| Citation: | Li Tian, Yu Liu, Yang Wu, Zimei Feng, Dan Hu, Zhengwang Zhang. 2024: Migration pattern of a population of Barn Swallows (Hirundo rustica) breeding in East Asian tropical region. Avian Research, 15(1): 100192. DOI: 10.1016/j.avrs.2024.100192 |
Birds exhibit a high degree of migratory diversity, which is influenced by various ecological factors and life history strategies. Conducting studies on tropical bird migration, of which research is scarce, and comparing it with temperate birds can enhance our understanding of bird migration behaviour and its underlying mechanisms. In this study, we explored the migration behaviour of a breeding population of the Barn Swallow (Hirundo rustica) in Zhanjiang, southern China, a region located in the northern tropics, using light-level geolocators. From 2021 to 2023, we deployed geolocators on 92 breeding swallows and retrieved geolocators successfully from 23 individuals. These swallows all exhibited migratory behaviour, and wintering on various islands in Southeast Asia. They displayed sex differences in their wintering locations. All males concentrated in Borneo, while females primarily chose Borneo but also dispersed to the Philippines, South China Sea, and Vietnam for wintering. The studied swallow population adopted a seasonal migration pattern of "indirect in autumn, direct in spring", bypassing the ecological barrier of the South China Sea in autumn and tending to directly cross it in spring migration. Moreover, the distance and duration of autumn migration was significantly longer than those of the spring migration. Compared to temperate Barn Swallows, the Barn Swallow population breeding in Zhanjiang adopts a pattern of "intra-tropical migration" and initiates autumn migration earlier. The formation of their migration pattern may be limited by ecological and physiological factors.
Migratory birds exhibit a high degree of migratory diversity, at both individual and population levels (Gilroy et al., 2016). The long-term evolution of avian migratory behaviour is influenced by various ecological selection pressures (e.g., climate, food) and life history strategies (e.g., annual reproductive investment; Gordo, 2007; Jahn et al., 2020). For example, the Ruff (Calidris pugnax) migrating through western Europe exhibits sexual difference in wintering ground selection (Gill et al., 1995), which may be related to the different roles of sexes in reproduction or to their body size dimorphism (Newton, 2008). Analysis of long-term banding data on the European Robin (Erithacus rubecula) has shown that different geographic populations adopt either complete or partial migration strategies, while winter temperatures at the breeding sites in different climate regions influence their migration distances (Ambrosini et al., 2016). Apart from established migration patterns shaped by long-term evolution, individual birds might also modify their migration behaviour in response to environmental changes, potentially developing new migration patterns quickly (Gu et al., 2024). For instance, the Eurasian Blackcap (Sylvia atricapilla) developed new migratory routes within just a few generations (Berthold et al., 1992). Due to the high costs in terms of time and energy during migration, and even the risk of death, the migration strategies adopted by birds are critical to their fitness. Studying the migratory behaviour and its diversity is crucial for migratory bird conservation, and for understanding the evolution of migratory behaviour (Yong et al., 2021).
Knowledge of migratory diversity across different climate zones helps us understand the mechanisms that shape bird migration patterns. Unlike the stable temperate-tropical migration patterns in the Northern Hemisphere, the migration patterns of birds in the tropics are quite different: the climatic differences between different seasons are relatively small, making the predictability for migration initiation or duration lower (Stutchbury and Morton, 2001; Newton, 2008; Stutchbury et al., 2016). Temperature remains one of the factors influencing the migration of tropical birds, though its impact is less significant (Hockey, 2000) and always interacting with other climatic factors such as drought and rainfall (Dingle, 2006). However, research on the migration patterns of tropical bird is still relatively limited, leading to a poor understanding of how they differ from temperate populations and potential driving factors behind their migration strategies (Jahn et al., 2020).
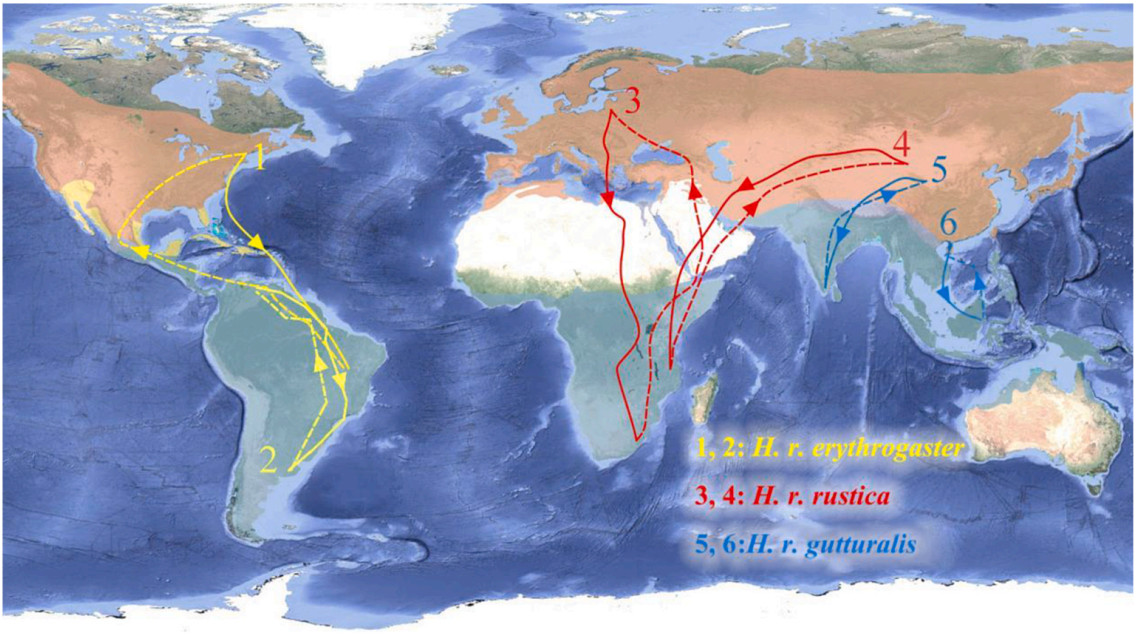
The Barn Swallow (Hirundo rustica) is a small insectivorous bird species distributed worldwide, mostly breeding in temperate regions and migrating to the tropics or the Southern Hemisphere to overwinter (Møller, 1984; Turner, 2006; Saino et al., 2017). Their breeding season in the temperate regions of the Northern Hemisphere spans from April to August, while in tropical regions, Barn Swallows breed from March to July (Saino et al., 2004; García-Pérez et al., 2014; García-Pérez and Hobson, 2014; Hobson et al., 2015a; Sicurella et al., 2016; Briedis et al., 2018; Pancerasa et al., 2018; Tian et al., 2023). In western China, two Barn Swallow groups located in the Asian temperate region migrate to East Africa (H. r. rustica) or South Asia (H. r. gutturalis) for wintering, which is associated with genetic differentiation (Scordato et al., 2020; Turbek et al., 2022). The Middle Eastern Asian subspecies (H. r. transitiva) of Barn Swallows only undertake short-distance migrations, while the Egyptian subspecies (H. r. savignii) are local residents (Turner, 2006). Furthermore, research has shown that a population of Barn Swallows in South America (H. r. erythrogaster) underwent a rapid shift in wintering and breeding areas several decades ago (Winkler et al., 2017). Thus, the migration patterns of the Barn Swallow exhibit a high level of diversity, making it an ideal species for studying the differences in formation of migration patterns and migratory driving factors across different environments, such as temperate and tropical regions.
Barn Swallows (H. r. gutturalis) are present throughout the year in the northern tropical regions of Asia, such as Zhanjiang City in South China, with both breeding populations (Tian et al., 2023) and wintering populations (China Bird Report Center, http://www.birdrecord.cn/). However, there is a lack of studies on the migration behaviour of Barn Swallows in this region. In this study, we aim to identify the migration patterns of a breeding population of Barn Swallows in Zhanjiang to address the following questions: (1) Do Barn Swallows in this region migrate? (2) If they migrate, where do they go for the winter and what are their migration patterns like? We predict that: (1) Based on the population size of Barn Swallows is lower during winter compared to summer in the study area (personal observation by L.T.), at least part of the breeding population of Barn Swallows in the study site exhibit migratory behaviour; (2) Due to that the study area is located south of the Tropic of Cancer and its tropical maritime monsoon climate (Cao et al., 2013) is suitable for the survival of the Barn Swallow throughout the year, Barn Swallows in this region experience an extended period suitable for breeding, resulting in earlier migration in spring and later migration in autumn phenologically: (3) Due to their different roles in reproduction, male and female swallows exhibit varying migration patterns, e.g., males return to the breeding grounds earlier. Furthermore, we will compare the migration behaviour of tropical Barn Swallows with those breeding in temperate regions.
The study was conducted in Zhanjiang City, Guangdong Province, South China, which is located in the northern tropical region, from 2020 to 2023. The main study sites included five villages in Mazhang District (21.26°N, 110.22°E) and Fucheng Town (20.88°N, 110 0.15°E, Appendix Fig. S1). Zhanjiang is located south of the Tropic of Cancer and is the southernmost coastal city in Chinese mainland. It is bordered by the South China Sea to the east, the Qiongzhou Strait to the south, the Beibu Gulf to the west and faces Vietnam across the gulf. Due to the influence of tropical maritime warm and humid air currents, the local climate in the area is characterised as tropical monsoon climate, with extreme minimum temperatures of 1.4 ℃, extreme maximum temperatures of 38.1 ℃, an annual average temperature of 23.3 ℃, and an annual precipitation of 1640 mm with distinct dry and wet seasons (Xu et al., 2019, Xue et al., 2014). The occurrence time of the Barn Swallow slightly varies at the two study sites, with sightings in Mazhang District from February to October and sightings in Fucheng Town throughout the year.
During the breeding seasons from March to July in 2021–2023, monitoring of the breeding process is conducted on each swallow nest, involving nest inspections every 3–5 days to ascertain the dates of egg-laying and hatching. We used mist nets or sweep nets to capture and band adult Barn Swallows roosting in their nests, usually on the 5th or 6th day of the nestling period. We deployed light-level geolocators (FLXX5 fLight tag, 0.33 g, Lotek Ltd., UK; Intigeo P30Z11-7-DIP-NOT, 0.36 g, Migrate Technology Ltd., UK) on randomly selected individuals weighing more than 15.00 g. When equipping Barn Swallows with light-level geolocators, a 0.70 mm diameter jewellery thread (Stretch Magic, Pepperell, USA) was used to fix activated geolocators on their backs using a harness attachment method (Rappole and Tipton, 1991). We searched and recaptured the tagged individuals in the following year and retrieved the geolocators for data offload and analysis.
In 2021, we banded 221 adult breeding individuals (102 males and 119 females), among which 72 (36 males and 36 females) were deployed with geolocators. In 2022, we banded 100 individuals (46 males and 54 females), among which 20 individuals (8 males and 12 females) were deployed with geolocators (Appendix Table S1).
Our analyses were conducted in R language (V4.3.1, R Foundation for Statistical Computing, Vienna, Austria; R Core Team, 2023) and RStudio (V2023.06.0, Posit Software, PBC, Boston, MA; RStudio Team, 2020). We used the IntigeoIF software (v1.15, Migrate Technology Ltd, UK) to offload the light intensity data recorded by the light-level geolocators, and pre-processed the data using the R package TwGeos (functions "readMTlux", "preprocessLight", "twilightEdit", "readMTlux", v0.1.2). We determined sunrise and sunset from daily light measurements by defining a threshold (typically 1.5) which was above the baseline of nocturnal sensor values (Lisovski et al., 2020). The R package FLightR (v0.5.2) was used to analyse the light intensity data, and the calibration dates were cross-checked using the built-in functions (function "make.calibration"). The "plot_slopes_by_location" function was used to visualise the calibration period. Models were constructed with one million particles (functions "make.prerun.object" and "run.particle.filter"), and the parameter was set to a maximum flight distance of 1000 km in a day (López-Calderón et al., 2021). The "stationary.migration.summary" function was used to detect stationary periods, with a minimum probability of movement set to 0.15 and a minimum duration of stationary periods set to 5 days. Finally, the migration routes of Barn Swallows were plotted (function "map.FLightR.ggmap"), and the dates of autumn departure from the breeding site, arrival at the wintering site, spring departure from the wintering site, and arrival at the breeding site were recorded, along with the locations of stopover sites and wintering sites. Besides, for each individual, we calculated migration distances (D) and durations (T) for both migration seasons, and also the average speed during the journey (V, V = D/T; Lindström et al., 2019).
We tested whether there were differences in recapture rates between tagged and untagged individuals, or between sexes, by fitting binomial generalised linear models (GLMs). We fitted both sex and geolocator attachment status as fixed effects, and their interaction to identify whether sexes responded differently to the geolocator attachment. We also used generalised linear mixed models (GLMMs) to compare some migration parameters of Barn Swallows between different migration seasons (autumn migration, spring migration) and different sexes (male, female), with sex, season, and year as fixed effects, and with ID of the bird as a random factor. These parameters included migration distance, duration, and speed. Furthermore, GLMMs were applied to compare differences between sexes and years in departure dates from wintering sites and arrival dates at breeding sites. The normality of regression residuals of the GLMMs was assessed, and a log transformation was applied to the dependent variable (i.e., migration distance) of the model whose regression residuals did not meet the normality requirement. The wintering area of individual Barn Swallows was calculated using the function "kernelHD", package "adhabitatHR" (v0.4.21) based on daily distribution points during the wintering period, with a selected area of 95% kernel density estimation. T-tests were used to compare the differences between sexes in terms of the range of wintering area. The distribution point heatmap was drawn using QGIS (v3.30; Calenge, 2011).
In this study, 23 Barn Swallows (9 males and 14 females) equipped with geolocators were recaptured in 2022 and 2023, with a recapture rate of 25.0% (20.5% for males and 29.2% for females). Among retrieved geolocators, one was damaged and unable to offload data, one had no battery power to offload data temporarily, and another one failed to construct migration routes due to poor data quality. Therefore, the subsequent analyses in this study were based on the data obtained from the remaining 20 geolocators (8 males and 12 females), among which one battery depleted during the wintering period, resulting in missing data for its spring migration. Additionally, 41 Barn Swallows with only bands (21 males and 20 females) were recaptured, with a recapture rate of 17.9% (20.2% for males and 16.0% for females; Appendix Table S1, Fig. S2).
No significant difference was found between the recapture rate of Barn Swallows with and without geolocators (GLM: β = 0.71, SE = 0.40, t = 1.79, p = 0.07), or between males and females (GLM: β = 0.40, SE = 0.33, t = 1.19, p = 0.24; Appendix Fig. S2).
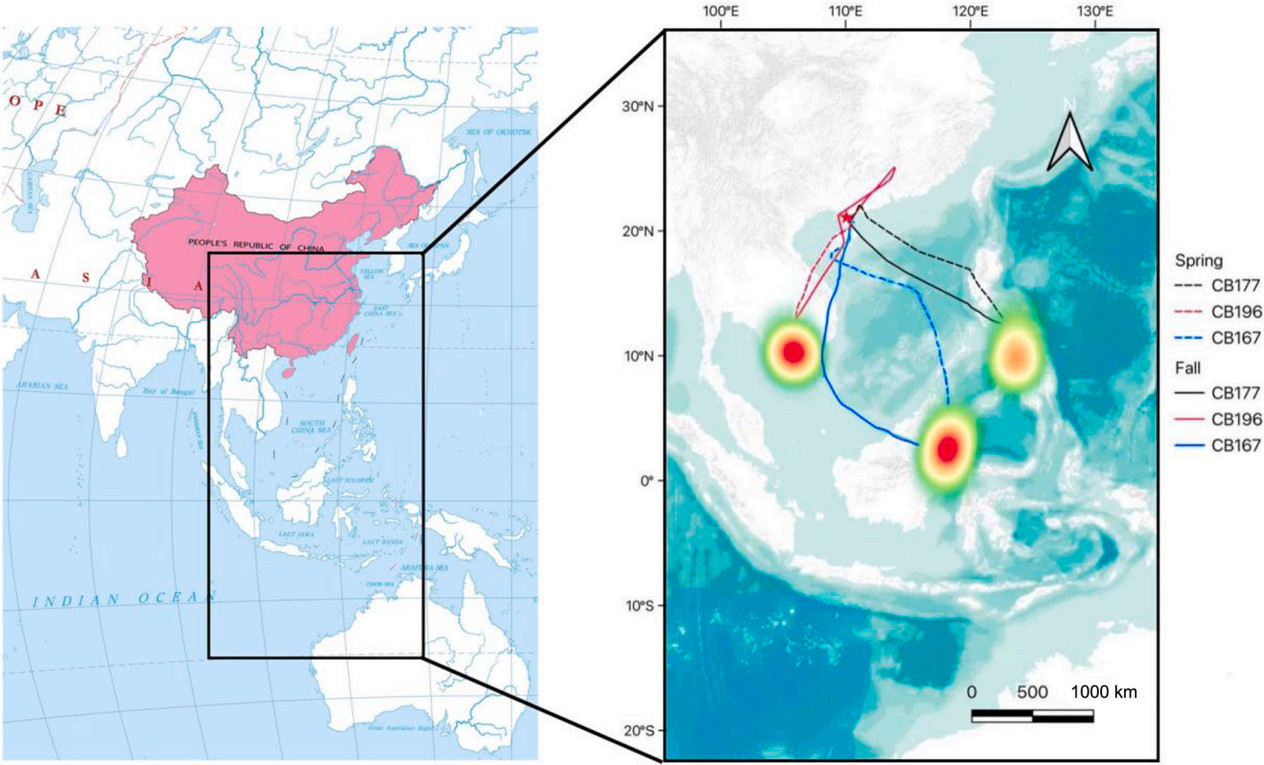
Analysis of the geolocator data from 20 Barn Swallows breeding in Zhanjiang revealed that all individuals exhibited migratory behaviour. There were three main autumn migration routes for Zhanjiang Barn Swallows: (1) Most individuals (17/20) made stopovers in Hainan Island, China, and Vietnam, before reaching their wintering grounds in Borneo (Indonesia, Malaysia) and the surrounding seas (e.g., CB167; Fig. 1); (2) Some individuals (2/20) either made a stopover in Hainan Island or directly crossed the South China Sea to reach their wintering grounds in the Philippines (e.g., CB177; Fig. 1); (3) One individual (1/20) made a stopover in Hainan Island and then crossed the Beibu Gulf to reach the wintering grounds in Vietnam and Cambodia (CB196; Fig. 1). In spring, the swallows primarily followed a direct route across the South China Sea from Borneo back to their breeding grounds, while only a single individual took a route from Borneo through Vietnam before returning to their breeding grounds.
The breeding period of Barn Swallows in Zhanjiang was from March to July, and the departure date of autumn migration after breeding lasted from June to September. 75.0% (15/20) of individuals chose to start migration in July, and 65.0% (13/20) of individuals arrived at their wintering grounds in August. The departure date of spring migration was from February to March, with 63.2% (12/19) of individuals leaving their wintering grounds in February. 47.37% (9/19) of individuals arrived at their breeding grounds in February, and 52.6% (10/19) of individuals arrived in March (Appendix Fig. S3).
The annual migration distance of Barn Swallows in this study was 5280 ± 920 km, with the autumn migration distance (2980 ± 980 km) significantly longer than the spring migration distance (2280 ± 650 km; GLMM: β = −0.41, SE = 0.13, t = −3.17, p < 0.01). The duration of autumn migration (30 ± 24 days) was significantly longer than the duration of spring migration (14 ± 9 days; GLMM: β = −25.54, SE = 6.24, t = −4.09, p < 0.01). Meanwhile, the spring average speed was significantly faster than the autumn average speed during the journey (GLMM: β = 128.00, SE = 42.34, t = 3.02, p = 0.01).
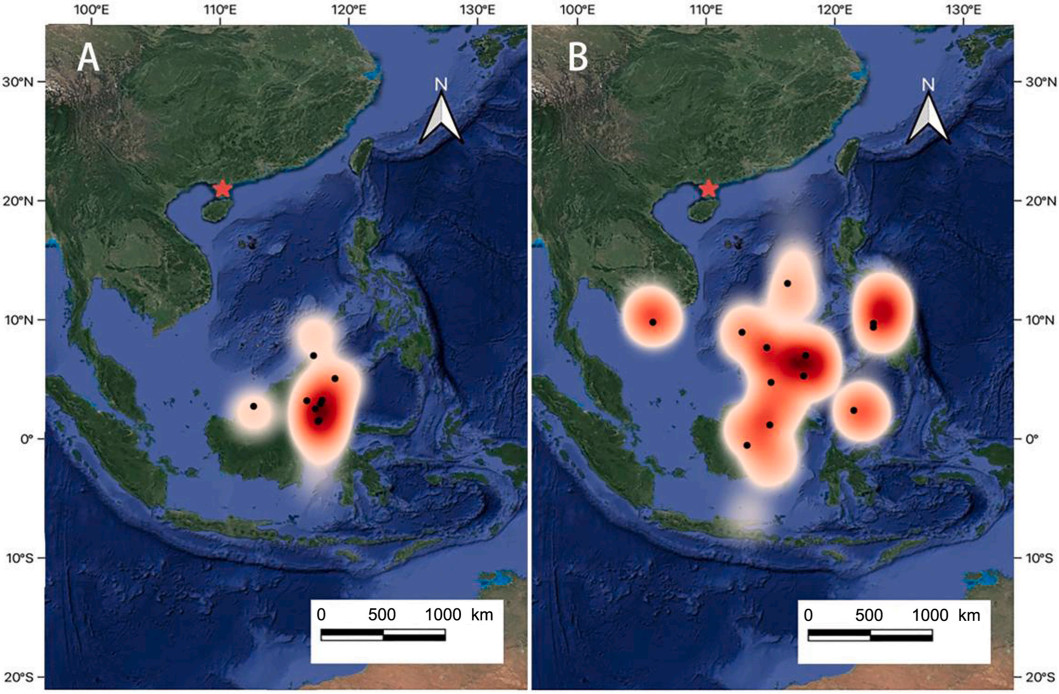
The wintering areas chosen by the Barn Swallows breeding in Zhanjiang were primarily various islands in Southeast Asia: 70.0% (14/20) of the swallows chose to winter in Borneo and its surrounding seas (−0.64–8.04°N, 115.45–119.24°E; Fig. 2), while a few individuals (3/20) wintered on the islands in the South China Sea (9.44–13.78°N, 113.10–116.72°E), in the Philippines (2/20, 7.32–10.28°N, 118.17–123.68°E), and in Vietnam (1/20, 10.32°N, 105.82°E). The average straight-line distance from the breeding areas to the wintering areas was 2000 ± 360 km, with the shortest distance being 1310 km and the longest being 2420 km. The average duration of stay in the wintering areas was 178 ± 27 days (see Fig. 3).
There were some differences in the selection of wintering areas between male and female swallows: all males concentrated in Borneo for wintering, while females primarily chose Borneo but also dispersed to the Philippines, South China Sea, and Vietnam. The total range of wintering areas for all females was 4, 650, 000 km2, with an average individual range of 297, 850 ± 320, 216 km2. The total range of wintering areas for all males was 808, 000 km2, with an average individual range of 232, 864 ± 252, 604 km2. The wintering range of females was 5.75 times that of males, but there was no significant difference in the average individual range between males and females (t = −0.33, df = 16.80, p = 0.75).
There were no significant differences between males and females in the average dates of autumn migration departure (GLMM: β = 8.33, SE = 6.28, t = 1.33, p = 0.20) and spring arrival (GLMM: β = −5.37, SE = 9.59, t = −0.56, p = 0.58; Fig. 3). There were also no sex differences in the distance (GLMM: β = −0.10, SE = 0.14, t = −0.71, p = 0.48) and average speed (GLMM: β = −60.05, SE = 56.52, t = 1.06, p = 0.30) during the journey of spring or autumn migration (Table 1, Fig. 4), but the duration of autumn migration was significantly longer for females than males (GLMM: sex: β = −20.64, SE = 7.27, t = −2.84, p = 0.01; sex * season interaction: β = 23.16, SE = 9.49, t = 2.44, p = 0.03). Regarding migration routes, males exhibited relatively concentrated and consistent migration routes, while females displayed more dispersed migration routes (Fig. S4).
| Duration of wintering period (days) | All | Female | Male |
| 178±27 (n=19) | 176±33 (n=11) | 184±9 (n=8) | |
| Direct distance (km) | 2000±360 (n=20) | 18, 670±390 (n=11) | 2170±190 (n=9) |
| Migration distance (km) | 5280±920 (n=19) | 5260±1010 (n=11) | 5290±70 (n=8) |
| Distance of autumn migration (km) | 2980±980 (n=20) | 3160±1180 (n=11) | 2750±440 (n=9) |
| Duration of autumn migration (days) | 30±24 (n=20) | 38±28 (n=11) | 17±7 (n=9) |
| Average speed during the autumn journey (km/day) | 144±78 (n=20) | 122±69 (n=11) | 185±73 (n=9) |
| Distance of spring migration (km) | 2280±650 (n=19) | 2100±660 (n=11) | 2520±500 (n=8) |
| Duration of spring migration (days) | 13±8 (n=19) | 12±7 (n=11) | 14±9 (n=8) |
| Average speed during the spring journey (km/day) | 233±130 (n=19) | 250±186 (n=11) | 254±144 (n=8) |
| Departure from Zhanjiang | 2021/07/24±15 (n=18) | 2021/07/20±11 (n=10) | 2021/07/29±18 (n=8) |
| Arrival at wintering area | 2021/08/23±26 (n=18) | 2021/08/29±32 (n=10) | 2021/08/15±12 (n=8) |
| Departure from wintering area | 2022/02/14±19 (n=18) | 2022/02/18±13 (n=10) | 2022/02/09±26 (n=8) |
| Arrival at Zhanjiang | 2022/03/12±20 (n=17) | 2022/03/15±22 (n=10) | 2022/03/06±18 (n=7) |
In our study, there were no significant differences in the recapture rates of Barn Swallows, regardless of geolocator deployment or sex. This result suggests that the deployment of geolocators did not influence the recapture rates of Barn Swallows, nor did it lead to differences in recapture rates between sexes.
Birds in tropical regions were once believed to stay year-round without having distinctive seasonal movements (Zimmer, 1938). Similarly, Barn Swallows are documented as permanent residents and summer residents in Guangdong Province, China (MacKinnon and Phillipps, 2000; Zheng, 2023). Although Barn Swallows can be observed throughout the year in our study site, our research indicates that breeding individuals in this area, at least those adults deployed with geolocators, migrate to tropical regions in lower latitudes for wintering after breeding. Furthermore, during the winters of 2020–2023 (January to early February), none of the 74 captured wintering Barn Swallows (adult: n = 21, young: n = 50, unknown age: n = 3) in the study area (Fucheng Town) were found to be banded ones (adult: n = 779, young: n = 956) from the breeding population (L.T., unpublished work). Therefore, we believe that the Barn Swallow population breeding in Zhanjiang adopts a pattern of "intra-tropical migration", i.e., the migration within the region between the Tropic of Capricorn and the Tropic of Cancer (Jahn et al., 2020). Compared to the migration of temperate Barn Swallows, the migration distance of tropical Barn Swallows appears to be shorter (Fig. 4).
Like numerous migratory songbirds in East Asia, the Barn Swallow migrates along the East Asian-Australasian Flyway. Among these songbirds, the majority (170 species, 67%) undertake long-distance migration, from Arctic Russia and western Alaska to Asian tropics and/or the lower latitudes of East Asia; while others engage in short-distance or altitudinal migration (Yong et al., 2015). Compared to the long-distance migrations of other East Asian Barn Swallow populations, such as those banded in Shandong Province, China found wintering in Malaysia, the Zhanjiang Barn Swallow population adopts a pattern of tropical intra-migration (Zhang and Yang, 1997). Furthermore, some species with distributions that straddle temperate and tropical regions may have non-migratory and migratory populations, such as the Indian Paradise Flycatcher (Terpsiphone paradisi), resulting in leapfrog migration patterns (Yong et al., 2015). Based on the geolocator data and surveys of the wintering population, we believe that Barn Swallows in Zhanjiang include summer residents, winter residents, as well as migration passengers throughout the year. This finding supports the hypothesis of a chain migration scenario in Barn Swallows in East Asia: northern breeding populations tend to winter further north, while southern breeding populations tend to winter further south (Åkesson et al., 2020).
Barn Swallows in Northern Hemisphere temperate regions generally migrate from August to mid-October in autumn, and their spring migration starts from mid-March to mid-April (Saino et al., 2013; Liechti et al., 2015; Turbek et al., 2022). Our study found that the population of Barn Swallows at lower latitudes in the northern tropical region departed from the breeding areas earlier (June to September) than the temperate populations and initiated their spring migration as early as February. This result is contrary to our expectation that the favourable year-round climate of our study area would to lead to a later autumn migration compared to regions at higher latitudes. This discrepancy may be attributed to the trade-offs in timing and energy allocation among crucial events such as breeding, migration, and moulting throughout the annual cycle.
The moulting of the Barn Swallow primarily occurs at the wintering sites, starting with the body feathers and wings (Jenni and Winkler, 1994; Turner, 2006). Research has shown that the moulting process of Barn Swallows takes at least 135 days (Jenni and Winkler, 1994), and Liechti et al. (2015) found that Barn Swallows spend an average of 157 days in their wintering areas south of the Sahara. In this study, Barn Swallows had an average stay of 178 days (128–209 days, n = 20) in their wintering areas. The breeding individuals of Barn Swallows in the study site start laying eggs as early as March (Tian et al., 2023), indicating that they may need to complete their entire moult in the wintering areas before mid-February in order to engage in breeding activities as soon as they arrive at the breeding areas. Throughout the annual cycle, Barn Swallows need to balance the timing allocation of "breeding-migration-moult-migration", resulting in earlier autumn migration initiation dates for Barn Swallows breeding in low latitudes.
Crossing ecological barriers is one of the most energy-demanding and risky stages in bird migration (Rubolini et al., 2002). Previous studies have found that Barn Swallow populations in Italy and Switzerland typically follow a "clockwise loop migration" pattern, crossing barriers through the central Mediterranean and the Sahara Desert during autumn migration, but shifting to a more western, longer route during spring migration, bypassing the barriers (Pancerasa et al., 2022). In this study, we found that the migratory pattern of the breeding population of Barn Swallows in Zhanjiang exhibited a contrary "indirect in autumn, direct in spring" pattern, with the majority of individuals choosing a western route bypassing the South China Sea to reach the wintering grounds via Vietnam during autumn migration, while during spring migration, they directly crossed the South China Sea, taking a shorter route. One possible reason for adopting this pattern is that birds need to quickly return to their breeding grounds and establish territories after spring migration (Winger et al., 2019). For instance, the spring migration duration of Common Swifts (Apus apus pekinensis) is significantly shorter than that of autumn migration, and they also make fewer stopovers in spring (Zhao et al., 2022). On the other hand, the migration of Barn Swallows may also be influenced by ecological factors such as wind. Studies have shown that wind can influence migration strategies (Liechti, 2006; Horton et al., 2016). For example, Common Swifts utilise wind during migration and may make detours due to wind direction and foraging sites, ultimately adopting a mixed fly-and-forage migration strategy (Åkesson et al., 2012). Shearwaters adjust their migration routes to optimise the wind conditions they encounter (González-Solís et al., 2009), and Arctic Terns (Sterna paradisaea) likely benefit from wind during northward migration, resulting in faster speed compared to southward migration (Hromádková et al., 2020). During autumn migration (from July to September) in the South China Sea, the prevailing winds are south-easterly, which creates headwinds for Barn Swallows. Therefore, it is likely that they choose to avoid the ecological barrier of the sea and take a detour westward through Vietnam to reach Borneo. In contrast, during spring migration (from February to March), the sea still experiences predominantly south-easterly winds, creating tailwinds. As a result, Barn Swallows may choose a shorter route directly crossing the South China Sea under the influence of wind.
In some migratory species, males and females adopt different migration strategies (Morbey and Ydenberg, 2001). Many studies have found sex differences in the timing of arrival during spring migration in migratory birds, with a certain degree of protandry before breeding (Stewart et al., 2002; Møller, 2004; Rubolini et al., 2004; Saino et al., 2015). Protandry has also been observed in temperate Barn Swallows when returning to their breeding grounds (Balbontín et al., 2007; Liechti et al., 2015), but we did not find this difference in our study. Some studies suggest that migratory birds in tropical regions generally experience lower selection pressure on arriving early at breeding grounds compared to birds in the northern temperate regions (Jahn et al., 2020). In this study, Barn Swallows were moving within the tropical region, which may result in lower selection pressure on arriving early at breeding grounds, explaining the absence of the protandry phenomenon. Furthermore, the timing of arrival at breeding areas may also be influenced by local temperatures: the temperature in February at the breeding site remains relatively low (minimum temperature of 6.6 ℃ in February 2022, monthly average minimum temperature of 12.1 ℃; minimum temperature of 11.7 ℃ in February 2023, monthly average minimum temperature of 16.5 ℃) and is unstable, requiring males to balance the benefits and risks of early arrival.
Barn swallows breeding in Zhanjiang also displayed sex differences in their wintering locations. Male individuals tend to concentrate in wintering areas on Kalimantan Island and nearby islands, while females were more widely dispersed, locating in Kalimantan Island, islands in the South China Sea, Vietnam, and the Philippines. Similar patterns have been observed in Ruffs (Calidris pugnax) migrating across Western Europe, where males winter in Europe and females winter south of the Sahara in Africa (Gill et al., 1995). Research indicates that sex variations in migration are linked to three aspects of species biology: (1) distinct roles in reproduction, potentially connected to their migration timing; (2) timing of other events in the annual cycle, especially molting, which can again affect the timing of departure from breeding areas; (3) differences in body size and dominance, which often vary between sexes and age groups and may be associated with migration timing and distance (Newton, 2008). Further analysis is needed to explore the specific factors driving the divergence in wintering locations between sexes in the studied population, with an increased sample size and additional research. In addition, our results found that the migration duration of males in autumn was shorter than that of females, which may also be influenced by the more scattered overwintering areas of females.
In this study, we described the migratory behaviour of the Barn Swallow within the tropics for the first time, and compared it with that of temperate populations. We showed that breeding individuals of Barn Swallows in Zhanjiang, a region located in the northern tropics, all exhibit migratory behaviour, and their autumn migration started earlier than northern temperate populations and followed "indirect in autumn, direct in spring" pattern. The formation of migration strategies may be restricted by physiological factors such as moulting and influenced by meteorological factors such as wind direction. We hope this study will enhance our understanding of the driving factors behind migration behaviour in different climate zones, and provide insights for further exploring the evolution of avian migration.
All the methods were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, revised in March 2017). The study was approved by the Ethical and Animal Welfare Committee (Approval No.: CLS-EAW-2021-018).
The data presented in this study are available upon request from the corresponding author.
Li Tian: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. Yu Liu: Supervision, Conceptualization, Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. Yang Wu: Formal analysis, Software, Writing – original draft. Zimei Feng: Investigation. Dan Hu: Formal analysis. Zhengwang Zhang: Supervision, Conceptualization, Writing – review & editing, Funding acquisition, Resources.
During the preparation of this work the authors used ChatGPT in order to improve language and readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Linna Xiao for plotting Fig. 4 and the many volunteers that helped with data collection over the three years of study. We are thankful to National Bird Banding Center of China, and Guangdong Forestry Department for providing permissions of bird capture, banding and geolocator installation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100192.
|
Cao, J., Zhong, W., Xue, J., Ouyang, J., Yin, H., 2013. Variation and mechanism of aleoclimate in the last glaciation in the tropic Northern Leizhou Peninsula in South China. Acta Geol. Sin. 87, 1178–1192 (in Chinese with English abstract).
|
|
Jahn, A.E., Cueto, V.R., Fontana, C.S., Guaraldo, A.C., Levey, D.J., Marra, P.P., et al., 2020. Bird migration within the neotropics. Auk 137, 1–23.
|
|
Jenni, L., Winkler, R., 1994. Moult and Ageing of European Passerines. Academic Press, London.
|
|
López-Calderón, C., Magallanes, S., Marzal, A., Balbontín, J., 2021. The migration system of Barn Swallows Hirundo rustica breeding in southwestern Spain and wintering across west Africa. ARDEOLA 68, 335–354.
|
|
MacKinnon, J., Phillipps, K., 2000. A Field Guide to the Birds of China. Oxford University Press, Oxford.
|
|
Newton, I., 2008. The Migration Ecology of Birds. Academic Press, London.
|
|
R Core Team, 2023. R: A Language and Environment for Statistical Computing.
|
|
Rappole, J.H., Tipton, A.R., 1991. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337.
|
|
RStudio Team, 2020. RStudio: Integrated Development Environment for R.
|
|
Stutchbury, B.J.M., Morton, E.S., 2001. Behavioral Ecology of Tropical Birds. Academic Press, New York.
|
|
Tian, L., Liu, Y., Zhou, Z., Zhou, H., Lu, S., Zhang, Z., 2023. Reproductive success of a tropical Barn Swallow Hirundo rustica population is lower than that in temperate regions. Animals 13, 62.
|
|
Turner, A., 2006. The Barn Swallow. T. & A. D. Poyser, London.
|
|
Xu, Y.X., Wang, Z.C., Zhu, W.K., Du, A.P., 2019. Litterfall and nutrient cycling of eucalyptus plantation with different ages on Leizhou Peninsula. J. Trop. Subtropical Bot. 27, 359–366.
|
|
Xue, J., Huang, X., Zhong, W., 2014. Precipitation change and the relationship with ENSO events in northern leizhou peninsula during the last 50 years. J. South China Normal Univ. (Soc. Sci. Ed.) 46, 112–117.
|
|
Zhang, F.Y., Yang, R.L., 1997. Bird Migration Research of China. China Forestry Publishing House, Beijing.
|
|
Zheng, G.M., 2023. A Checklist on the Classification and Distribution of the Birds of China, 4rd ed. Science Press, Beijing.
|
| Duration of wintering period (days) | All | Female | Male |
| 178±27 (n=19) | 176±33 (n=11) | 184±9 (n=8) | |
| Direct distance (km) | 2000±360 (n=20) | 18, 670±390 (n=11) | 2170±190 (n=9) |
| Migration distance (km) | 5280±920 (n=19) | 5260±1010 (n=11) | 5290±70 (n=8) |
| Distance of autumn migration (km) | 2980±980 (n=20) | 3160±1180 (n=11) | 2750±440 (n=9) |
| Duration of autumn migration (days) | 30±24 (n=20) | 38±28 (n=11) | 17±7 (n=9) |
| Average speed during the autumn journey (km/day) | 144±78 (n=20) | 122±69 (n=11) | 185±73 (n=9) |
| Distance of spring migration (km) | 2280±650 (n=19) | 2100±660 (n=11) | 2520±500 (n=8) |
| Duration of spring migration (days) | 13±8 (n=19) | 12±7 (n=11) | 14±9 (n=8) |
| Average speed during the spring journey (km/day) | 233±130 (n=19) | 250±186 (n=11) | 254±144 (n=8) |
| Departure from Zhanjiang | 2021/07/24±15 (n=18) | 2021/07/20±11 (n=10) | 2021/07/29±18 (n=8) |
| Arrival at wintering area | 2021/08/23±26 (n=18) | 2021/08/29±32 (n=10) | 2021/08/15±12 (n=8) |
| Departure from wintering area | 2022/02/14±19 (n=18) | 2022/02/18±13 (n=10) | 2022/02/09±26 (n=8) |
| Arrival at Zhanjiang | 2022/03/12±20 (n=17) | 2022/03/15±22 (n=10) | 2022/03/06±18 (n=7) |