| Citation: | Monika Homolková, Petr Musil, Diego Pavón-Jordán, Dorota Gajdošová, Zuzana Musilová, Šárka Neužilová, Jan Zouhar. 2024: Changes in the adult sex ratio of six duck species breeding populations over two decades. Avian Research, 15(1): 100187. DOI: 10.1016/j.avrs.2024.100187 |
Despite all efforts, long-term changes in the adult sex ratios of breeding duck populations are still unclear; this uncertainty is especially true for male-bias populations, which are often under the scrutiny of researchers lacking convenient results for the active protection of endangered species. Species with male-bias populations are usually strongly affected by a decline in population size that leads to a higher extinction risk. In this study, we examined our long-term data of the abundance of breeding populations in six duck species (Mallard Anas platyrhynchos, Gadwall Mareca strepera, Red-crested Pochard Netta rufina, Common Pochard Aythya ferina, Tufted Duck Aythya fuligula, and Common Goldeneye Bucephala clangula) from fishponds in South Bohemia, Czechia, between 2004 and 2022. This evidence was used to assess long-term changes in the adult sex ratio in these breeding populations and investigate the possible effects of the NAO index (North Atlantic Oscillation index) on them, indicating climate conditions in winter. We determined a long-term decrease of the proportion of females in the breeding season in two of the six examined species: Common Pochard and Red-crested Pochard, which is driven by the long-term increase in the number of males in contrast to the decreasing or stable number of females likely caused by different migration behaviours between females and males. In the case of Common Pochard, in breeding populations, we estimated 60–65% of males in the early 2000s rising to 75–80% in the early 2020s. However, we establish no significant effects linked to climate conditions of the previous winter in these species as a crucial cause of the changes of the proportion of females in the breeding population.
Although waterfowl species typically present an even hatching sex ratio (Swennen et al., 1979; Blums and Mednis, 1996; Cooch et al., 1997; Donald, 2007; Lehikoinen et al., 2008), breeding populations often reveal a male-biased sex ratio (McKinney, 1985; Baldassarre and Bolen, 1994; Donald, 2007; Székely et al., 2014). Increasing male-bias in a population mainly occurs due to the lower survival of the females (Donald, 2007), typically linked to ecological and life-history traits, such as: (ⅰ) higher energy expenditure of females during reproduction (Sargeant et al., 1984; Thomson et al., 1998; Post and Götmark, 2006) and brood rearing (Owen and Black, 1990; Liker and Székely, 2005); (ⅱ) higher predation pressure on incubating females (Owen and Black, 1990; Donald, 2007; Lehikoinen et al., 2008); (ⅲ) lower resistance to parasites (Bize et al., 2005) or diseases (Swennen et al., 1979) at the juvenile stage; and (ⅳ) a differing migratory strategy (Owen and Black, 1990; Carbone and Owen, 1995; Marra, 2000; Newton, 2008; Alves et al., 2013) potentially linked to a different tolerance to cold (Calder, 1974; Carbone and Owen, 1995; Evans, 2001). In Western Europe, females migrate further south in winter than males, which is a trade-off between facing higher hunting pressure (Gunnarsson et al., 2012) and lead shot poisoning (Mateo, 2009) and being less exposed to cold weather events (cold spells) that may increase over-winter mortality (Ridgill and Fox, 1990; Adam et al., 2015). Such differences in migratory behaviour could also affect the adult sex ratio (hereafter ASR) through possible differences in suitable food availability (Baastrup-Spohr et al., 2013; Rodrigo et al., 2015) and habitat changes (Rendón et al., 2008) in different wintering areas.
The observed increasing male-bias has far-reaching consequences for waterfowl population dynamics and has been shown to significantly affect the population growth rate of several species (Lee et al., 2011). For example, low productivity in male-biased populations could be associated with a failure in pair formation, which is especially alarming in species with a severe population decline (Lee et al., 2011). Also, a large proportion of males (compared to the number of females) increases the pressure of males on females during the mating period, causing higher stress levels in females. All these factors contribute to the unfavourable conservation status often found in strongly male-biased populations (Donald, 2007; Székely et al., 2014; Morrison et al., 2016; Brides et al., 2017; Pöysä et al., 2019).
A clear example of this phenomenon is the Common Pochard (Aythya ferina), which is the waterfowl species with the most pronounced male-biased population in the Western Paleartic (Carbone and Owen, 1995; Brides et al., 2017; Frew et al., 2018; Wood et al., 2021). This species has dramatically declined in numbers over the last 30 years, losing 30–49% of the entire European breeding population within three generations (Fox et al., 2016) and is listed as Vulnerable in IUCN's Red List (BirdLife International, 2017). Thus, from a conservation point of view, increasing our understanding of the temporal changes in ASR is critical, especially in long-lived, Red-listed species that have a high extinction risk due to the Allee effect (Allee et al., 1949; Engen et al., 2003).
Here, we used a long-time series (2004–2022) of data on waterfowl abundance during the breeding season in 173 fishponds within and around the Třeboň Biosphere Reserve (South Bohemia, Czech Republic) to assess long-term changes in the ASR in the breeding populations of six sympatric duck species: Common Pochard, Tufted Duck (Aythya fuligula), Gadwall (Mareca strepera), Mallard (Anas platyrhynchos), Red-crested Pochard (Netta rufina) and Common Goldeneye (Bucephala clangula). Moreover, we investigated whether changes over time (i.e., trends) in the observed ASR as well as in the number of females and males, are linked to the general weather conditions during the preceding winter in Europe, represented by the North Atlantic Oscillation (NAO) index (Hurrell, 1995; Hurrell and Deser, 2010). The severity of winter weather conditions (expressed here by the NAO index) will cause a later departure from wintering sites (Ridgill and Fox, 1990; Elmberg et al., 2014) as well as higher bird overwinter mortality (Ridgill and Fox, 1990; Adam et al., 2015) and are linked to the number of individuals arriving at the breeding grounds the following season (Pavón-Jordán et al., 2017).
We formulated two study questions based on previous studies linked to the ASR: (a) do we observe a long-term decrease in the proportion of breeding females in the six waterbird species considered in this study? and (b) do we find a negative impact of the preceding winter's severity on the proportion of females in the breeding populations?
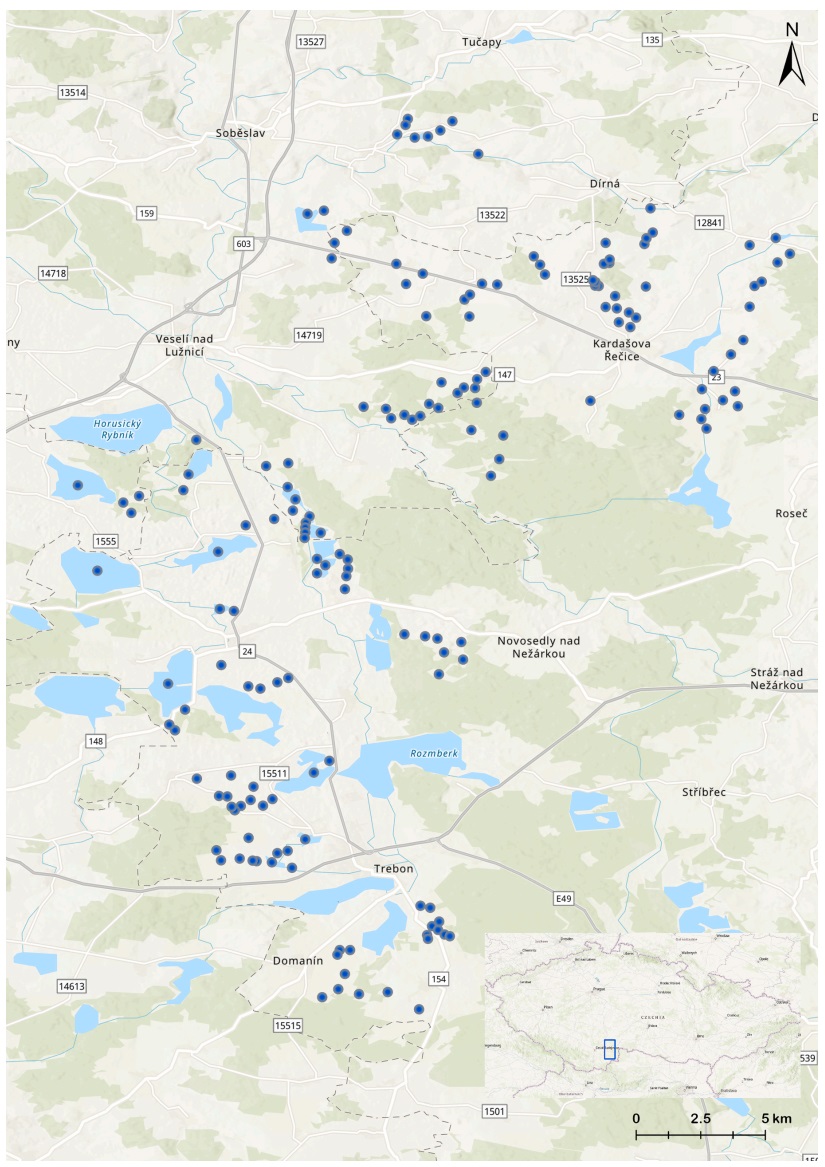
The numbers of males and females of the six most abundant duck species (Mallard, Gadwall, Red-crested Pochard, Common Pochard, Tufted Duck, and Common Goldeneye) were monitored at the beginning of each breeding season (Musil and Fuchs, 1994; Musil, 2000; Musil and Neužilová, 2009) on 173 fishponds located in the Třeboň Biosphere Reserve and its surrounding area (South Bohemia, Czech Republic, 48.9685–49.2649° N, 14.6622–14.9007° E; Fig. 1) from 2004 to 2022. Birds were counted from a fixed number of points covering the entire water surface of each of the 173 water bodies, with observers investing sufficient time to record all birds present, thus requiring different survey effort according to bird abundance, species and water body size (Bibby et al., 2000; Čehovská et al., 2019). We carried out full surveys (counts) of the entire study area (173 water bodies) every two weeks (i.e., nine surveys between April and August). All individuals of all waterbird species are counted (although, for this analysis, we only used data on the six most abundant duck species). We used data from the late April count for the early breeding species (Mallard and Common Goldeneye) and data from the late May count for the late breeding species (Gadwall, Red-crested Pochard, Common Pochard and Tufted Duck) (Musil et al., 2023). We consider abundance during this period to be the best indicator of the number of males and females and the breeding population size based on information gathered from marked individuals in the study area. This period coincides with an early start of the breeding season (before the start of incubation) after the end of spring migration and before the movements of males after pair separation for these species (Šťastný and Hudec, 2016; Čehovská et al., 2019; Musil et al., 2023). We calculated the bias in the sex ratio (i.e., ASR) as the proportion of females over the total number of individuals with identified sex (males or females). Sex was identified in almost all individuals except for a few cases due to bad conditions for the observation, e.g., strong wind, fog or back-lighting. The proportion of individuals with unidentified sex ranged between 0.3 and 2.7%, depending on the species.
We used the winter North Atlantic Oscillation (NAO) index as a proxy of winter harshness (see MacKenzie and Köster, 2004; Pavón-Jordán et al., 2019). Monthly NAO data were downloaded from https://crudata.uea.ac.uk/cru/data/nao/values.htm. The mean values for the previous winter months (December, January, February) were calculated for each breeding season. The NAO is closely associated with general weather conditions over the main wintering areas of the six species investigated in this paper (Hurrell and Deser, 2010; Wetlands International, 2019). The NAO index is calculated as the difference between the normalised sea level pressures in Reykjavik (Iceland) and Lisbon (Portugal) since 1864 (Hurrell and NCARS, 2016). Winters with high (positive) values of NAO reflect mild and wet winter weather conditions in Western and Northern Europe, which in turn can be associated with more benevolent winter conditions for waterbirds in these areas. Furthermore, positive values of NAO are also associated with drier winters in Southern Europe compared to years with mean or negative NAO values, as it is characterised by an atmospheric circulation that transports precipitation from the southwest towards the northeast (see Hurrell, 1995 for further details on the NAO index). Negative NAO, on the other hand, represents colder temperatures in Northern and Western Europe (Hurrell, 1995; Hurrell and Deser, 2010; Hurrell and NCARS, 2016), which can be associated with more unfavourable winter conditions for waterbirds in these regions.
We modelled the long-term changes (i.e., trend) in the ASR of the six most abundant duck species during the breeding season in the Třeboň Biosphere Reserve and surrounding area as a function of the general weather conditions in the preceding winter (ASR model). Our response variable is a 'proportion' (i.e., proportion of females in the breeding population), hence we specified our models with a binomial error distribution (Zuur et al., 2009). We included the fixed covariates Year (continuous covariate), NAO index (continuous covariate) and Species (categorical variable with six levels, one per species). In addition, we included the two two-way interactions of interest, Species × Year and Species × NAO. We also include an autoregressive (AR1) covariance structure (Zuur et al., 2009) to account for the temporally autocorrelated nature of waterbird counts. Its general notation is:
Proportion of femalesi, j, t ~ Yeart + NAOt + Speciesj + Yeart × Speciesj + NAOt × Speciesj (1)
where Proportion of femalesi, j, t is the ith observed number of females in relation to the number of males in the breeding population of species j (Common Pochard, Tufted Duck, Gadwall, Mallard, Red-crested Pochard, and Common Goldeneye) in year t and t = 2004, …, 2022. NAOt is the NAO index during the winter preceding the breeding season in year t.
We also modelled both the number of females and the number of males (females model and males model, respectively) using the same model structure and covariates (including the autoregressive covariance structure) as in (1), but with a Poisson error distribution as our response variable is a count (Zuur et al., 2009). All three models (ASR, females, and males models) were built with the glmmTMB (Brooks et al., 2017) package in R version 4.2.2 (R Core Team, 2022).
We then carried out two post-hoc analyses on the model results (for the three models separately). First, to estimate and compare the species-specific trends in ASR (ASR model), number of females (females model) and number of males (males model) (hereafter, trend analysis). With the second post-hoc analysis, we estimated the species-specific relationship between the proportion of females (ASR model), number of females (females model), and number of males (males model) with the gradient of NAO (hereafter, NAO analysis). All post-hoc analyses were carried out using the emtrends function in the emmeans package (Lenth, 2023) in R version 4.2.2 (R Core Team, 2022).
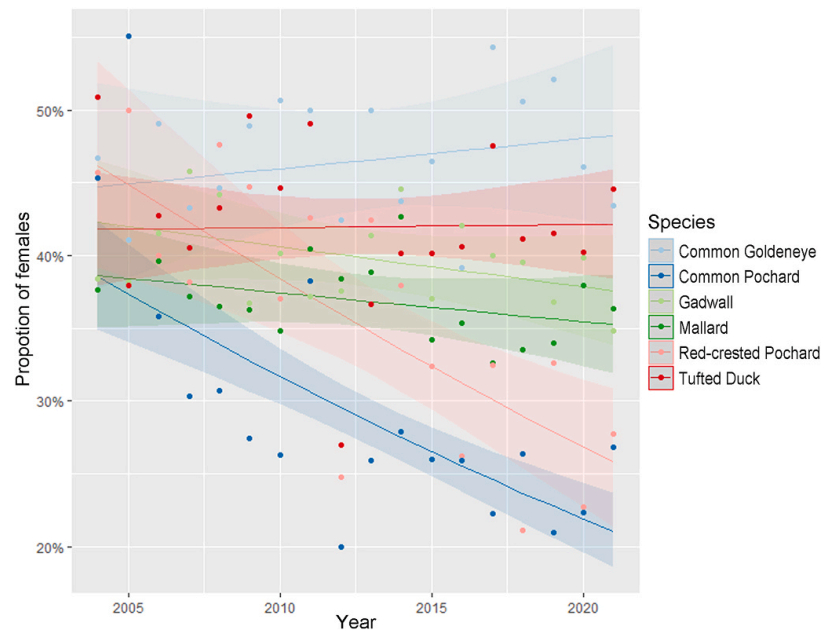
Over the study period, the numbers of females increased in two species (Mallard and Common Goldeneye), and its changes were not significant in Gadwall, Red-crested Pochard, Tufted Duck and Common Pochard (Table 1). The numbers of males increased in all species except for the Tufted Duck (Table 2, Fig. 2). The average yearly proportion of females over the study period varied between 0.296 (Common Pochard) and 0.471 (Common Goldeneye) (Fig. 3).
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.011 | 0.010 | −0.031 | 0.009 | −1.102 | 0.270 |
| Gadwall | 0.0170 | 0.010 | −0.003 | 0.037 | 1.663 | 0.096 |
| Common Goldeneye | 0.0452 | 0.013 | 0.019 | 0.071 | 3.416 | 0.001 |
| Mallard | 0.0200 | 0.010 | 0.001 | 0.039 | 2.035 | 0.042 |
| Red-crested Pochard | 0.0170 | 0.014 | −0.010 | 0.043 | 1.224 | 0.221 |
| Tufted Duck | 0.0040 | 0.010 | −0.016 | 0.023 | 0.355 | 0.7227 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | 0.040 | 0.009 | 0.022 | 0.0584 | 4.336 | < 0.001 |
| Gadwall | 0.029 | 0.010 | 0.010 | 0.048 | 2.994 | 0.003 |
| Common Goldeneye | 0.034 | 0.012 | 0.0101 | 0.058 | 2.780 | 0.005 |
| Mallard | 0.029 | 0.009 | 0.011 | 0.047 | 3.110 | 0.002 |
| Red-crested Pochard | 0.071 | 0.012 | 0.048 | 0.093 | 6.069 | < 0.001 |
| Tufted Duck | 0.002 | 0.009 | −0.017 | 0.0202 | 0.176 | 0.860 |
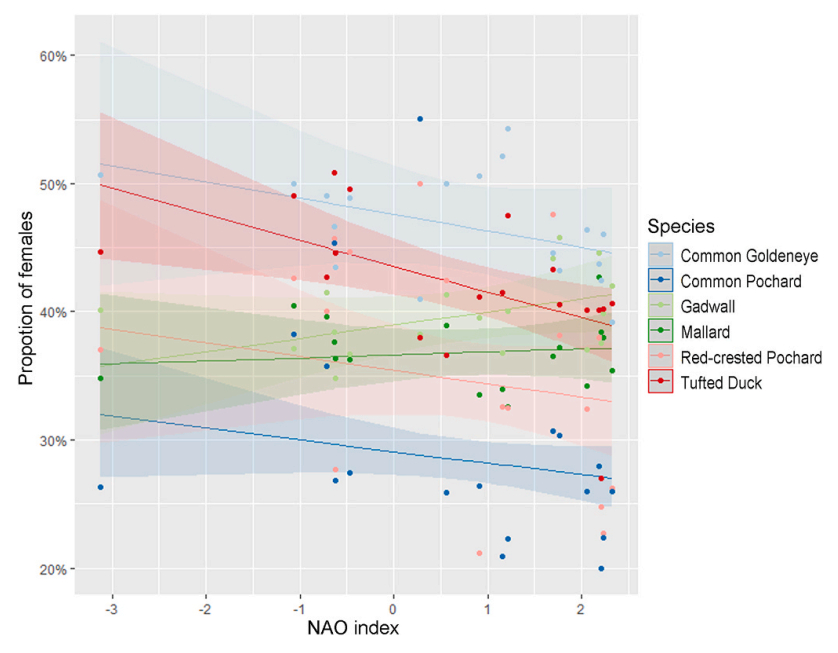
The trend analysis based on the ASR model showed significant negative long-term trends in the proportion of females in Common Pochard and Red-crested Pochard (Fig. 4, Table 3) over the study period. The NAO analysis showed a significant negative relationship between the proportion of Tufted Duck females and the NAO index in the preceding winter (the higher the NAO index the smaller the proportion of females in the following breeding season; Table 4). This relationship was not statistically significant in the other five species (Fig. 5, Table 4).
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.050 | 0.008 | −0.066 | −0.034 | −6.205 | < 0.001 |
| Gadwall | −0.012 | 0.009 | −0.029 | 0.006 | −1.316 | 0.188 |
| Common Goldeneye | 0.008 | 0.014 | −0.019 | 0.035 | 0.602 | 0.547 |
| Mallard | −0.008 | 0.008 | −0.024 | 0.007 | −1.084 | 0.278 |
| Red-crested Pochard | −0.053 | 0.014 | −0.080 | −0.026 | −3.807 | < 0.001 |
| Tufted Duck | 0.001 | 0.008 | −0.015 | 0.017 | 0.101 | 0.919 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.044 | 0.029 | −0.101 | 0.014 | −1.488 | 0.137 |
| Gadwall | 0.043 | 0.030 | −0.016 | 0.102 | 1.428 | 0.153 |
| Common Goldeneye | −0.051 | 0.048 | −0.146 | 0.043 | −1.061 | 0.289 |
| Mallard | 0.010 | 0.029 | −0.047 | 0.066 | 0.339 | 0.734 |
| Red-crested Pochard | −0.046 | 0.050 | −0.144 | 0.052 | −0.926 | 0.355 |
| Tufted Duck | −0.082 | 0.029 | −0.139 | −0.025 | −2.833 | 0.005 |
The NAO analysis from the females model and the males model showed a significant positive relationship between the number of Mallard females and the NAO index values (see Fig. 6, Table 5 for females; Fig. 7, Table 6 for males). The results show no statistically significant relationship between the number of females or males and the NAO index values in the other five species (Tables 5, 6).
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.006 | 0.039 | −0.083 | 0.072 | −0.143 | 0.887 |
| Gadwall | 0.047 | 0.040 | −0.030 | 0.125 | 1.193 | 0.233 |
| Common Goldeneye | −0.040 | 0.049 | −0.136 | 0.056 | −0.816 | 0.415 |
| Mallard | 0.087 | 0.039 | 0.010 | 0.163 | 2.213 | 0.027 |
| Red-crested Pochard | 0.047 | 0.052 | −0.055 | 0.149 | 0.907 | 0.365 |
| Tufted Duck | −0.055 | 0.0393 | −0.133 | 0.022 | −1.411 | 0.158 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | 0.030 | 0.036 | −0.040 | 0.100 | 0.841 | 0.400 |
| Gadwall | 0.004 | 0.037 | −0.068 | 0.077 | 0.120 | 0.904 |
| Common Goldeneye | 0.015 | 0.046 | −0.075 | 0.104 | 0.320 | 0.749 |
| Mallard | 0.080 | 0.036 | 0.010 | 0.151 | 2.228 | 0.026 |
| Red-crested Pochard | 0.071 | 0.044 | −0.016 | 0.157 | 1.603 | 0.109 |
| Tufted Duck | 0.019 | 0.037 | −0.052 | 0.091 | 0.532 | 0.595 |
The importance of monitoring data on ASR in breeding populations to inform decision-making in the management and conservation policies of declining species has been widely acknowledged for decades (Sheldon, 1998). However, obtaining such valuable data is a challenging task, requiring both a long-term monitoring scheme (Clausen et al., 2013; Frew et al., 2018; Pöysä et al., 2019) and a large amount of resources with detailed knowledge of the study population to properly conduct censuses at optimal times (Čehovská et al., 2019; Musil et al., 2023).
In this study, we analysed a unique long-term (2004–2022) dataset on the proportion of males and females in six abundant duck species in intensively managed man-made wetlands (fishponds), which represent the most important breeding habitat in Czechia, where there are no other wetland habitats suitable for the breeding of waterbirds (Musil et al., 2001, 2023).
We found evidence of an overall long-term decline in the proportion of breeding females in two of the six species included in the analysis: Common Pochard and Red-crested Pochard. Such male-biased populations in Common Pochard have been previously shown in other regions (Brides et al., 2017; Frew et al., 2018; Wood et al., 2021), and our estimates (from 60 to 65% of males in the early 2000s to 75–80% in the early 2020s) draw a similar alarming picture as previously reported. Brides et al. (2017) shows changes in the winter-time proportions of males of Common Pochard in 13 European countries between 0.617 (0.614–0.620) in 1989–1990 and 0.707 (0.705–0.710) in 2016. Also, Frew et al. (2018) and Wood et al. (2021) found increasing male bias among adult Common Pochard populations in the UK. However, Pöysä et al. (2019) did not find any change in the ASR for Common Pochard breeding in Finland when comparing data from the 1950s–1970s and the 1995–2015 periods. Furthermore, the male-biased ASR and its long-term changes in winter populations of Common Pochard are one of the longest-known in the Western Paleartic (Carbone and Owen, 1995; Brides et al., 2017; Frew et al., 2018; Wood et al., 2021). Besides the known issue with the Common Pochard, we also show, for the first time, empirical evidence of the long-term change in the ASR in Red-crested Pochard. Although both species show an increasing trend in the total number of adults in the study area, the declining trends in the proportion of females in Common Pochard and Red-crested Pochard are driven by the long-term increase in the number of males and the stable numbers of females in the past two decades. This trend reveals a crucial fact: the real population status cannot be assessed only by looking at trends in the total number of adults. We must consider males and females separately to uncover possible issues for the viability of populations.
The long-term decrease in the proportion of females could be critical even in the context of increasing the total number of adults in a population. We can expect several issues linked to the higher pressure of males on females during the mating period, causing higher stress levels in females (Reale et al., 1996; Marchesan, 2002; Le Galliard et al., 2005) or changes in population genetic diversity due to less genes being spread through the breeding population (Hughes et al., 2008; Chiba et al., 2023).
NAO-driven changes were observed only in the proportion of females (ASR) of Tufted Duck. The proportion of females of Tufted Duck during the breeding season decreased (i.e., the ASR was more male-biased) with an increasing NAO index in the preceding winter. However, unlike in Finland (Pöysä et al., 2019), we did not find significant trends in either females or males in our study region and no effect of winter NAO on either number of males or females. Pöysä et al. (2019) also did not find evidence of changes in the proportion of Tufted Duck females linked to harsh winters that would contribute to the decline in the proportion of females. In general, males try to winter at the northern edge of the wintering distribution, closer to the breeding grounds, whereas females migrate further south (Ridgill and Fox, 1990; Newton, 1998; Lehikoinen et al., 2013) as males have a larger body mass than females and are less sensitive to lower temperatures (Gunnarsson et al., 2012). This behaviour exposes males to harsher winter conditions and the vagaries of harsh cold spells compared to the weather conditions experienced by females further down the flyway. The abovementioned lower sensitivity of males to harsh winter weather conditions compared to females, coupled with the known within-winter movements observed in many species as a response to cold spells, suggests that the increase in the proportion of females after harsh winters in our study region may not be related to a higher mortality of males wintering at the northern edge of the wintering range. Instead, low (negative) NAO index values may increase the survival of females wintering at more southern parts of the wintering range, where low (negative) NAO index values denote wetter winter weather conditions and hence better conditions in wetland habitats around the Mediterranean Basin and SW Europe compared to winters with higher (positive) NAO index values, which are characterised by severe droughts (López-Moreno et al., 2011). Another possible (non-exclusive) explanation may be that during cold winters (low NAO), more males migrate further away from their breeding grounds in our study area, where they have a higher probability to pair with females from more diverse origins than when they winter closer to the breeding grounds. Due to the high site-fidelity known in duck females, our local males may follow these 'non-local' females outside our study region.
The NAO analysis for females and males only showed a positive relationship of NAO and the number of male and female Mallards. The number of both males and females increased with increasing NAO index values. Interestingly, although we found a negative relationship between NAO and the ASR in the Tufted Duck, we did not detect any evidence of the relationship between NAO and the number of females or males in this species.
However, there are other possible explanations for increasing male bias in populations. One of the most probable causes of changes in ASR is higher predation pressure on females, especially during incubation (Owen and Black, 1990; Donald, 2007; Pöysä et al., 2019). A possible increase in the predation rate of females could be linked to the appearance of alien predators such as the Raccoon Dog (Nyctereutes procyonoidesor) and American Mink (Neovison vison) in Europe (Brzeziński et al., 2020; Pöysä et al., 2023). Moreover, our unpublished data (Homolková et al., unpublished data) on predator presence at duck nests indicate a higher number of Raccoon (Procyon lotor) compared to American Mink, with no observations of the Raccoon Dog in our study area. Nevertheless, the Raccoon is a potentially dangerous alien predator with increasing population trends and range expansion in central Europe (Salgado, 2018; Stope, 2023).
According to our findings, we must point out the importance of estimating the adult sex ratio because the total number of adult individuals does not indicate the real population status in waterfowl (Frew et al., 2018). Although total population size indicates the state of danger for population viability, we here provide evidence of hidden threats, such as skewed sex ratios. In the case of Common Pochard and Red-crested Pochard, the total breeding population size (i.e., numbers of adults at the beginning of the breeding season) in the study area has been increasing for the past two decades while the number of females has remained stable. Our findings suggest that winter weather conditions are not an important driver of the observed changes in the ASR of the species considered here, except perhaps in the case of the Tufted Duck.
It still remains unknown in which life stage this change in the proportion of males and females occurs. Hence, we highlight the imperative need for extensive monitoring to record male and female counts for an appropriate determination of the actual population structure. As we have shown here, estimating ASR in local populations is of paramount importance for a complete assessment of population viability.
Monika Homolková: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Petr Musil: Writing – review & editing, Visualization, Validation, Supervision, Resources, Methodology, Investigation, Data curation, Conceptualization. Diego Pavón-Jordán: Writing – review & editing, Visualization, Validation, Software, Investigation, Formal analysis. Dorota Gajdošová: Conceptualization, Data curation, Formal analysis, Visualization, Writing – review & editing. Zuzana Musilová: Writing – review & editing, Project administration, Funding acquisition. Šárka Neužilová: Writing – review & editing. Jan Zouhar: Visualization, Software, Methodology, Formal analysis.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are very grateful to all co-workers involved in the fieldwork in South Bohemia in 2004–2022. Among many others, we thank Markéta Čehovská, Milan Haas, Tereza Kejzlarová, Blanka Kuklíková, Anna Langrová, Michaela Nachtigalová, Klára Poláková and Adéla Šenkýřová for their help in the field. We are also grateful to Steve Ridgill for language improvements. Our thanks also go to reviewers, whose comments greatly improved previous versions of the manuscript.
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.011 | 0.010 | −0.031 | 0.009 | −1.102 | 0.270 |
| Gadwall | 0.0170 | 0.010 | −0.003 | 0.037 | 1.663 | 0.096 |
| Common Goldeneye | 0.0452 | 0.013 | 0.019 | 0.071 | 3.416 | 0.001 |
| Mallard | 0.0200 | 0.010 | 0.001 | 0.039 | 2.035 | 0.042 |
| Red-crested Pochard | 0.0170 | 0.014 | −0.010 | 0.043 | 1.224 | 0.221 |
| Tufted Duck | 0.0040 | 0.010 | −0.016 | 0.023 | 0.355 | 0.7227 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | 0.040 | 0.009 | 0.022 | 0.0584 | 4.336 | < 0.001 |
| Gadwall | 0.029 | 0.010 | 0.010 | 0.048 | 2.994 | 0.003 |
| Common Goldeneye | 0.034 | 0.012 | 0.0101 | 0.058 | 2.780 | 0.005 |
| Mallard | 0.029 | 0.009 | 0.011 | 0.047 | 3.110 | 0.002 |
| Red-crested Pochard | 0.071 | 0.012 | 0.048 | 0.093 | 6.069 | < 0.001 |
| Tufted Duck | 0.002 | 0.009 | −0.017 | 0.0202 | 0.176 | 0.860 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.050 | 0.008 | −0.066 | −0.034 | −6.205 | < 0.001 |
| Gadwall | −0.012 | 0.009 | −0.029 | 0.006 | −1.316 | 0.188 |
| Common Goldeneye | 0.008 | 0.014 | −0.019 | 0.035 | 0.602 | 0.547 |
| Mallard | −0.008 | 0.008 | −0.024 | 0.007 | −1.084 | 0.278 |
| Red-crested Pochard | −0.053 | 0.014 | −0.080 | −0.026 | −3.807 | < 0.001 |
| Tufted Duck | 0.001 | 0.008 | −0.015 | 0.017 | 0.101 | 0.919 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.044 | 0.029 | −0.101 | 0.014 | −1.488 | 0.137 |
| Gadwall | 0.043 | 0.030 | −0.016 | 0.102 | 1.428 | 0.153 |
| Common Goldeneye | −0.051 | 0.048 | −0.146 | 0.043 | −1.061 | 0.289 |
| Mallard | 0.010 | 0.029 | −0.047 | 0.066 | 0.339 | 0.734 |
| Red-crested Pochard | −0.046 | 0.050 | −0.144 | 0.052 | −0.926 | 0.355 |
| Tufted Duck | −0.082 | 0.029 | −0.139 | −0.025 | −2.833 | 0.005 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | −0.006 | 0.039 | −0.083 | 0.072 | −0.143 | 0.887 |
| Gadwall | 0.047 | 0.040 | −0.030 | 0.125 | 1.193 | 0.233 |
| Common Goldeneye | −0.040 | 0.049 | −0.136 | 0.056 | −0.816 | 0.415 |
| Mallard | 0.087 | 0.039 | 0.010 | 0.163 | 2.213 | 0.027 |
| Red-crested Pochard | 0.047 | 0.052 | −0.055 | 0.149 | 0.907 | 0.365 |
| Tufted Duck | −0.055 | 0.0393 | −0.133 | 0.022 | −1.411 | 0.158 |
| Species | Trend estimate | SE | Lower confidence limit | Upper confidence limit | Z | P |
| Common Pochard | 0.030 | 0.036 | −0.040 | 0.100 | 0.841 | 0.400 |
| Gadwall | 0.004 | 0.037 | −0.068 | 0.077 | 0.120 | 0.904 |
| Common Goldeneye | 0.015 | 0.046 | −0.075 | 0.104 | 0.320 | 0.749 |
| Mallard | 0.080 | 0.036 | 0.010 | 0.151 | 2.228 | 0.026 |
| Red-crested Pochard | 0.071 | 0.044 | −0.016 | 0.157 | 1.603 | 0.109 |
| Tufted Duck | 0.019 | 0.037 | −0.052 | 0.091 | 0.532 | 0.595 |