scotocerca and Erythrocercus, the Scotocercidae (a monotypic family) and the Erythrocercidae (with three species), respectively, and formally proposed the name Macrosphenidae for the "Sphenoeacus group". They also tentatively recommended the use of Hyliidae for Hylia and Pholidornis, and argued against inclusion of Paridae, Remizidae and Stenostiridae in Sylvioidea. In total, their classification comprised 23 families in Sylvioidea (Fig. 1), containing over 1200 species in more than 220 genera (Gill and Donsker, 2013).
Sylvioidea is essentially an Old World radiation. Most of the families are either absent or represented by just a few species in the New World. A striking exception concerns the family Hirundinidae, which is well represented on all continents (excluding Antarctica), likely the result of the extraordinary flight capability of its members.
The present review focuses on studies of relationships among the families within Sylvioidea, as well as on relationships within genera, but only cursorily deals with taxonomic studies at the species level. For the families Bernieridae and Phylloscopidae, new analyses based on previously published sequence data are presented. Recent advances in the species-level taxonomy of Indian subcontinent birds have been reviewed by Rasmussen (2012), which see for additional information.
In the study by Alström et al. (2006), based on two loci, one mitochondrial and one nuclear, most of the deep internal nodes were unresolved. However, three of the relationships among the 10 clades they proposed to be recognized at the family level had high posterior probability (0.95–1.00): the Megaluridae + Donacobius + Acrocephalidae clade, the Phylloscopidae + Aegithalidae + Cettiidae + Hirundinidae + Pycnonotidae clade, and the sister relationship between Alaudidae + Panurus and the other Sylvioidea. However, none of these clades obtained >50% parsimony bootstrap support, so they were not considered reliable. Johansson et al. (2008a), utilizing three nuclear introns (including the one used by Alström et al., 2006), found strong support for a Megaluridae + Donacobius + Bernieridae clade, as well as for the sister relationships between these and Acrocephalidae. They also found strong support for the position of the Alaudidae + Panurus clade and Nicator outside of the other Sylvioidea, but no other strongly supported relationships among families.
The analysis of six nuclear and one mitochondrial loci by Fregin et al. (2012; Fig. 1) corroborated the Locustellidae (formerly Megaluridae; see below) + Donacobiidae (treated at family rank; see below) + Bernieridae clade, but found no statistical support for a close relationship between these and Acrocephalidae. They also found the Alaudidae + Panuridae clade and Nicatoridae (all treated at family level; see below) in a polytomy with the rest of Sylvioidea (the Sylvioidea clade excluding Alaudidae, Panuridae and Nicatoridae receiving 1.00 posterior probability and 74% maximum likelihood bootstrap support). Moreover, the Cettiidae + Scotocercidae clade and the Cettiidae + Scotocercidae + Erythrocercidae + Aegithalidae + Phylloscopidae clade were strongly supported both in the Bayesian and maximum likelihood analyses. The Cettiidae + Scotocercidae grouping had previously been shown to be supported by morphological characters (Alström et al., 2011c). However, other relationships among families were generally unsupported, or strongly supported by Bayesian inference but with poor or moderate maximum likelihood bootstrap support. Most of the deep nodes with poor or conflicting support were very short, suggesting rapid divergence resulting in difficulties in reconstruction of relationships (e.g. Lewis et al., 2005; Rokas and Carroll, 2006).
With respect to the relationships among the five primary "babbler" clades, using partly different loci, Gelang et al. (2009), Fregin et al. (2012) and Moyle et al. (2012) recovered the same topology, i.e. ((((Leiotrichidae/-inae, Pellorneidae/-inae), Timaliidae/-inae), Zosteropidae/-inae), Sylviidae/-inae) (cf. Fig. 1). The latter study was the only one that obtained unanimously strong support for these relationships, whereas the overall weakest support was found in the study with the largest number of unlinked loci (Fregin et al., 2012). The comparatively poor support (especially for the Leiotrichidae/-inae + Pellorneidae/-inae clade) in the Fregin et al. (2012) study might have been the result of the small number of species in each clade compared to the other studies.
The Alaudidae (larks) are found on all continents, although by far the largest number of species are found in Africa, and only one species each is found in the New World and Australasia (de Juana et al., 2004). This is one of the least studied of the families within Sylvioidea. The circumscription of this family has not been disputed, and it seems unlikely that its inclusiveness will change. However, the relationships within and among the different genera are uncertain, and the number of genera and their composition have fluctuated dramatically over the years (e.g. Roberts, 1940; Meinertzhagen, 1951; Vaurie, 1951; Macdonald, 1952a, b, 1953; Verheyen, 1958; Peters, 1960a; Clancey, 1966, 1980; Harrison, 1966; Maclean, 1969; Wolters, 1979; Dean et al., 1992; Dickinson, 2003; Pätzold, 2003; de Juana et al., 2004). Certain genera, notably Mirafra, have acted as "dumping grounds", whereas several monotypic genera (e.g. Pseudalaemon, Lullula, Ramphocoris) with uncertain affinities have been erected.
Until recently, only one phylogeny of the Alaudidae, based on mitochondrial sequences from a small number of mostly African species, has been published (Tieleman et al., 2003). However, a phylogeny including 81 of the 97 species and representatives of all 21 recognized genera, based on two mitochondrial and three nuclear loci has just appeared (Alström et al., 2013). The latter has revealed many unpredicted relationships, including some non-monophyletic genera (Calandrella, Mirafra, Melanocorypha, Spizocorys), and proposed a revised generic classification. For example, the genus Calandrella is shown to be separated into two non-sister clades, with C. brachydactyla, C. cinerea and C. acutirostris being closely related to Eremophila, whereas the other Calandrella species (e.g. C. rufescens and C. cheleensis) are more closely related to a clade containing the two monotypic genera Eremalauda and Chersophilus; the close affinity between Eremalauda and Chersophilus was also totally unexpected. Alström et al. (2013) proposed that the name Alaudala be used for the second of these traditional Calandrella clades. Another surprising result from Alström et al.'s (2013) multilocus analysis was that Melanocorypha leucoptera and M. mongolica are not closely related, hence it was proposed that the former be moved to the genus Alauda.
Lark taxonomy based on morphology has received much attention in Africa (Meinertzhagen, 1951; Winterbottom, 1957; Lawson, 1961; Clancey, 1989; Ryan and Bloomer, 1997; García et al., 2008) and Eurasia (Meinertzhagen, 1951; Vaurie, 1951, 1954; Dickinson and Dekker, 2001). Recent studies have revealed considerable hidden diversity in some taxa, such as Mirafra assamica, which was proposed to be split into four species based mainly on vocal, behavioral and morphological data (Alström, 1998; corroborated by molecular study by Alström et al., 2013); the Calendulauda (previously Certhilauda) albescens complex, which was suggested to consist of three instead of two species based on mitochondrial, morphological and vocal data (Ryan et al., 1998); the Certhilauda curvirostris complex, which was recommended to be treated as five instead of one species based on mitochondrial and morphological evidence (Ryan and Bloomer, 1999); and Galerida cristata, which was shown to consist of two partly sympatric and largely reproductively isolated species in Morocco, G. cristata sensu stricto and G. macrorhyncha (Guillaumet et al., 2005, 2006, 2008). In an analysis of mitochondrial data, Alström et al. (2013) found several unexpectedly deep divergences between taxa presently treated as conspecific (e.g. within Ammomanes cinctura, Ammomanes deserti, Calandrella brachydactyla, Eremophila alpestris), but also some shallow splits between currently recognized species (e.g. Certhilauda brevirostris–C. semitorquata–C. curvirostris [cf. Ryan and Bloomer, 1999]; Calendulauda barlowi–C. erythrochlamys [cf. Ryan et al., 1998]; Mirafra cantillans–M. javanica). Moreover, Zink et al. (2008) found a deep divergence in mitochondrial DNA between eastern and western populations of Alauda arvensis. It seems likely that the total number of recognized lark species is underestimated, and we predict that future studies based on molecular and/or vocal data will show that many of the currently recognized subspecies warrant recognition as separate species.
The family Panuridae contains the single species Panurus biarmicus, which is widely distributed across the temperate parts of Eurasia. It was previously considered a babbler, closely related to parrotbills (e.g. Deignan, 1964a; Dickinson, 2003), but molecular studies have unanimously recovered it as sister to the Alaudidae (Ericson and Johansson, 2003; Alström et al., 2006; Johansson et al., 2008a; Fregin et al., 2012).
The realization that this exclusively African family exists as a distinct evolutionary lineage has gradually grown out of molecular evidence, which piece by piece brought together a morphologically diverse assemblage of species as a monophyletic group. Sefc et al. (2003) found that Sylvietta, which was placed in Acrocephalidae by Sibley and Monroe (1990), was not closely related to any other warbler taxa in their analysis of mitochondrial markers. Beresford et al. (2005) included Achaetops, Bradypterus victorini, Macrosphenus, Sphenoeacus and Sylvietta in their analysis of nuclear RAG1 and RAG2 sequences and identified a clade consisting of these species as the "Sphenoeacus group". The position of Bradypterus victorini, removed from other species in the genus, prompted a reversion to its older generic name Cryptillas. Fuchs et al. (2006) confirmed the close association of Sylvietta, Macrosphenus and Sphenoeacus based on one nuclear and one mitochondrial marker, and found that Melocichla was also a member of the "Sphenoeacus group"; Alström et al. (2006) also recovered a clade including Sylvietta and Melocichla. Johansson et al. (2008a) corroborated the existence of the "Sphenoeacus group" in their analysis of three nuclear loci by finding a clade consisting of Cryptillas (Bradypterus) victorini, Macrosphenus, Sphenoeacus and Sylvietta, but they did not consider Achaetops or Melocichla. Fregin et al. (2012) used one mitochondrial and six nuclear markers and again found support for a clade consisting of Cryptillas victorini, Macrosphenus, Melocichla, Sphenoeacus and Sylvietta, but did not include Achaetops in their analysis. Fregin et al. (2012) erected the family Macrosphenidae for this clade, although it lacks known diagnostic morphological features. In total, 18 species in 6 genera are presently placed in this family (Gill and Donsker, 2013).
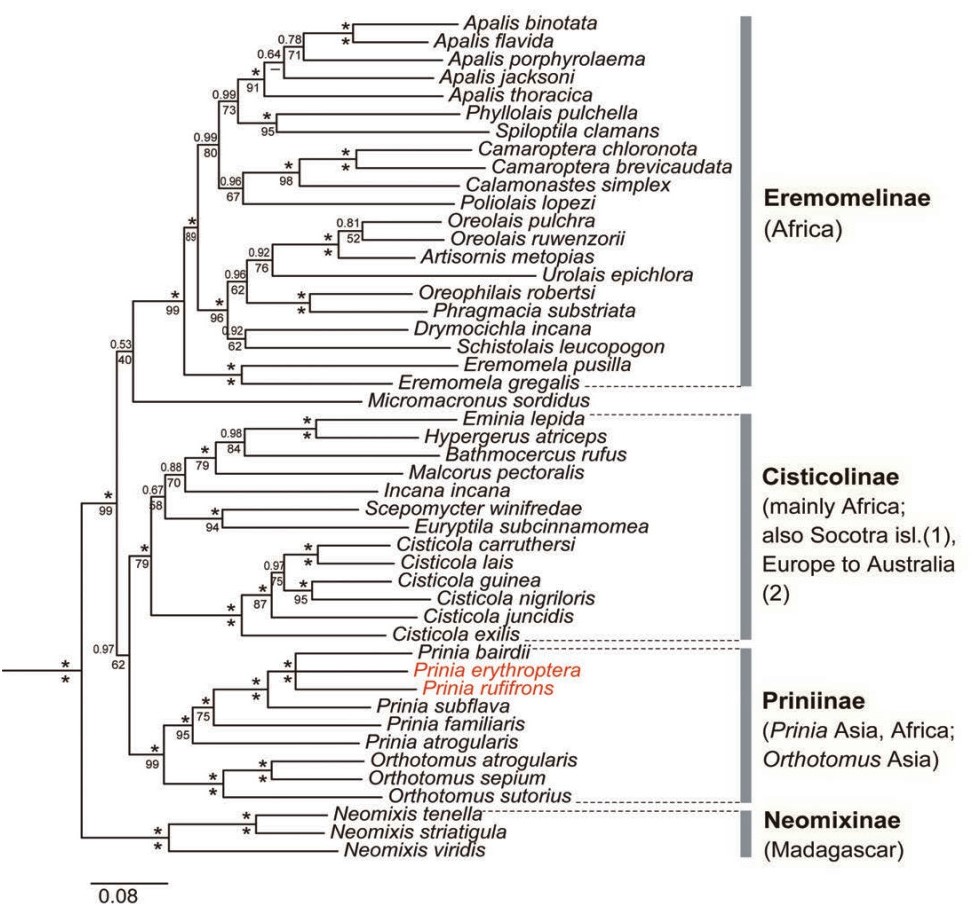
The family Cisticolidae (cisticolas, prinias, apalises and allies) is the largest of the sylvioid families, with 160 species in 27 genera; 14 of the genera are monotypic, whereas the largest, Cisticola, contains more than 50 species (Gill and Donsker, 2013). All of the genera are found mainly or exclusively in Africa, except Neomixis (Madagascar), Incana (Socotra island) and Orthotomus (Asia), and the vast majority of the species occur in Africa (Ryan et al., 2006). The Cisticolidae was first identified by Sibley and Ahlquist (1990) based on DNADNA hybridization data, revealing a previously unanticipated cluster of warbler genera. The speciose genera Apalis, Cisticola and Prinia comprise the core of the family (Sibley and Monroe, 1990). Several later studies have largely corroborated Sibley and Monroe's (1990) circumscription of Cisticolidae (Cibois et al., 1999; Sefc et al., 2003; Beresford et al., 2005; Alström et al., 2006; Nguembock et al., 2007, 2008, 2012; Johansson et al., 2008a; Alström et al., 2011c; Fregin et al., 2012). However, these later studies have also shown that the genera Bathmocercus, Eremomela, Neomixis, Orthotomus, Poliolais and Scepomycter, which were placed in Sylviidae by Sibley and Monroe (1990), belong to Cisticolidae. In contrast, the two monotypic genera Rhopophilus and Scotocerca, which were placed in Cisticolidae by Sibley and Monroe (1990) based on non-molecular data, have been shown by molecular analyses to have other affinities; the former is a "babbler" (Alström et al., 2006; Gelang et al., 2009; Olsson et al., 2013a), the latter sister to Cettiidae (Alström et al., 2011c; Fregin et al., 2012; placed in the monotypic family Scotocercidae by latter, see below).
Nguembock et al. (2007, 2012), who analyzed 15 and 17 cisticolid genera, respectively, using one to three mitochondrial and one nuclear markers, and Olsson et al. (2013a; Fig. 2), who analyzed representatives of all genera believed to belong in Cisticolidae using two mitochondrial and two nuclear markers, are the most complete studies to date. These authors identified four main clades: (1) the speciose genus Apalis and several smaller (including monotypic) genera, all with exclusively African distributions; (2) the large genus Cisticola and several smaller (including monotypic) genera, of which all except two species of Cisticola are restricted to Africa; (3) the fairly large genera Prinia (Africa, Asia) and Orthotomus (Asia) and the two monotypic African genera Heliolais and Urorhipis; and (4) the genus Neomixis containing three species, all restricted to Madagascar. Olsson et al. (2013a) classified these clades as Eremomelinae, Cisticolinae, Priniinae and Neomixinae, respectively. A fifth lineage, containing the Philippine Micromacronus sordidus, which was shown by Oliveros et al. (2012) to be part of Cisticolidae, was in an incertae sedis position within Cisticolidae in the study by Olsson et al. (2013a).
Studies using molecular markers have found several of the cisticolid genera to be non-monophyletic, resulting in taxonomic revisions. The genus Apalis was shown by Nguembock et al. (2007) to be non-monophyletic, as Apalis ruwenzorii and A. pulchra were more closely related to Artisornis metopias than to other Apalis species, leading to erection of the genus Oreolais for the two former species (Nguembock et al., 2008). Moreover, the two African taxa currently placed in Artisornis have previously been placed in Orthotomus (e.g. Hall and Moreau, 1970; Watson et al., 1986), but this has been shown to be incorrect as A. metopias is not closely related to Orthotomus (Nguembock et al., 2007, 2008, 2012; Fregin et al., 2012; Olsson et al., 2013a). Recently, Orthotomus cuculatus 1 was moved from Orthotomus to Phyllergates, as it has been shown to be part of Cettiidae (Alström et al., 2006, 2011b; Fuchs et al., 2006; Nguembock et al., 2007, 2012). Olsson et al. (2013a) synonymized Urorhipis and Heliolais with Prinia. They also showed that Prinia burnesii is a babbler (Pellorneidae sensu Fregin et al., 2012), and reinstated the generic name Laticilla for this species.
1 Note that the frequently used spelling cucullatus is apparently incorrect (Gill and Donsker, 2013).
Sheldon et al. (2012) reconstructed the phylogeny of all Orthotomus species based on mitochondrial and nuclear DNA. They revealed e.g. a deep split and nonmonophyletic relationships within O. castaneiceps, and showed that several taxa previously considered subspecies of O. atrogularis were highly divergent.
Prinia fluviatilis was recognized as a species due to its distinctive song (Chappuis, 1974), and P. superciliaris was split from P. atrogularis on the basis of morphological and vocal evidence (Rasmussen and Anderton, 2005, 2012). Several studies underway suggest that the genus Prinia contains considerably more species than presently recognized, and this seems likely to be the case also in the speciose genus Cisticola.
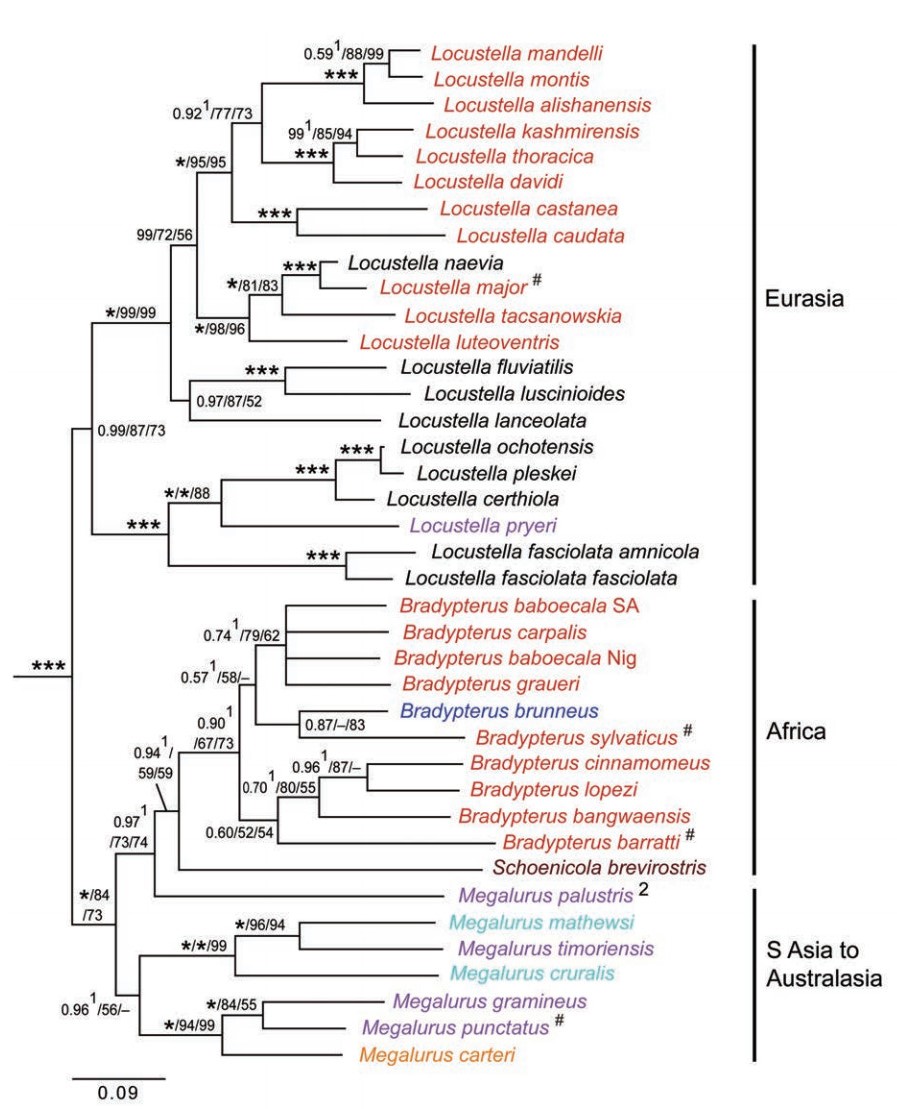
The family Locustellidae (grassbirds, bush warblers, grasshopper warblers and allies) is widely distributed across Africa, Eurasia and Australasia, with a total of 57 species in 9 genera (Gill and Donsker, 2013). It was first recognized by Sibley and Monroe (1990), partly based on DNA-DNA hybridization work by Sibley and Ahlquist (1990), as the subfamily Megalurinae, containing the genera Megalurus, Cincloramphus, Eremiornis, Amphilais, Megalurulus, Buettikoferella, Chaetornis, Graminicola and Schoenicola. Based on DNA sequence data from a broad selection of sylvioid genera, Alström et al. (2006) and Johansson et al. (2008a) recognized Megaluridae at the family level, comprising the genera Megalurus, Bradypterus, Locustella and Dromaeocercus. Hence, their circumscription included three of the genera placed in Acrocephalinae by Sibley and Monroe (1990). Moreover, molecular studies also revealed that the monotypic genus Graminicola is a babbler (Alström et al., 2006; Gelang et al., 2009; Olsson et al., 2013a), and that the aberrant Bradypterus victorini is not related to this clade (Beresford et al., 2005; now placed in Cryptillas – see under Macrosphenidae above). Alström et al. (2011a) noted that Locustellidae has priority over Megaluridae (as stated by Bock, 1994: 152).
The first comprehensive study of Locustellidae was published by Alström et al. (2011a; Fig. 3). It was based on a dataset comprising one mitochondrial and four nuclear loci for most of the species believed to belong in this family. The phylogeny strongly disagreed with earlier classifications at the generic level. All of the genera with more than one representative were found to be non-monophyletic: Bradypterus was separated into an Asian and an African clade, with Locustella and Megalurus pryeri nested within the former, and the monotypic Malagasy Dromaeocercus within the latter; only two of the five Megalurus species formed a clade that did not include other genera, and both Cincloramphus and Eremiornis were nested in one of the Megalurus clades. The non-monophyly of Bradypterus and Locustella had previously been found based on mitochondrial ND2 sequences of all Locustella, two Asian and three African Bradypterus and two Megalurus (M. pryeri and M. gramineus) (Drovetski et al., 2004). Moreover, the affinity of M. pryeri to Locustella had previously been suggested based on morphology (Morioka and Shigeta, 1993). Alström et al. (2011a) proposed a revised classification recognizing four instead of seven genera (Fig. 3). They acknowledged the non-monophyly of one of these genera (Megalurus), but stressed that the classification was tentative and took account of the phylogenetic uncertainty (i.e. conflict between their results and a study by Beresford et al., 2005 using another locus for five Locustellidae species, as well as morphology and vocalizations).
A recent study by Oliveros et al. (2012) unexpectedly found that the monotypic Sulawesi genus Malia was nested in Locustellidae and that the Philippine Robsonius was sister to Locustellidae. Five putative Locustellidae genera remain to be studied genetically: Amphilais (monotypic, Madagascar), Megalurulus (four species, Melanesia), Buettikoferella (monotypic, Timor), Chaetornis (monotypic, Indian Subcontinent) and Elaphrornis (monotypic, Sri Lanka) (Gill and Donsker, 2013).
The species in the traditional genera Locustella and Bradypterus are renowned for being difficult to identify by morphological characters, and there has been much taxonomic confusion over the years (see Bairlein et al., 2006; Kennerley and Pearson, 2010). One new species, Locustella (Bradypterus) alishanensis, was described as recently as 2000 from Taiwan, where it had been known to exist for many years but with confused taxonomy (Rasmussen et al., 2000). Dickinson et al. (2000) revised the poorly known Bradypterus seebohmi complex based on morphology and vocalizations, and tentatively recommended recognition of four species: B. seebohmi sensu stricto (s.s.), B. timorensis, B. montis and B. mandelli (placed in Locustella by Alström et al., 2011). Alström et al. (2011a) analyzed mitochondrial cytochrome b from different populations of the latter two, and suggested that they were so similar that their status as separate species needs to be re-evaluated. Locustella timorensis (previously Bradypterus timorensis), until recently only known from two specimens collected in 1932 on Timor (Dickinson et al., 2000), was recently rediscovered, along with a putative undescribed taxon from nearby Alor (Trainor et al., 2012; Verbelen and Trainor, 2012). Another undescribed taxon in the same complex has recently been found on Taliabu island, Indonesia (Rheindt, 2010). The L. thoracica (previously B. thoracicus) complex was revised by Alström et al. (2008) based on multiple independent datasets, and they proposed that three instead of one species be recognized. Locustella amnicola has usually been treated as a subspecies of L. fasciolata (e.g. Dickinson, 2003; Kennerley and Pearson, 2010), but has also been given species status (Watson et al., 1986 [with a note by Ernst Mayr stating that "The status of this species is in doubt."]; Stepanyan, 1990). Both Drovetski et al. (2004) and Alström et al. (2011a) showed that these two are markedly different genetically, supporting treatment as separate species. Alström et al. (2011a) also found deep cytochrome b divergences among some other taxa currently treated as conspecific, and suggested that species status might be warranted if corroborated by independent data: B. baboecala tongensis + B. b. transvaalensis vs. B. b. centralis + B. b. elgonensis; B. lopezi mariae + B. l. usambarae vs. B. l. ufipae; and M. palustris toklao vs. M. p. forbesi. Conversely, Alström et al. (2011a) and an earlier study by Drovetski et al. (2004), found slight divergences between L. pleskei and L. ochotensis, and noted that their status as separate species needs to be studied further.
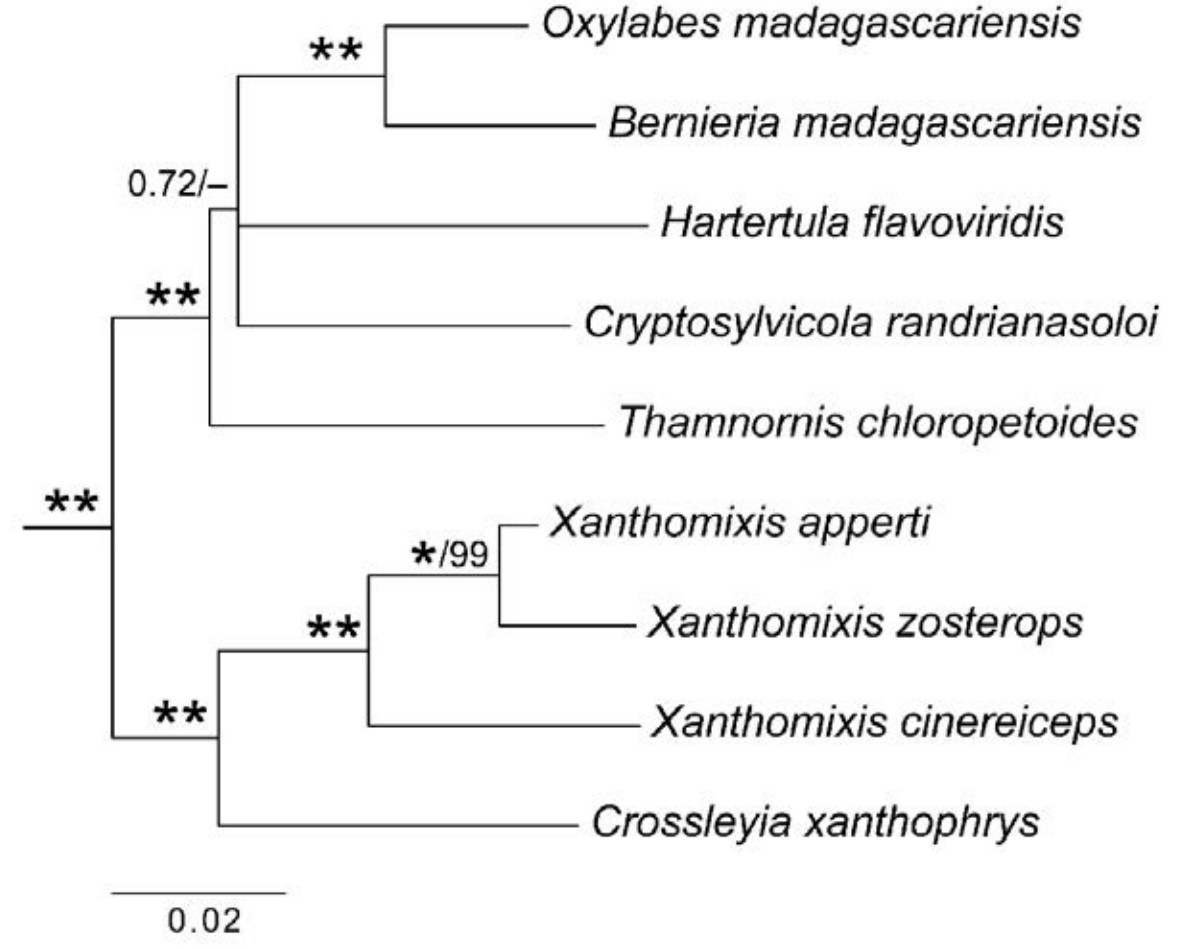
The family name Bernieridae was proposed by Cibois et al. (2010) for a clade of Malagasy "warblers". These were previously classified in the traditional families Timaliidae, Sylviidae and Pycnonotidae, but were shown by mitochondrial DNA to form an endemic Malagasy radiation (Cibois et al., 1999, 2001; Fjeldså et al., 1999). Cibois et al. (2010) recognized 10 species in seven genera: Bernieria madagascariensis, Xanthomixis zosterops, X. cinereiceps, X. apperti, X. tenebrosa, Oxylabes madagascariensis, Thamnornis chloropetoides, Crossleyia xanthophrys, Hartertula flavoviridis and Cryptosylvicola randrianasoloi. Dickinson (2003) and Gill and Donsker (2013) included also Randia pseudozosterops in Bernieridae, although this species has not yet been included in any phylogenetic study. The first four species listed above have often been placed in the bulbul (greenbul) genus Phyllastrephus, and H. flavoviridis has been placed in the "warbler" genus Neomixis (e.g. Delacour, 1946).
The study by Fregin et al. (2012) is the most complete analysis of this family thus far with respect to number of loci, although the study by Alström et al. (2011), based on four nuclear and one mitochondrial loci, investigated one additional monotypic genus (Cryptosylvicola), and the mitochondrial study by Cibois et al. (2001) included two additional species in the genus Xanthomixis. The tree in Fig. 4, which is based on all previously published sequence data, is well resolved and well supported, except for some uncertain relationships within a clade containing Oxylabes + Bernieria, Hartertula, Cryptosylvicola and Thamnornis.
The family Donacobiidae was proposed by Aleixo and Pacheco (2006). It contains the single species Donacobius atricapilla, which occurs in Central and South America. This taxon has a chequered history: it was placed in the family Mimidae by e.g. Mayr and Greenway (1960), and later in the subfamily Troglodytinae by Sibley and Ahlquist (1990) and Sibley and Monroe (1990). However, Barker et al. (2004) found evidence for an association with Zosterops and Prinia based on one mitochondrial and one nuclear marker, and Alström et al. (2006) confirmed that it was firmly nested within Sylvioidea also using one mitochondrial and one nuclear marker (different ones from those used in Barker et al., 2004). Johansson et al. (2008a) found strong support for the position of Donacobius in a clade with Locustellidae and Bernieridae based on three nuclear loci, and this was corroborated by Fregin et al. (2012) based on seven molecular markers. However, the relationships among these three families are uncertain, probably due to a short time between their separations, as indicated by the short internode between Donacobius and Bernieridae. From a biogeographical point of view, the distribution of Donacobius is remarkable, as all of its closest relatives, Locustellidae and Bernieridae, occur in the Old World.
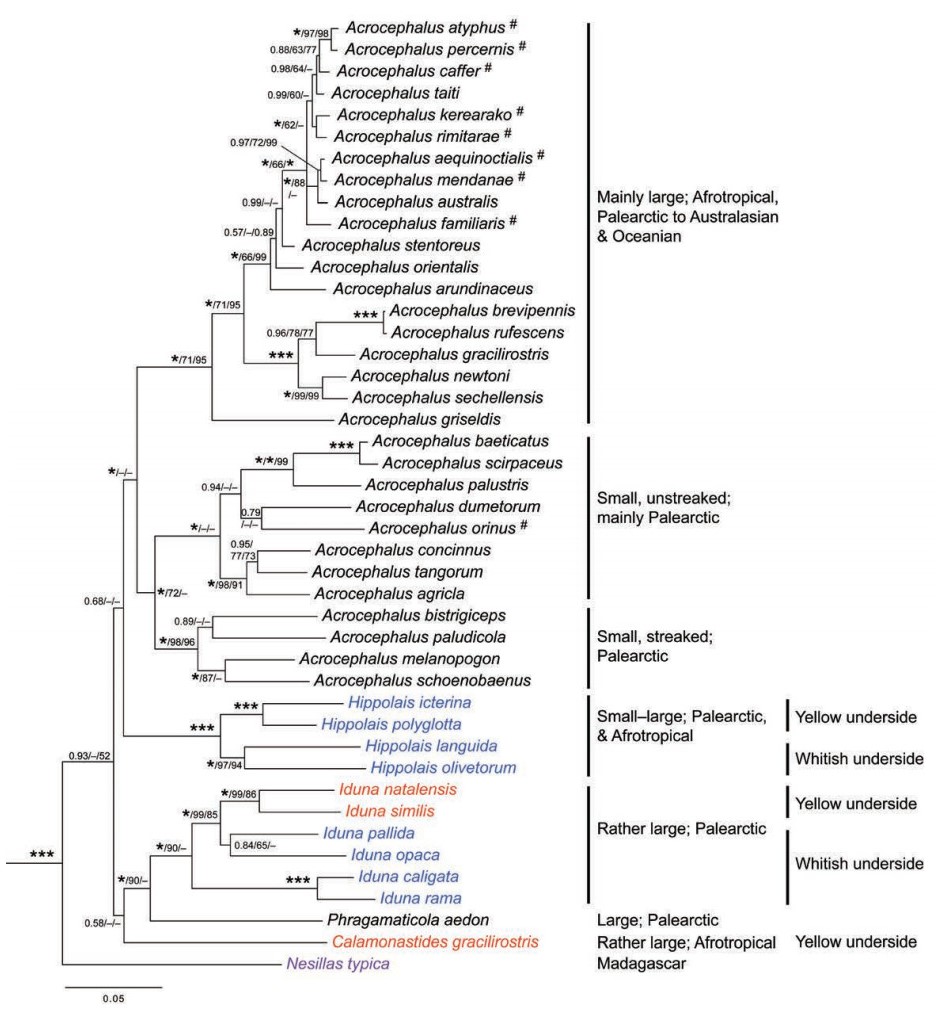
The relationships among the four genera in Acrocephalidae sensu Johansson et al. (2008a) and Fregin et al. (2009, 2012) [Acrocephalus ("reed warblers"), Hippolais ("tree warblers"), Chloropeta ("yellow warblers") and Nesillas ("brush warblers")] and their relationships to other taxa have long been debated based on morphology and oology (Hartert, 1909; Voous, 1977; Schönwetter, 1979; Wolters, 1982; Watson et al., 1986; Haffer, 1991; Cramp, 1992; Dickinson, 2003) as well as DNA (e.g. Sibley and Ahlquist, 1990; Leisler et al., 1997; Helbig and Seibold, 1999; Beresford et al., 2005; Alström et al., 2006; Johansson et al., 2008a; Fregin et al., 2009, 2012). The three genera Acrocephalus, Hippolais and Chloropeta have been split into several genera and/or subgenera, and there has been much disagreement regarding the classification of these taxa, especially Acrocephalus (Grant and Mackworth-Praed, 1941; Wolters, 1982; Watson et al., 1986; Sibley and Monroe, 1990; Dickinson, 2003; Haffer, 1991; Leisler et al., 1997; Helbig and Seibold, 1999). Leisler et al. (1997) and Helbig and Seibold (1999) came to nearly identical conclusions based on analyses of cytochrome b sequence data. Neither of them found the genera Acrocephalus and Hippolais to be monophyletic. Leisler et al. (1997) discussed the option of dividing these into a number of genera, and Helbig and Seibold (1999) proposed a classification into a number of subgenera. Helbig and Seibold (1999) investigated only one species of Chloropeta, C. gracilirostris, which was shown to belong in the clade with Acrocephalus and Hippolais, albeit in an unresolved position; Leisler et al. 1997 did not study any Chloropeta.
Fregin et al. (2009) revised the family Acrocephalidae based on one mitochondrial and three nuclear loci for 32 out of the 38 species of Acrocephalus (mitochondrial data only for nine species), all eight Hippolais, all three Chloropeta and one Nesillas (Fig. 5). They recovered all Acrocephalus except A. aedon in one clade. The latter was sister to a clade with C. natalensis and C. similis as sisters to H. pallida and H. opaca, with H. caligata and H. rama as sisters to these four, and the generic name Iduna was erected for these seven species, with the suggested option of placing I. aedon in the monotypic genus Phragamaticola; the latter was adopted by Kennerley and Pearson (2010). The third species of Chloropeta, C. gracilirostris, was in an effectively unresolved position, and the monotypic genus Calamonastides was reinstated for it. The other four Hippolais (H. icterina, H. polyglotta, H. olivetorum and H. languida) formed a clade with two sister pairs, and the generic name Hippolais was suggested to be restricted to these four species.
At the species level, several taxa have been the subject of taxonomic debate. Acrocephalus baeticatus has been considered conspecific with A. scirpaceus (Dowsett-Lemaire and Dowsett, 1987) or regarded as a distinct species because of different migratory behavior and associated wing structure (Kennerley and Pearson, 2010). Acrocephalus dumetorum has been suggested to be a subspecies of A. baeticatus (Fry et al., 1974), although this suggestion has been refuted by molecular data (Leisler et al., 1997; Helbig and Seibold, 1999; Fregin et al., 2009). Acrocephalus tangorum has been treated as a subspecies of A. bistrigiceps (Williamson, 1968; Wolters, 1982; Watson et al., 1986; Sibley and Monroe, 1990) or A. agricola (Vaurie, 1959; Alström et al., 1991; Sibley and Monroe, 1993), but based on molecular data (Helbig and Seibold, 1999; Leisler et al., 1997; Fregin et al., 2009) is now generally recognized as a separate species (Dickinson, 2003; del Hoyo et al., 2006; Gill and Donsker, 2013). Acrocephalus arundinaceus, A. orientalis, A. griseldis and A. stentoreus have been considered conspecific at some stage (cf. Salomonsen, 1929; Mayr, 1948; Stresemann and Arnold, 1949; Vaurie, 1959; Williamson, 1968; Watson et al., 1986; Cramp, 1992; Eck, 1994). Acrocephalus stentoreus was first split off, as it was found to breed sympatrically with A. arundinaceus in central Asia and Israel (Stresemann and Arnold, 1949; Zahavi, 1957). Acrocephalus griseldis was shown to be distinct morphologically (Pearson and Backhurst, 1988) and on the basis of molecular markers (Leisler et al., 1997; Helbig and Seibold, 1999; Fregin et al., 2009; cf. Fig. 5), and also A. orientalis was shown to be genetically distinct (Leisler et al., 1997; Helbig and Seibold, 1999; Fregin et al., 2009; cf. Fig. 5).
The Acrocephalus warblers occurring on different islands in eastern Polynesia were previously considered a single widespread species, A.caffer (Holyoak and Thibault, 1984; Dickinson, 2003), whereas some authors suggested splitting this species into three, mainly because of wide geographical separation: A. caffer s.s. on the Society Islands, A. atyphus on Tuamotu, and A. mendanae in the Marquesas (Pratt et al., 1987; BirdLife International, 2000; Cibois et al., 2011a, b). Using mitochondrial sequence data, Cibois et al. (2007) showed that A. mendanae formed two independent lineages, which they proposed be treated as specifically distinct: A. percernis (closely related to A. atyphus) and A. mendanae s.s. (sister to A. aequinoctialis). In a later study, Cibois et al. (2008) analyzed mitochondrial DNA from extinct Acrocephalus taxa from different Society Islands, and concluded that A. caffer s.l. was more appropriately treated as one extant species, A. caffer s.s. (Tahiti), and two extinct ones, A. longirostris (Mo'orea) and A. musae (Raiatea, Huahine). A comprehensive study of Pacific Acrocephalus warblers (Cibois et al., 2011a) revealed complex colonization histories for several taxa, and revealed that A. luscinius is seprated into three distantly related clades, calling for a taxonomic revision.
Until recently, Acrocephalus orinus was only known from a single specimen collected in winter in India in 1867, and its validity as a species was controversial. A series of studies, and the surprise finding of a live individual in Thailand in March 2006, gradually led to the discovery of a breeding population at high elevation in north-eastern Afghanistan and adjacent Tajikistan, and has been found during the breeding season, and almost certainly also breeds, in Kyrgyzstan, eastern Uzbekistan and south-eastern Kazakhstan (Bensch and Pearson, 2002; Round et al., 2007; Pearson et al., 2008; Svensson et al., 2008, 2010; Timmins et al., 2010; Ayé et al., 2010; Koblik et al., 2011).
The four Palearctic Iduna species (previously Hippolais) have long been treated as two species, H. pallida and H. caligata (e.g. Watson et al., 1986), although the former was suggested to be split into H. pallida s.s. and H. opaca based on morphological, vocal and behavioral characteristics (Svensson, 2001; Parkin et al., 2004), and the latter has often been split into H. caligata s.s. and H. rama on the same grounds, as well as sympatric breeding distributions (e.g. Stepanyan, 1990; Svensson, 2001; Parkin et al., 2004). Helbig and Seibold (1999) found deep divergences within both H. pallida s.l. and H. caligata s.l. in mitochondrial DNA, later confirmed by Ottosson et al. (2005) in an extensive study that also included morphological data, and by Fregin et al. (2009) using mitochondrial and nuclear loci (Fig. 5), further supporting the splits based on non-molecular data.
The globally distributed family Hirundinidae (swallows and martins) form a morphologically and ecologically rather homogeneous taxon, constrained by their aerial foraging behavior (Turner, 2004). In total, 88 species are recognized (Gill and Donsker, 2013). Mayr and Bond (1943) analyzed the swallows and martins based on morphology and nest-building characteristics, and judged that they could be divided into ten natural groups. Phedina was judged to be "possibly related to Hirundo". White-backed Swallow (Cheramoeca), Greyrumped Swallow (Pseudhirundo) and the African sawwings (Psalidoprogne) were judged to be "so clear-cut and isolated that they require no further comment". The Barn and Cliff Swallow group consisted of Delichon, Hirundo and Petrochelidon. The genus Riparia was judged "a primitive genus without close relatives", while the crag martins (Ptyonoprogne); the rough-wing swallow group (Alopochelidon, Neochelidon and Stelgidopteryx); the Atticora group (Atticora, Notiochelidon, Orochelidon and Pygochelidon); the tree swallows (Tachycineta, then split also into Callichelidon, Lamprochelidon, Iridoprocne) and the purple martin group (Progne) each qualified as a distinct group.
Sheldon and Winkler (1993) performed the first molecular study of the family, using the DNA-DNA hybridization to estimate the phylogeny of a limited number of species, and later used the phylogeny to superimpose nest construction data. Their results largely corroborated the groups defined by Mayr and Bond (1943). Winkler and Sheldon (1993) reconstructed the nestexcavating Psalidoprogne as sister to all other swallows, which were divided into two monophyletic groups, the mud-nesting Hirundo group, and a clade containing both nest excavating and cavity-nesting species. The ancestral mode of nest-building in this clade appeared to be nest-excavating, as cavity nesting is reconstructed as a recent apomorphy, which is only found in two lineages out of three in a polytomy containing the nestexcavating Riparia riparia, the cavity-nesting New World endemics (Atticora, Haplochelidon, Neochelidon, Progne, Pygochelidon and Stelgidopteryx) and the cavitynesting New World tree swallows (Tachycineta). The nest-excavating Cheramoeca, Phedina, Pseudhirundo and Riparia cincta all trace their ancestry to nodes basal to the cavity-nesting species.
The most comprehensive molecular study of swallow phylogeny (Sheldon et al., 2005) identified the two river martins Pseudochelidon as sisters to all other swallows, albeit based on mitochondrial sequence data only. The combined mitochondrial and nuclear data available for a wide range of other swallows reconstructed two large clades comprising the remaining species, which Sheldon et al. (2005) termed "core martins, mud nesters and basal relicts". The core martin clade was dominated by Progne, Riparia and Tachycineta, and also included the less speciose Alopochelidon, Atticora, Haplochelidon, Neochelidon, Notiochelidon, Phedina, Pygochelidon and Stelgidopteryx. The mud-nesters contained Cecropsis, Delichon, Hirundo, Petrochelidon and Ptyonoprogne. The basal relicts were placed as sisters to the mudnesters and consisted of the groups judged by Mayr and Bond (1943) to be highly distinct and isolated from the others: White-backed Swallow (Cheramoeca), Greyrumped Swallow (Pseudhirundo) and the African sawwings (Psalidoprogne). Although Sheldon et al. (2005) were unable to determine the exact branching sequence of the "core martins, mud nesters and basal relicts", they concluded that excavation of nests is the ancestral condition in swallows, and that the mud-nesters constitute a monophyletic radiation. Similarly, the core martins constituted a monophyletic group, within which two clades are cavity-nesters. The position of these two clades was unresolved in relation to the nest-excavating sand martins, so it was not possible to determine whether cavity-nesting arose once or twice.
Beside these major analyses, several studies focusing on particular genera have further clarified the phylogeny of swallows. Babin (2005) analyzed the geographic variation and speciation in the rough-winged swallows (Stelgidopteryx), shedding light on a complex picture of ancestral polymorphism not yet sorted among lineages. Dor et al. (2010) analyzed the Hirundo clade, arriving at results that are entirely congruent with Sheldon et al. (2005) but with the addition of H. megaensis and H. nigrorufa, which were not investigated in the latter analysis. Dor et al. (2010) and Zink et al. (2006) also undertook phylogeographical studies of H. rustica. Moyle et al. (2008) estimated a phylogeny of the New World martins (Progne), and discovered remarkably intricate and close relationships, polyphyly and possible hybrid introgression, and came to the conclusion that a population genetic perspective would probably be required to clarify the taxonomy, particularly among Amazonian populations.
Whittingham et al. (2002), Cerasale et al. (2012) and Dor et al. (2012) studied the phylogeny of Tachycineta using different approaches. Whittingham et al. (2002) used concatenated mitochondrial loci, Cerasale et al. (2012) used complete mitochondrial genomes, while Dor et al. (2012) used 16 nuclear introns to compare concatenation to a coalescent-based species tree inference method. All these approaches yielded different topologies, particularly concerning the positions of the North American T. bicolor, Peruvian T. stolzmanni, Hispaniolan T. euchrysea and Bahamian endemic T. cyaneoviridis. Dor et al. (2012) argued that the method of extracting information from the variability in coalescence times between independent gene genealogies outperforms other methods of analysis, thus advocating their species tree as the currently most reliable hypothesis. Similarly, Kirchman et al. (2000) analyzed relationships among different populations of the Petrochelidon fulva complex, and confirmed previous suggestions that the South American populations are distinct (P. rufocollaris).
A phylogeographical study of Riparia riparia and R. diluta, which were previously considered a single species (e.g. Peters, 1960b), was undertaken by Pavlova et al. (2008). It corroborated fieldwork suggesting that these are specifically distinct (Gavrilov and Savchenko, 1991; Goroshko, 1993), and suggested that they diverged sometime between the late Pliocene and middle Pleistocene. It also suggested a Pleistocene split between samples from central Siberia and Mongolia. However, no molecular studies have been carried out yet on the southern, mainly Chinese, populations, which have usually been treated as conspecific with R. diluta.
The Pycnonotidae comprises approximately 150 species (Gill and Donsker, 2013) distributed across Africa and southern Asia. The first major molecular study of bulbuls (27 species; Pasquet et al., 2001), using mitochondrial sequence data, focused on the genus Criniger, but their taxon sampling allowed general inferences about the family as a whole. Their results found a basal division into an African and a mainly Asian clade, and revealed that African and Asian clades of Criniger were not closely related. Warren et al. (2005) showed that the bulbuls on Madagascar and on the islands of the Indian Ocean (Hypsipetes) originated from Asian ancestors. Moyle and Marks (2006) followed up by using also nuclear genes and additional taxa (in total 57 species). Their phylogeny provided further detail and corroborated the two major clades, of which only the Asian clade included taxa also occurring in Africa, namely representatives of the genus Pycnonotus. In addition to the two main clades, Moyle and Marks (2006) found the monotypic African genus Calyptocichla to be sister to the Asian clade, but with weak support. A study by Johansson et al. (2007a) further clarified the phylogeny of the Afrotropical clade, among other things placing Calyptocichla in this clade, and proposing a revised taxonomy in which the genus Atimastillas was split from Chlorocichla, and the polyphyletic Andropadus was divided into four genera, Andropadus, Arizelocichla, Eurillas and Stelgidillas. Oliveros and Moyle (2010) studied the origin of the Philippine bulbuls, adding substantial resolution to the Asian clade. They found a number of instances of high genetic divergence within species, e.g. three deeply divergent lineages within Ixos philippinus (confirmed for two of these by Silva-Iturriza et al., 2010), and non-monophyly of the genus Ixos. Their preferred taxonomic solution was to include the genus Microscelis and Philippine members of Ixos in Hypsipetes, a genus to which they have previously been allocated (e.g. Rand and Deignan, 1960).
The phylogenetic position of Neolestes torquatus, which was originally described as an aberrant malaconotid by Cabanis (1875), has been studied genetically and morphologically, and has been shown to be a bulbul, belonging in the Afrotropical clade (Dowsett et al., 1999; Moyle and Marks, 2006; Johansson et al., 2007a; Oliveros and Moyle, 2010; Zuccon and Ericson, 2010). Johansson et al. (2007a) and Zuccon and Ericson (2010) found good support for Calyptocichla in a sister group to the Afrotropical clade, in a clade that also contained Andropadus importunus and A. gracilirostris. Today, all generally recognized genera in Pycnonotidae have been included in molecular phylogenies, but much work remains to be done at the intra- and interspecific levels, as illustrated by Fuchs et al. (2011), who found significant biometric differences between lowland and montane populations of Phyllastrepus debilis in Tanzania.
The "babblers" and allies, as presently circumscribed, comprise in total more than 450 species in five families, spread across Africa, Eurasia and Australasia, with particularly high diversity in temperate and sub-tropical parts of the Sino-Himalayan region; only one species (Chamaea fasciata) is found in the New World (Gill and Donsker, 2013). They are ecologically highly diverse, and exhibit a multitude of morphological and behavioral adaptations that have confounded morphologybased taxonomic judgment in the past. Reflecting the fact that the babblers were used as a "scrap basket" (Mayr and Amadon, 1951) for taxa that were difficult to classify with confidence, several taxa previously placed in this group have been removed as a result of molecular evidence. Recent molecular work on Passerida (Johansson et al., 2008a) and Sylvioidea (Alström et al., 2006; Fregin et al., 2012) in general, on the large scale phylogeny of babblers (Cibois, 2003a; Gelang et al., 2009; Moyle et al., 2012), and on several specific groups of babblers (e.g. Cibois et al., 2002; Pasquet et al., 2006; Reddy and Cracraft, 2007; Zhang et al., 2007; Zou et al., 2007; Luo et al., 2009; Dong et al., 2010a, b; Reddy and Moyle, 2011) have gradually clarified the phylogeny of this complex group. These studies have revealed that the traditional babblers (e.g. Deignan, 1964b; Dickinson, 2003) do not constitute a monophyletic group, and some taxa have been removed from the babbler assemblage (e.g. the Australasian "babblers" Garritornis and Pomatostomus [Sibley and Ahlquist, 1990]; Malagasy "babblers" Mystacornis, Oxylabes, Hartertula and Neomixis [Cibois et al., 1999; Johansson et al., 2008a, b; Fregin et al., 2012]; Chaetops frenatus [Ericson and Johansson, 2003; Barker et al., 2004; Beresford et al., 2005]; Panurus biarmicus [Ericson and Johansson, 2003; Alström et al., 2006; Fregin et al., 2012]; Erpornis [previously Yuhina] zantholeuca [Cibois et al., 2002; Barker et al., 2004]; Eupetes macrocerus [Jønsson et al., 2007]; Robsonius, Micromacronus, Leonardina and Malia [Moyle et al., 2012; Oliveros et al., 2012]; Kakamega [Johansson et al., 2008a]; Pnoepyga [Gelang et al., 2009; Fregin et al., 2012]; and Pteruthius [Cibois, 2003; Reddy and Moyle, 2011]), whereas some additional taxa have been shown to belong there (e.g. Sylvia and Zosterops [Cibois, 2003; Alström et al., 2003; Gelang et al., 2009; Fregin et al., 2012; Moyle et al., 2012]; Chamaea fasciata [Sibley and Ahlquist, 1990; Cibois, 2003; Gelang et al., 2009; Fregin et al., 2012; Moyle et al., 2012]; Lioptilus nigricapillus [Johansson et al., 2008a; Moyle et al., 2012]; Parophasma galinieri [Gelang et al., 2009; Moyle et al., 2012]; Rhopophilus pekinensis [Alström et al., 2006; Gelang et al., 2009], Graminicola bengalensis [Alström et al., 2006; Gelang et al., 2009]; Laticilla [previously Prinia] burnesii [Olsson et al., 2013a]).
Five primary clades have been identified in the babbler group, three of which are made up of traditional babblers, one of a mixture of babblers (mainly Yuhina) and white-eyes (latter traditionally Zosteropidae), and one containing babblers and Sylvia warblers (Cibois, 2003a; Alström et al., 2006; Gelang et al., 2009; Fregin et al., 2012; Moyle et al., 2012). The three most recent of these papers all recovered the same five primary clades, but differed in their taxonomic recommendations. Gelang et al. (2009) separated Sylviidae and Timaliidae at the family level, and treated Leiothrichinae, Pellorneinae, Timaliinae and Zosteropinae as subfamilies within Timaliidae. Fregin et al. (2012) recognized all five clades at the family level: Leiothrichidae, Pellorneidae, Timaliidae, Sylviidae and Zosteropidae. Moyle et al. (2012) took a third position in recognizing Sylviidae, Timaliidae and Zosteropidae at the family level, with Leiothrichinae, Pellorneinae and Timaliinae as subfamilies in Timaliidae. We here follow Fregin et al. (2012) and Gill and Donsker (2013) and treat the five primary babbler clades at the rank of family, as we consider that to be in better agreement with the treatment of the other primary clades in Sylvioidea than the treatments of Gelang et al. (2009) and Moyle et al. (2012).
The Timaliidae clade is restricted to (southern) Asia, and comprises the genera Dumetia, Macronus (see below), Pomatorhinus (see below), Rhopocichla, Spelaeornis, Sphenocichla (see below), Stachyris (see below), Timalia and Xiphirhynchus (see below) (Cibois, 2003; Gelang et al., 2009; Moyle et al., 2012), in total c. 55 species (Gill and Donsker, 2013). Macronus, Pomatorhinus and Stachyris, as traditionally circumscribed (e.g. Deignan, 1964b; Dickinson, 2003), have all been found to be non-monophyletic, and Moyle et al. (2012) suggested a number of rearrangements: resurrection of Mixornis (for M. gularis, M. flavicollis and M. kelleyi; two latter unstudied by molecular methods); restricting Macronus to M. striaticeps and M. ptilosus; reinstatement of Cyanoderma for several species placed in Stachyris or Stachyridopsis (e.g. C. chrysaeum, C. erythropterum, C. ruficeps and C. rufifrons); restricting Stachyris to a group containing e.g. S. nigriceps, S. striolata, the recently described S. nonggangensis (Zhou and Jiang, 2008; not studied by molecular methods) and Sphenocichla; and splitting Pomatorhinus into Pomatorhinus s.s. (comprising most species, including P. [previously Xiphirhynchus] superciliaris) and Megapomatorhinus (new genus name, containing M. hypoleucos, M. erythrocnemis, M. erythrogenys and M. swinhoei).
The relationships within Pomatorhinus s.l. have recently been examined by Dong et al. (2010a) using two mitochondrial and four nuclear markers for a relatively small number of taxa and by Reddy and Moyle (2011) based on the same two mitochondrial markers for a larger number of taxa. Both studies, as well as Moyle et al. (2012), inferred Stachyris s.s. nested within Pomatorhinus s.l., although there was conflict between the studies regarding the position of the Stachyris s.s. clade in relation to the primary Pomatorhinus s.l. clades. Moreover, all these, as well as Cibois (2003a) and Gelang et al. (2009), recovered Xiphirhynchus (see above) deep inside the Pomatorhinus s.l. clade. Reddy and Moyle (2011) found multiple deep divergences within traditional species of Pomatorhinus s.l. as well as nonmonophyletic species, and also identified 27 lineages with diagnosable plumage differences (i.e. phylogenetic species sensu Cracraft, 1989). Previously, Collar (2006) and Collar and Robson (2007) suggested elevation of several taxa treated as subspecies to the rank of species based on morphology and vocalizations.
Also other Timaliidae, e.g. the genus Spelaeornis, were revised recently, by Rasmussen and Anderton(2005, 2012), Collar (2006) and Collar and Robson (2007) based mainly on morphological and vocal characters.
The genera Actinodura, Babax (see below), Cutia, Garrulax (see below), Heterophasia, Leiothrix, Liocichla, Minla, Turdoides, Kupeornis (see below) and Phyllanthus (see below) have been recovered as members of the Leiothrichidae clade (Cibois, 2003; Gelang et al., 2009; Moyle et al., 2012), in total c. 130 species (Gill and Donsker, 2013). They are widely distributed across (southern) Asia, although the Turdoides clade (including Kupeornis and Phyllanthus; see below) has radiated extensively in Africa.
The genera Garrulax, Actinodura, Minla, Heterophasia and Turdoides, as generally circumscribed (e.g. Deignan, 1964b; Dickinson, 2003), have been found to be non-monophyletic (Cibois, 2003; Gelang et al., 2009; Luo et al., 2009; Dong et al., 2010b; Moyle et al., 2012), and based on these results Moyle et al. (2012) suggested a taxonomic revision of this clade. They proposed that Garrulax be divided into Garrulax, Grammatoptila (monotypic: G. striata), Ianthocincla and Trochalopteron, and Babax synonymized with Ianthocincla. Moreover, they proposed that Kupeornis and Phyllanthus be subsumed in Turdoides, Heterophasia annectans moved to Minla, and Minla cyanouroptera and M. strigula transferred to Actinodura. However, it should be noted that no molecular study has included the type species of Garrulax, G. rufifrons, so the circumscription of this genus should be considered preliminary. The reinstatement of Trochalopteron was earlier proposed by Luo et al. (2009) based on molecular data as well as the observation that all species in that clade have speckled eggs, unlike any other traditional Garrulax (though eggs have not been studied for all species). Earlier, Rasmussen and Anderton (2005) and Collar and Robson (2007) proposed reclassifications based on morphological features, and some of these agree with the molecular results, whereas others do not, and some have not yet been tested by molecular markers; Rasmussen and Anderton (2012) followed various genetic analyses in further revising Garrulax and other laughingthrush genera.
The taxonomy of Garrulax canorus has been assessed by molecular markers by Li et al. (2006, 2009), who proposed that the Taiwanese taxon should be treated as specifically distinct, G. taewanus (placed in genus Leucodioptron in latter study). The same conclusion was previously reached by Collar (2006) based on morphology. Several other taxa were also elevated from the rank of subspecies to species by Rasmussen and Anderton(2005, 2012), Collar (2006) and Collar and Robson (2007), mainly based on morphology and vocalizations. A new species, Liocichla bugunorum, was described as recently as 2006 (Athreya, 2006).
The Pellorneidae clade comprises species in the genera Gampsorhynchus, Graminicola, Illadopsis, Jabouilleia (see below), Kenopia, Malacocincla, Malacopteron, Napothera, Pellorneum, Ptilocichla, Ptyrticus (see below), Rimator (see below), Schoeniparus (formerly Alcippe; see below), Trichastoma (see below) and Turdinus (Cibois, 2003; Gelang et al., 2009; Moyle et al., 2012), in total c. 70 species (Gill and Donsker, 2013). In addition, Laticilla (previously Prinia) burnesii was found by Olsson et al. (2013a) to belong in this clade. With the exception of the African Illadopsis, this family occurs only in Asia.
The genera Illadopsis, Napothera, Malacocincla and Pellorneum, as traditionally defined (e.g. Deignan, 1964b; Dickinson, 2003), have been inferred to be nonmonophyletic (Cibois, 2003; Moyle et al., 2012), and in the taxonomic revision suggested by Moyle et al. (2012) several taxa were synonymized: Ptyrticus with Illadopsis; Trichastoma with Pellorneum; and Jabouilleia and Rimator with Napothera. These authors also moved some Napothera and some Malacocincla to Turdinus, while the remaining Malacocincla were placed in Pellorneum. However, the name Turdinus seems to be misapplied. The type species of this genus, T. macrodactylus, was not included in the analysis by Moyle et al. (2012), but in the study by Gelang et al. (2009), it was sister to Graminicola, with strong support, while Malacocincla abbotti, which was also placed in Turdinus by Moyle et al. (2012), was in a different clade (with high posterior probability but low maximum likelihood bootstrap support). The revised classification by Collar and Robson (2007), which was based mainly on morphological traits, agrees in some respects but differs in others from the treatment by Moyle et al. (2012).
A new species, Jabouilleia naungmungensis, was recently described from Myanmar (Rappole et al., 2005), although this was considered a subspecies of J. (=Rimator) danjouei by Collar and Robson (2007) and Collar (2011). Graminicola bengalensis was recently suggested to be split into two species, G. bengalensis s.s. (Indian subcontinent) and G. striatus (southern China, northern South-East Asia) (Leader et al., 2010).
The circumscription of Sylviidae today is radically different from the traditional usage of this name (e.g. Watson et al., 1986; Sibley and Monroe, 1990; Dickinson, 2003; Bairlein et al., 2006; see introduction). The Sylviidae s.s. clade includes the genera Chamaea, Chrysomma, Conostoma (see below), Fulvetta (see below), Horizorhinus (see below), Lioparus (see below), Lioptilus, Moupinia (sometimes synonymized with Chrysomma), Myzornis, Paradoxornis (see below), Parisoma (see below), Parophasma, Pseudoalcippe (see below), Rhopophilus and Sylvia (Barhoum and Burns, 2002; Cibois, 2003; Gelang et al., 2009; Moyle et al., 2012), in total c. 70 species (Gill and Donsker, 2013). This family is geographically more widespread than the previous babbler clades. Although the majority occurs in southern Asia, Sylvia (including Horizorhinus, Parisoma and Pseudoalcippe; see below) is also distributed in northern Asia, Europe and Africa, Parophasma and Lioptilus are restricted to Africa, and Chamaea is restricted to western North America, as the only babbler representative in the New World.
The genus Paradoxornis has been shown to be nonmonophyletic, with at least Conostoma nested within Paradoxornis (Yeung et al., 2011). In a comprehensive analysis of the parrotbills, Yeung et al. (2011) identified three major clades in both mitochondrial and nuclear gene trees, further supported by body size and plumage coloration. The monotypic genus Conostoma was confirmed to belong in one of these clades. However, while trees inferred by combined nuclear sequences supported monophyly of the parrotbills, mitochondrial DNA suggested that one of the parrotbill clades was more closely related to Chamaea, Fulvetta and Lioparus (two latter treated in genus Alcippe) than to the other parrotbills; however, the latter topology received no maximum likelihood bootstrap support (but high posterior probability). Penhallurick and Robson (2009) proposed a reclassification of the parrotbills into eight genera (including a new genus name, Sinosuthora, containing S. alphonsiana, S. brunnea, S. conspicillata, S. przewalskii, S. webbiana and S. zappeyi), based on size, plumage and vocalizations, backed up by a published study of mitochondrial DNA (Yeung et al., 2006; see correction of one of these generic names in Penhallurick, 2010). Previously, King and Robson (2008) had suggested splitting Paradoxornis ruficeps into two species, P. ruficeps s.s. and P. bakeri.
Shirihai et al. (2001) and Voelker and Light (2011) inferred the relationships of the genus Sylvia based on mitochondrial DNA, and found that the African species previously placed in the genus Parisoma were nested within the essentially Palearctic Sylvia. The latter authors, and previously Voelker et al. (2009), also found that Horizorhinus dohrni and Pseudoalcippe abyssinica formed a sister clade to Sylvia borin, and placed both in the genus Sylvia; P. abyssinica had previously been found to be closely related to Sylvia (S. melanocephala) by Cibois (2003a). This was later corroborated by Moyle et al. (2012; using P. atriceps), who also found Lioptilus nigricapillus to be part of this clade. Several groups of Sylvia warblers have been the subject of taxonomic studies based on multiple character sets, usually including DNA, which have resulted in the recognition of a larger number of species, e.g. the splitting of S. crassirostris from S. hortensis (Shirihai et al., 2001), S. balearica from S. sarda (Shirihai et al., 2001), and S. subalpina (synonym S. moltonii) from S. cantillans (Brambilla et al., 2008a, b, c). The long-standing uncertainty over species limits in the S. curruca complex (e.g. Watson et al., 1986; Cramp, 1992; Martens and Steil, 1997; Shirihai et al., 2001; Loskot, 2001, 2005; Bairlein et al., 2006) was recently investigated using mitochondrial DNA (Olsson et al., 2013b), but although six long-separated clades representing the taxa althaea, blythi, curruca, halimodendri, margelanica and minula were recovered, the gene tree partly disagreed with morphological evidence, and it was concluded that independent data were required to resolve the issue.
The following genera have been found to belong in the Zosteropidae clade: Chlorocharis, Cleptornis, Heleia, Lophozosterops, Oculocincta, Rukia, Speirops, Sterrhoptilus, Yuhina, Woodfordia, Zosterops and Zosterornis (latter placed in Stachyris in earlier studies) (Cibois et al., 2002; Cibois, 2003a; Zhang et al., 2007; Gelang et al., 2009; Moyle et al., 2009, 2012). Most of these have been represented by single species in these analyses, but those that have been represented by two or more species (Lophozosterops, Yuhina, Zosterops and Zosterornis) have been found to be non-monophyletic. For example, Yuhina is separated into at least three (Moyle et al., 2012) or four (Zhang et al., 2007; Moyle et al., 2009) monophyletic groups, with Y. diademata being sister to all Zosteropidae, and one of these Yuhina clades is sister to the rest of the Zosteropidae. The main Zosterops clade, which also includes at least Chlorocharis, Rukia, Speirops and Woodfordia, has been termed a "great speciator" by Moyle et al. (2009), as it shows evidence of an exceptionally rapid speciation rate and expansion over a vast area covering subsaharan Africa, much of southern Asia and Australasia. The monotypic genus Hypocryptadius was previously considered related to Zosterops, but was recently shown to be a forest-adapted sparrow (Fjeldså et al., 2010). A thorough taxonomic revision of the Zosteropidae is obviously greatly needed.
Detailed studies in Zosterops have been carried out on both Australian (Degnan and Moritz, 1992), Philippine (Jones and Kennedy, 2008), Indian Ocean (Warren et al., 2006; Milá et al., 2010) and South African (Oatley et al., 2011, 2012) species complexes, often revealing complex patterns of genetic and morphological divergence.
The genus Alcippe provides one of the most extreme examples of the difference between past taxonomic judgments based on morphology (e.g. Deignan, 1964b; Dickinson, 2003) and recent molecular phylogenies. In a study based on mitochondrial DNA by Pasquet et al. (2006) the genus was found to consist of four different unrelated clades, the positions of which have gradually been clarified in multilocus analyses by Gelang et al. (2009) and Moyle et al. (2012). One clade, Schoeniparus (containing S. cinereus, S. castaneceps, S. rufogularis, S. brunneus and S. dubius), was found to be nested in Pellorneidae; two clades, Fulvetta (including e.g. F. cinereiceps, F. ruficapilla and F. vinipectus) and Lioparus (monotypic: L. chrysotis), were nested in Sylviidae; whereas the position of the Alcippe s.s. clade (comprising e.g. A. poioicephala and A. morrisonia) is somewhat uncertain. The latter clade was recovered by Gelang et al. (2009) as sister to Pellorneidae with good support, whereas Moyle et al. (2012) recovered it as sister to Leiothrichidae, also with good support. The latter authors remarked that the position of this clade is mainly weakly supported or even contradicted by single loci. More research is needed to establish where the Alcippe s.s. clade belongs in the babbler phylogeny.
Phylogeographical studies of the Alcippe morrisonia complex by Zou et al. (2007) and Song et al. (2009) revealed unexpectedly deep divergences between different geographical areas (oldest split dated to 11.6 million years ago by Song et al., 2009). Moreover, Zou et al. (2007) found that one of these clades (A. m. fratercula from Yunnan, China) is more closely related to A. peracensis annamensis (Vietnam) than to other taxa treated as A. morrisonia. Based on these results, Gill and Donsker (2013) split A. morrisonia into four species: A. morrisonia s.s. (Taiwan), A. davidi (Shaanxi, Gansu, Sichuan, Hunan, Guangxi Provinces in China, northern Vietnam), A. fratercula (Yunnan Province in China to central Myanmar and northern Thailand) and A. hueti (Fujian, Guangdong and Hainan Provinces, China). Collar (2006) and Collar and Robson (2007) revised the taxonomy of several species of Fulvetta and Alcippe s.s. based mainly on morphological characters; several of these have a contorted taxonomic history.
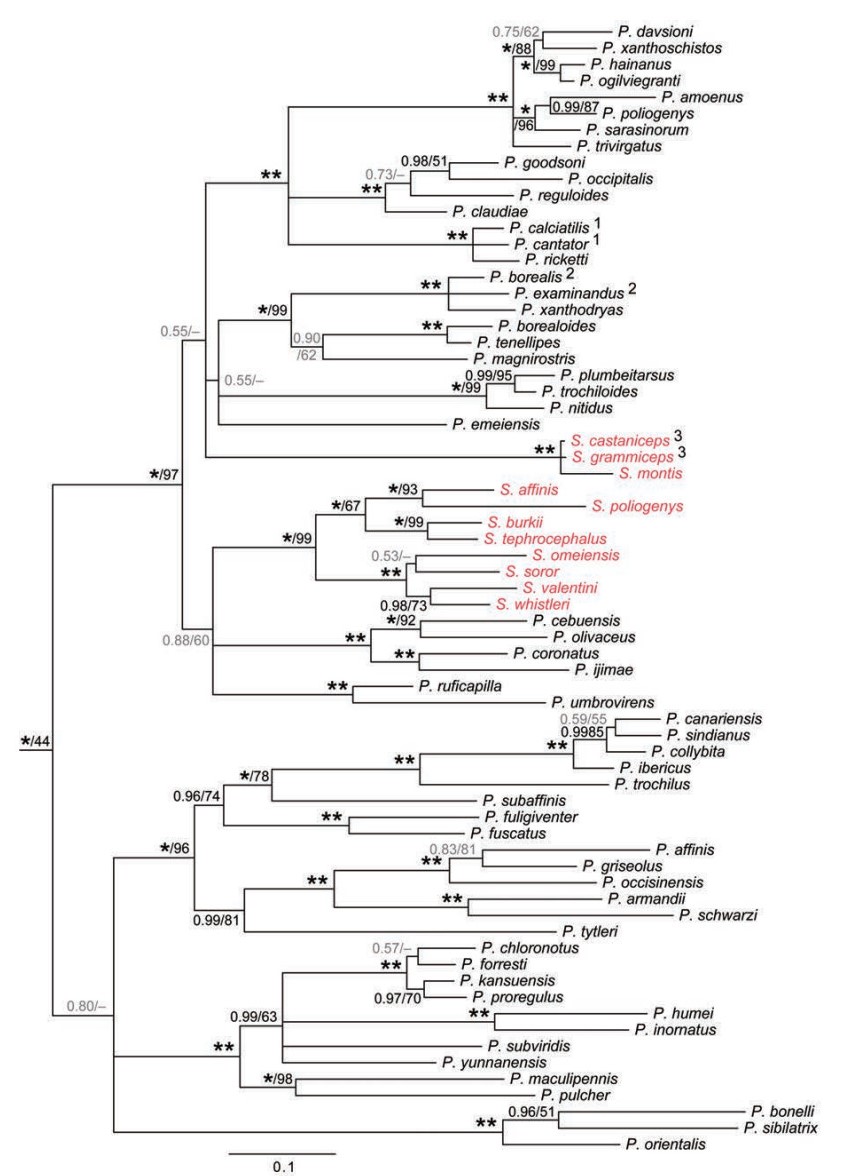
The family Phylloscopidae, as presently circumscribed, comprising the genera Phylloscopus and Seicercus, was proposed by Alström et al. (2006). Sibley and Ahlquist (1990) and Sibley and Monroe (1990) placed these genera in the subfamily Acrocephalinae together with many other genera, while Dickinson (2003) put them in the subfamily Phylloscopinae, together with Tickellia, Abroscopus, Eremomela, Sylvietta and Graueria. Neither of the classifications by Sibley and Ahlquist (1990), Sibley and Monroe (1990) or Dickinson (2003) are consistent with phylogenetic studies (Beresford et al., 2005; Alström et al., 2006; Johansson et al., 2007b; Fregin et al., 2012; Olsson et al., 2013a), although Graueria has not yet been studied phylogenetically. The most comprehensive phylogeny of Phylloscopidae to date (Johansson et al., 2007b) was based on two mitochondrial and one nuclear loci for 55 of the 68 species recognized at that time (Dickinson, 2003). Other studies based on smaller, but partly different datasets have been published (Richman and Price, 1992; Martens et al., 2004, 2008; Olsson et al., 2004, 2005; Päckert et al., 2004, 2009). Figure 6 shows a tree for all of the species in these studies based on the same sequences, but reanalyzed here. As has already been remarked in some of the previous studies, the tree shows that Seicercus is nested within Phylloscopus, rendering the latter genus non-monophyletic. The tree also suggests that Seicercus is non-monophyletic, although this has poor statistical support. As has also been suggested by previous authors, the taxonomic implications are that either Seicercus be synonymized with Phylloscopus or the complex be split into more than two genera. We await a more complete taxonomic study that is in preparation.
The genera Phylloscopus and Seicercus have undergone dramatic taxonomic changes in the past 20 years, due to studies of especially vocalizations and DNA. No fewer than six species new to science have been described in that period: P. hainanus (Olsson et al., 1993), P. emeiensis (Alström and Olsson, 1995), S. omeiensis (Martens et al., 1999), S. soror (Alström and Olsson, 1999), P. occisinensis (Martens et al., 2008) and P. calciatilis (Alström et al., 2010). Moreover, taxonomic revisions have led to the recognition of several new species: P. canariensis and P. ibericus (previously treated as subspecies of P. collybita; P. ibericus formerly incorrectly called P. brehmii) (Helbig et al., 1996; Salomon et al., 1997; Helbig et al., 2001; Bensch et al., 2002; Salomon et al., 2003); P. orientalis (previously subspecies of P. bonelli; Helbig et al., 1995); P. borealoides (previously synonym of P. tenellipes; Martens, 1988); P. chloronotus (previously subspecies of P. proregulus; Alström and Olsson, 1990; Martens et al., 2004); P. forresti (previously synonym of P. proregulus chloronotus; Martens et al., 2004); P. kansuensis (previously synonym of P. p. proregulus; Alström et al., 1997; Martens et al., 2004); P. yunnanensis (previously synonym of P. proregulus chloronotus; Alström et al., 1990, 1992 [described as P. sichuanensis in latter study]; Martens et al., 2004); P. claudiae, P. goodsoni and P. ogilviegranti (two former previously subspecies of P. reguloides, third previously subspecies of P. davisoni; Olsson et al., 2005; Päckert et al., 2009); P. examinandus and P. xanthodryas (previously subspecies of P. borealis; Saitoh et al., 2006, 2008, 2010; Reeves et al., 2008; Martens, 2010; Alström et al., 2011d); S. valentini, S. whistleri and S. tephrocephalus (previously subspecies of S. burkii; Alström and Olsson 1999, 2000; Martens et al., 1999; Olsson et al., 2004; Päckert et al., 2004). The taxonomic rank of some currently recognized species, e.g. P. subaffinis and P. humei, has been debated over the years, but has been settled due to recent studies of vocalizations and/or DNA (Alström and Olsson, 1992; Irwin et al., 2001b). In the case of the P. trochiloides–P. plumbeitarsus–P. nitidus complex, the debate is still ongoing due to different interpretations of the available data (Irwin, 2000; Irwin et al., 2001b, 2005, 2008; Knox et al., 2002; Dickinson, 2003; Gill and Donsker, 2013). One species, P. xanthoschistos, has been transferred from Seicercus to Phylloscopus (Olsson et al., 2005; Päckert et al., 2009), and one subspecies from one species to another, P. fuscatus weigoldi to P. fuligiventer weigoldi (Martens et al., 2008). See reviews by Irwin et al., (2001a), Rheindt (2006) and Martens (2010).
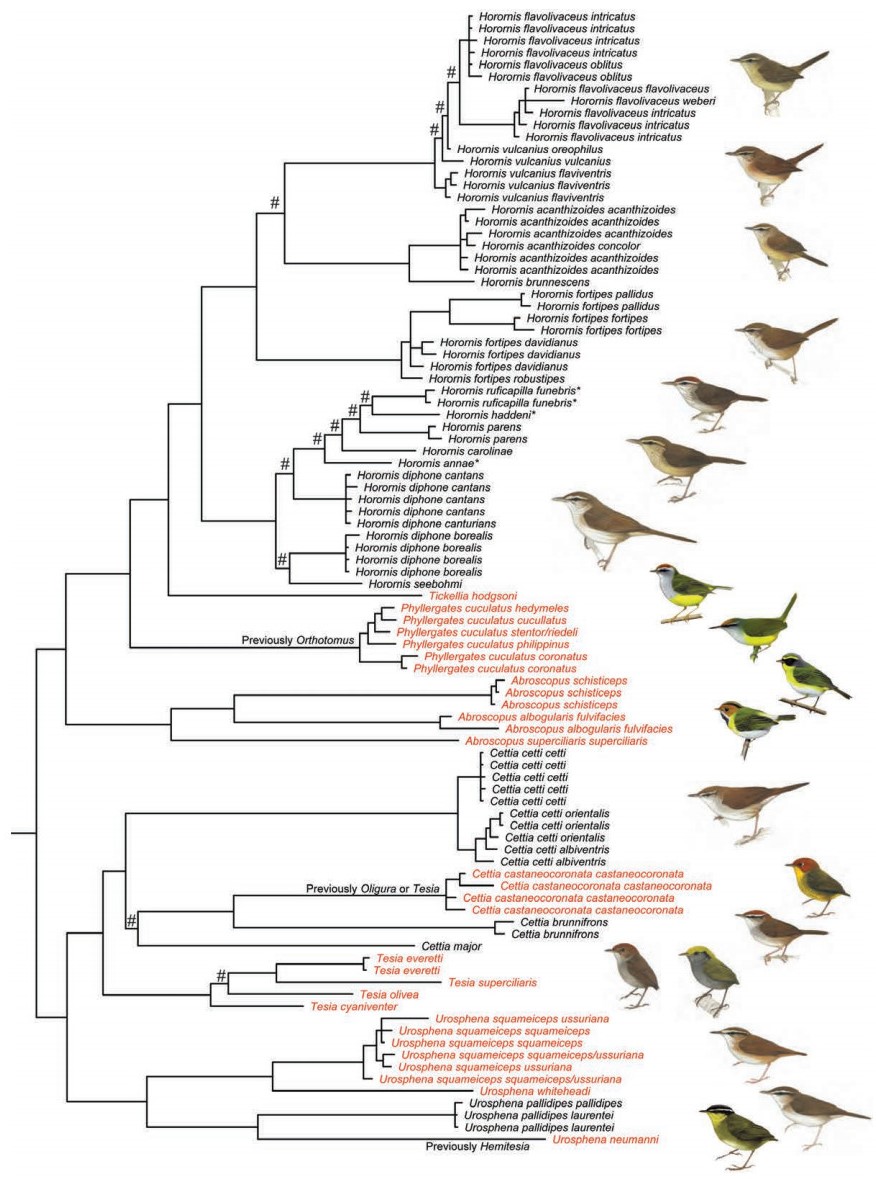
Alström et al. (2006) found that, based on sequence data from two loci, one mitochondrial and one nuclear, that two species of Cettia, one species each of Urosphena, Tesia, Abroscopus and Tickellia, and Orthotomus cuculatus formed a clade, well separated from a diverse number of other passerines. They proposed the family name Cettiidae for this group. This clade (limited to one species each of Cettia, Abroscopus and Tickellia) was corroborated by Johansson et al. (2008a) based on three nuclear loci. Irestedt et al. (2011) concluded, based on four loci, that Hemitesia is also part of this clade. Two of these studies (Alström et al., 2006; Irestedt et al., 2011) indicated that the genus Cettia is non-monophyletic. Alström et al. (2011a) revised the family based on one mitochondrial and three nuclear loci (Fig. 7). They confirmed the previous findings, including the strong non-monophyly of the genus Cettia, as this genus is scattered across the entire family tree. Based on these results, they proposed a revised classification in which Cettia is divided into Cettia s.s. and Horornis and one species (C. pallidipes) transferred to Urosphena (where it was sometimes placed previously); Oligura (Tesia) castaneocoronata moved to Cettia; Hemitesia synonymized with Urosphena; and Phyllergates reinstated as the generic name for O. cuculatus (Fig. 7). Previously, King (1989) proposed a new classification of some Tesia and Urosphena based on morphology, vocalizations and behavior, which is partly in agreement with the molecular study of Alström et al. (2011a).
Two of the species, Horornis carolinae and H. haddeni, were recently described (Rozendaal, 1987; LeCroy and Barker, 2006; respectively). Moreover, the taxonomy of several taxa has been much debated. Horornis diphone has variously been treated as a single species, or split into H. diphone s.s. and H. canturians, generally without explanation (cf. Delacour, 1942–1943; King and Dickinson, 1975; Morony et al., 1975; Watson et al., 1986; Sibley and Monroe, 1990; Inskipp et al., 1996; Baker, 1997; Dickinson, 2003; Bairlein et al., 2006; Kennerley and Pearson, 2010). According to mitochondrial DNA, the Japanese H. diphone cantans and central Chinese H. d. canturians were recently found to form a clade separate from the northern H. d. borealis (cf. Fig. 7). This is inconsistent with morphology, as the two latter are similar in plumage and structure, while the first one is more divergent. The authors concluded that a more comprehensive study was needed before any conclusions could be drawn. Furthermore, Horornis seebohmi has often been treated as a subspecies of H. diphone s.l. (e.g. Delacour, 1943; Watson et al., 1986; Baker, 1997), although some authors considered H. seebohmi to be a distinct species, based on unpublished differences in song and lack of the pronounced sexual size dimorphism of H. diphone/H. canturians (King and Dickinson, 1975; Inskipp et al., 1996; Dickinson, 2003). The specific status of H. seebohmi has recently been supported based on vocalizations and mitochondrial DNA (Hamao et al., 2008) as well as multilocus data (Alström et al., 2011a).
Horornis acanthizoides has recently been split into H. acanthizoides s.s. and H. brunnescens based on a study of morphology, vocalizations and mitochondrial DNA (Alström et al., 2007). Olsson et al. (2006) concluded, based on congruence of gene trees from one mitochondrial and one nuclear loci, that H. vulcanius is nested within H. flavolivaceus, and that some of the subspecies of H. flavolivaceus be moved to H. vulcanius. This was, however, contradicted in the study by Alström et al. (2011a; Fig. 7), which comprised a larger number of loci and samples (including all of the samples from Olsson et al., 2006). The latter study inferred deep divergences between two main H. flavolivaceus clades, with the taxon intricatus represented in both (Fig. 7). The authors concluded that more data, including unsampled subspecies, would be needed to resolve this issue. Deep divergences have also been found between different populations of Horornis fortipes (Alström et al., 2011a; cf. Fig. 7), although contradictory results from different analyses prevented taxonomic conclusions from being drawn. The same study also found that western and eastern populations of Cettia cetti were separated into rather divergent clades (cf. Fig. 7), calling for additional studies.
The family Scotocercidae was recently proposed, comprising the single species Scotocerca inquieta (Fregin et al., 2012). This was based on molecular (Alström et al., 2011c; Fregin et al., 2012) and morphological data (Alström et al., 2011c). The earlier of these studies also suggested that western and eastern populations might be different at the species level, and called for further studies.
The Afrotropical genus Erythrocercus has been reconstructed in an unresolved position in relation to Cettidae and Aegithalidae (Alström et al., 2011c; Fregin et al., 2012). The three species in the genus (Dickinson, 2003; Gill and Donsker, 2013) are morphologically and ecologically divergent from both Cettidae and Aegithalidae, and Fregin et al. (2012) proposed the family name Erythrocercidae based on molecular, morphological and ecological evidence.
The family Aegithalidae, bushtits (or long-tailed tits), is one of the smallest families within Sylvioidea, comprising only 13 species (Gill and Donsker, 2013). Nine of these are Eurasian Aegithalos, two are Asian Leptopoecile, one is the Javan endemic Psaltria, and one is the North and Central American Psaltriparus. Psaltria does not appear to have been investigated in any molecular phylogeny, but three multilocus studies have shown Aegithalos and Psaltriparus to be sisters, with Leptopoecile sister to these (Johansson et al., 2008a; Päckert et al., 2010; Fregin et al., 2012). Dai et al. (2010) analyzed mitochondrial DNA for four Chinese Aegithalos species, while the relationships among all Aegithalidae species except Psaltria were studied by a combination of mitochondrial and nuclear markers by Päckert et al. (2010). The latter study obtained a well-resolved and strongly supported tree, except for a surprising lack of differentiation among four of the species recognized by Gill and Donsker (2013): A. fuliginosus, A. iouschistos, A. bonvaloti and A. sharpei. Additionally, they found deep splits among different populations of especially A. concinnus. Dai et al. (2011, 2013) confirmed the existence of deeps splits within A. concinnus, and evaluated their causes. In a study of mitochondrial DNA of A. caudatus sampled across Russia, Zink et al. (2008) found no strong geographical structure.
Few taxa can show a record of taxonomic volatility as extreme as that of the Afrotropical Pholidornis, which has been placed in Dicaeidae, Estrildidae, Hyliidae, Meliphagidae, Nectariniidae, Remizidae and Sylviidae (Sefc et al., 2003 and references therein). The uncertainty regarding the Afrotropical Hylia has been less extreme, but opinion has been divided. The most favored positions have been to treat it as a sunbird (Sclater, 1930; Bannerman, 1948; Brosset and Erard, 1986) or a warbler (Chapin, 1953; Sibley and Monroe, 1990; Dowsett and Dowsett-Lemaire, 1993; Keith, 1997), but e.g. Meliphagidae (Beecher, 1953) and Paridae (Keith, 1997) have also been suggested. There is morphological evidence for a relationship between the two genera in the form of a brush-tipped tongue and a long hyoid with flattened epibranchial horns, characters that also suggest a relationship with Nectarinidae (Bannerman and Bates, 1924). These similarities prompted Bates (1930) to erect the family Hyliidae, but this was not widely accepted by subsequent authors, most of whom did not consider Pholidornis and Hylia as members of the same family. The phylogenetic position of both Hylia and Pholidornis continued to be contentious up to the first molecular analysis by Sefc et al. (2003), who showed that they were closely related and belong in Sylvioidea, later corroborated for Hylia by Beresford et al. (2005), Fuchs et al. (2006), Irestedt e al. (2011) and Fregin et al. (2012). Fregin et al. (2012) tentatively proposed that the family Hyliidae should be resurrected for Hylia and Pholidornis.
Marks (2010) revealed the existence of four (five) markedly divergent haplotype groups within Hylia prasina, indicating that further taxonomic studies are warranted.
The Asian Pnoepyga wren babblers were classified as babblers (Timaliidae s.l.) until Gelang et al. (2009) found that they were not closely related to any of the babbler clades, and erected the monotypic family Pnoepygidae. The family contains only four species (Gill and Donsker, 2013). The taxonomy of the Taiwanese endemic P. formosana has been debated, and it has variously been treated as conspecific with P. pusilla (Deignan, 1964b; Cheng, 1987) or with P. albiventer (Dickinson, 2003; Liu et al., 2010). Collar (2006) suggested, based on morphological evidence, that the Taiwanese taxon should be considered a distinct species, and the same conclusion was reached by Päckert et al. (2012) based on DNA sequence data and analyses of songs. Päckert et al. (2012) also found both molecular and acoustic evidence of a divergence within P. albiventer. They proposed that the Chinese taxon should be treated as a species, P. mutica, but refrained from suggesting any taxonomic action for a genetically equally divergent Myanmar population within the P. albiventer complex, for which they lacked acoustic data.
The Afrotropical genus Nicator, with just three species, has variously been placed in the shrike family Laniidae (Reichenow, 1902–1903; Sclater, 1930; Wolters, 1979) or with the Pycnonotidae (Chapin, 1953; Hall and Moreau, 1970; Rand and Deignan, 1960), based on morphological criteria. DNA-DNA hybridization studies (Sibley and Ahlquist, 1985) and feather protein electrophoresis (Hanotte et al., 1987) placed Nicator with Pycnonotidae. However, based on sequence data, Beresford et al. (2005), Johansson et al. (2008a) and Fregin et al. (2012) found Nicator chloris and the Alaudidae + Panuridae clade in unresolved sister positions to the rest of the Sylvioidea (cf. Fig. 1). Gill and Donsker (2013), based on the above-mentioned recent studies, placed Nicator in a monotypic family.
The relationships within the superfamily Sylvioidea have been enormously clarified in recent years thanks to a large number of molecular studies. These analyses have confirmed numerous conclusions drawn from earlier morphological studies, but they have also suggested multiple cases of previously unexpected relationships. Some of the surprising findings revealed by molecular methods have been supported by more careful reevaluation of morphological data (earlier morphologybased classifications have generally been based on superficial morphological similarity rather than thorough analyses). However, many of the results from molecular studies need to be corroborated by independent data. Most molecular phylogenies are based on a small number of loci (sometimes only mitochondrial), and even in multilocus analyses, strongly supported relationships frequently receive their support from a small fraction of the analyzed loci, with the majority of the loci neither supporting nor contradicting these relationships. As genomic data are becoming cheaper and more easily obtainable, many of the recent discoveries will be reevaluated by considerably larger datasets in the not too distant future. One of the challenges will be to resolve the relationships among the different families within Sylvioidea, which are still largely unresolved. However, if, as seems likely to be the case based on the presently available data, the lack of resolution results mainly from rapid divergence of the primary lineages (families), these relationships might remain obscure.
The total number of species within Sylvioidea has increased much in recent years, mainly due to studies applying molecular markers and/or vocalizations and other life-history data, and in many groups multiple cryptic species have been detected. It seems likely that the number of species is going to continue to increase, as new groups are being studied with modern integrative methods (using a combination of morphological, vocal, genetic, behavioural and other data). In particular, some polytypic species of larks, "warblers", bulbuls, and "babblers" are likely to comprise considerably more species than presently recognized. As good taxonomic assessments are a fundamental basis for many evolutionary studies, as well as for conservation purposes, continued efforts to revise the species-level taxonomy within Sylvioidea are much needed.
We are grateful to Nigel Collar, Silke Fregin, Margaret Koopman/Niven Library at the Percy FitzPatrick Institute of African Ornithology, Peter Kennerley, Lars Larsson, David Pearson, He Peng, Pamela Rasmussen, Craig Robson and an anonymous reviewer for comments on the manuscript or other assistance; and the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (No. 2011T2S04) to P.A., and NSFC (No. 31010103901) to F.L. and P.A.
|
Aleixo A, Pacheco JF. 2006. A family name for the monotypic oscine passerine genus Donacobius. Rev Brasil Ornitol, 14: 172–173.
|
|
Alström P, Fregin S, Norman JA, Ericson PGP, Christidis L, Olsson U. 2011a. Multilocus analysis of a taxonomically densely sampled dataset reveal extensive non- monophyly in the avian family Locustellidae. Mol Phylogenet Evol, 38: 381–397.
|
|
Alström P, Olsson U, Colston P. 1997. Re-evaluation of the taxonomic status of Phylloscopus proregulus kansuensis Meise. Bull Br Ornithol Club, 117: 177–193.
|
|
Alström P, Olsson U, Colston PR. 1990. Description of a possible new species of leaf warbler of the genus Phylloscopus from China. Bull Br Ornithol Club, 110: 43–47.
|
|
Alström P, Olsson U, Colston PR. 1992. A new species of Phylloscopus warbler from central China. Ibis, 134: 329–334.
|
|
Alström P, Olsson U, Round PD. 1991. The taxonomic status of Acrocephalus agricola tangorum. Forktail, 6: 3–13.
|
|
Alström P, Olsson U. 1990. Taxonomy of the Phylloscopus proregulus complex. Bull Br Ornithol Club, 110: 38–43.
|
|
Alström P, Olsson U. 1992. Taxonomic status of Phylloscopus affinis and P. subaffinis. Bull Br Ornithol Club, 112: 111–126.
|
|
Alström P, Olsson U. 1995. A new species of Phylloscopus warbler from Sichuan Province, China. Ibis, 137: 459–468.
|
|
Alström P, Olsson U. 1999. The Golden-spectacled Warbler: a complex of sibling species, including a previously undescribed species. Ibis, 141: 545–568.
|
|
Alström P, Olsson U. 2000. Golden-spectacled Warbler systematics. Ibis, 142: 495–500.
|
|
Alström P. 1998. Taxonomy of the Mirafra assamica complex. Forktail, 13: 97–107.
|
|
Athreya R. 2006. A new species of Liocichla (Aves: Timaliidae) from Eaglenest Wildlife Sanctuary, Arunachal Pradesh, India. Indian Birds, 2: 82–94.
|
|
Babin MJ. 2005. Geographic variation in rough-winged swallows (Aves: Hirundinidae: Stelgidopteryx. M.S. thesis. Louisiana State University and Agricultural and Mechanical College, Louisiana, USA.
|
|
Bairlein F, Alström P, Aymí R, Clement P, Dyrcz A, Gargallo G, Hawkins F, Madge S, Pearson D, Svensson L. 2006. Family Sylviidae (Warblers). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the Birds of the World. Volume 12. Lynx Edicions, Barcelona, pp 492–709.
|
|
Baker K. 1997. Warblers of Europe, Asia and North Africa. A & C Black, London.
|
|
Bannerman DA, Bates GL. 1924. On the birds collected in Northwestern and Northern Cameroon and parts of northern Nigeria – Part Ⅱ. Systematic Notes. Ibis, 66: 200–276.
|
|
Bannerman DA. 1948. The Birds of Tropical West Africa. Vol. 6. Crown Agents, London.
|
|
Bates GL. 1930. Handbook of the Birds of West Africa. Bale, Sons and Danielson, London.
|
|
Beresford P, Barker FK, Ryan PG, Crowe TM. 2005. African endemics span the tree of songbirds (Passeri): molecular systematics of several evolutionary 'enigmas'. Proc Roy Soc Lond B, 272: 849–858.
|
|
BirdLife International. 2000. Threatened Birds of the World. Lynx Edicions and BirdLife International, Barcelona and Cambridge, UK.
|
|
Bock WJ. 1994. History and nomenclature of avian family-group names. Bull Amer Mus Nat Hist, 222: 1–283.
|
|
Brosset A, Erard C. 1986. Les Oiseaux des Régions Forestiêres du Gabon, Vol. 1. Société National de Protection de la Nature, Paris
|
|
Cabanis J. 1875. [Neue Arten]. J Ornithol, 23: 234–238.
|
|
Chapin JP. 1953. The birds of the Belgian Congo. Part Ⅲ. Bull AMNH, 75A: 1–826.
|
|
Chappuis C. 1974. Illustration sonore de problèmes bioacoustiques posés par les oiseaux de la zone éthiopienne. Alauda, 42: 197–222.
|
|
Cheng T-H. 1987. A Synopsis to the Avifauna of China. Parey Scientific Publ., Hamburg, Germany.
|
|
Cibois A, Slikas B, Schulenberg TS, Pasquet E. 2001. An endemic radiation of Malagasy songbirds is revealed by mitochondrial DNA sequence data. Evolution, 55: 1198–1206.
|
|
Cibois A, Thibault J-C, Pasquet E. 2007. Uniform phenotype conceals double colonization by reed-warblers of a remote Pacific archipelago. J Biogeogr, 34: 1155–1166.
|
|
Cibois A. 2003b. Sylvia is a babbler: taxonomic implications for the families Sylviidae and Timaliidae. Bull Br Ornithol Club, 123: 257–261.
|
|
Clancey PA. 1980. S.A.O.S. Checklist of Southern African Birds. Southern African Ornithological Society, Johannesburg.
|
|
Clancey PA. 1989. Taxonomic and distributional findings on some birds from Namibia. Cimbebasia, 11: 111–133.
|
|
Collar NJ, Robson C. 2007. Family Timaliidae (Babblers). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the Birds of the World. Vol. 12. Picathartes to Tits and Chickadees. Barcelona: Lynx Edicions, pp 70–291.
|
|
Collar NJ. 2006. A partial revision of the Asian babblers (Timaliidae). Forktail, 22: 85–112.
|
|
Collar NJ. 2011. Taxonomic notes on some Asian babblers (Timaliidae). Forktail, 27: 100–102.
|
|
Cracraft J, Barker FK, Braun M, Harshman J, Dyke GJ, Feinstein J, Stanley S, Cibois A, Schikler P, Beresford P, García-Moreno J, Sorenson MP, Yuri T, Mindell DP. 2004. Phylogenetic relationships among modern birds (Neornithes). Toward an avian tree of life. In: Cracraft J, Donoghue MJ (eds), Assembling the Tree of Life. Oxford University Press, Oxford.
|
|
Cracraft J. 1989. Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation. In: Otte D, Endler JA (eds) Speciation and Its Consequences. Sinauer, Sunderland, Mass, pp 28–59.
|
|
Cramp S. 1992. The Birds of the Western Palearctic, Vol. 7. Oxford University Press, Oxford.
|
|
de Juana E, Suárez F, Ryan P, Alström P, Donald P. 2004. Family Alaudidae (Larks). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the Birds of the World, Vol. 9. Lynx Edicions, Barcelona, pp 496–601.
|
|
Dean WRJ, Fry CH, Keith S, Lack P. 1992. Family Alaudidae: Larks. In: Keith S, Urban EK, Fry CH (eds) The Birds of Africa, Vol. 4. Academic Press, London, pp 13–124.
|
|
Deignan H. 1964a. Subfamily Panurinae, parrotbills. In: Mayr E, Paynter RA (eds) Check-list of Birds of the World, Vol. X. Museum of Comparative Zoology, Cambridge, Mass, pp 430–442.
|
|
Deignan H. 1964b. Subfamily Timaliinae, babblers. In: Mayr E, Paynter RA (eds). Check-list of Birds of the World, Vol. X. Museum of Comparative Zoology, Cambridge, Mass, pp 240–427.
|
|
del Hoyo J, Elliott A, Christie DA. 2006. Handbook of the Birds of the World. Old World Flycatchers to Old World Warblers, Vol. 11. Lynx Edicions, Barcelona.
|
|
Delacour J. 1942–1943. The bush-warblers of the genera Cetti and Bradypterus, with notes on allied genera and species. Ibis, 6: 509–519.
|
|
Delacour J. 1943. A revision of the genera and species of the family Pycnonotidae (bulbuls). Zoologica, 28: 17–28.
|
|
Delacour J. 1946. Les timaliinés. L'Oiseau, 16: 7–36.
|
|
Dickinson E. 2003. The Howard and Moore Complete Checklist of the Birds of the World. Third ed. Helm, London.
|
|
Dickinson EC, Dekker RWRJ. 2001. Systematic notes on Asian birds. 11. A preliminary review of the Alaudidae. Zool Verh Leiden, 335: 61–84.
|
|
Dickinson EC, Rasmussen PC, Round PD, Rozendaal FG. 2000. Systematic notes on Asian birds. 1. A review of the russet bush-warbler Bradypterus seebohmi (Ogilvie-Grant, 1895). Zool Verh Leiden, 331: 12–64.
|
|
Dong F, Wu F, Liu LM, Yang XJ. 2010b. Molecular phylogeny of the barwings (Aves: Timaliidae: Actinodura), a paraphyletic group, and its taxonomic implications. Zool Stud, 49: 703–709.
|
|
Dowsett RJ, Dowsett-Lemaire F. 1993. A contribution to the distribution and taxonomy of Afrotropical and Malagasy birds. Tauraco Research Reports 5. Tauraco Press, Liège.
|
|
Dowsett RJ, Olson SL, Roy MS, Dowsett-Lemaire F. 1999. Systematic status of the Black-collared Bulbul Neolestes torquatus. Ibis, 141: 22–28.
|
|
Dowsett-Lemaire F, Dowsett RJ. 1987. European and African Reed Warblers, Acrocephalus scirpaceus and A. baeticatus: vocal and other evidence for a single species. Bull Br Ornithol Club, 107: 74–85.
|
|
Eck S. 1994. Die geographisch-morphologische Vikarianz der grossen palaearktischen Rohrsänger (Aves: Passeriformes: Sylviidae: Acrocephalus[arundinaceus]). Zool Abh Staatl Mus Tierkd Dresden, 48: 161–168.
|
|
Fjeldså J, Goodman SM, Schulenberg TS, Slikas B. 1999. Molecular evidence for relationships of Malagasy birds. In: Adams NJ, Slotow RH (eds) Proceedings of the 22nd International Ornithological Congress, Durban. BirdLife South Africa, Johannesburg, pp 3084–3094.
|
|
Fry CH, Williamson K, Ferguson-Lees IJ. 1974. A new subspecies of Acrocephalus baeticatus from Lake Chad and a taxonomic reappraisal of Acrocephalus dumetorum. Ibis, 116: 340–346.
|
|
Gavrilov EI, Savchenko AP. 1991. On species validity of the Pale Sand Martin (Riparia diluta Sharpe et Wyatt, 1893). Byul Mosk O-va Isp Prir Otd Biol, 96: 34–44. (in Russian)
|
|
Gelang M, Cibois A, Pasquet E, Olsson U, Alström P, Ericson PGP. 2009. Phylogeny of babblers (Aves, Passeriformes): major lineages, family limits and classification. Zool Scr, 32: 279–296.
|
|
Goroshko OA. 1993. Taxonomic status of the pale (sand?) martin Riparia (riparia?) diluta (Sharpe & Wyatt, 1893). Russ Ornit Zhurnal, 2: 303–323. (in Russian)
|
|
Haffer J. 1991. Familie Sylviidae-Zweigsänger (Grasmücken und Verwandte). In: von Blotzheim GUN, Bauer KM (eds) Handbuch der Vögel Mitteleuropas, Vol. 12. Aula-Verlag, Wiesbaden, pp 11–21 and 208–216.
|
|
Hall BP, Moreau RE. 1970. An Atlas of Speciation in African Passerine Birds. Trustees of the British Museum (Natural History), London.
|
|
Harrison CJO. 1966. The validity of some genera of larks (Alaudidae). Ibis, 108: 573–583.
|
|
Hartert E. 1909. Die Vögel der Paläarktischen Fauna. Verlag von R. Friedländer und Sohn, Berlin.
|
|
Holyoak DT, Thibault J-C. 1984. Contribution a l'eìtude des oiseaux de Polyneìsie orientale. Meìm. MNHN, Paris, 127: 1–209.
|
|
Inskipp T, Lindsey N, Duckworth W. 1996. An Annotated Checklist of the Birds of the Oriental Region. Oriental Bird Club, Sandy, Bedfordshire
|
|
International Commission on Zoological Nomenclature (ICZN). 1999. International Code of Zoological Nomenclature. Fourth Edition. The International Trust for Zoological Nomenclature, Padova.
|
|
Irwin DE. 2000. Song variation in an avian ring species. Evolution, 54: 998–1010.
|
|
Keith S. 1997. Genus Hylia Cassin, Hylia prasina (Cassin), Green Hylia. In: Urban EK, Fry CH, Keith S (eds) The Birds of Africa, Vol. 5. Academic Press, London, pp 428–430.
|
|
Kennerley P, Pearson D. 2010. Reed and Bush Warblers. Christopher Helm, London.
|
|
King B, Dickinson EC. 1975. A Field Guide to the Birds of SouthEast Asia. Collins, London.
|
|
King B, Robson C. 2008. The taxonomic status of the three subspecies of Greater Rufous-headed Parrotbill Paradoxornis ruficeps. Forktail, 24: 120–122.
|
|
King B. 1989. The avian genera Tesia and Urosphena. Bull Br Ornithol Club, 109: 162–166.
|
|
Leader PJ, Carey GJ, Olsson U, Baral HS, Alström P. 2010. The taxonomic status of Rufous-rumped Grassbird Graminicola bengalensis, with comments on its distribution and status. Forktail, 26: 121–126.
|
|
Liu X, Ding Z, Fang W, Lin W, Cai M, Yan C. 2010. The Avifauna of Taiwan. Taiwan Forestry Bureau, Taipei. (in Chinese)
|
|
Loskot VM. 2001. Taxonomic revision of the Hume's Whitethroat Sylvia althaea Hume, 1878. Avian Ecol Behav, 6: 41–42.
|
|
Loskot VM. 2005. Morphological variation and taxonomic revision of five south-eastern subspecies of Lesser Whitethroat Sylvia curruca (L. ) (Aves: Sylviidae). Zool Mededelingen Leiden, 79: 157–165.
|
|
Macdonald JD. 1952a. Notes on the Long-bill Lark Certhilauda curvirostris. Ibis, 94: 122–127.
|
|
Macdonald JD. 1952b. Notes on the taxonomy of the clapper larks of South Africa. Ibis, 94: 629–635.
|
|
Macdonald JD. 1953. Taxonomy of the Karoo and Red-back Larks of western South Africa. Bull Br Mus Nat Hist Zool, 1: 319–350.
|
|
Mackworth-Praed CW, Grant CHB. 1941. A survey of the African species placed in the genus Bradypterus. Ibis, 83: 441–455.
|
|
Maclean GL. 1969. South African Lark genera. Cimbebasia A, 1: 79–94.
|
|
Martens J, Eck S, Päckert M, Sun Y-H. 1999. The Golden-spectacled Warbler Seicercus burkii – a species swarm (Aves: Passeriformes: Sylviidae), part 1. Zool Abhandl Mus Dresden, 50: 281–327.
|
|
Martens J, Tietze DT, Eck S, Veith M. 2004. Radiation and species limits in the Asian Pallas's warbler complex (Phylloscopus proregulus s. l. ). J Ornithol, 145: 206–222.
|
|
Martens J. 2010. A preliminary review of the leaf warbler genera Phylloscopus and Seicercus. Br Orn Club Occas Publs, 5: 41–116.
|
|
Mayr E, Amadon D. 1951. A classification of recent birds. Amer Mus Novitat, 1496: 1–42.
|
|
Mayr E, Bond J. 1943. Notes on the generic classification of the swallows Hirundinidae. Ibis, 85: 334–341.
|
|
Mayr E, Greenway Jr JC. 1960. Check-list of Birds of the World, Vol. IX. Museum of Comparative Zoology, Cambridge, MA.
|
|
Morioka H, Shigeta Y. 1993. Generic allocation of the Japanese Marsh Warbler Megalurus pryeri (Aves: Sylviidae). Bull Nat Sci Mus Tokyo A, 19: 37–43. (in Japanese)
|
|
Morony JJ, Bock WJ, Farrand J. 1975. Reference List of the Birds of the World. American Museum of Natural History, New York.
|
|
Olsson U, Alström P, Colston PR. 1993. A new species of Phylloscopus warbler from Hainan Island, China. Ibis, 135: 2–7.
|
|
Päckert M, Martens J, Liang W, Hsu Y-L, Sun Y-H. 2012. Molecular genetic and bioacoustic differentiation of Pnoepyga WrenBabblers. J Ornithol, 154: 329–337.
|
|
Parkin DT, Collinson M, Helbig AJ, Knox AG, Sangster G, Svensson L. 2004. Species limits in Acrocephalus and Hippolais warblers from the Western Palaearctic. Brit Birds, 97: 276–299.
|
|
Pasquet E, Han L-X, Khobket O, Cibois A. 2001. Towards a molecular systematics of the genus Criniger, and a preliminary phylogeny of the bulbuls (Aves, Passeriformes, Pycnonotidae). Zoosystema, 23: 857–863.
|
|
Pätzold R. 2003. Kompendium der Lerchen Alaudidae. Jan Schimkat Medienpublizistik, Dresden.
|
|
Pearson DJ, Backhurst GC. 1988. Characters and taxonomic position of Basra Reed Warbler. Brit Birds, 81: 171–178.
|
|
Pearson DJ, Kennerley PR, Bensch S. 2008. A second museum specimen of large-billed reed warbler Acrocephalus orinus. Bull Br Ornithol Club, 128: 136–137.
|
|
Penhallurick J. 2010. A correction to Penhallurick & Robson (2009). Forktail, 26: 147–148.
|
|
Penhallurick J, Robson C. 2009. The generic taxonomy of parrotbills (Aves: Timaliidae). Forktail, 25: 137–141.
|
|
Peters JL. 1960a. Family Alaudidae. In: Mayr E, Greenway JC (eds) Check-list of Birds of the World, Vol. IX. Museum of Comparative Zoology, Cambridge, Mass, pp 3–80.
|
|
Peters JL. 1960b. Family Hirundinidae. In: Mayr E, Greenway JC (eds) Check-list of Birds of the World, Vol. IX. Museum of Comparative Zoology, Cambridge, Mass, pp 80–129.
|
|
Pratt HD, Bruner PL, Berrett DG. 1987. The Birds of Hawaii and the Tropical Pacific. Princeton University Press, Princeton, NJ.
|
|
Rand A L, Deignan HG. 1960. Family Pycnonotidae. In: Mayr E, Greenway JC (eds) Check-List of Birds of the World, Vol. IX. Museum of Comparative Zoology, Cambridge, pp 221–300.
|
|
Rasmussen PC, Anderton JC. 2005. Birds of South Asia: the Ripley Guide, First Ed. Lynx Edicions, Barcelona.
|
|
Rasmussen PC, Anderton JC. 2012. Birds of South Asia: the Ripley Guide, Second Ed. Lynx Edicions, Barcelona.
|
|
Rasmussen PC. 2012. Then and now: new developments in Indian systematic ornithology. J Bombay Nat Hist Soc, 109: 3–16.
|
|
Reichenow A. 1902–1903. Die Vögel Afrikas. Neudamm (J. Neumann).
|
|
Rheindt FE. 2006. Splits galore: the revolution in Asian leaf warbler systematics. Bird Asia, 5: 25–39.
|
|
Rheindt FE. 2010. New biogeographic records for the avifauna of Taliabu (Sula Islands, Indonesia), with preliminary documentation of two previously undiscovered taxa. Bull Br Ornithol Club, 130: 33–51.
|
|
Roberts A. 1940. The Birds of South Africa. HF & G. Witherby, London.
|
|
Round PD, Hansson B, Pearson DJ, Kennerley PR, Bensch S. 2007. Lost and found: the enigmatic large-billed reed warbler Acrocephalus orinus rediscovered after 139 years. J Avian Biol, 38: 133–138.
|
|
Rozendaal F. 1987. Description of a new species of bush warbler of the genus Cettia Bonaparte, 1834 (Aves: Sylviidae) from Yamdena, Tanimbar Islands, Indonesia. Zool Mededel, 61: 177–202.
|
|
Ryan PG, Dean WRJ, Madge SC, Pearson DJ. 2006. Family Cisticolidae. In: del Hoyo J, Elliott A, Christie DA (eds). Handbook of the Birds of the World. Vol 11. Old World Flycatchers to Old World Warblers. Lynx Edicions, Barcelona, pp 378–491
|
|
Ryan PG, Hood I, Bloomer P, Komen J, Crowe TM. 1998. Barlow's Lark: a new species in the Karoo Lark Certhilauda albescens complex of southwest Africa. Ibis, 140: 605–619.
|
|
Saitoh T, Nishiumi I, Alström P, Olsson U, Ueda K. 2006. Deep phylogeographical divergences among far eastern populations of the widespread Arctic Warbler. J Ornithol, 147: 242.
|
|
Salomon M, Bried J, Helbig AJ, Riofrio J. 1997. Morphometric differentiation between male Common Chiffchaffs, Phylloscopus (c. ) collybita Vieillot, 1817, and Iberian Chiffchaffs, P. (c. ) brehmii Homeyer, 1871, in a secondary contact zone (Aves). Zoologischer Anzeiger, 236: 25–36.
|
|
Salomon M, Voisin JF, Bried J. 2003. On the taxonomic status and denomination of the Iberian Chiffchaffs. Ibis, 145: 87–97.
|
|
Schönwetter M. 1979. Handbuch der Oologie. Band Ⅱ. Akademie-Verlag, Berlin.
|
|
Sclater WL. 1930. Systema Avium Aethiopicarum. British Ornithologists' Union, London
|
|
Shirihai H, Gargallo G, Helbig AJ. 2001. Sylvia warblers. Identification, taxonomy and phylogeny of the genus Sylvia. Christopher Helm, London.
|
|
Sibley CG, Ahlquist JE. 1990. Phylogeny and ClassiWcation of Birds. A Study in Molecular Evolution. Yale University Press, New Haven and London.
|
|
Sibley CG, Monroe Jr BL. 1990. Distribution and Taxonomy of Birds of the World. Yale University Press, New Haven.
|
|
Sibley CG, Monroe Jr BL. 1993. A Supplement to Distribution and Taxonomy of Birds of the World. Yale University Press, New Haven.
|
|
Silva-Iturriza A, Ketmaier V, Tiedemann R. 2010. Mitochondrial DNA suggests multiple colonizations of central Philippine islands (Boracay, Negros) by the sedentary Philippine bulbul Hypsipetes philippinus guimarasensis (Aves). J Zool Syst Evol Res, 48: 269–278.
|
|
Stepanyan LS. 1990. Konspekt ornitologicheskoy fauny SSSR. Nauka, Moscow.
|
|
Stresemann E, Arnold J. 1949. Speciation in the group of Great Reed-Warblers. J Bombay Nat Hist Soc, 48: 428–443.
|
|
Svensson L. 2001. Identification of western and eastern olivaceous, booted and Sykes's warblers. Bird World, 14: 192–219.
|
|
Timmins RJ, Ostrowski S, Mostafawi N, Noori H, Rajabi AM, Svensson L, Olsson U, Poole CM. 2010. New information on the Large-billed Reed Warbler Acrocephalus orinus, including its song and breeding habitat in north-eastern Afghanistan. Forktail, 26: 9–23.
|
|
Turner A. 2004. Family Hirundinidae (swallows and martins). In: Del Hoyo J, Elliott A, Christie D (eds) The Birds of the World, Vol. 9, Lynx Edicions, Barcelona, Spain, pp 602–685
|
|
Vaurie C. 1951. A study of Asiatic larks. Notes from the Walter Koelz collections number 11. Bull AMNH, 97: 435–526.
|
|
Vaurie C. 1954. Systematic Notes on Palearctic birds. No. 7. Alaudidae and Motacillidae (genus Anthus). Amer Mus Novit, 1672: 1–13.
|
|
Vaurie C. 1959. The Birds of the Palearctic Fauna. Passeriformes. Witherby, London.
|
|
Verbelen F, Trainor CR. 2012. Rediscovery of the Timor Bush Warbler Locustella (Bradypterus) timorensis on Alor and Timor, Wallacea, Indonesia. Bird Asia, 17: 47–48.
|
|
Verheyen R. 1958. Contribution a l'anatomie de base et la systematique des Alaudidae (Passeriformes). Alauda, 26: 1–25.
|
|
Voelker G, Spellman GM. 2003. Nuclear and mitochondrial DNA evidence of polyphyly in the avian superfamily Muscicapoidea. Mol Phylogenet Evol, 30: 386–394.
|
|
Voous KH. 1977. List of Recent Holarctic Bird Species, Second Ed. British Ornithologists' Union, London.
|
|
Watson GE, Traylor MA, Jr Mayr E. 1986. In: Mayr E, Cottrell GW (eds) Check-list of Birds of the World. Vol. 11. Museum of Comparative Zoology, Cambridge, Massachusetts.
|
|
Williamson K. 1968. Identification for Ringers. The genera Cettia, Locustella, Acrocephalus and Hippolais, Third Ed. British Trust for Ornithology, Tring.
|
|
Winterbottom JM. 1957. The problem of status of the Dusky Bush Lark Mirafra nigricans. Ostrich, 28: 240–241.
|
|
Wolters HE. 1979. Die Vogelarten der Erde. Paul Parey, Hamburg and Berlin.
|
|
Wolters HE. 1982. Die Vogelarten der Erde. 7. Lieferung Paul Parey, Hamburg and Berlin.
|
|
Yeung CKL, Lei F, Yang X, Han L, Lin M, Li SH. 2006. Molecular phylogeny of parrotbills. Paper presented at the 24th International Ornithological Congress of 2006, Hamburg, Germany. Abstract J Orn, 147(Suppl. 1): 87–88.
|
|
Zahavi A. 1957. The breeding birds of the Huleh swamp and lake (northern Israel). Ibis, 99: 606.
|
|
Zhang S, Yang L, Yang X, Yang J. 2007. Molecular phylogeny of the yuhinas (Sylviidae: Yuhina): a paraphyletic group of babblers including Zosterops and Philippine Stachyris. J Ornithol, 148: 417–426
|
|
Zink RM, Pavlova A, Rohwer S, Drovetski SV. 2006. Barn swallows before barns: population histories and intercontinental colonization. Proc R Soc London B, 273: 1245–1252.
|