| Citation: | Bruce E. LYON, John M. EADIE. 2013: Patterns of host use by a precocial obligate brood parasite, the Black-headed Duck: ecological and evolutionary considerations. Avian Research, 4(1): 71-85. DOI: 10.5122/cbirds.2013.0008 |
The Black-headed Duck (Heteronetta atricapilla) is unique among obligate avian brood parasites because its highly precocial young leave the host nest shortly after hatching and impose no post-hatching costs on their hosts. Accordingly, we might expect host-parasite interactions in this parasite to differ strikingly from those of other brood parasites — they should be able to parasitize a broad diversity of hosts and be highly successful with these hosts. We conducted the second detailed study ever completed on patterns of host use in Black-headed Ducks. Based on four years of systematic searches of multiple marshes in Argentina, we found no evidence that Black-headed Ducks ever had nests of their own, confirming the previous conclusion that Black-headed Ducks are, indeed, true obligate brood parasites. Contrary to expectation, however, we found that Heteronetta is ecologically dependent on a surprisingly small number of host species — two species of coots and a gull — all of which are widespread and locally abundant species. Other species are numerically less important as hosts either because they are relatively uncommon, or because they are avoided by the ducks. In the three main host species, hatching success of the duck eggs was also surprisingly low (≤ 28%), based on expectations for a precocial parasite, mainly due to host rejection or neglect. Mortality due to predation on host nests, in contrast, was low for all three primary host species. These observations corroborate Weller's observations from a single-year study. The combination of a dependence on few primary hosts and a relatively low hatching success are inconsistent with some previous hypotheses for the evolution of obligate brood parasitism in Heteronetta. Instead, our observations, and those of Weller, suggest that intense nest predation in Austral wetlands, coupled with an abundance of a few common host species that aggressively defend their nests and obtain high nest success rates, may have been an important factor in the evolution of obligate parasitism in this enigmatic duck.
Obligate avian brood parasites depend entirely on other species to raise their offspring. This form of avian brood parasitism has evolved independently seven times and, with subsequent diversification in most of these lineages, there are now some one hundred extant species of obligate avian brood parasites (Davies, 2000). The relationship between obligate brood parasites and the host species they parasitize is usually antagonistic because the parasitic offspring compete with the host offspring for limited parental food and/or because the parasitic adults or chicks directly kill some or all of the hosts eggs or chicks (Rothstein, 1990; Davies, 2000; Massoni and Reboreda, 2002; Spottiswoode and Koorevaar, 2012). The fitness costs to hosts imposed by this competition and mortality have fueled co-evolutionary arms races in some taxa, making brood parasitism a model system for studying co-evolution (Payne, 1977; Brooke and Davies, 1988; Rothstein, 1990; Stokke et al., 2002). Egg rejection and egg mimicry provide one particularly well-studied aspect of this interaction (Brooke and Davies, 1988; Davies, 2000), but other traits that are potential targets of co-evolution include chick rejection and mimicry (Redondo and Arias de Reyna, 1988; Langmore et al., 2003, 2010), and host nest defense and brood parasitic traits involved in egg laying and access to host nests (Feeney et al., 2012).
The Black-headed Duck (Heteronetta atricapilla; Fig. 1) is an obligate brood parasite found in southern South America, primarily Argentina and Chile (Weller, 1968; Lyon and Eadie, 2004; Cofré et al., 2007). This species is unique among the avian obligate brood parasites in that its completely precocial offspring leave the host nest within a day of hatching and then raise themselves without any assistance from the hosts (Weller, 1968; Lyon and Eadie, 2004; Fig. 1). This independence is in striking contrast to all other obligate brood parasites whose altricial young require a great deal of parental care, including extensive amounts of food provided by the host parents. Thus, unlike all other brood parasites, Black-headed Ducks rely on their hosts only for incubating and protecting their eggs, not for raising their offspring after they hatch. We might therefore expect host-parasite interactions in this precocial parasite to differ strikingly from those of other brood parasites. Parasitism should be less costly to hosts and we would expect that hosts would therefore generally lack defensive behaviors that reduce the reproductive success of parasites. In addition, because incubation is a fairly general form of parental care, in theory a wide range of species should be able to serve as potential hosts, given that the incubation periods of most potential hosts are as long as those of the Black-headed Duck (Weller, 1968).
The patterns of host use by Black-headed Ducks, and the hatching success of their eggs in the different host species, are of special interest because of their ecological and evolutionary implications. The ecological persistence of brood parasites depends in large part on their annual reproductive success, which in turn is determined by the average fecundity of individual females, the hatching success of the eggs, and juvenile survival. Because Heteronetta ducklings are independent after hatch, patterns of host use will affect hatching success, and perhaps also the fecundity realized by parasites (if hosts are limiting), but not duckling survival. These same issues are integral to understanding the evolution of obligate brood parasitism in the Black-headed Duck. Indeed, the evolution of obligate brood parasitism can be viewed from the framework of clutch size evolution (Lyon and Eadie, 1991). We argued previously that obligate brood parasitism is most common in species with altricial young because clutch size in such species is limited by food available for offspring. When food for offspring limits clutch size (Lack, 1947) — rather than egg-laying capacity per se — clutch sizes of nesting individuals can be several times smaller than the maximum number of eggs a female can lay based on egg production constraints. Thus, we suggested that most obligate brood parasites are species with altricial offspring because parasites in these taxa gain a huge increase in fecundity by forgoing the constraints of parental care and having other birds raise their chicks (Lyon and Eadie, 1991).
In contrast, clutch size in precocial birds whose young feed themselves is limited not by food for offspring, but by other factors, including egg laying capacity (Lack, 1968; Winkler and Walters, 1983). In general, precocial taxa are predicted to gain far less of a fecundity increment from brood parasitism than altricial species (Lyon and Eadie, 1991). The evolution of obligate brood parasitism in the Black-headed Duck is therefore enigmatic and of special interest.
Patterns of host use, total individual fecundity by individual females, and the hatching success rates of the eggs are central to understanding why this species has completely forsaken parental care to become a complete brood parasite. Only three detailed studies have been conducted on the breeding biology and natural history of the Black-headed Duck (Weller, 1968; Lyon and Eadie, 2004; Cofré et al., 2007), and so we lack a full understanding of these key demographic questions. Weller (1968) provided the first detailed study of patterns of host use and reproductive success in Heteronetta, with a pioneering one-year study on a series of interconnected wetlands near General Lavalle, Buenos Aires Province, Argentina. More recently, we conducted a detailed study of brood parasitism in Black-headed Ducks at some of the same sites as Weller, which permits a comparison of patterns of parasitism across a broad time span (25 years). Moreover, we studied Black-headed Ducks over four breeding seasons (1992–1994, 1997), and across a broad survey of wetlands, which should enable us to determine whether any of Weller's findings were specific to the particular wetlands he studied, or perhaps the year in which he conducted his study. Most recently, Cofré et al. (2007) used a correlational approach to identify host species that are likely important to Black-headed Ducks in Chile: they searched for correlations between the abundance of various potential host species on individual wetlands and the abundance of Black-headed Ducks on those same wetlands.
Our goal here is to examine general patterns of host use by Black-headed Ducks, with a consideration of the ecological and evolutionary implications of these patterns. We previously published a detailed investigation of the interaction between Heteronetta and its two main hosts, two species of coots (Fulica spp.; Lyon and Eadie, 2004), but the information presented here on general patterns of host use is new.
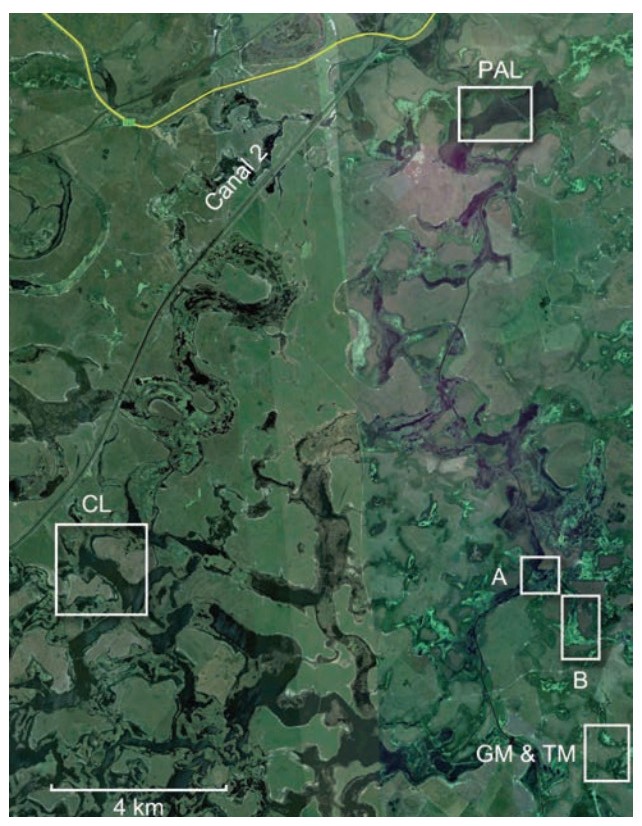
Our study wetlands were located within 30 km of General Lavalle (mainly W and SW), Buenos Aires Province, Argentina (Fig. 2). Wetlands are either identified by the name of estancia (ranch) on which they are located (e.g., Palenque in 1992, 1994; Los Ingleses in 1992; Cari-Lauquen in 1994, 1997), or by designated names when we studied two different wetlands on the same estancia (e.g., Marshes "A" and "B" on Estancia Real Viejo (10 km S of Palenque) in 1993, 1997 and "Gull" and "Tern" Marshes on Estancias Mal Abrigo and Don Manuel (10 km S of Palenque) in 1993). All of these marshes were vegetated almost entirely by the bulrush Schoenoplectus californicus, which varied in density from sparse to moderately dense (Fig. 3). To find and monitor potential or actual host nests, we conducted extensive surveys of the marshes every two to four days, either on foot or by canoe. To ensure systematic searches that could reveal virtually all potential host nests, we marked the marshes into quadrates (ca. 1 ha) with tall bamboo poles. All nests were tagged (nearby) with numbered flagging tape and all eggs in each nest were given a unique number with a permanent marker. In 1992 spot checks were done to determine the occurrence of parasitism, but the fates of nests or duck eggs were not systematically determined. In 1993 and 1994, we followed most nests through to hatching. In 1997 we conducted spot checks and egg mimicry experiments, but did not monitor nest or egg fates. In 1997 we also focused nonrandomly on searching for nests of one host species, the Red-gartered Coot (Fulica armillata), which can create a bias in patterns of host use if not taken into consideration (see below).
Nests were identified to species by observing the nest owner leaving the nest or alarm calling at our presence. Parasitism was easy to detect because the duck eggs differed dramatically in appearance from the eggs of all of the major hosts (Figs. 1 and 4), with the exception of the Rosy-billed Pochard (Netta peposaca). However, although Black-headed Duck eggs are similar in color and shape to those of the pochard, their surface texture differs dramatically, making identification straightforward. Pochard eggs are smooth and somewhat shiny, whereas Black-headed Duck eggs have a rough surface texture (Weller, 1968). To find potential host nests, we systematically and thoroughly checked large tracts of similar habitat of varying vegetation density every two to three days, and we ensured that a given quadrat was searched by different individuals to increase the likelihood that nests were not missed. Because the large nests of all species were conspicuous and easily found (with the exception of Rosy-billed Pochards, which hide their nests in dense vegetation), we believe that sample size of nests of each species is a reasonably good proxy for the relative abundance of the different potential host species, at least locally within our plots. Similar nest search approaches with the American Coot (Fulica americana), a close relative the two main hosts and with similar nesting behavior and habitat, revealed that > 99% of successful nests are discovered by these systematic search methods, virtually all in early laying stages (Lyon, 1993). However, for species that did not occur in high densities on our focal study plots, we also censused all nests found in non-focal areas. Including these nests in the totals slightly inflates the relative abundance of some of these rarer species compared to the common host species.
The fates of duck eggs were determined by the following criteria: pipped eggs were assumed to have hatched, as were cases where shells from hatched eggs were found in the lining of the host nest (given that the host nest was still active and not depredated); eggs found buried in host nests or that disappeared too early to have hatched were considered rejected; eggs that were rotten and liquid were considered addled, in contrast to eggs that were "leftover" because they were laid too late in the host's laying cycle to successfully hatch.
Despite extensive, systematic searches of the study wetlands, we never found any Black-headed Duck nests; all duck eggs were found in the nests of other species. In addition, despite very frequent observations of adult Black-headed Ducks, we never saw these birds attending broods of duckling. Our study thus corroborates Weller's (1968) conclusion and those of earlier naturalists that Black-headed Ducks are obligate brood parasites. Parasitism by Black-headed Ducks was common on the study wetlands and almost 1000 duck eggs were found during the course of the study (Table 1).
| Host species | Number of duck eggs | % of all eggs | ||||
| 1992 | 1993 | 1994 | 1997 | All years | ||
| Red-gartered Coot | 32 | 233 | 139 | 37 | 441 | 45.0 |
| Red-fronted Coot | 18 | 274 | 45 | 4 | 341 | 34.8 |
| Brown-hooded Gull | 52 | 51 | 20 | 123 | 12.5 | |
| Black-necked Swan | 9 | 4 | 5 | 18 | 1.8 | |
| White-faced Ibis | 16 | 16 | 1.6 | |||
| Coscoroba | 0 | 6 | 2 | 8 | 0.8 | |
| Rosy-billed Pochard | 11 | 11 | 1.1 | |||
| Limpkin | 10 | 10 | 1.0 | |||
| Black-crowned Nightheron | 6 | 6 | 0.6 | |||
| Snail Kite | 5 | 0 | 5 | 0.5 | ||
| Southern Screamer | 0 | 2 | 0 | 0 | 2 | 0.2 |
| Totals | 72 | 580 | 245 | 84 | 981 | |
We found Heteronetta eggs in 11 species of host nests, spanning a broad variety of taxonomic groups (Table 1, Figs. 4 and 5). From a numerical perspective, however, the ducks are fairly specialized parasites and there were three main host species at our study area — two species of coots and a gull. Together these three species accounted for 92% of all duck eggs and all other species each accounted for at least an order of magnitude fewer duck eggs than did each of the main hosts (Table 1). Almost half of all eggs were laid in nests of a single species, the Red-gartered Coot, and 80% of all eggs were laid in the two species of coots (Table 1).
Several factors could explain why a few host species accounted for most of the duck parasitism. First, hosts might vary in abundance, with the commonly parasitized species simply being the most abundant species on the wetlands. Second, irrespective of host abundance, the ducks might not parasitize all host species equally, but preferentially parasitize some species and avoid others. Both factors are important in our study (Table 2). Potential host species vary tremendously in abundance, which accounts for some of the observed patterns of host use. The three main host species — the two coots and the gull — were the most abundant species on our study wetlands (Table 2). In terms of the two most important hosts, the Red-gartered and Red-fronted Coots (Fulica rufifrons), we obtain different estimates of their relative frequencies of parasitism depending on which data are included. In 1997, we focused almost entirely on Red-gartered Coots because their nests are easier to find and we sought to conduct egg rejection experiments. While both species overlap in some habitats, more open vegetation is often dominated by Red-gartered Coots and in 1997 we focused our searches in those habitats. However, 1997 was also a year in which Black-headed Ducks were scare, evidenced by the low number of duck eggs we found overall. Thus, including data from 1997 results in a much lower estimate of parasitism frequency for Red-gartered Coots relative to Red-fronted Coots (Table 2). We believe that the estimate obtained by excluding 1997, which indicates very similar parasitism rates of the two species (Table 2), is a more accurate estimate.
| 1992 | 1993 | 1994 | 1997 | 1992–1994 combined | All years combined | |
| Host species | ||||||
| Red-gartered Coot | 42.4 (33) | 77.1 (144) | 37.5 (216) | 8.6 (266) | 52.4 (393) | 34.7 (659) |
| Red-fronted Coot | 53.8 (26) | 61.8 (238) | 33.0 (100) | 57.1 (7) | 53.3 (364) | 53.4 (371) |
| Brown-hooded Gull | — | 17.4 (207) | 26.8 (168) | 8.9 (202) | 21.6 (375) | 17.2 (577) |
| Black-necked Swan | — | 60.0 (5) | 30.8 (13) | 100 (1) | 38.9 (18) | 42.1 (19) |
| White-faced Ibis | — | — | — | 7.0 (200) | — | 7.0 (200) |
| Coscoroba Swan | 0 (1) | — | 42.9 (3) | 50.0 (4) | 37.5 (8) | 41.7 (12) |
| Rosy-billed Pochard | 66.7 (3) | — | — | — | 66.7 (3) | 66.7 (3) |
| Limpkin | — | 100 (2) | — | — | 100 (2) | 100 (2) |
| Black-crowned Night-heron | 24.0 (25) | — | — | — | 24.0 (25) | 24.0 (25) |
| Snail Kite | 57.1 (7) | — | 0 (2) | — | 44.4 (9) | 44.4 (9) |
| Southern Screamer | 0 (6) | 16.7 (12) | 0 (20) | 0 (12) | 5.3 (38) | 4.0 (50) |
| Unparasitized species | ||||||
| Snowy-crowned Tern | — | 0 (58) | — | — | 0 (58) | 0 (58) |
| Great Egret | 0 (≈ 100) | — | — | — | 0 (100) | 0 (100) |
| Snowy Egret | 0 (≈ 20) | — | — | — | 0 (20) | 0 (20) |
| Chimango Caracara | 0 (2) | 0 (3) | 0 (1) | — | 0 (6) | 0 (6) |
In contrast to the main hosts, several species were unimportant hosts — not because they were rarely parasitized but because they were uncommon (Table 2, Fig. 5): e.g. parasitism rates of Rosy-billed Pochard, Black-necked Swan (Cygnus melanocoryphus), Coscoroba Swan (Coscoroba coscoroba), Limpkin (Aramus guarauna) and Snail Kites (Rostrhamus sociabilis) exceeded 40% (Table 2) but all of these species are far less abundant than coots and gulls, and each accounted for only 1% to 2% of all duck eggs (Table 1).
The abundance of potential host species, however, is not the entire explanation for patterns of host use. A few species were moderately abundant but rarely or never parasitized (Table 2, Fig. 5; e.g. Southern Screamer (Chauna torquata), White Faced Ibis (Plegadis chihi), Snowycrowned Tern (Sterna trudeauii), Great Egret (Ardea alba), Snowy Egret (Egretta thula)). The low parasitism rates of these species could result if ducks avoid them because they are low quality hosts, or, alternatively, because host nest defense behaviors or nest location prevents the ducks from gaining access to nests. The suitability of these species as hosts could be determined by experimentally parasitizing them with real, viable duck eggs. Finding very low hatching rates of experimentally added duck eggs in these species would support the hypothesis that they are avoided because they are unsuitable hosts; observing a high hatching success would support the idea that host characteristics (nest defense, nest location) prevent parasitism from occurring. In terms of nest location, nest height does not seem to be a critical factor — Snail Kite nests were considerably higher above the water than other species (typically > 1m), but they were nonetheless parasitized at a moderate frequency (Table 2).
The lack of parasitism of Snowy-crowned Terns (Table 2, Fig. 5) is particularly intriguing because this species nests in mixed colonies with Brown-hooded Gulls (Larus maculipennis) and their nests were difficult for us to distinguish without measuring eggs (the tern eggs are significantly smaller). Terns were never parasitized despite the fact than gull nests a meter or two away often were. Although terns in general are well known for their aggressive nest defense, gulls are aggressive nest defenders as well (Burger, 1974) — we often saw gulls chasing predators such as Chimango Caracaras (Milvago chimango) or Long-winged Harriers (Circus buffoni) away from their colonies. Thus, it seems unlikely that terns prevent ducks from gaining access to their nests while gulls fail to do so, but observations of interactions between ducks and these two hosts at mixed colonies would be worthwhile. Given the small size of tern eggs, we suggest that terns may not be suitable hosts and so Black-headed Ducks avoid parasitizing this species, a hypothesis that could be tested with experimental parasitism of tern nests. If Black-headed Ducks avoid parasitizing tern nests because they are unsuitable hosts, it would indicate that they are indeed highly adept 'birdwatchers', able to identify and discriminate among two host species that nest in close proximity and whose nests and eggs are very difficult to distinguish.
The low parasitism rate of Southern Screamers (Table 2, Fig. 5) is also puzzling. Nest location and large body size seem unlikely explanations because their nest structure, nest location and body size is similar to the two species of swans, both of which are parasitized at fairly high rates (Table 1). Screamers are heavily armed with sharp double spurs on their wings, weapons that could be used to aggressively defend their nests and potentially prevent Black-headed Ducks from attempting to lay parasitic eggs. Risk of injury could also potentially explain the complete lack of parasitism of Great and Snowy Egrets. These two egret species nested in a mixed-species rookery located in the marsh vegetation, and most nests were fairly close to the water and certainly lower than most nests we found of Snail Kites, which were heavily parasitized. The fact that Black-crowned Night-herons (Nycticorax nycticorax) nesting in this same rookery had a moderate parasitism rate also indicates that Black-headed Ducks visited the rookery. Egrets have strong sharply pointed beaks, which they readily use as weapons — this, coupled with the fact that they nested at much higher densities than night-herons, could explain their complete lack of parasitism. Finally, the low frequency of parasitism of White-faced Ibis — a patchy but locally abundant species where nesting colonies occur — may reflect a swamping effect of a very high density and nesting synchrony (Weller, 1968) that limit the ability of individual brood parasites to fully capitalize on the large number of potential host nests. Weller (1968) observed a much larger ibis colony than we did (18000 nests compared to 200 nests) and he also observed a somewhat lower parasitism rate (1.5% compared to 7%).
Despite the expectation that a precocial brood parasite should experience a high hatching success rate, the hatching success of Black-headed Duck eggs was fairly low in its three main hosts: Red-gartered Coot (28.4% of 155 eggs hatched), Red-fronted Coots (15.8% of 148 eggs) and Brown-hooded Gulls (18.3% of 71 eggs). Sources of mortality varied among species but egg rejection was the most important source of mortality for duck eggs in all three of the main host species. The low hatching success of duck eggs in Red-fronted Coots was mainly due to high rates of egg rejection: this species rejected 67% of the duck eggs laid in its nest, whereas Red-gartered Coots rejected 39% of the duck eggs (Lyon and Eadie, 2004). The coots rejected eggs by burying them in their nests (Lyon and Eadie, 2004), something Weller observed as well.
Gulls also rejected a moderate fraction of duck eggs (26.8% of 71 eggs) but there was a striking difference in how individuals in our three focal gull colonies responded to duck eggs. Addled eggs were almost entirely restricted to gull nests (85% of addled eggs occurred in gulls) and these were found primarily at one wetland (10 of the 11 addled eggs in gull nests occurred at the Cari Lauquen site, comprising 24% of all 41 duck eggs laid on this wetland (Fig. 6). Interestingly, rates of egg rejection were extremely low at this wetland (1 of 41 eggs (2.4%)) compared to the two other wetlands with gulls (18 of 30 eggs rejected (60.0%)); while addled eggs were very rare at the two wetlands where rejection was common (1 of 30 eggs addled (3.3%); Fisher Exact p = 0.0003 for comparison of number of addled versus rejected eggs between Cari Lauquen and the other two wetlands combined). These differences suggests that these two sources of mortality may be due to alternative host responses: outright rejection (at two of the wetlands) versus neglect (at Cari Lauquen). Although the gulls at Cari Lauquen did not reject Heteronetta eggs, duck eggs were often off to the side and cold to the touch when we visited the gull nests, indicating that the gulls were not incubating the duck eggs (their own eggs were warm).
Finally, mortality due to nest predation was low for all three hosts; < 10% of duck eggs were lost to predation in each of three host species. In part, the low mortality due to nest predation simply reflects the high nest survival rates of the main hosts. Weller (1968) also noted the high nest success rates for the two species of coots in his study.
Anecdotal observations of two aspects of brood parasitism lend further insight on the manner by which Black-headed Ducks interact with their hosts: the behavior of the ducks while seeking host nests to parasitize and interactions between ducklings and their hosts. We often observed Heteronetta in the marshes and we mostly observed a male and female pair rather than solitary individuals. We also frequently observed more than one pair in the same general location, at times very close together and often clearly using the same areas of marsh, without signs of aggression. These observations would suggest that Black-headed Ducks are neither territorial, nor do they defend areas of hosts nests, even temporarily.
We also on occasion observed pairs of ducks that clearly seemed to be assessing host nests (Fig. 7). At a wetland adjacent to downtown Buenos Aires, La Costanera Sur, we observed two pairs of ducks apparently checking several nests of Common Moorhens (Gallinula chloropus) and White-winged Coots (Fulica leucoptera), typically passing within 3 m of the nests. The two duck pairs overlapped in parts of the wetland they were using, at times coming close to each other. On another occasion we used a floating blind to follow a pair of Heteronetta for almost an hour at one of our focal wetlands (Marsh B). The male did most of the leading (Fig. 7), and several times the female changed direction to follow him — it is possible that males play a role in finding host nests and perhaps even facilitating access to host nests. Eventually this pair came close to an occupied Red-gartered Coot nest (Fig. 7), whereupon the female swam back and forth 5 m from the nest before eventually being chased off by the attending coot.
We also made observations of the interaction between hatchling ducklings and their hosts. We hatched ducklings in captivity to obtain blood samples for genetic analyses and then released a few of these ducklings to active nests of some host species, using a floating blind to add the duckling and to observe the hosts' response. We also observed several interactions between naturally-hatched ducklings and their actual hosts. One duckling added to a Brown-hooded Gull nest was attacked repeatedly by the attending gull and the gull prevented it from returning to the nest by mantling the nest with outstretched wings (Fig. 8). We rescued the duckling and released it on its own. We observed a second similar attack of gull on a duckling. This aggressive host behavior contrasts strikingly with an observation of a duckling that hatched naturally in a gull nest; this duckling was brooded by the gull and treated similarly to the gull's own chick (Fig. 8). We also added ducklings to a Red-fronted Coot nest, where the parent coot gently pecked the duckling three times but then accepted it in the nest. In another natural parasitism of a Red-fronted Coot nest, we observed a duckling interacting with the hosts over two days. On the first day, the parent coot went off the nest and called to the duckling with solicitation calls. The duckling left the nest but eventually returned to be brooded. The following morning the duckling was still at this nest, being brooded. As on the previous day, the parent left the nest and the duckling followed (Fig. 8), and the adult coot even tried to feed the duckling twice (coots feed their own offspring). Finally, we added a ducking to a Red-gartered Coot nest, where it was accepted. However, the duckling left the nest when the attending adult coot left the nest to fight with a neighbor, and it then swam to an adjacent territory with a Red-gartered Coot nest with coot chicks. The duckling approached the nest but the two adult coots aggressively lunged at it with wings outspread, and they gave it a couple of pecks, and the duckling then swam off into dense vegetation. These observations indicate that many hosts will not tolerate already hatched ducklings joining their nests. Once ducklings leave their original host nests, it seems unlikely that they can regain access to those nests, or the nests of other individuals, to be brooded.
Various early naturalists concluded that Black-headed Ducks are obligate brood parasites because nests were never found. However, prior to Weller's (1968) study, systematic searches of wetlands had not been conducted, and so it was conceivable the Black-headed Ducks were mainly parasitic, but that under some ecological conditions some females nested. Sorenson's (1991) work with the facultative interspecific brood parasite, the Redhead (Aythya americana), revealed that the degree to which females allocate eggs to brood parasitism versus nesting can vary strikingly with ecological conditions. In one year of his study, the majority of breeding females (79%) laid only parasitic eggs and completely relinquished nesting, while in another year only 29% of the breeding females did so (Sorenson, 1991: 780). This striking year-to-year variation in parasitic frequency — and the fact that in some years the population rates of pure parasitism in Redheads approach obligate parasitism levels — suggests that Heteronetta could feasibly initiate their own nests in some years, perhaps when host availability is particularly low. Thus, even though Weller's systematic study of large areas of marsh failed to turn up evidence of Heteronetta nests or broods, his single year study may not have been sufficient to rule out the possibility that nesting takes place in some years but not others. Our four-year systematic study of large areas of wetlands also failed to detect evidence for nesting and so our results provide strong support for the conclusion that Heteronetta is an obligate brood parasite.
Three different studies all point to the importance of coots as the most important hosts of Heteronetta, but there are also differences in conclusions about which species of coot is most important (Weller, 1968; Cofré et al., 2007; Lyon and Eadie, 2004 and this study). Weller concluded that Red-fronted Coots were the main species used by Heteronetta at the two wetlands he studied near General Lavalle, Argentina. At one wetland with dense vegetation, Estancia Vanini, Red-gartered Coots appeared to be largely absent. However, at a second wetland, Estancia Palenque, both species occurred at high densities, yet the parasitism rate of Red-fronted Coot nests was more than triple that of Red-gartered Coots nests (54% of nests parasitized versus 16%). By contrast, in our study in this same region, the overall parasitism rates for all wetlands combined were nearly identical for both species (when the potential bias from focusing on one species in 1997 is taken into account; Table 2). However, we also found that for the same wetland that Weller studied at Estancia Palenque, the relative parasitism rates of the two coot species varied across years: in one year (1992) Red-fronted Coots were parasitized at a slightly higher rate (54% of nests) than Red-gartered Coots (42% of nests), while in a second year (1994), Red-fronted Coots were parasitized at a considerably lower rate (23%) than Red-gartered Coots (62%). Clearly, the parasitism rates for the two coot species vary across wetlands and across years, and it seems that Weller may not have obtained a representative picture of the broad patterns of parasitism of the two species of coots from his single year study of two wetlands.
In a broad geographic survey of wetlands in Chile, Cofré et al. (2007) used a correlational approach to infer the importance of different potential host species to Heteronetta. They surveyed the densities of Heteronetta and several potential host species on 19 wetlands and then used the strength of correlations between population densities of Heteronetta and different potential host species to identify potentially important host species. The densities of four species were strongly correlated (r ≥ 0.48) with Heteronetta densities: Red-fronted Coots, Red-gartered Coots, Rosy-billed Pochards and Black-crowned Night-herons (Cofré et al., 2007). In support of conclusions from the two Argentinian studies (Weller, 1968; Lyon and Eadie, 2004 and this study) the correlational approach identified the two species of coots as the best predictors of Heteronetta densities, but in contrast to the Argentinian studies, the Chilean study suggested that pochards and night-herons could potentially also be very important hosts. The main problem with this study is that the densities of the different host species could be correlated with each other (for example if different species show the same habitat preferences) so that correlations with some species might be a consequence of habitat (and other hosts), and not the effect of the density of the potential host species per se. It would be interesting to more directly assess patterns of host use in these Chilean wetlands. Regardless, an important conclusion of all studies combined is that coots are the most important hosts over large part of Heteronetta's breeding range.
With respect to several less important host species, we found largely similar patterns to Weller (1968): Rosy-billed Pochards are uncommon but heavily parasitized (both studies), egrets are avoided completely (both studies), screamers are rarely parasitized (this study) or never parasitized (Weller, 1968). Two host species differed in importance between the two studies. We found that Brown-hooded Gulls were moderately important hosts in terms of the proportion of all duck eggs that were laid in this species (Table 1). Weller found a single parasitized gull nest (but he only found a very small number of gull nests overall). In contrast, we found three large gull colonies, where a reasonable proportion of nests were parasitized (Table 2), which results in gulls being the third most important host in terms of number of duck eggs in our study (Table 1). Weller (1968) also found that the White-faced Ibis was an important host, at least locally, in contrast to our observations. In terms of parasitized nests (rather than total parasitic eggs) 27% of all parasitized nests found by Weller were ibis nests. We found that a tiny fraction of all duck eggs were laid in ibis nests (Table 1), but two factors suggest that this estimate should be viewed with caution. First, as Weller (1968) noted, White-faced Ibis colonies are regionally rare — the fact that ibis happened to nest in our study areas might have led to an overestimate of their importance as hosts on a regional scale. Moreover, we specifically targeted the ibis colony for study because we knew from Weller's study that it was a potentially important host. Second, the only year that ibis nested in our study areas, 1997, was also a year with very low numbers of Black-headed Ducks, and markedly lower parasitism rates of their main host, the Red-gartered Coot (Table 2). Thus, our observation that relatively few duck eggs were laid in ibis nests could reflect the low densities of the brood parasites in that year rather than the lack of importance of ibis as hosts. This bias could also potentially explain the differences between our study and Weller's study in the importance of White-faced Ibis as a host species. More generally, a large-scale regional survey of all potential host species would be needed to properly assess the importance of patchy but high density species like gulls and ibis as duck hosts.
A consistent picture is now emerging from Wellers's study and our own that helps elucidate the pathways by which obligate parasitism may (or may not) have evolved in this species. It is clear that, while the Black-headed Duck can use a variety of hosts, it is actually quite specialized in the sense that it depends on very few hosts (i.e., 92% of all eggs are found in nests of just three species, 80% in two species). In addition, and somewhat unexpectedly for a precocial host that imposes little cost on its host, hatching success was lower than might have been expected. These results bear on two possible hypotheses that could explain why obligate parasitism has evolved in Heteronetta. The first hypothesis poses that, as a precocial bird whose young require little species-specific parental care, Heteronetta can use a wide range of hosts and this, in turn, would lead to a large number of host nests being available (Davies, 2000). The abundance of diverse suitable hosts could facilitate the evolution of an obligate mode of parasitism (Lyon and Eadie, 1991). Our results indicate that relatively few host species are used by Heteronetta, although other potential host species apparently are not utilized. Nonetheless, it is not simply the number (diversity) of host species that determines host availability, but rather the number of host nests, or more precisely, the ratio of population sizes of hosts and parasites, and the temporal availability of the hosts as well. Given how abundant coots are in the marshes we studied, hosts may be far from limiting for individual female Heteronetta. Moreover, the ducks also have a reasonably long period of host availability (e.g. six weeks at our study site, see also Weller 1968). Hence, the simple idea that obligate parasitism is driven by the diversity of host species is not supported, but host availability might still play an important role given that coots nests are so abundant and available over a reasonable period of time.
A second hypothesis is that because a precocial bird should impose few costs on its hosts, host defenses should be rare or absent and the duck should enjoy exceptionally high hatching success. However, we found that hatching success was not exceptionally high, largely because of rejection or neglect by hosts. Elsewhere, we show that egg recognition and rejection has been selected for in the two main hosts species (Red-fronted and Red-gartered Coots) as a defense against frequent conspecific brood parasitism (coots parasitizing each other), which entails significant costs for hosts (Lyon and Eadie, 2004). Hence, the non-mimetic white eggs of Heteronetta (Figs. 1 and 4) are rejected or buried as incidental "collateral damage" of this intraspecific arms race. Experiments indicate that even with more mimetic eggs — mimetic within a range of realistically feasible options for the first stages of mimicry — Black-headed Ducks would not be able to improve their hatching success (Lyon and Eadie, 2004). Thus, the Black-headed Duck is trapped in the sense that high hatching success cannot be fully realized, even in the absence of any direct costs to the host. Switching to other hosts also appears to not be an option (Table 2).
What additional factors might influence the unusual nesting behavior of this enigmatic bird? One possibility is that, via complete emancipation of any form of parental care, the fecundity of Heteronetta could be enhanced (Lyon and Eadie, 1991). Certainly with a six-week egg-laying window (Weller, 1968; our study) and plentiful hosts, a parasitic female has the opportunity to lay many more eggs than the typical clutch size of a nesting duck. Unfortunately, we know very little about actual fecundities or even egg-laying potential of this species. This is an area for future research, which could be significantly aided by new techniques in molecular ecology, including the ability to determine maternity of unhatched eggs (Andersson and Åhlund, 2000).
We also know little about how females might select among possible hosts or whether they target certain host species or nests at suitable stages of laying. Perhaps one of the greatest remaining mysteries is what happens to the ducklings after they leave the nest of the host, presumably to rear themselves alone. Despite thousands of person-hours spent in the marshes, we have encountered ducklings only twice. What is the survival of ducklings and why do we never see them? Perhaps they are nocturnal and consequently experience exceptionally high duckling survival, which might help to raise the fitness of the parasitic strategy. Preliminary data suggest that ducklings may indeed be nocturnal — we hatched several dozen Heteronetta eggs in captivity for genetic studies and it was our impression these captive-hatched ducklings were more active at night. Clearly, there are a number of key life history parameters that must yet be explored for this strange duck before we can fully know which life history components — fecundity, hatching success, juvenile survival and adult survival — have been sufficiently increased by emancipation from parental care to favor the evolution of obligate parasitism (Lyon and Eadie, 1991).
One final factor that could be important in favoring obligate parasitism is the role of nest predation and the enhanced success gained by using hosts that achieve relatively high hatching success through aggressive nest defense. Weller (1968:36) commented on the fact that main hosts of Heteronetta all have fairly high hatching success, and likely much higher success than the nest success of ducks — this difference in nest success may have been key to evolution of obligate parasitism. Specifically, if a female Heteronetta were to nest she would experience low nesting success typical of marsh nesting ducks. However, by laying parasitically in the nests of species with aggressive nest defense, mortality from nest predation could be greatly reduced, perhaps enough to compensate for other sources of mortality such as egg rejection by hosts or mismatched timing with the hosts laying cycle. Nest predators occur at high densities in all of the marshes we studied and in an artificial nest experiment, 100% of experimental nests were depredated in a very short time interval (< 48 h, Lyon and Eadie, unpubl. data). Despite this, nest predation was a minor source of mortality for Heteronetta eggs in our study — less than 10% of duck eggs perished due to nest predation in each of the three main host species. The contrast between the extreme predation rates of eggs in unattended experimental nests and the low predation rates of duck eggs in host nests suggests that nest defense by hosts could substantially improve the hatching success of Black-headed Ducks over that which might be realized had they nested on their own. Because Heteronetta no longer builds nests of its own, we cannot know what hatching success they would enjoy were they to nest. However, as over-water nesters, Rosy-billed Pochards might provide a reasonable proxy, given that the ancestral species that gave rise to Heteronetta was likely to have been an over-water nester (stifftail ducks (Livezey, 1986), all of which nest over water). Weller's data show that the hatching success of Rosy-billed Pochards in Argentine marshes is very low (16% of 6 nests) compared to coots (83% for Red-fronted Coots), although his sample size for pochards is admittedly very small. It would be well worthwhile to obtain a better understanding of the patterns of nest mortality of over-water nesting ducks in South American wetlands. This information, in combination with an investigation of the fecundities of individual Heteronetta females, will lead to a more complete understanding of the life history tradeoffs that are likely to have been central to the evolution of obligate brood parasitism in this fascinating duck.
We thank W. Liang for the invitation to write this paper; the National Geographic Society and the British Broadcasting Corporation (D. Attenborough's Life of Birds) for funding; the Flores family, J. Echerran, M. Beade and J.C. Reboreda for logistic support; and V. Meuhter, G. Goggin and A. Carminati for assistance in the field. During part of the study, B.E.L. was supported by the Kananaskis Field Stations of the University of Calgary and University of California, and J.M.E. by the Dennis G. Raveling Endowment.
|
Davies NB. 2000. Cuckoos, Cowbirds and Other Cheats. T & AD Poyser, London.
|
|
Lack D. 1947. The significance of clutch-size, parts 1 and 2. Ibis, 89: 302–352.
|
|
Lack D. 1968. Ecological Adaptations for Breeding in Birds. Methuen, London.
|
|
Langmore N, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2010. Visual mimicry of host nestlings by cuckoos. Proc R Soc B, 278: 2455–2463.
|
|
Redondo T, Arias de Reyna L. 1988. Vocal mimicry of hosts by great spotted cuckoo Clamator glandiarius: further evidence. Ibis, 130: 540–544.
|
|
Stokke BG, Moksnes A, Røskaft E. 2002. Obligate brood parasites as selective agents for evolution of egg appearance in passerine birds. Evolution, 56: 199–205.
|
|
Weller MW. 1968. The breeding biology of the parasitic black-headed duck. Living Bird, 7: 169–208.
|
|
Winkler DW, Walters JR. 1983. The determination of clutch size in precocial birds. Curr Ornithol, 1: 33–68.
|
| Host species | Number of duck eggs | % of all eggs | ||||
| 1992 | 1993 | 1994 | 1997 | All years | ||
| Red-gartered Coot | 32 | 233 | 139 | 37 | 441 | 45.0 |
| Red-fronted Coot | 18 | 274 | 45 | 4 | 341 | 34.8 |
| Brown-hooded Gull | 52 | 51 | 20 | 123 | 12.5 | |
| Black-necked Swan | 9 | 4 | 5 | 18 | 1.8 | |
| White-faced Ibis | 16 | 16 | 1.6 | |||
| Coscoroba | 0 | 6 | 2 | 8 | 0.8 | |
| Rosy-billed Pochard | 11 | 11 | 1.1 | |||
| Limpkin | 10 | 10 | 1.0 | |||
| Black-crowned Nightheron | 6 | 6 | 0.6 | |||
| Snail Kite | 5 | 0 | 5 | 0.5 | ||
| Southern Screamer | 0 | 2 | 0 | 0 | 2 | 0.2 |
| Totals | 72 | 580 | 245 | 84 | 981 | |
| 1992 | 1993 | 1994 | 1997 | 1992–1994 combined | All years combined | |
| Host species | ||||||
| Red-gartered Coot | 42.4 (33) | 77.1 (144) | 37.5 (216) | 8.6 (266) | 52.4 (393) | 34.7 (659) |
| Red-fronted Coot | 53.8 (26) | 61.8 (238) | 33.0 (100) | 57.1 (7) | 53.3 (364) | 53.4 (371) |
| Brown-hooded Gull | — | 17.4 (207) | 26.8 (168) | 8.9 (202) | 21.6 (375) | 17.2 (577) |
| Black-necked Swan | — | 60.0 (5) | 30.8 (13) | 100 (1) | 38.9 (18) | 42.1 (19) |
| White-faced Ibis | — | — | — | 7.0 (200) | — | 7.0 (200) |
| Coscoroba Swan | 0 (1) | — | 42.9 (3) | 50.0 (4) | 37.5 (8) | 41.7 (12) |
| Rosy-billed Pochard | 66.7 (3) | — | — | — | 66.7 (3) | 66.7 (3) |
| Limpkin | — | 100 (2) | — | — | 100 (2) | 100 (2) |
| Black-crowned Night-heron | 24.0 (25) | — | — | — | 24.0 (25) | 24.0 (25) |
| Snail Kite | 57.1 (7) | — | 0 (2) | — | 44.4 (9) | 44.4 (9) |
| Southern Screamer | 0 (6) | 16.7 (12) | 0 (20) | 0 (12) | 5.3 (38) | 4.0 (50) |
| Unparasitized species | ||||||
| Snowy-crowned Tern | — | 0 (58) | — | — | 0 (58) | 0 (58) |
| Great Egret | 0 (≈ 100) | — | — | — | 0 (100) | 0 (100) |
| Snowy Egret | 0 (≈ 20) | — | — | — | 0 (20) | 0 (20) |
| Chimango Caracara | 0 (2) | 0 (3) | 0 (1) | — | 0 (6) | 0 (6) |