| Citation: | Lorena Cruz-Bernate, Camilo Espinosa-Bravo, Héctor Fabio Rivera-Gutiérrez. 2023: Does cryptic dichromatism exist in the Saffron Finch (Sicalis flaveola)? Colorimetric variables and the avian visual model. Avian Research, 14(1): 100127. DOI: 10.1016/j.avrs.2023.100127 |
Sexual dichromatism, a particular type of sexual dimorphism, occurs in several species and has been associated with sexual selection. In some cases, the differences are so small that they are imperceptible to humans, but possibly detected by birds. The objective measurement of color with spectrophotometers and detailed analyses according to the perception ability of the avian eye have revealed that some species that were once considered to be monochromatic, are in fact dichromatic and able to perceive these differences. In the tropics, the Saffron Finch (Sicalis flaveola) does not present marked sexual dimorphism in coloration, which makes studies in behavioral ecology, natural history and population dynamics difficult. To assess whether there is dichromatism in the species, the reflectance (between 300 and 700 nm) of ten body regions was measured in 196 wild adults in Cali-Colombia. Sex was determined using the CHD1 gene on the sex chromosomes. Reflectance spectra were analyzed using: colorimetric variables and the avian visual model. We found that reflectance shows a bimodal curve in all body regions, except the crown. Males presented higher reflectance at long wavelengths, while for females this occurred in ultraviolet wavelengths. For the visual model, we found that there are significant intrasexual differences; however only in crown coloration is there a possible perceived difference between sexes. We conclude that in the Saffron Finch there are color differences between the sexes in all regions considering the physical phenomenon (reflectance), but in general, when evaluating color perception (avian visual model), there are no differences between the sexes in most of the body regions. The intrasexual differences are significant, indicating the possibility of these being signals that influence social interactions in the species.
Dichromatism, differences in the expression of color between the sexes, presents a pattern similar to that of dimorphism, in which sexual selection is the main selective pressure that modulates manifestation (Darwin, 1871). The classical view has attributed unidirectional sexual selection pressures only upon males, where ornaments develop as a signal indicating health and mate quality (Hill and McGraw, 2006). Whilst in the case of females, cryptic color is related to the adaptive value that they have in the reproductive stage (Martin and Badyaev, 1996). However, in several species color expression is similar between the sexes and it has been proposed that female plumage may also be subject to the same sexual selection pressures as males (Amundsen, 2000).
In different mating systems of birds, it can be seen how the pressure of sexual selection has shaped different degrees of dichromatism in species. The most marked differences are found in species that exhibit polygyny and "leks" (Webster, 1992; Székely et al., 2000), while the smallest differences tend to be in monogamous species (Delestrade, 2001; Radford and Du Plessis, 2004). Several species, which based on their life history and reproductive biology would appear to be monochromatic, are now recognized as dichromatic (Tubaro et al., 2005; Hofmann et al., 2007; Bridge et al., 2008; Masello et al., 2009). Given the differences in the two visual systems, humans are unable to discriminate the same color differences that birds perceive (Cuthill et al., 2000; Håstad and Ödeen, 2008; Bergeron and Fuller, 2018) and thus some species that are truly dichromatic, have been misclassified as monochromatic (Eaton, 2005, 2007).
Tanagers (family Thraupidae) typically have a monogamous mating system (Skutch and Gardner, 1989), however, genetic studies in six species have reported a percentage of extra-pair paternity of 15–63% in nests and 8–50% in nestlings (Sánchez et al., 2018). Tanagers are noted for the great diversity of color in their plumage (Brush, 1990). This family includes species that vary from opaque monochromatic to different degrees of dichromatism (Burns, 1998). Their colors are mainly produced by carotenoids, melanins, structural color and/or a combination of both (Burns and Shultz, 2012; Mason et al., 2014; Barreira et al., 2016; Price-Waldman et al., 2020).
In the particular case of carotenoids—which are not synthesized by the body—they must be acquired based on dietary intake (Hill, 2000; McGraw, 2006); implying effort in the search for this resource. Given the energetic cost of displaying these colors (Hill, 2000; Møller et al., 2000; McGraw et al., 2005) and the intraspecific color differences that occur (Hill, 2000; Siefferman and Hill, 2003), the classical perspective of the resource trade-off hypothesis considers the expression of carotenoid-based coloration to be a sign of health condition and individual quality (Hill, 2000; McGraw and Ardia, 2004; Hill and McGraw, 2006). More recent studies have shown this may not be the case (Koch et al., 2019) and as an alternative, the Shared-Pathway Hypothesis has been proposed, which states that the color would reflect the metabolic rate, since carotenoid ketolation and cell respiration could share biochemical pathways (Hill, 2011; Powers and Hill, 2021). These honest signals may be subject to the pressures of social selection and sexual selection that over time, could exert a differential effect in color between the sexes (Irwin, 1994; Burns, 1998; Dale et al., 2015; McCoy et al., 2021). In tanagers, it is believed that the evolution of sexual dichromatism does not correspond to the classic vision of sexual selection processes that fall only on males (Burns, 1998), but rather is a more complex process that can include various types of selection pressures on individuals (Shultz and Burns, 2017).
In the case of the Saffron Finch (Sicalis flaveola, Thraupidae), plumage coloration is carotenoid-based, it is a social species and is observed in large groups made up of adults and juveniles that feed in open areas (Marcondes-Machado, 1982, 1997). The Saffron Finch has a socially monogamous reproductive system (Burnham and Cruz-Bernate, 2020), but extra-pair paternity has been reported in 31.8% of nestlings and 51.8% of nests in a population from Argentina (Benítez Saldivar et al., 2019). Unlike subspecies distributed in southern South America, those from the tropics (flaveola group) do not show marked sexual dimorphism in the coloration of their plumage, nor do they show seasonal plumage changes throughout the year. Both sexes have an orange crown and throat, and there is a general yellow coloration with olive tones on the back, wings and tail (Hilty and Brown, 2001). Although it has been reported from human observations that the female is duller than the male in crown color (Hilty and Brown, 2001; Rising et al., 2011), it is unknown if there are quantitative colors differences between sexes of the Saffron Finch in the tropics, and if so, whether these differences are used for recognition between individuals. Most researches addressing this issue has focused on species from North America and Europe (Hill, 1990; McGraw and Hill, 2000a, 2000b; Delhey, 2005) with few studies on species from tropical areas (Tubaro et al., 2005; Bridge et al., 2008; Masello et al., 2009; Diniz et al., 2016). Due to the fact that an objective analysis of color and its possible ecological implications in terms of life history has not been carried out in the flaveola group, our objective was to determine, for the first time in the north of South America, whether there are in fact differences in plumage color between individuals and if these differences allow them to discriminate by sex among their congeners. The analysis and comparison of plumage colorimetric characteristics between body regions is also presented, in order to determine how this sexual characteristic is expressed within the species. This research contributes to the understanding of the evolution of cryptic sexual dichromatism in tropical birds.
The research was carried out between January 2017 and December 2019 in two areas of the department of Valle del Cauca, Colombia, South America, located in the municipalities of Santiago de Cali and Jamundí. The first area, the campus of Universidad de Valle (3°22′ N, 76°32′ W, 970 m), is classified as a tropical dry forest (bs-T) (Espinal, 1967), with average annual temperature of 24.1 ℃ and average annual rainfall of 1096 mm (IDEAM, Institute of Hydrology http://dhime.ideam.gov.co/atencionciudadano/ May 14, 2021). The second zone corresponding to the urban area of the municipality of Jamundí (3°15′ N, 76°33′ W, 998 m) is classified as Tropical Humid Forest (bh-T) (Espinal, 1967), with average annual temperature of 24.8 ℃ and average annual rainfall of 2152 mm (La Independencia Meteorological Station, CVC, years 2017–2018).
To capture individuals, the following were used: a) mist nets, b) baited traps and c) nest boxes. a) Mist nets: ten mist nets (12 m × 2.5 m and 30 mm mesh) were periodically installed between 07:00–15:00 on the campus of Universidad del Valle and between 17:00–19:00 in Jamundí. b) Baited traps: were cube-shaped (height 50 cm, width 60 cm and depth 80 cm), without a floor, with a mobile door and walls of translucent material, which allowed for observation of the food placed inside them. c) Nest boxes: 235 nest boxes were installed in the university campus in order to provide breeding sites for the species. Adults were captured by installing a mobile door at the entrance of the nest box, which was activated and closed remotely once the individual entered to feed the nestlings. All captured adults were measured, weighed and ringed for individual recognition with three colored rings and one metallic ring. The individuals were released at the same capture sites.
Molecular sex determination was carried out and a 10 μL blood sample was taken from the brachial vein and preserved in Quenn lysis buffer (Seutin et al., 1991 https://www2.unbc.ca/genetics/protocols-recipes#queen), following the molecular method proposed by Ellegren (1996) and implemented in the species by Espinosa et al. (2017) consisting of the amplification of an intron of the CDH1 gene (chromo-helicase domain) located on the sex chromosomes of birds (W, Z). For the DNA polymerase chain reaction (PCR) the primers 2550F–2718R (Fridolfsson and Ellegren, 1999) were used and PCR products were separated and visualized in 3% agarosa gels.
The reflectance of individuals that presented adult plumage in ten body regions was measured: crown, throat, breast, belly, thigh, mandible: lower mandible, coverts, nape, mantle and rump. For each region, five different points were selected, except in thigh, throat and mandible, where three were taken (Fig. 1), and averaged them to produce a single spectrum for the region. Plumage reflectance was measured between 300 and 700 nm with a Flame spectrophotometer (Ocean Optics) of deuterium-tungsten halogen light (DH-2000-BAL) in the ultraviolet (300–400 nm) and visible (400–700 nm) wavelengths (Cuthill et al., 2000; Eaton, 2005) at 1 nm intervals. Measurements were taken perpendicularly (90° angle) and at a fixed distance of 6 mm from the surface. To keep this distance constant, a black hard cover fitted to the end of the spectrometer's fiber optic probe was used (Delhey, 2005). The reflectance measurements were obtained relative to a white standard (WS-2) and to dark which were measured before each individual. In order to avoid possible ambient light interference during the measurements, the birds captured with adult plumage were taken to a dark room located in the Ornithology and Animal Behavior Laboratory-OYCA.
Reflectance spectra were generated for each body region using OceanView (version 1.6.7) Software (Ocean Optics). They were processed and analyzed with pavo package (Maia et al., 2013, 2019) in R software version 4.0.5 (R Core Team, 2021). The two spectral data analysis approaches used were: a) analysis based on the shape of the reflectance spectrum (physical phenomenon of light reflectance), from which the colorimetric variables are obtained; b) analysis that take into account the avian visual system of the organism that receives the color signal (Maia et al., 2019).
A total of 23 colorimetric variables (Montgomerie, 2006) were calculated for each body region per individual (Maia et al., 2013, 2019). Due to the large number and varied sets of colorimetric variable combinations used in the literature (Andersson, 1999; Keyser and Hill, 2000; Saks et al., 2003; Andersson and Prager, 2006; Montgomerie, 2006; Butler et al., 2011), it was decided to design a variable selection procedure.
This procedure was carried out taking into account aspects such as: a) how informative it was, b) redundancy and c) academic recommendation and frequency of use in the literature (Andersson and Prager, 2006; Montgomerie, 2006). To determine: a) which variables were informative and therefore had a significant effect in explaining plumage coloration, the partial least squares regression for generalized linear models (PLS-Rgml) method with cross-validation and resampling was used (1000 iterations) (Bertrand et al., 2014). For the number of significant predictors within each component, the Bastien et al. (2005) and Bastien and Tenenhaus (2001) were used. Confidence intervals (CI) were generated for each colorimetric variables (predictors) which included the probabilities obtained with resampling and the variables that had the greatest weight, according to the interval and the weight in each component, were chosen (Appendix Fig. S1); b) Redundancy: Those variables with a value equal to or greater than 0.8 in the correlation were considered redundant, so only one of them was chosen for the analysis (de Smith, 2018). The Pearson or Spearman correlation was used depending on whether the assumption of normality was met (de Smith, 2018); c) Academic recommendation, the advantages and disadvantages of using the available variables were consulted and the scientific recommendations were accepted (Andersson and Prager, 2006; Montgomerie, 2006). For example, we included colorimetric variables in the ultraviolet range (320–400 nm) because the coloration is based on carotenoids (MacDougall and Montgomerie, 2003; Hill and McGraw, 2004; Mays et al., 2004; Bleiweiss, 2005).
The PLS-Rgml selected colorimetric variables were: Brightness (B2), calculated as the average reflectance in all the wavelengths measured. UV Brightness (UV–B3), maximum reflectance at the UV Peak (320–400 nm). Saturation (S7), calculated as the difference between the relative reflectance before and after the wavelength at which reflectance is halfway between its minimum and its maximum. Carotenoid chroma (S9), ratio of reflectance between 450 and 700 nm in relation to total reflectance. UV Chroma (UV–S1), proportion of the total reflectance between 320 and 400 nm and the total reflectance. Hue (H3), wavelength at the midpoint of the maximum and minimum reflectance of the reflectance spectrum. UV Hue (UV–H1), corresponds to the wavelength at the maximum value of the UV Peak (Montgomerie, 2006).
To determine how robust the metrics chosen in the PLS-Rgml are, repeatability was estimated with the rptR package (Stoffel et al., 2017). On average, the repeatability was: B2 (R = 0.58 ± 0.18), UV-B3 (R = 0.54 ± 0.19), UV-H1 (R = 0.71 ± 0.11), S7 (R = 0.29 ± 0.09), S9 (R = 0.47 ± 0.19), UV-S1 (R = 0.56 ± 0.17) and H3 (R = 0.36 ± 0.13). Variables S7 and H3 were discarded due to their low repeatability. When evaluating the redundancy between the variables, it was found that two variables presented a high correlation with UV-B3: B2 (r = 0.81 ± 0.1) and UV-S1 (r = 0.77 ± 0.1). UV-B3 was discarded to avoid redundancy.
Considering the above, three colorimetric variables were chosen: Brightness (B2) as the achromatic variable, UV Hue (UV–H1) for the UV region due to its robustness (R = 0.71) in the measurement, and carotenoid chroma (S9) for being a chromatic variable associated with long wavelengths which reflects the carotenoid content of feathers (Andersson and Prager, 2006). These colorimetric variables are similar to those used by Benítez Saldivar and Massoni (2018) in a previous study with the species.
For the visual model of the Saffron Finch and given that there was no physiological information about its visual system, the psychophysical models of color vision of the Blue Tit (Cyanistes caeruleus) were considered as the reference parameter (Hart, 2001). This species was chosen because it is phylogenetically in the same order as the Saffron Finch (Paseriformes) (Clements et al., 2021). The characteristics of the photoreceptors show little variation in passeriformes, including sensitivity in the ultraviolet range (UVS) (Ödeen and Håstad, 2003; Eaton, 2005; Cuthill, 2006; Casalía et al., 2020). In addition, this species is found in open habitats; an ecological characteristic shared by the Saffron Finch. Furthermore, the Blue Tit visual system has already been applied for various passerines (Eaton, 2005), including the Saffron Finch, albeit in the southern temperate zone corresponding to the subspecies S. f. pelzeni (Benítez Saldivar and Masoni, 2018).
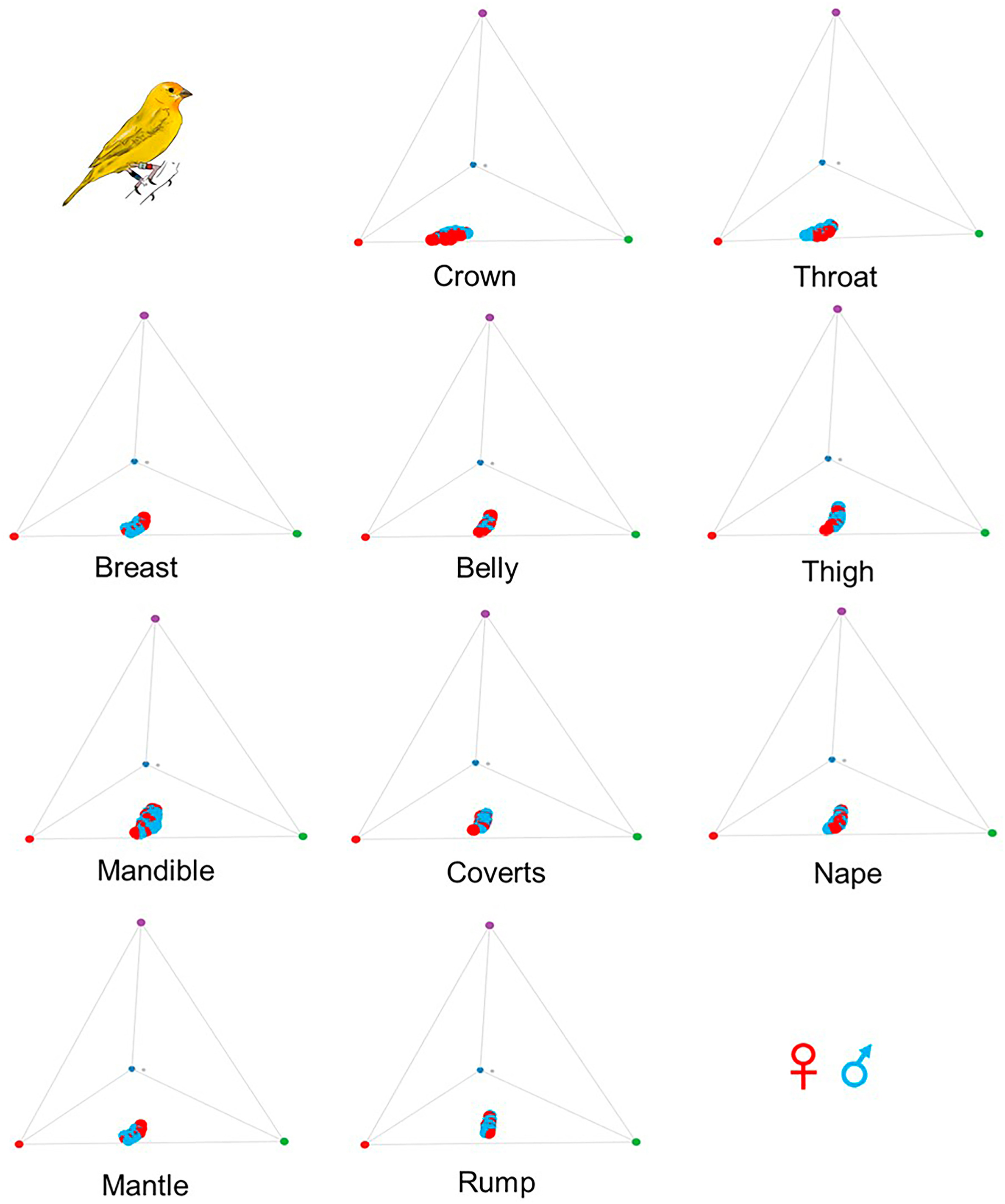
The visual model was calculated with the pavo package (Maia et al., 2019) and had the following specifications: a) cones that are sensitive to very short (VS), short (S), medium (M) and long (L) wavelengths (Eaton, 2005), the wavelengths for the values of greatest sensitivity in each of the photoreceptors were 371, 448, 502 and 563 nm (Hart et al., 2000; Hart and Vorobyev, 2005); b) relative photoreceptor densities (u = 1, s = 1.9, m = 2.68, l = 2.7); c) Weber fraction of 0.1 for the long-wavelength sensitive photoreceptor; d) Illuminant conditions for open habitats = D65; and e) Transmission of the Ocular Media = 0.5 to 1.0 gradually from 317 to 700 nm (Hart et al., 2000). The function vismodel calculates the amount of "quantums" captured by each type of pigment in the photoreceptors in the avian retina. A plot of each region was made in tetrahedral color space.
With the estimation of receptor stimulation, the chromatic distances intra and intersexual with noise based on relative photoreceptor densities con la function coldist (Maia et al., 2019). Chromatic distances (ΔS) have "Just Noticeable Differences" (JNDs) as measurement units and distances greater than 1 JND between spectra are theoretically considered to be discernible (Maia et al., 2019).
To establish whether the species could discriminate color differences in the different body regions between males and females, the procedure outlined by Maia and White (2018) was followed (see 2.6. Statistical analysis).
The Mahalanobis distance was used to identify multivariate outliers (Tabachnick and Fidell, 2001; Montgomerie, 2006), both for the analysis of the Colorimetric variables (23 variables) and for the analysis of chromatic coordinates of the visual model.
Tests of the mean differences between the sexes were carried out (t-test, Welch-t, Wilcoxon Mann–Whitney or Randomization test), according to compliance with the assumptions of normality and homogeneity of variance. For the evaluation of normality, the Anderson–Darling test (de Smith, 2018) was used and for homogeneity of variance, the F-test or Levene's test (de Smith, 2018) were used.
For the comparisons of chromatic distances between sexes, the Permutational multivariate analysis of variance (PERMANOVA) was used (Maia and White, 2018). PERMANOVA was performed with the adonis function in the R package vegan and configured with 999 permutations (Anderson, 2005). Prior to PERMANOVA, the assumption of homogeneity of variance of the chromatic distances was validated with the betadisper function in the R package vegan (Anderson et al., 2006). The dispersion of the groups was also examined when the assumption of homogeneity was not fulfilled, which was corroborated with the Tukey test (de Smith, 2018). To determine if the differences in the chromatic distances between the sexes was discriminated by the birds, a Bootstrap based on 1000 runs was performed with the bootcoldist function to estimate the confidence interval of chromatic distances (ΔS) between the centroids of color samples in multivariate space (Maia and White, 2018). To determine if the differences in chromatic distances between the sexes was above the discrimination threshold. For this study, a value of ≥1 JND was established as the reference threshold (Kelber et al., 2003; Siddiqi et al., 2004; Olsson et al., 2015), due to the ecological and light conditions of the habitats occupied by the species in the tropical region and for being a widely adopted threshold (Amat et al., 2018; Maia and White, 2018; Demko et al., 2020; Aguilar et al., 2022).
For all statistical analyses, the R version 4.0.5 software (R Core Team, 2021) was used, and probabilities less than 0.05 were considered statistically significant results.
The mean and standard deviation of the reflectance spectra (n = 8624) of 196 adult individuals (87 females and 109 males) are shown for ten body regions in the Saffron Finch (Fig. 2). The reflectance spectrum had a bimodal behavior, with a higher peak between 600 and 700 nm and a lower one between 320 and 400 nm in all body regions, except the crown.
Males and females presented differences in all body regions in at least one of the three colorimetric variables evaluated, statistically significant differences were observed (Table 1). A differential pattern was observed between the sexes for the UV region and the visible region. The UV hue of females was significantly higher than that of males in all body regions. Overall, males showed higher values for the colorimetric variables in visible wavelengths (Brightness and Carotenoid chroma) (Table 1).
| Colorimetric variables by body region | Females (Mean ± SD) | Males (Mean ± SD) | Test‡ | Estimate Std | df(t) | P-value | |||||||
| Crown | n = 79 | n = 99 | |||||||||||
| Brightness | B2 | 14.388 ± 4.818 | 14.603 ± 3.949 | t | −0.327 | 176 | 0.744 | ||||||
| Carotenoid chroma | S9 | 0.928 ± 0.019 | 0.915 ± 0.013 | W | 0 | 0.062 | |||||||
| UV Hue | UV-H1 | 333.899 ± 11.453 | 327.485 ± 10.291 | W | 2576 | 0.000† | |||||||
| Throat | n = 76 | n = 99 | |||||||||||
| Brightness | B2 | 18.668 ± 5.463 | 16.829 ± 5.856 | t | 2.119 | 173 | 0.035† | ||||||
| Carotenoid chroma | S9 | 0.951 ± 0.014 | 0.945 ± 0.018 | t(R) | 2.257 | 0.024† | |||||||
| UV Hue | UV-H1 | 338.789 ± 5.646 | 334.535 ± 6.200 | t | 4.675 | 173 | 0.000† | ||||||
| Breast | n = 77 | n = 99 | |||||||||||
| Brightness | B2 | 22.984 ± 3.499 | 24.527 ± 3.681 | W | 30.976 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.915 ± 0.037 | 0.926 ± 0.024 | W | 13.552 | 0.039† | |||||||
| UV Hue | UV-H1 | 346.468 ± 4.624 | 343.121 ± 6.207 | t(R) | 4.097 | 0.000† | |||||||
| Belly | n = 75 | n = 97 | |||||||||||
| Brightness | B2 | 30.589 ± 5.137 | 33.309 ± 3.991 | W | 29.584 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.888 ± 0.052 | 0.894 ± 0.043 | W | 12.900 | 0.037† | |||||||
| UV Hue | UV-H1 | 349.48 ± 2.617 | 346.453 ± 4.890 | t(R) | 52.066 | 0.000† | |||||||
| Thigh | n = 59 | n = 78 | |||||||||||
| Brightness | B2 | 30.051 ± 7.742 | 31.933 ± 7.555 | W | 18.769 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.816 ± 0.087 | 0.836 ± 0.077 | W | 8.083 | 0.044† | |||||||
| UV Hue | UV-H1 | 348.237 ± 4.84 | 344.961 ± 7.014 | t(R) | 3.231 | 0.001† | |||||||
| Mandible | n = 60 | n = 78 | |||||||||||
| Brightness | B2 | 20.305 ± 11.561 | 22.376 ± 10.357 | W | 19, 044 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.741 ± 0.145 | 0.757 ± 0.114 | t | −0.757 | 136 | 0.450 | ||||||
| UV Hue | UV-H1 | 355.833 ± 10.44 | 349.67 ± 12.977 | W | 1609 | 0.002† | |||||||
| Coverts | n = 74 | n = 97 | |||||||||||
| Brightness | B2 | 15.405 ± 2.986 | 16.447 ± 2.88 | W | 29, 241 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.799 ± 0.051 | 0.822 ± 0.034 | t(R) | −3.426 | 0.029† | |||||||
| UV Hue | UV-H1 | 344.838 ± 6.182 | 341.927 ± 6.997 | t(R) | 28.795 | 0.004† | |||||||
| Nape | n = 73 | n = 99 | |||||||||||
| Brightness | B2 | 13.28 ± 2.075 | 14.507 ± 2.69 | t(R) | −3.376 | 0.001† | |||||||
| Carotenoid chroma | S9 | 0.874 ± 0.046 | 0.886 ± 0.036 | W | 12, 556 | 0.014† | |||||||
| UV Hue | UV-H1 | 345.685 ± 4.079 | 341.363 ± 6.302 | t(R) | 5.448 | 0.000† | |||||||
| Mantle | n = 62 | n = 80 | |||||||||||
| Brightness | B2 | 11.966 ± 1.452 | 12.461 ± 1.499 | t | −1.98 | 140 | 0.050 | ||||||
| Carotenoid chroma | S9 | 0.79 ± 0.061 | 0.822 ± 0.049 | W | 8804 | 0.061 | |||||||
| UV Hue | UV-H1 | 346.677 ± 3.749 | 343.787 ± 5.013 | t(R) | 3.929 | 0.000† | |||||||
| Rump | n = 69 | n = 91 | |||||||||||
| Brightness | B2 | 15.56 ± 2.481 | 16.356 ± 2.201 | t | −2.146 | 158 | 0.033† | ||||||
| Carotenoid chroma | S9 | 0.791 ± 0.082 | 0.811 ± 0.063 | W | 11, 040 | 0.031† | |||||||
| UV Hue | UV-H1 | 347.391 ± 4.535 | 345.087 ± 6.214 | t(R) | 2.71 | 0.006† | |||||||
| † Significant differences. ‡ Statistic test: t = t-Student; t(W) = t-Welch; t(R) = Randomization test; W = Wilcoxon rank. | |||||||||||||
In the visual model, individuals are located between long and medium wavelengths. In all body regions, overlapping of individuals by sex is observed, and a sectorized and adjacent distribution is evident only in the crown (Fig. 3).
The chromatic distances (ΔS) calculated from the visual model, both intra- and intersexually, were above the threshold of perception (ΔS values ≥ 1 JND) in all body regions (Table 2). The mandible, thigh, throat and nape were the body regions with the greatest chromatic distances (Table 2). The chromatic distances between males and females are statistically significant in five body regions: crown, breast, coverts, nape and mantle (Table 3).
| Body region | Females | Males | Females vs. males | |||||
| Mean ± SD | Min–max | Mean ± SD | Min–max | Mean ± SD | Min–max | |||
| Crown | 3.077 ± 2.978 | 0.041–14.518 | 1.848 ± 1.184 | 0.089–10.409 | 2.804 ± 2.630 | 0.129–15.745 | ||
| Throat | 2.95 ± 2.063 | 0.048–14.567 | 3.436 ± 2.215 | 0.110–12.74 | 3.258 ± 2.111 | 0.106–15.903 | ||
| Breast | 2.808 ± 1.945 | 0.085–12.002 | 2.633 ± 1.837 | 0.043–13.349 | 2.799 ± 2.001 | 0.059–15.840 | ||
| Belly | 3.03 ± 2.425 | 0.086–15.786 | 2.688 ± 1.826 | 0.053–9.923 | 2.884 ± 2.127 | 0.036–14.964 | ||
| Thigh | 3.65 ± 2.822 | 0.127–17.132 | 3.264 ± 2.27 | 0.069–13.054 | 3.468 ± 2.517 | 0.001–18.002 | ||
| Mandible | 4.892 ± 3.663 | 0.044–18.967 | 4.265 ± 3.197 | 0.054–17.924 | 4.566 ± 3.414 | 0.038–18.596 | ||
| Coverts | 2.117 ± 1.455 | 0.081–10.515 | 1.761 ± 1.155 | 0.028–8.256 | 2.008 ± 1.350 | 0.054–10.574 | ||
| Nape | 2.931 ± 2.169 | 0.051–13.568 | 2.972 ± 2.147 | 0.078–13.746 | 3.027 ± 2.203 | 0.088–14.327 | ||
| Mantle | 1.979 ± 1.34 | 0.000–7.663 | 2.217 ± 1.726 | 0.018–11.555 | 2.283 ± 1.612 | 0.056–11.142 | ||
| Rump | 2.426 ± 1.696 | 0.055–10.183 | 2.373 ± 1.655 | 0.037–10.039 | 2.430 ± 1.704 | 0.038–10.561 | ||
| Body region | df | df (residuals) | df (total) | Sums Sqs | F value | P-value |
| Crown | 1 | 182 | 183 | 152.347 | 28.132 | 0.001† |
| Throat | 1 | 183 | 184 | 18.584 | 2.470 | 0.111 |
| Breast | 1 | 181 | 182 | 43.947 | 8.068 | 0.006† |
| Belly | 1 | 177 | 178 | 8.417 | 1.348 | 0.261 |
| Thigh | 1 | 143 | 144 | 3.419 | 0.377 | 0.566 |
| Mandible | 1 | 136 | 137 | 4.524 | 0.281 | 0.645 |
| Coverts | 1 | 180 | 181 | 18.193 | 6.791 | 0.008† |
| Nape | 1 | 179 | 180 | 35.639 | 5.327 | 0.024† |
| Mantle | 1 | 144 | 145 | 39.723 | 11.451 | 0.001† |
| Rump | 1 | 164 | 165 | 14.328 | 3.358 | 0.061 |
| † Significant differences. | ||||||
In general, the Saffron Finch visual model cannot discriminate sex by color, with the possible exception of the crown, with a mean value of 1.818 JND and a confidence interval of 95% (1.193–2.54 JND) (Fig. 4).
Overall, and according to the colorimetric variables, males unlike females, show greater expression of plumage color in long wavelengths (brightness and carotenoid chroma) in most body regions. The difference reported by Hilty and Brown (2001) that females are slightly opaquer than males and less orange in crown, refers to the fact that the crown of males has a more intense (saturation) color in the crown compared to females. In contrast, female plumage reflects significantly more ultraviolet wavelengths than males (UV hue) in all body regions. Females could be transmitting information about the physicochemical composition and perhaps the "honesty" of the cost of producing a particular signal (Bleissweiss, 2004). Costs may be related both directly to carotenoids and indirectly to associated changes in feather structure (Bleiweiss, 2004).
It is suggested that in the Saffron Finch there could be differences in both the structure of the feather and in the concentration of pigments between sexes, as occurs with other anatomical and physiological characteristics in birds (Wetmore, 1936; Grubb et al., 1991; Hill, 1995; Owen et al., 2005; Norte et al., 2009). Macro, micro, and nanostructural differences in feathers have been shown to influence expressed color variation between males and females of the same species (Maia et al., 2012; Hegyi et al., 2018; McCoy et al., 2021). This is the case of the Large-billed Crow (Corvus macrorhynchos), where males had a higher density of barbs, but shorter length of prongs compared to females (Lee et al., 2009a). Also, the density of melanin granules and their orderly arrangement in the barbules was another factor to which Lee et al. (2009b) attributed the difference in iridescent coloration between the sexes. It is also likely that sexual variation in color expression in the Saffron Finch is not only due to differential productions of feather structure, but also physiological capacity for carotenoid transport, fixation, and utilization (Hill et al., 1994; Hill, 1995; Figuerola and Gutiérrez, 1998; Webb, 2021). These molecules fulfill varied and important functions within organisms such as improving color discrimination, serving as a photoprotective barrier in the eye, vitamin A precursor, antioxidant activity, and enhancing the immune system (McGraw and Ardia, 2003; Hill and Johnson, 2012; Toomey et al., 2015; Toomey and Ronald, 2021).
Given that males and females normally have sex-specific carotenoid distribution priorities (Webb, 2021), due to the physiological functions associated with carotenoids, there is a need to maintain a balance with color expression (Møller et al., 2003). It is expected that in the reproductive season, females have high costs associated with their immune system and reproductive success in which carotenoids intervene (Møller et al., 2003), accordingly, carotenoids are a limiting resource during this season and could lead to differential use, depending on the conditions of the female and the environment (Hõrak et al., 2002; Møller et al., 2003; Navara et al., 2006). In the male Saffron Finch, greater saturation of the plumage can be partially explained by the role it plays in the reproductive cycle. We know that the male is the one who guards and defends the nest during incubation and emits his territorial song daily at dawn (Unpublished data, pers. obs.). The high activity of the male would be reflected in a high metabolic rate, which requires a greater amount of carotenoids, associated with the metabolic pathways of cellular respiration and the avoidance of oxidative stress (Kelly et al., 2012; Powers and Hill, 2021).
All the body regions of the Saffron Finch show important intrasexual chromatic variation. According to Delhey et al. (2017), and as expected for conspicuous ornaments based on carotenoids, the presence of large variations can be informative, and play a role as a social signal in the species. Mutual ornamentation has been explained by processes: mate choice (mutual sexual selection) and social competition over nonsexual resources (social selection) (Kraaijeveld et al., 2007). In tanagers, intraspecific competition between females has been considered as one of the factors responsible for changes in dichromatism (Burns, 1998). It has been shown both in captivity and experimentally that plumage color influences dominance interactions (Marcondes-Machado, 1997; Espinosa, 2019; Araújo-Silva, 2021; Araújo-Silva et al., 2022).
Whilst it cannot be ruled out that the coloration of the females corresponds to non-direction pressures, given that both sexes share a large part of their genome (Amundsen, 2000), the chromatic differences found in this research support the evidence that plumage color corresponds to a sexual signal, and plays a role in mate choice (Espinosa, 2019) and that in wildlife there is assortative mating by color (Data submitted for publication). On the effect of color on dominance, Araújo-Silva et al. (2022) found that Saffron Finch males and females with more conspicuous plumage coloration tended to win aggressive interactions against over individuals of the same sex. Therefore, it is suggested that the intraspecific differences in the color of the body regions may be affected by social and sexual selection pressures, and that the ornamentation of both sexes are visual signals that affect the interactions in the species.
Apart from social and sexual selection, it appears that the evolution of ornaments is strongly and differentially associated with morphological and natural history variables (Dale et al., 2015). Natural history traits, such as seasonality, small clutch size, and low latitudes, have been linked to the plumage similarity of females to males (Dale et al., 2015). This could explain the differences in color between the subspecies: while in the flaveola group there is mutual ornamentation, in the pelzeni group the females are opaque. It is known that the flaveola group has differences in its reproductive biology (smaller clutch size and longer incubation and chick rearing periods) with respect to the pelzeni group distributed in the southern hemisphere (Mason, 1985; Quiroga et al., 2003; Palmerio and Massoni, 2009, 2011; Palmerio, 2012; Orozco Valor et al., 2016; Espinosa et al., 2017).
The results suggest a possible ability of the Saffron Finch to distinguish sex by color, as found in other tanagers (Burns and Shultz, 2012). However, although in some species monochromatic for humans such as the Chestnut Thrush (Turdus rubrocanus) (Lou et al., 2022), Great Tit (Parus major) (Hofmann et al., 2007) and Black Bulbul (Hypsipetes leucocephalus) (Hung et al., 2017) dichromatism has been observed in several body regions simultaneously, in the Saffron Finch chromatic discriminability occurs in a single region: the crown. Although it is only a single region, the crown has been considered important as a social and sexual signal in other bird species (Parsons and Baptista, 1980; Siitari et al., 2002; Hegyi et al., 2007; Pryke, 2007; Lou et al., 2022), and color differences have been found in this region for species that were considered monochromatic species by humans (Hunt et al., 1998; Tubaro et al., 2005; Valdez and Benitez-Vieyra, 2016; Morales et al., 2020).
Color differences between the sexes found in the crown of the Saffron Finch are below conservative thresholds (2–4 JND), recommended because they may be more biologically relevant (Kelber et al., 2003; Osorio et al., 2004; Martin Schaefer et al., 2007; Olsson et al., 2015). However, the ability to discriminate color according to the psychophysical visual models (Vorobyev and Osorio, 1998) depends on the lighting conditions, and it is known that when the light intensity is high, the noise relative to the signal is small (Olsson et al., 2015). In the tropics, light conditions are stable throughout the year, with high irradiance compared to temperate zones (Kopp, 2023). In addition, it is known that some behaviors are associated with a specific light intensity threshold (Wilkinson and Palmer, 2022). The aforementioned could be the case of the Saffron Finch, when in the wild, the courtship of the male is recorded from the first hour of dawn light until noon (pers. obs.). We propose to maintain the theoretical threshold of 1 JND (Vorobyev and Osorio, 1998) for this species in its tropical distribution, taking into account the conditions of the habitat it occupies and its behavioral strategy. We consider ecological and behavioral studies a priority to validate the proposal of the crown as a possible intersex signaling region in the species.
Our results suggest that there are differences in color between the sexes in all body regions in relation to the physical phenomenon (reflectance) according to the colorimetric variables, and these differences are possibly associated with variations in plumage structure, concentration and types of carotenoids between the sexes. However, when evaluating color perception, the psychophysical model indicates that although there is chromatic variation in all body regions; this variation does not allow individuals to discriminate their conspecifics by sex, with the possible exception of the crown. The intrasexual differences found in plumage color indicate a possible role as a signal, as occurs in the subspecies S. flaveola brasiliensis. Further research on plumage color in the Saffron Finch is required in order to understand the evolutionary process of color differences in females of the different subspecies.
LCB: conceptualization and designed the study, taking reflectance spectra, statistical analyzes, writing-original draft and editing, funding acquisition (lead), supervision and project administration (lead). CEB: conceptualization, taking reflectance spectra, helped with statistical analyses, writing-original draft and editing. HFR: funding acquisition (supporting), writing editing (supporting). All authors read and approved the final manuscript.
This study was evaluated by the Animal Ethics Committee of Universidad del Valle (Act No 008-2016) and had the permission as required by Colombian legislation (Resolution 149 of 25 June 2015). In addition, it is covered by the framework permit for the collection of specimens of wild species for non-commercial scientific research purposes from the National Environmental Licensing Authority-ANLA, which covers Universidad del Valle in its scientific research activities (Resolution 1070 of 28 August 2015).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
To the Department of Biology and the Postgraduate Biology Department, Faculty of Natural and Exact Sciences of the Universidad del Valle, Cali-Colombia. To Humberto Álvarez-López, James Montoya Lerma, Manuel A. Galeano Márquez, Isabel Castro for their great support and accompaniment. To Michelle Galeano Cruz for Sicalis illustration. To Juan Manuel Galeano Cruz for his great support with R software. To Wilmar Torres for his statistical advice. Helen Burnham for translation of the manuscript. To all the members of the Ornithology and Animal Behavior Laboratory-OYCA of Universidad del Valle for their invaluable help in the field. To the Research Vice-chancellor of Universidad del Valle for the financing of the project, Call 4–2016. We are grateful for the constructive comments of anonymous referees that helped improve the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100127.
|
Anderson, M.J., 2005. Permutational multivariate analysis of variance. J. Geosci. Environ. Protect. 26, 32–46.
|
|
Andersson, S., Prager, M., 2006. Quantifying colors. In: Hill, G.E., McGraw, K.J. (Eds. ), Bird Coloration, Volume 1: Mechanisms and Measurements. Harvard University Press, Cambridge, pp. 41–89.
|
|
Araújo-Silva, B.M., 2021. Coloração da plumagem, agressividade e dominância social em Sicalis flaveola brasiliensis. Doctoral Thesis. Universidade Vila Velha, Vila Velha.
|
|
Bastien, P., Tenenhaus, M., 2001. PLS generalised linear regression. Application to the analysis of life time data. In: Proceedings of the PLS'01 International Symposium, Paris.
|
|
Bertrand, F., Meyer, N., Maumy-Bertrand, M., 2014. Partial least squares regression for generalized linear models. R package version.
|
|
Cuthill, I.C., 2006. Analyzing colors. In: Hill, G.E., McGraw, K.J. (Eds. ), Bird Coloration, Volume 1: Mechanisms and Measurements. Harvard University Press, Cambridge, pp. 3–40.
|
|
de Smith, M.J., 2018. Statistical Analysis Handbook. The Winchelsea Press, Drumlin Publications, Drumlin Security Ltd, UK.
|
|
Delhey, K., 2005. Sexual Selection and Blue Tit (Parus caeruleus) Crown Coloration. Doctoral Thesis. Ludwig-Maximilians-Universität, München.
|
|
Espinal, L.S., 1967. Visión ecológica del departamento del Valle del Cauca. Santiago de Cali, Universidad del Valle.
|
|
Espinosa, C., 2019. Relación entre coloración de la coronilla, elección de pareja y dominancia en Sicalis flaveola (Aves: Thraupidae). Universidad del Valle, Colombia.
|
|
Figuerola, J., Gutierrez, R., 1998. Sexual differences in levels of blood carotenoids in cirl buntings Emberiza cirlus. Ardea 86, 245–248.
|
|
Grubb Jr., T.C., Waite, T.A., Wiseman, A.J., 1991. Ptilochronology: induced feather growth in Northern Cardinals varies with age, sex, ambient temperature, and day length. Wilson Bull. 103, 435–445.
|
|
Hill, G.E., McGraw, K.J., 2006. Bird Coloration, vol. 2. Function and Evolution. Harvard University Press, Cambridge.
|
|
Hilty, S.L., Brown, W.L., 2001. Guía de las aves de Colombia. American Bird Conservancy, Cali, Colombia.
|
|
Horak, P., Surai, P.F., Moller, A.P., 2002. Fat-soluble antioxidants in the eggs of great tits Parus major in relation to breeding habitat and laying sequence. Avian Sci. 2, 123–130.
|
|
Marcondes-Machado, L.O., 1997. Comportamento social de Sicalis flaveola brasiliensis em cativeiro (Passeriformes, Emberizidae). Iheringia Ser. Zool. 82, 151–158.
|
|
McGraw, K.J., 2006. Mechanism of carotenoid-based coloration. In: Hill, G.E., McGraw, K.J. (Eds. ), Bird Coloration, Volume 1: Mechanisms and Measurements. Harvard University Press, Cambridge, pp. 177–242.
|
|
Møller, A.P., Biard, C., Blount, J.D., Houston, D.C., Ninni, P., Saino, N., et al., 2000. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability. Avian Biol. Res. 11, 137–159.
|
|
Montgomerie, R., 2006. Analyzing colors. In: Hill, G.E., McGraw, K.J. (Eds. ), Bird Coloration, Volume 1: Mechanisms and Measurements. Harvard University Press, Cambridge, pp. 90–147.
|
|
Orozco Valor, P.M., Santillan, M.A., Bragagnolo, L.A., Rebollo, M.E., López, F.G., Martínez, P.A., 2016. Aportes a la biología reproductiva del Chirigüe Azafrán (Sicalis flaveola) en cajas nido en un bosque semiárido del centro de Argentina. Rev. Chi. Ornitol. 22, 165–170.
|
|
Palmerio, A.G., 2012. Maduración tardía del plumaje y costo reproductivo en el Jilguero Dorado Sicalis flaveola. Doctoral Thesis. Universidad de Buenos Aires, Buenos Aires.
|
|
Quiroga, M., del Barco, O., Agostelli, F., 2003. First approaches to de reproductive biology of Sicalis flaveola birds: Emberizidae at the alluvial valley of Paraná River, Argentina. FAVE 2, 35–40.
|
|
Rising, J.D., Jaramillo, A., Copete, J.L., Ryan, P.G., Madge, S., 2011. Tanagers to new world blackbirds. In: del Hoyo, J., Elliott, A., Christie, D. (Eds. ), Handbook of the Birds of the World, Vol. 16. Lynx Edicions, Barcelona, pp. 381–382.
|
|
Skutch, A.F., Gardner, D., 1989. Life of the Tanager. Comstock Publishing Associates,
Ithaca.
|
|
Tabachnick, B.G., Fidell, L.S., 2001. Using Multivariate Statistics. Pearson, Boston.
|
|
Webb, E.A., 2021. Where Have the Carotenoids Gone? Physiology of Carotenoid Absorption and Distribution in Birds. Doctoral Thesis. Arizona State University, Tempe.
|
| 1. | Takeshi Honda, Hironori Ueda. Why Mammals do Not Damage Entire Farmlands Like Insect Pests Do? A Review from a Behavioral Perspective. Mammal Study, 2023, 48(2) DOI:10.3106/ms2022-0054 |
| 2. | Luqin Yin, Cheng Wang, Wenjing Han, et al. Birds’ Flight Initiation Distance in Residential Areas of Beijing Are Lower than in Pristine Environments: Implications for the Conservation of Urban Bird Diversity. Sustainability, 2023, 15(6): 4994. DOI:10.3390/su15064994 |
| 3. | Melissa Ardila-Villamizar, Gustavo Alarcón-Nieto, Adriana A. Maldonado-Chaparro. Fear in urban landscapes: conspecific flock size drives escape decisions in tropical birds. Royal Society Open Science, 2022, 9(11) DOI:10.1098/rsos.221344 |
| 4. | Swaroop Patankar, Ravi Jambhekar, Kulbhushansingh Ramesh Suryawanshi, et al. Which Traits Influence Bird Survival in the City? A Review. Land, 2021, 10(2): 92. DOI:10.3390/land10020092 |
| 5. | Takeshi Honda. Geographical personality gradient in herbivorous animals: Implications for selective culling to reduce crop damage. Ecological Research, 2021, 36(1): 145. DOI:10.1111/1440-1703.12186 |
| 6. | Takeshi Honda, Yohichi Kubota, Yutaka Ishizawa. Ungulates-exclusion grates as an adjoining facility to crop damage prevention fences. European Journal of Wildlife Research, 2020, 66(1) DOI:10.1007/s10344-020-1362-7 |
| 7. | Daniel T. Blumstein. What chasing birds can teach us about predation risk effects: past insights and future directions. Journal of Ornithology, 2019, 160(2): 587. DOI:10.1007/s10336-019-01634-1 |
| 8. | Jenna L. Van Donselaar, Jenna L. Atma, Zachary A. Kruyf, et al. Urbanization alters fear behavior in black-capped chickadees. Urban Ecosystems, 2018, 21(6): 1043. DOI:10.1007/s11252-018-0783-5 |
| 9. | Javier delBarco-Trillo. Shyer and larger bird species show more reduced fear of humans when living in urban environments. Biology Letters, 2018, 14(4): 20170730. DOI:10.1098/rsbl.2017.0730 |
| 10. | T. Honda, H. Iijima, J. Tsuboi, et al. A review of urban wildlife management from the animal personality perspective: The case of urban deer. Science of The Total Environment, 2018, 644: 576. DOI:10.1016/j.scitotenv.2018.06.335 |
| 11. | Matthew R. E. Symonds, Michael A. Weston, Wouter F. D. van Dongen, et al. Time Since Urbanization but Not Encephalisation Is Associated with Increased Tolerance of Human Proximity in Birds. Frontiers in Ecology and Evolution, 2016, 4 DOI:10.3389/fevo.2016.00117 |
| 12. | Bao-Sen Shieh, Shih-Hsiung Liang, Yuh-Wen Chiu, et al. Interspecific comparison of traffic noise effects on dove coo transmission in urban environments. Scientific Reports, 2016, 6(1) DOI:10.1038/srep32519 |
| Colorimetric variables by body region | Females (Mean ± SD) | Males (Mean ± SD) | Test‡ | Estimate Std | df(t) | P-value | |||||||
| Crown | n = 79 | n = 99 | |||||||||||
| Brightness | B2 | 14.388 ± 4.818 | 14.603 ± 3.949 | t | −0.327 | 176 | 0.744 | ||||||
| Carotenoid chroma | S9 | 0.928 ± 0.019 | 0.915 ± 0.013 | W | 0 | 0.062 | |||||||
| UV Hue | UV-H1 | 333.899 ± 11.453 | 327.485 ± 10.291 | W | 2576 | 0.000† | |||||||
| Throat | n = 76 | n = 99 | |||||||||||
| Brightness | B2 | 18.668 ± 5.463 | 16.829 ± 5.856 | t | 2.119 | 173 | 0.035† | ||||||
| Carotenoid chroma | S9 | 0.951 ± 0.014 | 0.945 ± 0.018 | t(R) | 2.257 | 0.024† | |||||||
| UV Hue | UV-H1 | 338.789 ± 5.646 | 334.535 ± 6.200 | t | 4.675 | 173 | 0.000† | ||||||
| Breast | n = 77 | n = 99 | |||||||||||
| Brightness | B2 | 22.984 ± 3.499 | 24.527 ± 3.681 | W | 30.976 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.915 ± 0.037 | 0.926 ± 0.024 | W | 13.552 | 0.039† | |||||||
| UV Hue | UV-H1 | 346.468 ± 4.624 | 343.121 ± 6.207 | t(R) | 4.097 | 0.000† | |||||||
| Belly | n = 75 | n = 97 | |||||||||||
| Brightness | B2 | 30.589 ± 5.137 | 33.309 ± 3.991 | W | 29.584 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.888 ± 0.052 | 0.894 ± 0.043 | W | 12.900 | 0.037† | |||||||
| UV Hue | UV-H1 | 349.48 ± 2.617 | 346.453 ± 4.890 | t(R) | 52.066 | 0.000† | |||||||
| Thigh | n = 59 | n = 78 | |||||||||||
| Brightness | B2 | 30.051 ± 7.742 | 31.933 ± 7.555 | W | 18.769 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.816 ± 0.087 | 0.836 ± 0.077 | W | 8.083 | 0.044† | |||||||
| UV Hue | UV-H1 | 348.237 ± 4.84 | 344.961 ± 7.014 | t(R) | 3.231 | 0.001† | |||||||
| Mandible | n = 60 | n = 78 | |||||||||||
| Brightness | B2 | 20.305 ± 11.561 | 22.376 ± 10.357 | W | 19, 044 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.741 ± 0.145 | 0.757 ± 0.114 | t | −0.757 | 136 | 0.450 | ||||||
| UV Hue | UV-H1 | 355.833 ± 10.44 | 349.67 ± 12.977 | W | 1609 | 0.002† | |||||||
| Coverts | n = 74 | n = 97 | |||||||||||
| Brightness | B2 | 15.405 ± 2.986 | 16.447 ± 2.88 | W | 29, 241 | 0.000† | |||||||
| Carotenoid chroma | S9 | 0.799 ± 0.051 | 0.822 ± 0.034 | t(R) | −3.426 | 0.029† | |||||||
| UV Hue | UV-H1 | 344.838 ± 6.182 | 341.927 ± 6.997 | t(R) | 28.795 | 0.004† | |||||||
| Nape | n = 73 | n = 99 | |||||||||||
| Brightness | B2 | 13.28 ± 2.075 | 14.507 ± 2.69 | t(R) | −3.376 | 0.001† | |||||||
| Carotenoid chroma | S9 | 0.874 ± 0.046 | 0.886 ± 0.036 | W | 12, 556 | 0.014† | |||||||
| UV Hue | UV-H1 | 345.685 ± 4.079 | 341.363 ± 6.302 | t(R) | 5.448 | 0.000† | |||||||
| Mantle | n = 62 | n = 80 | |||||||||||
| Brightness | B2 | 11.966 ± 1.452 | 12.461 ± 1.499 | t | −1.98 | 140 | 0.050 | ||||||
| Carotenoid chroma | S9 | 0.79 ± 0.061 | 0.822 ± 0.049 | W | 8804 | 0.061 | |||||||
| UV Hue | UV-H1 | 346.677 ± 3.749 | 343.787 ± 5.013 | t(R) | 3.929 | 0.000† | |||||||
| Rump | n = 69 | n = 91 | |||||||||||
| Brightness | B2 | 15.56 ± 2.481 | 16.356 ± 2.201 | t | −2.146 | 158 | 0.033† | ||||||
| Carotenoid chroma | S9 | 0.791 ± 0.082 | 0.811 ± 0.063 | W | 11, 040 | 0.031† | |||||||
| UV Hue | UV-H1 | 347.391 ± 4.535 | 345.087 ± 6.214 | t(R) | 2.71 | 0.006† | |||||||
| † Significant differences. ‡ Statistic test: t = t-Student; t(W) = t-Welch; t(R) = Randomization test; W = Wilcoxon rank. | |||||||||||||
| Body region | Females | Males | Females vs. males | |||||
| Mean ± SD | Min–max | Mean ± SD | Min–max | Mean ± SD | Min–max | |||
| Crown | 3.077 ± 2.978 | 0.041–14.518 | 1.848 ± 1.184 | 0.089–10.409 | 2.804 ± 2.630 | 0.129–15.745 | ||
| Throat | 2.95 ± 2.063 | 0.048–14.567 | 3.436 ± 2.215 | 0.110–12.74 | 3.258 ± 2.111 | 0.106–15.903 | ||
| Breast | 2.808 ± 1.945 | 0.085–12.002 | 2.633 ± 1.837 | 0.043–13.349 | 2.799 ± 2.001 | 0.059–15.840 | ||
| Belly | 3.03 ± 2.425 | 0.086–15.786 | 2.688 ± 1.826 | 0.053–9.923 | 2.884 ± 2.127 | 0.036–14.964 | ||
| Thigh | 3.65 ± 2.822 | 0.127–17.132 | 3.264 ± 2.27 | 0.069–13.054 | 3.468 ± 2.517 | 0.001–18.002 | ||
| Mandible | 4.892 ± 3.663 | 0.044–18.967 | 4.265 ± 3.197 | 0.054–17.924 | 4.566 ± 3.414 | 0.038–18.596 | ||
| Coverts | 2.117 ± 1.455 | 0.081–10.515 | 1.761 ± 1.155 | 0.028–8.256 | 2.008 ± 1.350 | 0.054–10.574 | ||
| Nape | 2.931 ± 2.169 | 0.051–13.568 | 2.972 ± 2.147 | 0.078–13.746 | 3.027 ± 2.203 | 0.088–14.327 | ||
| Mantle | 1.979 ± 1.34 | 0.000–7.663 | 2.217 ± 1.726 | 0.018–11.555 | 2.283 ± 1.612 | 0.056–11.142 | ||
| Rump | 2.426 ± 1.696 | 0.055–10.183 | 2.373 ± 1.655 | 0.037–10.039 | 2.430 ± 1.704 | 0.038–10.561 | ||
| Body region | df | df (residuals) | df (total) | Sums Sqs | F value | P-value |
| Crown | 1 | 182 | 183 | 152.347 | 28.132 | 0.001† |
| Throat | 1 | 183 | 184 | 18.584 | 2.470 | 0.111 |
| Breast | 1 | 181 | 182 | 43.947 | 8.068 | 0.006† |
| Belly | 1 | 177 | 178 | 8.417 | 1.348 | 0.261 |
| Thigh | 1 | 143 | 144 | 3.419 | 0.377 | 0.566 |
| Mandible | 1 | 136 | 137 | 4.524 | 0.281 | 0.645 |
| Coverts | 1 | 180 | 181 | 18.193 | 6.791 | 0.008† |
| Nape | 1 | 179 | 180 | 35.639 | 5.327 | 0.024† |
| Mantle | 1 | 144 | 145 | 39.723 | 11.451 | 0.001† |
| Rump | 1 | 164 | 165 | 14.328 | 3.358 | 0.061 |
| † Significant differences. | ||||||