| Citation: | Nyanasengeran Movin, Tatjana Gamova, Sergei G. Surmach, Jonathan C. Slaght, A.A. Kisleiko, James A. Eaton, Frank E. Rheindt. 2022: Using bioacoustic tools to clarify species delimitation within the Blakiston's Fish Owl (Bubo blakistoni) complex. Avian Research, 13(1): 100021. DOI: 10.1016/j.avrs.2022.100021 |
Although Blakiston's Fish Owl (Bubo blakistoni) is widely treated as a single species, marked differences in the structure of pair duets between continental and insular populations have been documented. However, no study has quantitatively assessed these vocal differences. We obtained 192 duets from 22 pairs of Blakiston's Fish Owl: 15 pairs of B. b. blakistoni from the Japanese island of Hokkaido and the Russian Kuril island of Kunashir, and seven pairs of B. b. doerriesi from Primorye on the Russian mainland. This is a sizeable dataset for such a large, retiring, and rare owl. We conducted bioacoustic examinations of 14 vocal parameters using principal component analysis and the Isler criterion to quantitatively test species boundaries within the B. blakistoni complex. We found that the insular populations on Hokkaido and Kunashir emerged as vocally similar to each other but markedly different from the continental populations of B. blakistoni, corresponding closely with presently accepted subspecies limits. Bioacoustic differences in the duets of the insular and continental groups are greater than the pairwise comparisons of territorial vocalisations between other sympatric owl species. Based on the reproductive importance of vocal duets in owl biology, we propose the taxonomic elevation of the continental subspecies to species level as Northern Fish Owl B. doerriesi. Our study corroborates the importance of bioacoustics in ascertaining species boundaries in owls and has important implications for the management of the two newly delimited species, each likely to be assessed as Endangered. Both species should be managed independently to optimise conservation outcomes.
Owls (Strigidae), with their nocturnal habits, distinct vocalisations and striking appearance, have captured human imagination for millennia (Hussain, 2021). Various cultures have considered them symbols of wisdom, good fortune, protection, and often portents of death (Talebinejad and Dastjerdi, 2015). However, human fascination for owls belies our poor understanding of their ecology and diversity. In recent decades, the use of bioacoustics has considerably improved our understanding of the taxonomy of many owl species complexes (Howell and Robbins, 1995; King, 2002; Rasmussen et al., 2012; Pons et al., 2013; Sangster et al., 2013; Flint et al., 2015; Sadanandan et al., 2015; Krabbe, 2017; Gwee et al., 2019b). Yet much of this work has focused on the smaller-bodied members of the family while the taxonomy of the largest owl genus, Bubo, has been relatively static, apart from conflicting treatments of Fraser's (Bubo poensis) and Usambara Eagle-owl (Bubo vosseleri) based on discrepancies in the interpretation of bioacoustic data (Turner et al., 1991; del Hoyo and Collar, 2014; Turner and Pearson, 2015). The genus Bubo comprises 17–20 of the largest owl species with representatives from the Holarctic to the tropics occupying every major continent apart from Australia and Antarctica (del Hoyo and Collar, 2014; Winkler et al., 2020).
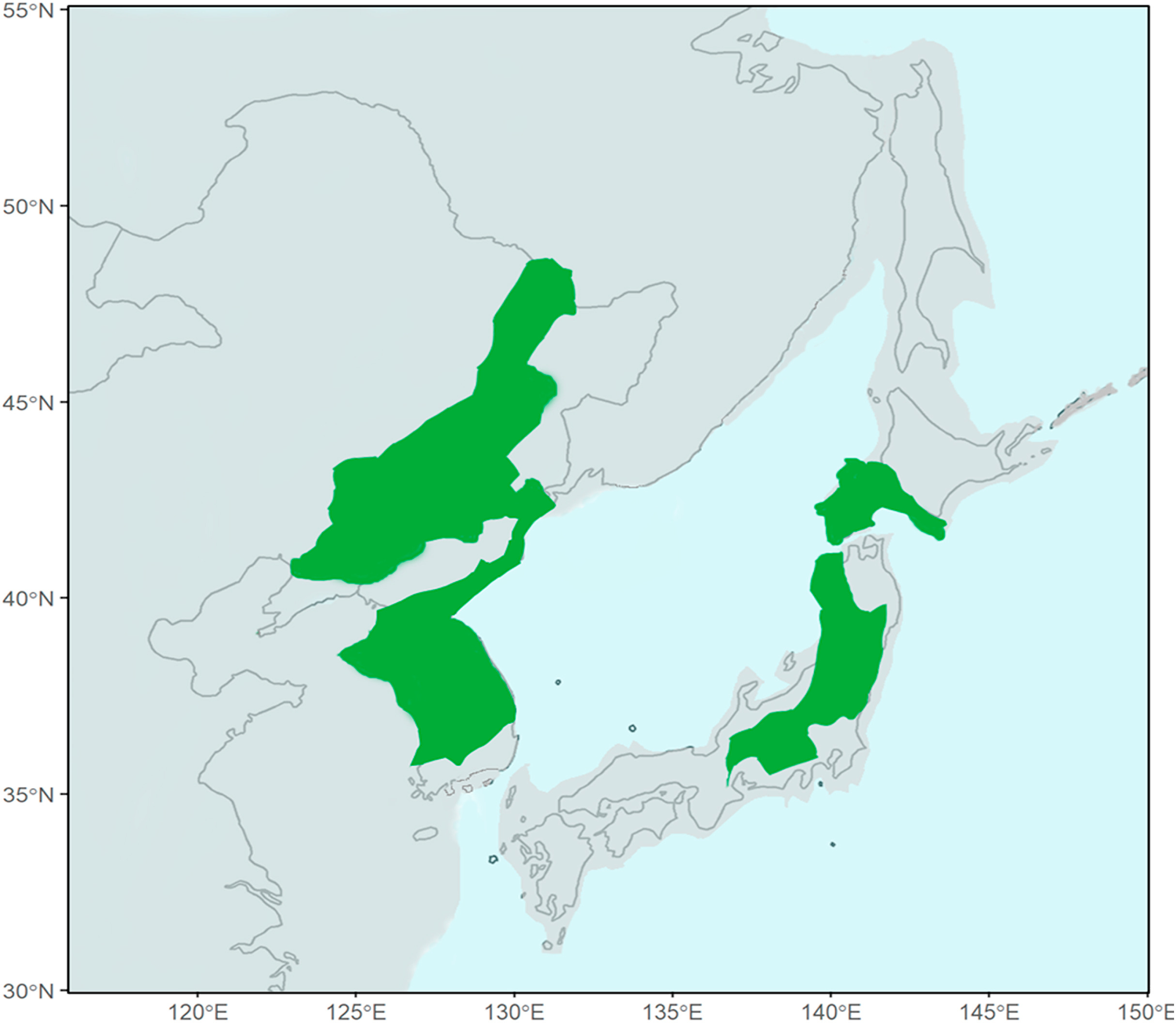
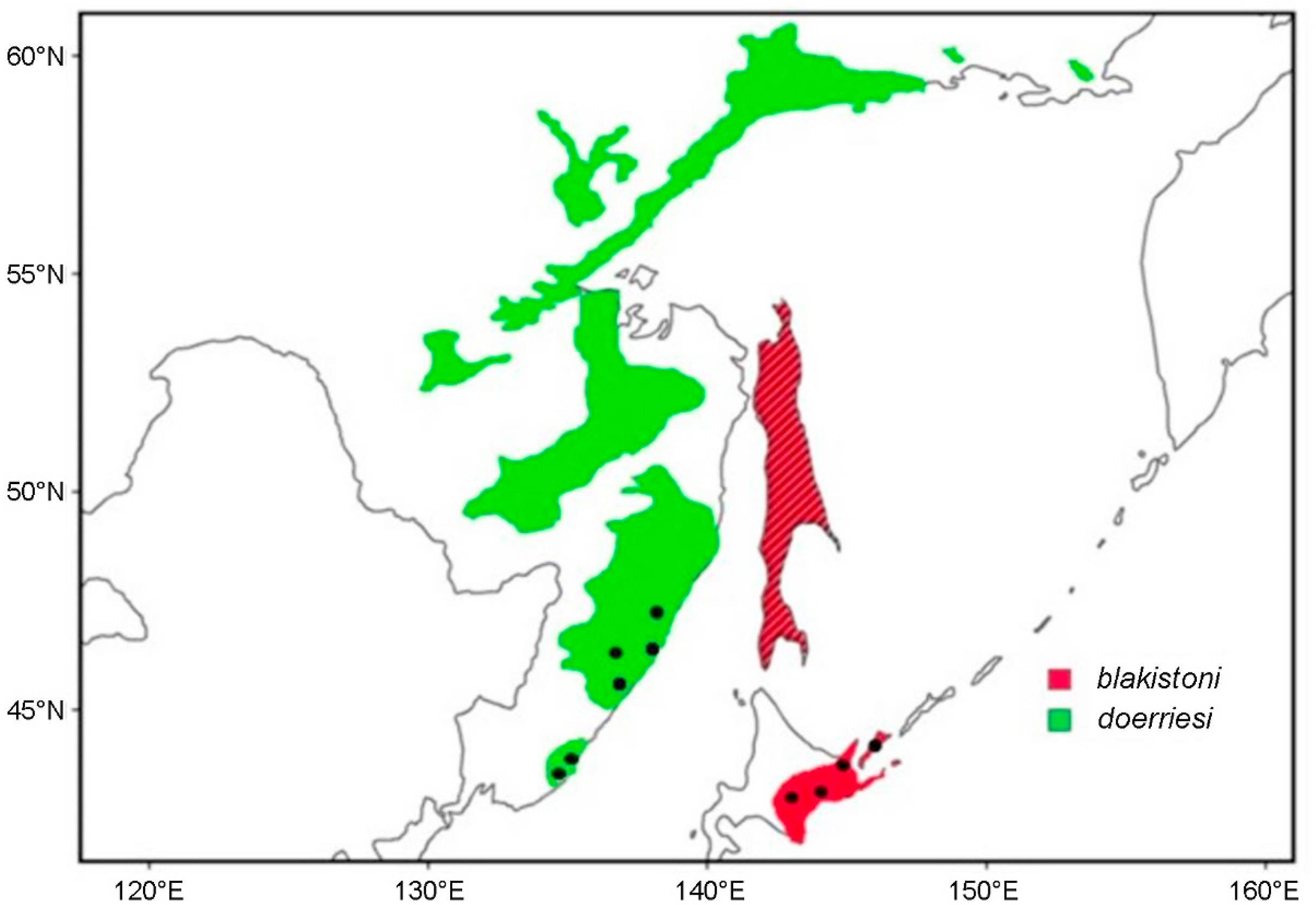
Blakiston's Fish Owl (Bubo blakistoni) is the largest species of Bubo on average, exceeding 70 cm in length, possessing a wingspan reaching 180 cm and attaining a weight of up to 4.6 kg (Taczanowski, 1891; Nechaev, 1991; Yamamoto, 1999; Slaght et al., 2018). This species is an inhabitant of higher latitude riparian forests where it predominantly feeds on salmonid fish and other aquatic prey (Slaght et al., 2018; Takenaka, 2018). Two subspecies of Blakiston's Fish Owl are presently recognised. The nominate subspecies which has an entirely insular distribution on the Japanese island of Hokkaido and the Russian islands of Kunashir and Shikotan, part of the southern Kuril Island chain (Dykhan and Kisleiko, 1988; Takenaka, 1998; Berzan, 2005; Slaght and Surmach, 2008). A potentially extirpated population of this form was also known from Sakhalin where it has remained unrecorded since the last sightings in 1974 (Nechaev, 1991) and 1976 (Bardin, 2006) (Fig. 1). The second subspecies, B. b. doerriesi, has a continental distribution in Northeast Asia ranging from the western shores of the Sea of Japan to the northern coasts of the Sea of Okhotsk (Slaght et al., 2018) (Fig. 1).
Past studies have alluded to major differences in the pair duets and to minor plumage differences between the insular and continental subspecies of Blakiston's Fish Owl (Pukinskii, 1974; Brazil and Yamamoto, 1989; Slaght and Surmach, 2008), but no serious quantitative analysis of these purported differences has been presented. Recent work comparing the mitochondrial genomes of both subspecies found relatively minor mitochondrial divergence values between them (∼0.8%), which was interpreted as the signature of a relatively recent divergence time of 560–770 kyr BP (Omote et al., 2018). However, the avian mitochondrial clock rate is known to differ taxonomically and has been shown to be particularly slow in large-bodied birds with long generation times (Nunn and Stanley, 1998; Lovette, 2004). Therefore, the documented mitochondrial divergence may in fact point to a more ancient split between the two forms than hitherto assumed.
Here, we investigated species limits between the two subspecies of B. blakistoni using a quantitative bioacoustic analysis. Given the great importance of bioacoustic traits in owl evolution (Howell and Robbins, 1995; Rasmussen et al., 2012; Pons et al., 2013; Sangster et al., 2013; Flint et al., 2015; Sadanandan et al., 2015; Krabbe, 2017; Gwee et al., 2019b; Dantas et al., 2021), this approach allowed us to examine species delimitation even in the absence of a multilocus molecular dataset based on the premise that bioacoustic data show extensive congruence with molecular results across many birds (Gwee et al., 2017; Prawiradilaga et al., 2018; Isler et al., 2020), though some discordance has been observed in some diurnal bird species where plumage may be more important (Price et al., 2007). In our taxonomic assessment, we utilise the “yardstick approach” (Mayr and Ashlock, 1991), which compares the magnitude of differences between target populations to differences between members of related, well-studied species pairs where their status as independent species is accepted.
A total of 192 duets from 22 pairs of Blakiston's Fish Owl were collected from various sources, including our personal recordings, xeno-canto (http://www.xeno-canto.org), and the Bird Songs of Japan audio-disk. Of these, eight pairs were from Hokkaido, Japan, seven pairs were from Kunashir Island, Russia, and seven were from Primorye on the Russian mainland (Fig. 1). Prior to the analysis we ensured that all recordings were homologous and screened for the presence of at least one complete duet per pair.
Although recording equipment differed across recordists and sound libraries, inspection of the spectrograms revealed negligible equipment bias. Amongst recordings from the same source, variability in background noise and minor variations in note shape were equivalent to the variability amongst recordings from different sources. As such, differences in recording quality were more important than equipment differences in our dataset (Rheindt et al., 2011; van Balen et al., 2013; Gwee et al., 2017).
We analysed only the duets of the two subspecies of fish owls, as this species is known to have multiple vocalisations for different purposes (Slaght and Surmach, 2008). Duets in owls appear to be related to pair formation, copulation and territorial defence (Penteriani, 2002; Slaght et al., 2018) and as such are well-suited to unravelling taxonomic insights.
The duets of the Russian mainland population of Blakiston's Fish Owl differ markedly from those of the insular populations from Hokkaido and the Kurils (Fig. 2). Both duet types comprise a male and female motif. Insular duets have the two notes of the male motif uttered rapidly one after another, rapidly followed by the female motif that comprises a single or two weakly separated notes. In mainland duets, the two notes of the male motif are spaced further apart while the female motif also comprises two notes which closely follow the corresponding male notes (Fig. 2). A motif was defined as the entire male or female sequence that was present across the whole duet (Fig. 2).
Raven Pro Version 1.6 (Bioacoustics Research Program, Cornell Lab of Ornithology, Ithaca, NY, USA) was used to perform measurements and inspect descriptive parameters of the duets. Default settings of Raven Pro were retained except for window size, which was optimised to 550 after inspection of all spectrograms. A total of 14 characters composed of both frequency-based and temporal parameters were measured: (1) male motif duration (s), (2) female motif duration (s), (3) duration between first note of female motif and last note of male motif (s), (4) total duration of entire duet (s), (5) lowest frequency across male motif (Hz), (6) highest frequency across male motif (Hz), (7) bandwidth across male motif (Hz), (8) center frequency across male motif (Hz), (9) peak frequency across male motif (Hz), (10) lowest frequency across female motif (Hz), (11) highest frequency across female motif (Hz), (12) bandwidth across female motif (Hz), (13) center frequency across female motif (Hz), and (14) peak frequency across female motif (see Table 1, Table 2).
| Taxa | Male motif duration (s) | Female motif duration (s) | Duration between first note of female motif and last note of male motif (s) | Total duration of entire duet (s) | Lowest frequency across male motif (Hz) | Highest frequency across male motif (Hz) | Bandwidth across male motif (Hz) | Center frequency across male motif (Hz) | Peak frequency across male motif (Hz) | Lowest frequency across female motif (Hz) | Highest frequency across female motif (Hz) | Bandwidth across female motif (Hz) | Center frequency across female motif (Hz) | Peak frequency across female motif |
| blakistoni (Hokkaido) vs. blakistoni (Kunashir) | 336 | |||||||||||||
| blakistoni (Hokkaido) vs. doerriesi | ✓ | ✓ | ✓ | ✓ | 337 | |||||||||
| blakistoni (Kunashir) vs. doerriesi | ✓ | ✓ | ✓ | ✓ | 338 |
| Taxon | Male motif duration (s) | Female motif duration (s) | Duration between first note of female motif and last note of male motif (s) | Total duration of entire duet (s) | Lowest frequency across male motif (Hz) | Highest frequency across male motif (Hz) | Bandwidth across male motif (Hz) |
| blakistoni (Hokkaido) | 0.54 ± 0.04 | 0.55 ± 0.09 | 0.15 ± 0.14 | 1.03 ± 0.22 | 169.37 ± 15.06 | 272.7 ± 23.13 | 103.32 ± 30.75 |
| blakistoni (Kunashir) | 0.52 ± 0.05 | 0.54 ± 0.07 | 0.11 ± 0.04 | 0.94 ± 0.11 | 172.65 ± 17.27 | 257.47 ± 11.34 | 84.82 ± 23.43 |
| doerriesi | 2.48 ± 0.34 | 2.66 ± 0.31 | 2.20 ± 0.23 | 3.11 ± 0.39 | 144.79 ± 18.91 | 260.80 ± 9.16 | 116.02 ± 18.23 |
| Taxon | Center frequency across male motif (Hz) | Peak frequency across male motif (Hz) | Lowest frequency across female motif (Hz) | Highest frequency across female motif (Hz) | Bandwidth across female motif (Hz) | Center frequency across female motif (Hz) | Peak frequency across female motif (Hz) |
| blakistoni (Hokkaido) | 235.59 ± 15.17 | 236.49 ± 15.88 | 132.98 ± 19.48 | 215.57 ± 19.13 | 82.59 ± 26.91 | 187.74 ± 16.24 | 201.00 ± 20.3 |
| blakistoni (Kunashir) | 226.60 ± 7.22 | 230.44 ± 8.01 | 139.23 ± 14.27 | 211.56 ± 11.72 | 72.33 ± 22.42 | 185.19 ± 7.63 | 190.91 ± 10.87 |
| doerriesi | 229.25 ± 17.22 | 234.39 ± 10.84 | 126.41 ± 14.76 | 228.13 ± 9.51 | 101.73 ± 17.57 | 210.35 ± 15.92 | 215.64 ± 16.01 |
Most recordings contained multiple bouts of duets uttered by a single pair. For recordings containing more than 12 duets uttered by a single pair, the first 12 duets were measured and values were averaged to maximise accuracy. For pairs in which fewer than 12 duets were available, all available duets were measured and averaged. In total, 192 duets were measured from 22 pairs of fish owl (Table S1).
We used R Studio version 1.1.453 (https://www.rstudio.com) and R version 3.5.0 (R Core Team, 2018) to conduct principal component analysis (PCA) on the vocal dataset to distinguish discrete bioacoustic variations from clinal ones. We also utilised the criterion outlined by Isler et al. (1998) (henceforth referred to as the Isler criterion) to assess the vocal diagnosability of each of the characters measured. Though this test was specifically designed for suboscine passerines (Isler et al., 1998), it has been applied to a wide range of Asian passerine (Cros and Rheindt, 2017; Gwee et al., 2019a) and non-passerine species (Sangster and Rozendaal, 2004; Rheindt et al., 2011; Ng and Rheindt, 2016; Ng et al., 2016; Cros and Rheindt, 2017; Prawiradilaga et al., 2018), including owls (Gwee et al., 2017).
The Isler criterion is based on the following two conditions: (i) no overlap between the ranges of measurements of the two populations being compared and (ii) the means and standard deviations (SD) of the population with the smaller set of measurements (a) and the population with the larger set of measurements (b) have to meet the following requirement:
To the human ear, the duets of Blakiston's Fish Owls from the Asian mainland sound considerably different from those of the insular populations from Hokkaido and the southern Kuril islands, which in turn appear identical to one another. The presence of two notes in the female motif and widely separated notes of the male, in the former serve to distinguish it from the insular populations of the owl (Fig. 2). In addition, the call notes of the female motif in the continental population, consists of two notes with an inverted ‘tick shape’, ascending rapidly and descending at a steep angle (Fig. 2; Figs. S1–S3). In contrast, the note of the female motifs of insular populations has an overall flatter shape, also initially ascending rapidly but descending more gradually with an additional inflection in the middle of the descending portion of the note (Fig. 2; Figs. S1–S3).
Of the 14 bioacoustic characters compared, four were found to be Isler-diagnosable between the insular and continental populations of Blakiston's Fish Owl (Table 1, Table 2) while there were no Isler-diagnosable characters between the insular populations on Hokkaido and Kunashir.
A PCA plot across the 14 bioacoustic parameters supported these distinctions by showing that the mainland and insular populations cluster separately (Fig. 3). The Kunashir population is vocally indistinguishable from the Hokkaido population with a large spatial overlap (Fig. 3). Principal components 1 and 2 (PC1 and PC2) collectively account for 77.1% of the observed variability in the vocal data, while subsequent PCs were much less informative in accounting for variability.
Considerable taxonomic insight can be gleaned from the distinctive vocalisations of owls and other nocturnal birds as these traits are inherited and subjected to strong selective pressures (King, 2002), with a striking concordance between bioacoustic and multilocus genetic data (Gwee et al., 2017). Duets play an important role in facilitating mate guarding, pair bonding and the initiation of copulation (Penteriani, 2002; Slaght et al., 2018), thus having a disproportionate influence on reproduction. Vocal duets may be particularly useful for clarifying taxonomic affinities in owls. Our bioacoustic analysis yielded strong evidence for the presence of two distinct duet types in Blakiston's Fish Owl. One confined to the continental population on the East Asian mainland (subspecies doerriesi), is characterised by two, widely spaced notes produced each by the male and female owl where the female's notes closely following the notes uttered by the male. In the insular populations, the male instead utters two closely spaced notes that are followed by a single or two very closely spaced notes produced by the female.
The Isler criterion analysis demonstrated that four of the vocal parameters compared were completely diagnosable between the two populations of fish owls (Table 1). The Isler criterion is known to be particularly conservative and just small numbers of diagnosable differences have been found in the territorial calls of even sympatric owl species. For instance, only two completely diagnosable parameters were identified between the territorial vocalisations of the sympatric Barking Owl (Ninox connivens) and Southern Boobook (Ninox boobook) (Gwee et al., 2017), half the number we have diagnosed between the two populations of fish owl. Other studies have elevated bird populations to species based on fewer completely diagnosable parameters (Gwee et al., 2017; Prawiradilaga et al., 2018; Isler et al., 2020) than what we have identified here.
Indeed, a number of owl species presently accepted to be separate species have far more similar territorial vocalisations than our pair of fish owls. For instance, the Cloud Forest Screech Owl (Megasops marshalli) and the Cinnamon Screech Owl (Megascops petersoni) both share a highly similar territorial song comprising of a series of monotonic hoots but are still regarded as two species that occur in geographic proximity (Krabbe, 2017).
On the basis of these extreme vocal differences, when placed into the greater context of territorial vocalisations in owls and coupled with documented differences in plumage, the most pronounced being a white patch on the back of B. b. doerriesi that B. b. blakistoni lacks (Fig. 4) (Seebohm, 1895; Yakovlev, 1929; Pukinskii, 1993; Slaght et al., 2018; Takenaka, 2018) and the presence of ‘a nearly white tail when fully adult’ for B. b. doerriesi (Seebohm, 1895), we are comfortably proposing a split of Blakiston's Fish Owl into two species:
1.Blakiston's Fish Owl (B. blakistoni), comprising the nominate insular populations on Hokkaido, the southern Kuril islands of Shikotan and Kunashir, and Sakhalin island (where it may be extirpated);
2.Northern Fish Owl (B. doerriesi), comprising the continental population of the species, named after for the fact that this population occupies the highest latitude of all presently known fish owl taxa.
Our bioacoustic dataset lacks samples of Blakiston's Fish Owl duets from Sakhalin island, where populations have not been detected since 1974 (Slaght and Surmach, 2008). However, mtDNA places these populations firmly with the other insular populations from Hokkaido and the southern Kurils (Omote et al., 2018). Hence, we tentatively assign the population on Sakhalin to the insular species of Blakiston's Fish Owl.
The separation of Blakiston's Fish Owl into a continental and insular species is mirrored by multiple other avian taxa with similar distributions (Saitoh et al., 2015). Forest-dependent species pairs in particular display this pattern, such as Sakhalin Leaf Warbler (Phylloscopus borealoides) and Pale-legged Leaf Warbler (Phylloscopus tenellipes) (Sun et al., 2020), Arctic Warbler (Phylloscopus borealis) and Kamchatka Leaf Warbler (Phylloscopus examinandus) (Reeves et al., 2008), and Grey's Grasshopper Warbler (Locustella fasciolata) and Sakhalin Grasshopper Warbler (Locustella amnicola) (Drovetski et al., 2004). These divergences are likely due to the fragmentation of a previously contiguous broadleaf forest landscape by the southward spread of boreal forest (Weir and Schluter, 2004) and steppe-tundra (Courtin et al., 2021) during multiple glaciations in the mid to late Quaternary. Though land bridges repeatedly connected the islands of Sakhalin, Hokkaido and Kunashir with the mainland during the multiple sequential glacial maxima of the Quaternary (Harrison et al., 2001) (Fig. 5), much of this area lacked riparian broadleaf forests and instead comprised a boreal (Harrison et al., 2001; Weir and Schluter, 2004; Iwase et al., 2012; Binney et al., 2017; Chung et al., 2017; Wang et al., 2017.; Omote et al., 2018) or steppe-tundra (Courtin et al., 2021) landscape (Fig. 5), rendering it unsuitable for habitat specialists such as fish owls. The broadleaf forests that Blakiston's and Northern Fish Owls currently inhabit would have shifted southwards during this period, with a continental refuge in the Korean Peninsula and northeastern China, and a geographically disjunct insular refuge in southwestern Hokkaido and northern Honshu (Harrison et al., 2001; Iwase et al., 2012; Binney et al., 2017; Chung et al., 2017; Wang et al., 2017) (Fig. 5), spatially segregating these two populations. Barriers of unsuitable habitat to the north and the Sea of Japan between these refugia would have kept the two populations of fish owls farther apart than they are now, further reducing the likelihood of gene flow and driving the pronounced vocal differentiation in their duets.
Populations of both species of fish owl would have tracked the movement of their preferred broadleaf forest habitats northwards after the end of each glacial period, with present-day populations on Sakhalin potentially originating through the overland dispersal of populations from Hokkaido towards the tail-end of the last inter-glacial.
Despite the long geographic isolation of the two populations based on palaeo-historic vegetation maps, plumage and bioacoustic data, there is only a mitochondrial divergence of ∼0.8% between Blakiston's and Northern Fish Owls (Omote et al., 2018). Barcoding studies utilising the mitochondrial COI gene have generally documented divergences of around or greater than 3% in many avian sister species (Hebert et al., 2004; Kerr et al., 2007). However, this value must be interpreted with caution, as these studies have also identified numerous biological species pairs characterised by mitochondrial divergences far lower than the 3% threshold (see Table 1 in Kerr et al., 2007).
For long-lived, slowly reproducing species, mitochondrial divergence rates are known to be comparatively slower. Calibrating the rates of such species to those of faster reproducing taxa likely underestimates actual population divergence timings. Mutations in mitochondrial DNA accrue over reproductive cycles and fish owls, with their longer generation times (Bird et al., 2020) and unusually long post-fledging periods (Pukinskii, 1973; Slaught and Surmach, 2008), would accumulate fewer mutations in their mtDNA over a similar time scale when compared to taxa that breed at an earlier age and fledge faster. Accordingly, lower cytochrome b mutation rates have been found in larger bird species when compared to smaller bodied members of the same family (Nunn and Stanley, 1998). Blakiston's Fish Owl is arguably the most massive owl (Slaght et al., 2018) and would consequently be expected to have one of the lowest rates of mitochondrial evolution in its family. Low mitochondrial divergences in these owls must be interpreted accordingly.
Even if the slower mitochondrial evolutionary rate of fish owls were to be ignored, other factors could lead to artificially low mitochondrial divergence rates between taxa that are actually deeply differentiated. Rare hybridisation and gene flow events between the mainland and insular fish owl species could have resulted in genetic introgression which would effectively reset mitochondrial divergences between the two populations (Rheindt and Edwards, 2011). This scenario is possible for Blakiston's Fish Owl via sporadic overwater dispersal between the Russian mainland and Sakhalin island or through ephemeral broadleaf forest corridors that may have formed across the Russo-Sakhalin land bridge during some ice ages.
Mitochondrial evidence notwithstanding, we believe that the pronounced differences in the duets of these two populations are key in influencing mate choice and reproduction and should supersede mitochondrial divergence values in taxonomic considerations. Our application of the yardstick approach additionally corroborates that vocal differences between these two owl populations are at the species level (see above). Nevertheless, multilocus nuclear data would be a welcome addition to corroborate our taxonomic proposal.
Both the insular and mainland species of what used to be Blakiston's Fish Owl have been collectively assessed by the IUCN as Endangered based on possessing a global population of <2500 mature individuals, with <250 individuals in each subpopulation (BirdLife International, 2016). Splitting the mainland and insular populations of Blakiston's Fish Owl into separate species reduces the overall population sizes of each taxon and requires a re-assessment of their statuses. The mainland population comprising B. doerriesi is thought to number between 800 and 1600 mature individuals (Slaght et al., 2018), still meeting the IUCN criterion C2a(i) for Endangered. Though the insular populations (i.e., B. blakistoni in the narrow sense) are thought to be smaller, likely only totalling 166–182 mature individuals (Slaght et al., 2018), they have undergone an increasing population trend in recent years (Yoshii et al., 2018) and also fulfil IUCN criterion C2a(i) to be assessed as Endangered.
Based on our results we recommend the separate management of Blakiston's and Northern Fish Owls and do not support the relocation of individuals from the mainland to augment populations on the islands or vice versa. Instead, each of the newly delimited species should be managed separately, with conservation efforts specifically attuned to address regional causes for population declines and taking into account each lineage's unique evolutionary history and trajectory.
Recordings are available at the public sound library Xeno-canto (www.xeno-canto.org) and the audio-disc Wild Bird Songs of Japan by Hideo Ueda. Personal recordings were also provided by Yann Muzika, Chikara Otani, EEK, SS and JCS (available upon request).
NM conducted the measurements for the bioacoustics analysis. TG, AAK and SS assisted in choosing the key vocal parameters for the analysis. SS, AAK, JAE and JCS conducted fieldwork and obtained many of the sound recordings utilised in this study. All authors contributed equally to the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
We thank A. Ryzhov, S. Avdeyuk, A. Katkov, A. Bezrukov, I. Donchenko, and A. Popov for their help with data collection in the field in Russia. We are grateful to Otani Chikara and Yann Muzika for generously providing their sound recordings of Blakiston's Fish Owl duets from Hokkaido. We would also like to thank E. E. Kozlovsky for providing us with recordings from the Russian Mainland from his fieldwork. We acknowledge financial and logistical support from the Wildlife Conservation Society and the Amur-Ussuri Center for Avian Biodiversity, and financial support from Columbus Zoo and Aquarium. We also acknowledge a Singapore Ministry of Education Tier 2 grant for financial support (WBS R154-000-C41-112). NM was supported by the Lady Yuen Peng McNeice Graduate Fellowship during the execution of this project.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100021.
|
Bardin, A.V., 2006. Autumn encounter with a Blakiston’s fish owl Ketupa blakistoni on Sakhalin. Russkii Orn. Zhurnal Ekspress-vypusk. 15, 738-739 (in Russian)
|
|
Berzan, A.P., 2005. Analysis of modern distribution and population size of Blakiston’s fish owls in the southern Kuril Islands and Sakhalin. In: Volkov, S.V., Morozov, V.V., Sharikov, A.V. (Eds.), Owls of Northern Eurasia. Working Group of Birds of Prey and Owls, Moscow, Russia, pp. 447–449 (in Russian with English summary).
|
|
Brazil, M.A., Yamamoto, S., 1989. The behavioural ecology of Blakiston’s Fish Owl Ketupa blakistoni in Japan: calling behaviour. In: Meyburg, B.-U., Chancellor, R.D. (Eds.), Raptors in the Modern World: Proceedings of the III World Conference on Birds of Prey and Owls. World Working Group on Birds of Prey and Owls, Berlin, Germany, pp. 403–410.
|
|
Courtin, J., Andreev, A.A., Raschke, E., Bala, S., Biskaborn, B.K., Liu, S., et al., 2021. Vegetation changes in southeastern Siberia during the Late Pleistocene and the Holocene. Front. Ecol. Evol. 26, 9
|
|
del Hoyo, J., Collar, N.J., 2014. HBW and BirdLife International Illustrated Checklist of the Birds of the World. Non-passerines, vol. 1. Lynx Edicions, Barcelona.
|
|
Dykhan, M.B., Kisleiko, A.A., 1988. Number and distribution of Blakiston's fish owls on Kunashir Island during the breeding period. In: Litvinenko, N.M. (Ed.), Rare Birds of the Russian Far East and Their Protection. Dalnevostochnoe Otdeleniye Akademii Nauk SSSR, Vladivostok, Russia, pp. 29–32 (in Russian).
|
|
Howell, S.N.G., Robbins, M.B., 1995. Species limits of the least pygmy-owl (Glaucidium minutissimum) complex. Wilson Bull. 107, 7-25
|
|
Isler, M.L., Chesser, R.T., Robbins, M.B., Cuervo, A.M., Cadena, C.D., Hosner, P.A., 2020. Taxonomic evaluation of the Grallaria rufula (Rufous Antpitta) complex (Aves: Passeriformes: Grallariidae) distinguishes sixteen species. Zootaxa 4817, zootaxa-4817
|
|
King, B. 2002. Species limits in the Brown Boobook Ninox scutulata complex. Bull. Br. Ornithol. Club 122, 250-256
|
|
Krabbe, N.K. 2017. A new species of Megascops (Strigidae) from the Sierra Nevada de Santa Marta, Colombia, with notes on voices of New World screech-owls. Ornitol. Colomb. 16, 1-27
|
|
Lovette, I.J., 2004. Mitochondrial dating and mixed support for the “2% rule” in birds. Auk 121, 1-6
|
|
Mayr, E., Ashlock, P.D., 1991. Principles of Systematic Zoology, second ed. McGraw-Hill Inc, Now York, p. 475.
|
|
Nechaev, V.A., 1991. Birds of Sakhalin Island. Amur-Ussuri Center for Avian Biodiversity, Vladivostok (in Russian).
|
|
Pukinskii, Y.B., 1973. Ecology of Blakiston’s Fish Owl in the Bikin river basin. Byull. Mosk. O-va Ispyt. Prir. Otd. Biol. 78, 40-47 (In Russian with English summary)
|
|
Pukinskii, Y.B., 1974. Blakiston’s Fish Owl vocal reactions. Vestn. Leningr. Univ. 3, 35-39 (In Russian with English summary)
|
|
Pukinskii, Y.B., 1993. Blakiston’s fish owl – Ketupa blakistoni. In: Numerov, A.D. (Ed.), The Birds of Russia and Contiguous Regions: Pterocliformes, Columbiformes, Cuculiformes, Strigiformes. Nauka, Moskow, pp. 290–302 (in Russian).
|
|
Rasmussen, P.C., Allen, D.N.S., Collar, N.J., DeMeulemeester, B., Hutchinson, R.O., Jakosalem, P.G.C., et al., 2012. Vocal divergence and new species in the Philippine Hawk Owl Ninox philippensis complex. Forktail 28, 1-20
|
|
Sangster, G., Rozendaal, F.G., 2004. Systematic notes on Asian birds. Territorial songs and species-level taxonomy of nightjars of the Caprimulgus macrurus complex, with the description of a new species. Zool Verh Leiden. 350, 7-45
|
|
Seebohm, H., 1895. Bulletin of the British Ornithologists’ Club. Ibis 5, 4
|
|
Slaght, J.C., Takenaka, T., Surmach, S.G., Fujimaki, Y., Utekhina, I.G., Potapov, E.R., 2018. Global distribution and population estimates of Blakiston's Fish Owl. In: Nakamura, F. (Ed.), Biodiversity Conservation Using Umbrella Species. Ecological Research Monographs. Springer, Singapore, pp. 9–18.
|
|
Taczanowski, L., 1891. Faune Ornitologique de la Siberie orientale. Premiere partie. Memoris de l’Academie Imperiale des Sciences de St Petersbourg. Serie 7, 1278 (in French)
|
|
Takenaka, T., 1998. Distribution, Habitat Environments, and Reasons for Reduction of the Endangered Blakiston’s Fish Owl in Hokkaido, Japan. Ph.D thesis. Hokkaido University, Sapporo, Japan.
|
|
Takenaka, T., 2018. Ecology and conservation of Blakiston's fish owl in Japan. In: Nakamura, F. (Ed.), Biodiversity Conservation Using Umbrella Species. Ecological Research Monographs. Springer, Singapore, pp. 19–46.
|
|
Turner, D.A., Pearson, D.J., 2015. Systematic and taxonomic issues concerning some East African bird species, notably those where treatment varies between authors. Scopus 34, 1-23
|
|
Yakovlev, B.P., 1929. Animal world of Manchuria: birds. Obshchestvo Izucheniya Manchzhurskovo Kraiya, Kharbin. Serie A. 33, 1-51 (in Russian)
|
|
Yamamoto, S., 1999. The Blakiston's Fish Owl. Hokkaido Shinbun Press, Japan (in Japanese).
|
|
Yoshii, C., Yamaura, Y., Nakamura, F., 2018. Predicting future range expansions of Blakiston's fish owl subject to conservation efforts. In: Nakamura, F. (Ed.), Biodiversity Conservation Using Umbrella Species. Ecological Research Monographs. Springer, Singapore, pp. 221–236.
|
| Taxa | Male motif duration (s) | Female motif duration (s) | Duration between first note of female motif and last note of male motif (s) | Total duration of entire duet (s) | Lowest frequency across male motif (Hz) | Highest frequency across male motif (Hz) | Bandwidth across male motif (Hz) | Center frequency across male motif (Hz) | Peak frequency across male motif (Hz) | Lowest frequency across female motif (Hz) | Highest frequency across female motif (Hz) | Bandwidth across female motif (Hz) | Center frequency across female motif (Hz) | Peak frequency across female motif |
| blakistoni (Hokkaido) vs. blakistoni (Kunashir) | 336 | |||||||||||||
| blakistoni (Hokkaido) vs. doerriesi | ✓ | ✓ | ✓ | ✓ | 337 | |||||||||
| blakistoni (Kunashir) vs. doerriesi | ✓ | ✓ | ✓ | ✓ | 338 |
| Taxon | Male motif duration (s) | Female motif duration (s) | Duration between first note of female motif and last note of male motif (s) | Total duration of entire duet (s) | Lowest frequency across male motif (Hz) | Highest frequency across male motif (Hz) | Bandwidth across male motif (Hz) |
| blakistoni (Hokkaido) | 0.54 ± 0.04 | 0.55 ± 0.09 | 0.15 ± 0.14 | 1.03 ± 0.22 | 169.37 ± 15.06 | 272.7 ± 23.13 | 103.32 ± 30.75 |
| blakistoni (Kunashir) | 0.52 ± 0.05 | 0.54 ± 0.07 | 0.11 ± 0.04 | 0.94 ± 0.11 | 172.65 ± 17.27 | 257.47 ± 11.34 | 84.82 ± 23.43 |
| doerriesi | 2.48 ± 0.34 | 2.66 ± 0.31 | 2.20 ± 0.23 | 3.11 ± 0.39 | 144.79 ± 18.91 | 260.80 ± 9.16 | 116.02 ± 18.23 |
| Taxon | Center frequency across male motif (Hz) | Peak frequency across male motif (Hz) | Lowest frequency across female motif (Hz) | Highest frequency across female motif (Hz) | Bandwidth across female motif (Hz) | Center frequency across female motif (Hz) | Peak frequency across female motif (Hz) |
| blakistoni (Hokkaido) | 235.59 ± 15.17 | 236.49 ± 15.88 | 132.98 ± 19.48 | 215.57 ± 19.13 | 82.59 ± 26.91 | 187.74 ± 16.24 | 201.00 ± 20.3 |
| blakistoni (Kunashir) | 226.60 ± 7.22 | 230.44 ± 8.01 | 139.23 ± 14.27 | 211.56 ± 11.72 | 72.33 ± 22.42 | 185.19 ± 7.63 | 190.91 ± 10.87 |
| doerriesi | 229.25 ± 17.22 | 234.39 ± 10.84 | 126.41 ± 14.76 | 228.13 ± 9.51 | 101.73 ± 17.57 | 210.35 ± 15.92 | 215.64 ± 16.01 |