| Citation: | Xiaogang Yao, Neng Wu, Yan Cai, Canchao Yang. 2023: The effects of anthropogenic noise on nest predation with respect to predator species across different habitats and seasons. Avian Research, 14(1): 100121. DOI: 10.1016/j.avrs.2023.100121 |
Noise pollution is a major component of sensory pollution that can disrupt the well-being and functioning of living organisms, affect a variety of life history traits in animals, and reduce their reproductive success. In this study, we used artificial nest experiments with noise manipulation to investigate the influence of anthropogenic noise on nest predation during the breeding and non-breeding seasons and in different forest habitats. We found that the noise treatment did not alter the predation rates or survival probabilities of birds in artificial nests. However, the diversity and species composition of nest predators in artificial pine forests varied between breeding and non-breeding seasons, which may be explained by season-specific adaption of nest predators to bird nests or the unstable ecosystems of artificial forests. The diversity and species composition of nest predators differed between the different forests, probably because of differences in habitat heterogeneity. Predation time varied with treatment, season, and habitat, although most predators were nocturnal mammals. Niche segregation or changes in optimal foraging time may explain this phenomenon.
Sensory pollution refers to the overwhelming bombardment of the senses with noise, light, and other stimuli that are not naturally present in the environment (Swaddle et al., 2015). It causes disconnection by drowning out the stimuli that link animals to their surroundings and to one another (Dominoni et al., 2020). Sensory pollution affects wildlife and other organisms that rely on their senses to navigate the world around them (Senzaki et al., 2020). Many species, including birds and insects, rely on visual and auditory cues to find food, mates, and shelter. Artificial lighting and noise can interfere with these cues, causing confusion and disorientation (Gaston et al., 2017). This can have serious consequences for the survival of these species, as they may have difficulty finding food or mates or may become more vulnerable to predators. In some cases, sensory pollution can lead to changes in behavior and physiology. For example, studies have shown that birds exposed to artificial lighting exhibit altered migration patterns and breeding behaviors (Villain et al., 2016; Senzaki et al., 2020).
Noise pollution from humans is a major component of sensory pollution. Excessive exposure to noise can disrupt the well-being and functioning of living organisms (Halfwerk et al., 2011). It is not only an issue in urban areas, but also in rural and natural settings. Studies have demonstrated that noise pollution can cause immense harm to animals. For instance, chronic exposure to noise can affect the stress response and endocrine system of several organisms, such as birds, rodents, and primates (Francis et al., 2012; Newport et al., 2014; Raap et al., 2017). These disruptions may lead to reduced reproductive success, abnormal hormone levels, and changes in the immune system (Halfwerk et al., 2011). Furthermore, noise pollution can also trigger several physiological effects in animals, such as an increase in heart rate, respiration rate, and blood pressure, which could lead to health issues, including cardiovascular disease (Swaddle et al., 2015). Additionally, chronic exposure to noise can cause migratory birds to deviate from their usual migration paths, thereby disrupting their ecological functions and affecting the distribution of plant species (Villain et al., 2016; Dominoni et al., 2020).
Different nest types have different primary predators. The primary type of predator depends on the distribution of nest predators in the habitat and on their specific foraging behavior. For example, corvids feed almost exclusively on shrub nests, while mammals mainly feed on ground nests in the zone between forest and grassland; species that nest in the canopy may be more vulnerable to avian predators (Söderström et al., 1998; Reidy and Thompson, 2012). However, some diurnal nest predators, such as Long-tailed Macaques (Macaca nemimiina) and Green Cat Snakes (Boiga cyanea) in southeast Asian evergreen forests (Wesolowski and Tomialojć, 2005), exhibit no preferences related to nest type and height. Medium-sized mammals such as the Raccoon (Procyon lotor), Pine Martens (Martes), and Striped Skunks (Mephitis), usually associated with nocturnal predation, are the dominant nest predator in deciduous forests (Leimgruber et al., 1994; Weidinger, 2009). Rodents commonly prey on nests in deciduous forests, while snakes are more likely to prey on nests in brush and grassland (Thompson et al., 1999; Cox et al., 2012). Most nest predation studies are conducted during the bird breeding season. However, some researchers have used artificial nests to carry out nest-predation studies in the non-breeding season. For example, artificial-nest predation rates exhibited clear seasonal variation, being high in the breeding season and low in the non-breeding season (Husby and Hoset, 2018).
It is feasible to use artificial nests with noise manipulation to investigate the effects of anthropogenic noise on wildlife reproduction. Artificial nests have been increasingly used in scientific research to study avian nesting behavior and predation rates (Moore and Robinson, 2004). An artificial nest consists of a container or structure that simulates a natural nest site and is filled with either real or fake eggs. This method can be used to measure the effects of various factors on nest predation, such as habitat fragmentation, predation type, and other environmental factors (Major and Kendal, 2008). Nest predation, the destruction or consumption of eggs or young birds in their nests, is a major cause of reproductive failure in birds. By providing artificial nests, researchers can manipulate their location and design to determine how these factors affect predation rates (King, 1999; Lambrechts et al., 2010).
Artificial nests have several advantages over natural ones. First, they can be standardized, making it possible to control for potential confounding variables that could skew the data (Major and Kendal, 2008). For instance, researchers can vary the color, number, and size of eggs in the nests or modify the shape, height, or location of the nest sets to test the impacts of different factors on predation rates (Yang et al., 2016). This approach allows for replication of experiments, providing more reliable results and better comparisons between different sites and species than when using natural nests (Moore and Robinson, 2004). Second, artificial nests are easy to construct and can be placed in any suitable habitat, regardless of the availability or accessibility of naturally occurring nests. This feature makes it possible to study nesting behavior and predation in a wider range of habitats and species (Lambrechts et al., 2010). Furthermore, artificial nests can be used to study rare or threatened bird species without putting the actual nests at risk of disturbance or predation, thereby protecting birds from harm (Moore and Robinson, 2004). Another significant advantage of artificial nest research is that it can help identify the most common and effective predators of bird nests in various habitats. This information is valuable for identifying conservation management strategies to reduce nest predation and preserve avian biodiversity (Batáry et al., 2004). However, there are some drawbacks and limitations to the use of artificial nests in scientific research. One major concern is that they may not accurately simulate the natural behavior of birds when they interact with their real nests (King, 1999). For instance, some species may be less likely to visit or defend artificial nests than natural ones or may behave differently when they encounter a predator. This difference in bird behavior could result in inaccurate or unreliable results. In addition, artificial nests may be more susceptible to other forms of disturbance such as theft, vandalism, or weather damage (Moore and Robinson, 2004). These concerns require careful consideration and the design of experiments to minimize potential limitations.
In this study, we combined artificial nests with noise manipulation to test the effects of anthropogenic noise on nest predation. Infrared cameras were used to monitor each artificial nest to obtain accurate predation information. Furthermore, we investigated differences in the effects of noise between seasons (breeding or non-breeding season) and habitats (primary, secondary, and artificial forests). Because birds do not breed and nest in the non-breeding season, running the artificial nest experiment in the non-breeding season aims to reveal the season-specificity of nest predators rather than to simulate natural nests. In other words, some specialized nest predators may search for bird nests more specifically during the breeding season than during the non-breeding season (Husby and Hoset, 2018). We hypothesized: (1) that anthropogenic noise would alter nest predation (including predation rate and predator species) if the noise intimidates or attracts predators; (2) that some nest predators would adapt to the breeding season if predation rates and predator species change with season; and (3) that the predation rate and predator species would vary between the three habitat types, owing to differences in habitat heterogeneity.
This study was performed in the Kuankuoshui National Nature Reserve (107°02′23″–107°14′09″ E, 28°06′25″–28°19′25″ N), which is located in Southwest China. The nature reserve is characterized by a central subtropical humid monsoon climate with low temperature, frequent fog, and little sunshine (Yang et al., 2019). The annual average temperature is between 11.7 and 15.2 ℃, with a relative humidity >82%, and an annual precipitation between 1300 and 1350 mm (Ye et al., 2022). The total area of the nature reserve is 26,231 ha, with an altitude between 650 and 1762 m. These habitats include primary Fagus lucida forest, secondary mixed forest, artificial pine forests, shrubs, and tea plantations (Yang et al., 2020). The levels of vegetation cover around artificial nests are similar in the three habitats, with vegetation coverage of ca. 30% in the breeding season and 15% in the non-breeding season. In the artificial forest, the dominant species is Cedar (Cunninghamia lanceolata), which is distributed at an altitude of 1535–1620 m. The secondary forest is mixed forest, comprising mainly hardwood and broadleaved plants such as Pubescent Hornbeam (Carpinus pubescens), distributed at an altitude of 1552–1646 m. The primary forest is a mixed deciduous, evergreen, and broad-leaved forest dominated by Beech (Fagus lucida), distributed at an altitude of 1585–1676 m. We used artificial ground nests. The major bird species of conservation concern in the nature reserve are Phasianidae species such as the Golden Pheasant (Chrysolophus pictus), Reeves's Pheasant (Syrmaticus reevesii), Common Pheasant (Phasianus colchicus), Satyr Tragopan (Tragopan temminckii), Chinese Bamboo Partridge (Bambusicola thoracica), and Silver Pheasant (Lophura nycthemera).
We conducted an artificial nest experiment in primary, secondary, and artificial forests during the breeding (April–June) and non-breeding (September–November) seasons in 2022. The experiment was divided into two treatments: noise and control. For both treatments, an artificial nest containing two fresh Domestic Quail (Coturnix japonica) eggs and dry leaves as the nest lining was arranged on the forest ground. The artificial nests were not reused (eggs were not replaced after the artificial nests were predated). In the forests, six line transects at least 50 m away from the forest edges and roads were randomly selected for each habitat. The distance between adjacent transect transects was 50 m. The artificial nests were placed on the forest ground along the transects in an alternate arrangement (i.e., the treatment and control nests were placed alternately along the transect) with 30 m between nests, with 10 artificial nests per transect. This experimental design references previous predation studies in which the distance between adjacent treatments was less than 20 m (Cuthill et al., 2005; Stevens et al., 2008). The artificial nest was approximately 30 cm from the tree, where a loudspeaker (ShiDu P3, Shenzhen, China) was hidden at the bast of the trunk using leaves. An infrared camera (SG880V, Bestguarder, Beijing, China) was set up on the same tree as the loudspeaker, approximately 30 cm from the nest, and was set to take three consecutive photographs on detecting predator activity. Detection sensitivity (the interval between successive events) was set to 1 s. In this study, the infrared cameras succeed in detecting all predation cases; no cases of predation without predator detection were recorded. The only difference between the noise and control treatments was the sound recording from the loudspeaker. For the noise treatment, a 10 min anthropogenic noise recording of noise from the busy farmer's market in Suiyang County was played from 08:00 to 18:00 during the entire experiment (i.e., 15 d). This recording replicates the noise produced by crowd while shopping, talking, and walking. In the study area, such noises are produced by forest rangers and local residents. The peak frequency of the anthropogenic noise was 813 Hz and the volume was 44–64 dB, which is transmitted ca. 10 m. This ensures that the sound would not overlap between two adjacent treatments. For the control treatment, the procedure was the same, except that the recording was of the ambient sound of the nature reserve. The peak frequency was 625 Hz and the volume was 36–40 dB. Although the volume slightly differed between the two treatments, we emphasize that the control treatment was designed to control for the effect of the loudspeakers playing itself, rather than of the sound volume. Furthermore, playing the ambient sound at the same volume as the noise would considerably violate the natural volume of the ambient sound itself. After 15 d of the experiment, the cameras were retrieved to extract predation information, including the survival duration of the nest, predation time, and the species of predators. Although this duration (15 d) is similar to the incubation period of quail eggs (16 d), it was designed to provide a consistent time between treatments, rather than to simulate the incubation period of any bird species. There were 30 samples for each treatment in each season and habitat. Therefore, 360 artificial nests were used in this study. The positions of the artificial nests between the breeding and non-breeding seasons in a habitat was the same; therefore, position was used as a random effect for statistical analyses.
Generalized linear mixed models based on the Markov Chain Monte Carlo technique (MCMC-GLMMs) were used to analyze the treatment effects and their seasonal and habitat differences. The outcome of predation was the response variable (i.e., predated or not predated). The treatment, season, and habitat were the fixed effects; interactions between treatment and season and between treatment and habitat were also tested for seasonal and habitat effects. Nest position was used as a random effect. Cox proportional-hazards models (Cox models) were used to analyze the survival probabilities for the artificial nests, with the predation rate and survival duration as the response variables, and treatment, season, habitat, and the interactions between treatment and season and between treatment and habitat as fixed effects. Artificial nest position was also controlled in these models. Kaplan–Meier curves were used to determine the survival probabilities of the artificial nests. To compare the diversity of predator species between treatments, seasons, or habitats, Shannon–Wiener diversity indices were calculated and compared using Hutcheson's t-test. Differences in predation time between treatments, seasons, and habitats were compared using the Watson–William's test, which is specifically used for circular data. The MCMC-GLMMs, Cox models, Kaplan–Meier curves, Hutcheson's t-tests, and the Watson–William's tests were performed using the ‘MCMCglmm’, ‘survival’, ‘survminer’, ‘ecolTest’, and ‘circular’ packages in R v.4.1.0 for Windows (https://www.r-project.org/). All statistical tests were two-tailed, and statistical significance was set at P < 0.05.
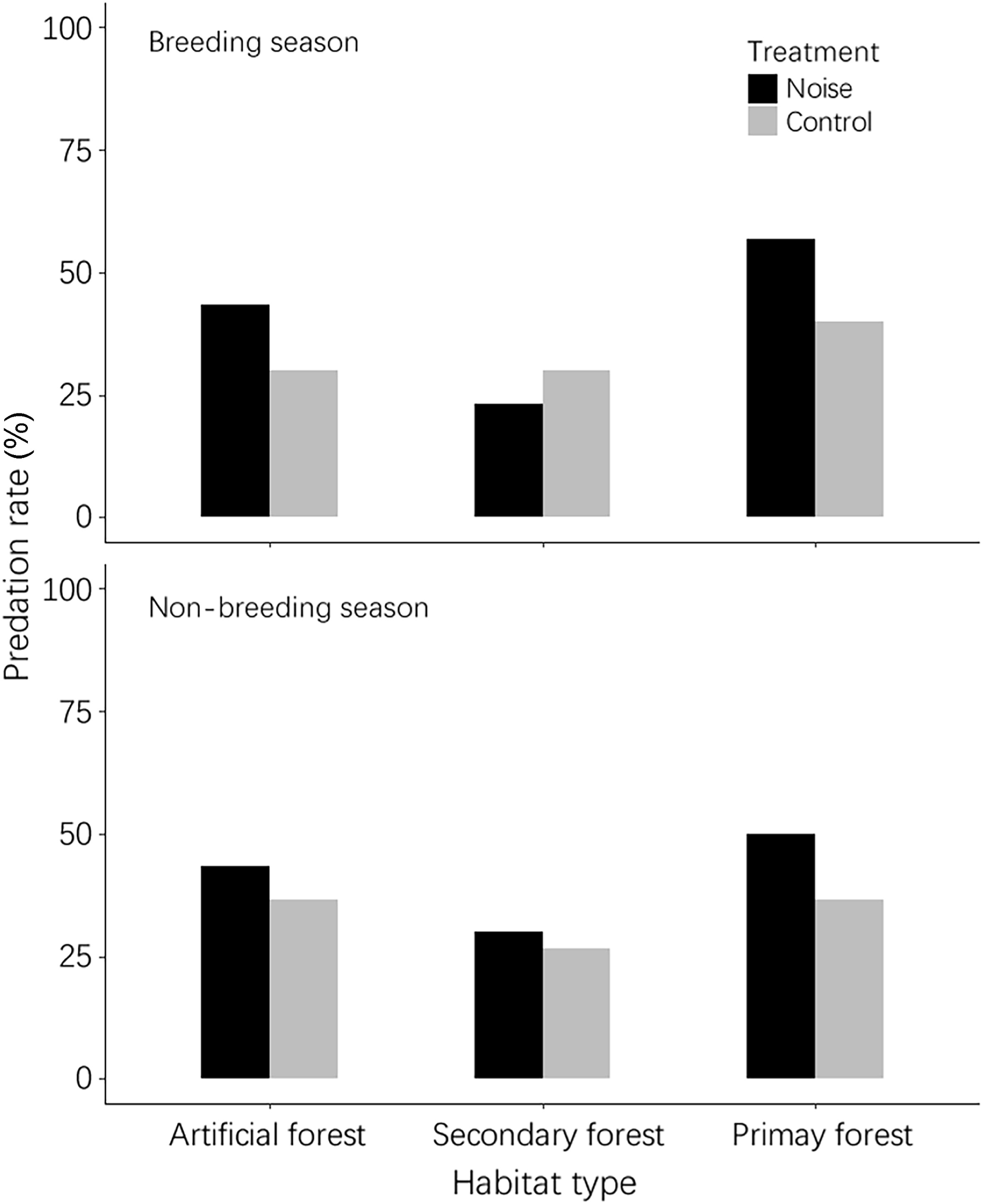
According to the results, the predation rates for artificial nests during the breeding season in the artificial pine forest were 43.33% (n = 30) and 30% (n = 30) for the noise and control treatments, respectively (Fig. 1). The corresponding values during the non-breeding season were 43.33% (n = 30) and 36.67% (n = 30), respectively. For the breeding season in the secondary mixed forest, the predation rates were 23.33% (n = 30) and 30% (n = 30) in the noise and control treatments, respectively. The values for the non-breeding season were 30% (n = 30) and 26.67% (n = 30), respectively. Finally, the predation rates for the noise and control treatments in the primary forest were 56.67% and 40% during the breeding season and 50% and 36.67% during the non-breeding season, respectively (Fig. 1). The results of MCMC-GLMMs indicate that neither the treatment, season, nor habitat predicted the predation rates of artificial nests (treatment: P-MCMC = 0.342; season: P-MCMC = 0.950; habitat: P-MCMC = 0.064 and 0.202). The interactions between treatment and season and between treatment and habitat did not show significant results (Table 1). Furthermore, the Cox model showed that none of the effects succeeded in predicting the survival probabilities for artificial nests (Table 2). Further analyses using a separate Cox model for each treatment achieved the same results; the 95% confidence intervals of all Kaplan–Meier curves largely overlapped between the treatments (Fig. 2).
| Effect | Reference variable | Compared variable | Posterior mean | Lower 95% CI | Upper 95% CI | P-MCMC |
| Intercept | 0.427 | 0.288 | 0.559 | <0.001 | ||
| Treatment | Noise | Control | −0.093 | −0.279 | 0.103 | 0.342 |
| Season | Breeding | Non-breeding | 0.004 | −0.125 | 0.137 | 0.950 |
| Habitat | Artificial forest | Secondary forest | −0.161 | −0.323 | 0.006 | 0.064 |
| Primary forest | 0.104 | −0.071 | 0.266 | 0.202 | ||
| Interaction | Noise × breeding | Control × non-breeding | −0.002 | −0.216 | 0.177 | 0988 |
| Noise × artificial | Control × secondary | 0.113 | −0.130 | 0.352 | 0.346 | |
| Control × primary | −0.054 | −0.263 | 0.205 | 0.636 | ||
| The random effect was the position of the artificial nests. Here, the 95% CI refers to the 95% credible interval of the Bayesian model. | ||||||
| Effect | Reference variable | Compared variable | Coefficient | S.E. | Z | P |
| Treatment | Noise | Control | 0.699 | 0.339 | −1.056 | 0.291 |
| Season | Breeding | Non-breeding | 0.945 | 0.234 | −0.241 | 0.809 |
| Habitat | Artificial forest | Secondary forest | 0.547 | 0.318 | −1.896 | 0.058 |
| Primary forest | 1.414 | 0.265 | 1.306 | 0.191 | ||
| Interaction | Noise × breeding | Control × non-breeding | 1.075 | 0.348 | 0.208 | 0.835 |
| Noise × artificial | Control × secondary | 1.465 | 0.458 | 0.833 | 0.405 | |
| Control × primary | 0.861 | 0.405 | −0.371 | 0.711 |
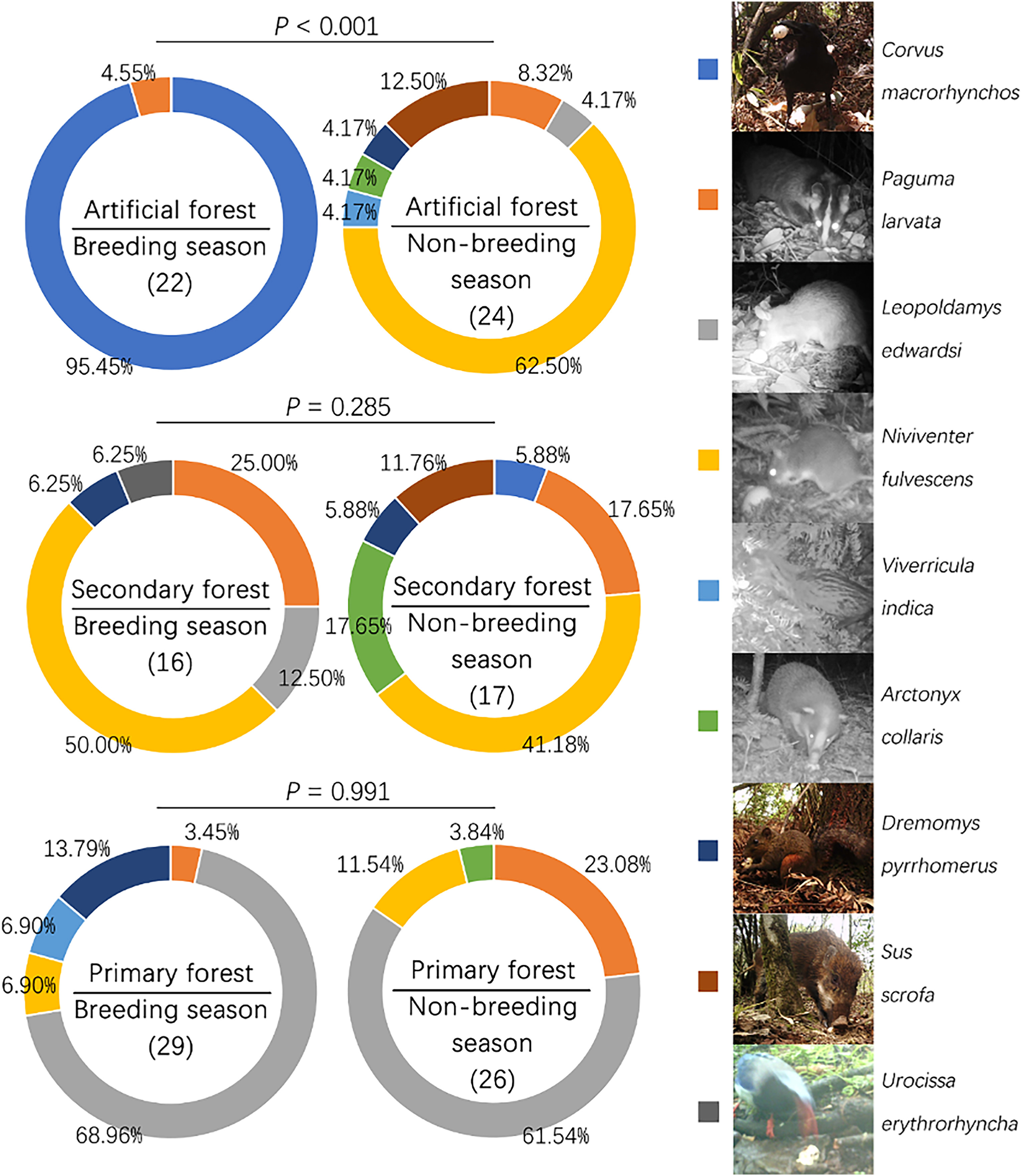
Nine species of nest predators were identified in this study: Large-billed Crow (Corvus macrorhynchos), Red-billed Blue Magpie (Urocissa erythrorhyncha), Chestnut White-bellied Rat (Niviventer fulvescens), Edwards' Long-tailed Giant Rat (Leopoldamys edwardsi), Himalayan Palm Civet (Paguma larvata), Small Indian Civet (Viverricula indica), Greater Hog Badger (Arctonyx collaris), Red-hipped Squirrel (Dremomys pyrrhomerus), and Wild Boar (Sus scrofa). Seven out of nine (77.8%) of these predators were mammals. The diversity of nest predators did not differ between the noise and control treatments (t = 0.526, df = 123.07, P = 0.600, Hutcheson's t-test). However, the predator diversity of the artificial forest was significantly higher in the non-breeding season than in the breeding season (t = −4.216, df = 38.96, P < 0.001, Hutcheson's t-test). The Large-billed Crow was the dominant nest predator during the breeding season, but seven of the other species were important predators during the non-breeding season (Fig. 3). During the non-breeding season, more than half of the predation cases involved Chestnut White-bellied Rats, while the other six mammal species accounted for the rest (Fig. 3). No significant differences were found between the breeding and non-breeding seasons for the secondary or primary forests (secondary forest: t = −1.088, df = 32.07, P = 0.285; primary forest: t = 0.012, df = 53.53, P = 0.991, Hutcheson's t-test; Fig. 3). Moreover, for both seasons combined, predator diversity was higher in secondary than primary forest (t = 2.291, df = 76.08, P = 0.025, Hutcheson's t-test), while it did not differ between artificial and secondary forests (t = −1.001, df = 73.42, P = 0.320, Hutcheson's t-test) or between artificial and primary forests (t = 1.355, df = 99.66, P = 0.179, Hutcheson's t-test). Additionally, the species of major predators differed between the habitats. The dominant nest predator in the secondary forest was the Chestnut White-bellied Rat, while it was Edwards's Long-tailed Giant Rat in the primary forest (Fig. 3). Finally, although most of these nest predators are nocturnal, the timing of nocturnal predation differed between treatments (F1,132 = 4.190, P = 0.043, Watson–William's test) and seasons (F1,132 = 8.404, P = 0.004, Watson–William's test) (Fig. 4). For the habitats, the timing of predation did not differ between the secondary and primary forests (F1,86 = 1.458, P = 0.231, Watson–William's test), while it differed between them and the artificial forests (Fig. 5).
Our results indicate that noise treatment did not alter the predation rates and survival probabilities of artificial nests, relative to the control treatment. Furthermore, the diversity of nest predators was not different between the two treatments. Therefore, our first hypothesis was not supported, i.e., anthropogenic noise did not intimidate or attract nest predators. This was a reasonable conclusion because most of the nest predators in this study were nocturnal mammals. The only two diurnal predators were the Large-billed Crow and Red-billed Blue Magpie, and the magpie occurred in only a few cases of predation during the breeding season in the secondary forest. Moreover, the experimental treatments were applied only during the daytime (08:00–18:00); thus, the treatments were unlikely to affect nocturnal predators. Additionally, the use of ground nests as artificial nests may also contribute to this result, because ground nests are more vulnerable to mammalian predators than tree nests (Söderström et al., 1998). Previous studies have reported that predators are more likely to detect ground nests because of their increased visibility, whereas shrub nests provide better concealment (Angelstam, 1986). However, the main predators in our study area are nocturnal, which seems to contradict this, as such predators are more likely to use olfactory cues than visual cues to hunt. A possible explanation may be that they forage mostly on the ground or close to the ground.
With the exception of the artificial forest, the diversity and major species of nest predators were consistent between seasons within habitats, but differed between habitats. This result may be due to the fact that the different forest habitats were occupied by different dominant species. This is reasonable because niche competition and segregation are common phenomena in nature (Austin, 1985; Brightsmith, 2005; Ye et al., 2019). Furthermore, it is possible that the predators may remember nest locations, and visit them in both seasons. However, this is highly unlikely because the time interval between the two seasons for our experiment was ca. Five months. It is hard to image that predator would visit the same location repeatedly in the five months after their first detection. Nevertheless, both the diversity and species composition of nest predators differed significantly between the breeding and non-breeding seasons in the artificial pine forests. Therefore, this result supports our second hypothesis, in that some nest predators may adapt to the breeding season. Alternatively, this result implies that species composition may not be as stable in artificial forests as in natural forests, i.e., seasonal changes in species composition may be more drastic in artificial forests than in natural forests. Previous studies have indicated that seasonal changes in the composition of tree species are more pronounced in artificial forests than in natural forests because artificial forests are usually composed of a few fast-growing and shade-tolerant species, whereas natural forests have a more diverse and complex structure (Kang et al., 2017). For animals, seasonal variation in population dynamics is greater in artificial forests than in natural forests because of habitat homogeneity, human activities, and mono-dominance (del Monte-Luna et al., 2004; Kazmierczak et al., 2016).
The third hypothesis of our study was partly supported by the fact that the diversity and species composition of nest predators mostly differed between the habitats. This is understandable because habitats differ in various ecological factors, such as habitat heterogeneity (Tews et al., 2004; Seibold et al., 2016). In addition, although most predation cases occurred at night, the predation time still differed between treatments, seasons, and some habitats. First, this may further contribute to the explanation of niche competition and segregation between nocturnal animals. Second, the optimal foraging time may vary between seasons because of environmental factors (including climate), leading to differences in predation time (Paez et al., 2018). The slight difference in predation time between the noise and control treatments is unlikely to be explained by the noise effect, because the noise playback was during in the daytime. However, whether or not noise has a delayed effect remains unknown; further studies are required to confirm this in the future. The third hypothesis was only partly supported, in that the predation rates did not differ significantly between the habitats. The density of the surrounding vegetation greatly impacts nest predation (Chotprasertkoon et al., 2017; Somsiri et al., 2019). Therefore, the lack of differences in predation rates between the habitats may be because we designed the experiment to have similar vegetation cover in the habitats, potentially reducing their differences in predation rates.
Finally, it is worth mentioning that snakes were not detected as nest predators in this study, even though snakes have previously been recorded as important nest predators (Weatherhead and Blouin-Demers, 2004; Sperry et al., 2008; DeGregorio et al., 2014). A possible reason for this is that the cooled eggs of artificial nests may not be attractive to snakes that rely on thermal detection to find prey. This is therefore another disadvantage of using artificial nests, and should be addressed in future studies.
CY conceived and designed the experiments, XY and NW conducted the experiments. YC performed data analyses and wrote the draft manuscript, and CY improve the manuscript. All authors read and approved the final manuscript.
The experiments reported here comply with the current laws of China. Fieldwork was carried out under permission from Kuankuoshui National Nature Reserves. Experimental procedures were in agreement with the Ethical Evaluation Group for Animal Behavior Study (protocol code: EEGABS-003).
The data presented in this study are available on request from the corresponding author.
The authors declare they have no competing interests.
We would like to thank Kuankuoshui National Nature Reserve for their help and cooperation. We thank the anonymous reviewers who have provided valuable suggestion to improve our manuscript.
|
Batáry, P., Winkler, H., Báldi, A., 2004. Experiments with artificial nests on predation in reed habitats. J. Ornithol. 145, 59–63.
|
|
Del Monte-Luna, P., Brook, B.W., Zetina-Rejón, M.J., Cruz-Escalona, V.H., 2004. The carrying capacity of ecosystems. Global Ecol. Biogeogr. 13, 485–495.
|
|
Dominoni, D., Smit, J.A.H., Visser, M.E., Halfwerk, W., 2020. Multisensory pollution: Artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environ. Pollut. 256, 113314.
|
|
Kang, D., Wang, X., Li, S., Li, J., 2017. Comparing the plant diversity between artificial forest and nature growth forest in a giant panda habitat. Sci. Rep. 7, 3561.
|
|
King, D.I., 1999. Do predation rates on artificial nests accurately reflect predation rates on natural bird nests? J. Field Ornithol. 70, 257–262.
|
|
Lambrechts, M.M., Adriaensen, F., Ardia, D.R., Artemyev, A.V., Atiénzar, F., Bańbura, J., et al., 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: A review of methodological inconsistencies and potential biases. Acta Ornithol. (Wars.) 45, 1–26.
|
|
Major, R.E., Kendal, C.E., 2008. The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138, 298–307.
|
|
Raap, T., Pinxten, R., Casasole, G., Dehnhard, N., Eens, M., 2017. Ambient anthropogenic noise but not light is associated with the ecophysiology of free-living songbird nestlings. Sci. Rep. 7, 2754.
|
|
Söderström, B., Pärt, T., Rydén, J., 1998. Different nest predator faunas and nest predation risk on ground and shrub nests at forest ecotones: an experiment and a review. Oecologia 117, 108–118.
|
|
Somsiri, K., Gale, G.A., Pierce, A.J., Khamcha, D., Sankamethawee, W., 2019. Habitat structure affects nest predation of the Scaly-crowned Babbler (Malacopteron cinereum) by macaques and snakes in a Thai-seasonal evergreen forest. J. Ornithol. 161, 389–398.
|
|
Swaddle, J.P., Francis, C.D., Barber, J.R., Cooper, C.B., Kyba, C.C., Dominoni, D.M., et al., 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560.
|
|
Villain, A.S., Fernandez, M.S., Bouchut, C., Soula, H.A., Vignal, C., 2016. Songbird mates change their call structure and intrapair communication at the nest in response to environmental noise. Anim. Behav. 116, 113–129.
|
|
Weatherhead, P.J., Blouin-Demers, G., 2004. Understanding avian nest predation: why ornithologists should study snakes, J. Avian Biol. 35, 185–190.
|
|
Weidinger, K., 2009. Nest predators of woodland open-nesting songbirds in central Europe. Ibis 151, 352–360.
|
|
Yang, C., Wang, J., Liang, W., 2016. Blocking of ultraviolet reflectance on bird eggs reduces nest predation by aerial predators. J. Ornithol. 157, 43–47.
|
|
Yang, C., Ye, P., Huo, J., Møller, A.P., Liang, W., Feeney, W.E., 2020. Sparrows use a medicinal herb to defend against parasites and increase offspring condition. Curr. Biol. 30, R1411-R1412.
|
|
Ye, P., Cai, Y., Wu, N., Yao, X., Li, G., Liang, W., et al., 2022. Egg rejection based on egg size recognition as a specific strategy against parasitic cuckoos. Curr. Zool. 69, zoac037.
|
| Effect | Reference variable | Compared variable | Posterior mean | Lower 95% CI | Upper 95% CI | P-MCMC |
| Intercept | 0.427 | 0.288 | 0.559 | <0.001 | ||
| Treatment | Noise | Control | −0.093 | −0.279 | 0.103 | 0.342 |
| Season | Breeding | Non-breeding | 0.004 | −0.125 | 0.137 | 0.950 |
| Habitat | Artificial forest | Secondary forest | −0.161 | −0.323 | 0.006 | 0.064 |
| Primary forest | 0.104 | −0.071 | 0.266 | 0.202 | ||
| Interaction | Noise × breeding | Control × non-breeding | −0.002 | −0.216 | 0.177 | 0988 |
| Noise × artificial | Control × secondary | 0.113 | −0.130 | 0.352 | 0.346 | |
| Control × primary | −0.054 | −0.263 | 0.205 | 0.636 | ||
| The random effect was the position of the artificial nests. Here, the 95% CI refers to the 95% credible interval of the Bayesian model. | ||||||
| Effect | Reference variable | Compared variable | Coefficient | S.E. | Z | P |
| Treatment | Noise | Control | 0.699 | 0.339 | −1.056 | 0.291 |
| Season | Breeding | Non-breeding | 0.945 | 0.234 | −0.241 | 0.809 |
| Habitat | Artificial forest | Secondary forest | 0.547 | 0.318 | −1.896 | 0.058 |
| Primary forest | 1.414 | 0.265 | 1.306 | 0.191 | ||
| Interaction | Noise × breeding | Control × non-breeding | 1.075 | 0.348 | 0.208 | 0.835 |
| Noise × artificial | Control × secondary | 1.465 | 0.458 | 0.833 | 0.405 | |
| Control × primary | 0.861 | 0.405 | −0.371 | 0.711 |