| Citation: | Jorge Garrido-Bautista, Carmen Hernández-Ruiz, José Luis Ros-Santaella, Eliana Pintus, Nicola Bernardo, Mar Comas, Gregorio Moreno-Rueda. 2023: Habitat-dependent breeding biology of the Blue Tit (Cyanistes caeruleus) across a continuous and heterogeneous Mediterranean woodland. Avian Research, 14(1): 100109. DOI: 10.1016/j.avrs.2023.100109 |
Mediterranean woodland environments are characterised by high spatial and temporal heterogeneity, which means the inhabiting species face a wide variety of selective pressures. Species may respond differently to habitat heterogeneity and so distinct eco-evolutionary scenarios may be responsible for the inter-habitat variability in reproductive strategies observed in certain species. The inter-forest variability of some reproductive traits in passerines has been examined by comparing forest patches or separated fragments. However, there is still little information regarding how such highly mobile animals adjust their breeding performance across continuous and heterogeneous woodlands. Here we studied the reproductive performance of a population of Blue Tits (Cyanistes caeruleus) in an area of continuous Mediterranean woodland that included two mountain slopes and four different types of forest, ranging from deciduous oak forests to perennial non-oak forests. We studied the habitat heterogeneity and inter-forest phenotypic variation in terms of reproductive performance and adult and nestling biometry, besides also exploring the effects of ectoparasites on Blue Tit reproduction. Eggs were laid earliest in deciduous Pyrenean Oak (Quercus pyrenaica) forests, while clutch size and the number of fledglings were highest in the humid Pyrenean Oak forest, which had the greatest tree coverage and most humid climate, and lowest in the coniferous Scots Pine (Pinus sylvestris) forest. There were no inter-forest differences in hatching (percentage of nests with at least one egg hatched) and fledging (percentage of nests in which at least one nestling fledged) success. Similarly, there were no inter-forest differences in adult and nestling biometry, but adults that raised more fledglings had a lower body mass, while males whose females laid larger clutches had smaller tarsi. Most ectoparasites did not affect Blue Tit reproduction, although Culicoides had a negative impact on nestling body mass. These results suggest that Blue Tits can adjust their reproductive effort to the forest where they breed even across a very small spatial scale. Different eco-evolutionary scenarios, such as phenotypic plasticity or genetic structuring and local adaptation, might explain the phenotypic differentiation in the reproductive strategies observed over small areas in woodlands.
Reproductive isolation is a key factor in speciation, as it prevents interbreeding between populations and allows for the accumulation of differences (Mayr, 1942). While morphological features are implicated in reproductive isolation across most animals, bioacoustic characters can be of primary importance in some bird families (Isler et al., 1998; Rheindt et al., 2008; Ng et al., 2016), especially in nocturnal ones such as owls (Strigidae) in which optical cues are less relevant (Howell and Robbins, 1995; King, 2002; Sangster et al., 2013; Pons et al., 2013; Flint et al., 2015; Krabbe, 2017; Gwee et al., 2017, 2019; Dantas et al., 2021; Movin et al., 2022).
Properly diagnosing species-specific differences in vocalizations is key in correct species delimitation in optically cryptic assemblages such as owls. Making sense of the geographic variation in bioacoustic traits can be challenging when such characters are distributed in complex ways, such as when they form a leapfrog pattern (Remsen, 1984). A leapfrog pattern is one in which two populations at opposing ends of a distribution range resemble each other in certain character traits, but are separated by intervening populations that differ in these traits, either along a geographic continuum or in a non-clinal fashion (Remsen, 1984). While most leapfrog patterns documented by evolutionary biologists refer to morphological characters, bioacoustic leapfrog patterns are known from various tropical bird groups (Cadena and Cuervo, 2010; Rheindt et al., 2011).
Little is known about the mechanisms that create leapfrog patterns; they may be implicated in speciation events (Remsen, 1984; Rheindt et al., 2011). The traits of the terminal populations that are geographically distant may reflect the ancestral character state, while populations in the middle may be on their way to becoming a different species (if they have not already speciated), and hence diverge in specific characters from populations on either side. Studying bioacoustic leapfrog pattern could be a promising avenue to provide insights into the mechanisms of speciation in cryptic bird groups such as owls. In this study, we uncover a vocal leapfrog pattern in the Collared Scops Owl (Otus bakkamoena) complex that has long been overlooked, and we shed light on the turn-over of bioacoustic traits in this confusing group, which has been marked by much taxonomic confusion.
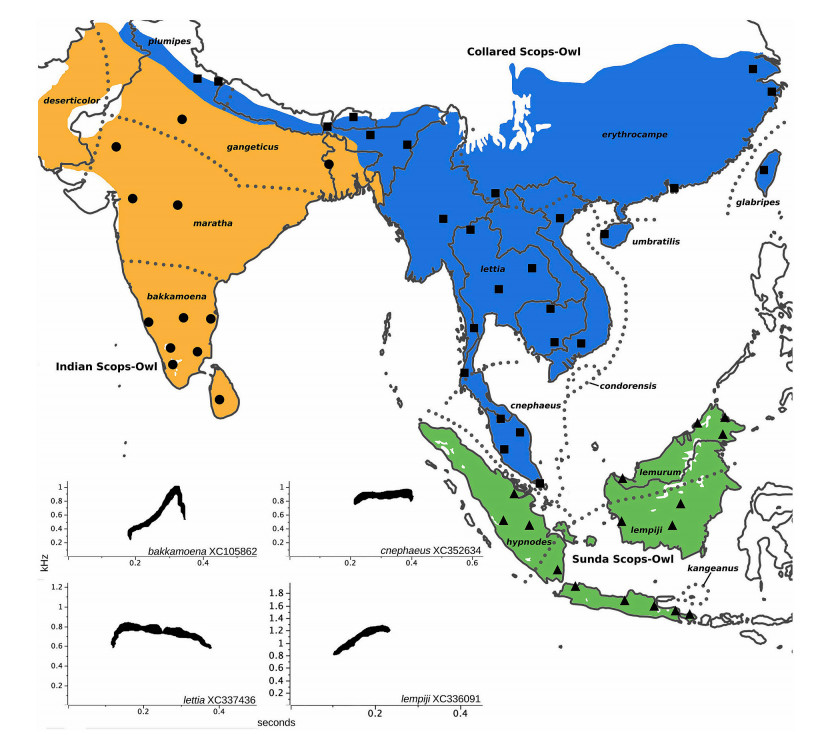
The Collared Scops Owl complex, which has been one of the taxonomically most controversial owl assemblages, ranges across tropical and subtropical Asia. It comprises roughly 15 subspecies-level taxa (Fig. 1), often divided into anywhere between 1 and 3 species-level lineages including the Indian Scops Owl (O. bakkamoena) from the Indian subcontinent, the Collared Scops Owl (O. lettia) from the Himalayan foothills and large parts of subtropical East and Southeast Asia, and the Sunda Scops Owl (O. lempiji) from tropical Southeast Asia (Fig. 1) (Robson and Allen, 2005; Eaton et al., 2021; Clements et al., 2022; Handbook of the Birds of the World & BirdLife International, 2022). An alternative taxonomic treatment has divided the complex into four species, with the 3-species split outlined above in addition to the separation of cnephaeus from the Malay Peninsula and Singapore into a monotypic species (König and Weick, 2008).

Vocal variation within the Collared Scops Owl complex has been described in multiple ornithological treatises and studies (Najmi-Hanis et al., 2016; Yee et al., 2016; Eaton et al., 2021), but has thus far not resulted in one unequivocal taxonomic interpretation. In this study, we used a quantitative bioacoustic approach to characterize the territorial vocalisations of the Collared Scops Owl complex across its range. We used these new insights to revise the taxonomy of the entire complex, emulating work on multiple other owl complexes (Howell and Robbins, 1995; King, 2002; Rasmussen et al., 2022; Pons et al., 2013; Sangster et al., 2013; Flint et al., 2015; Sadanandan et al., 2015; Krabbe, 2017; Gwee et al., 2019; Dantas et al., 2021; Movin et al., 2022) and other Asian bird species (Ng et al., 2016; Ng and Rheindt, 2016; Prawiradilaga et al., 2018; Rheindt et al., 2011).
We compiled 213 sound recordings from the online sound library xeno-canto (https://www.xeno-canto.org) filed under O. lempiji, O. lettia and O. bakkamoena (Appendix Table S1). To ensure measurements of vocal homologs across the complex, we focused only on their territorial call—a single short note. Recordings that did not feature the territorial call were removed. Many birds, including Otus owls, are known to return to the same breeding site with the same partner for many years (Greenwood, 1980; Linkhart and Reynolds, 2007). To avoid duplicating vocalisations of the same individual, only one recording made by the same person at the same site and time was retained, and we additionally retained only one out of multiple recordings within a five-year period if they were taken at a narrow specific locality. In total we measured 122 recordings of the main territorial call for quantitative vocal analysis, with each recording representing one individual, after removal of poor-quality recordings (Fig. 1; Appendix Table S1).
We used Raven Pro 1.5 (Bioacoustics Research Program, Cornell Laboratory of Ornithology, Ithaca, NY, USA) to inspect and measure parameters of each recording. Although different recording equipment was used among recordists, spectrogram inspection revealed that there was negligible equipment bias. All notes made by the same individual were measured up to 20 times, when available, to account for within-individual variation. With the exception of the window size being set to 1000 to obtain the best resolution across all recordings, default settings of Raven Pro were used. The contrast and brightness of the sonograms were adjusted to ensure measurements were carried out under similar settings across all recordings. We measured a total of six parameters: (1) lowest frequency of the call; (2) highest frequency of the call; (3) duration of the call; (4) peak frequency of the call; (5) change in peak frequency between the first third and second third of the call; (6) change in peak frequency between the second third and the last third of the call. As preliminary inspection of the sonograms revealed distinct element shapes in different populations (Fig. 1), we used (5) and (6) to quantify this change.
R 3.3.2 (R Core Team, 2009) was used to conduct principal component analysis (PCA). We also computed Isler et al.’s (1998) vocal diagnosability criterion, commonly known as the Isler criterion. This criterion has been used in bioacoustic species delimitation across a wide variety of Asian birds, including owls (Gwee et al., 2017, 2019; Movin et al., 2022). The Isler criterion uses the standard deviation of the sample points rather than the taxon mean, and as such is substantially more discriminating than the t-test or the Mann-Whitney U test (Isler et al., 1998; Rheindt et al., 2011; Ng and Rheindt, 2016; Gwee et al., 2017).
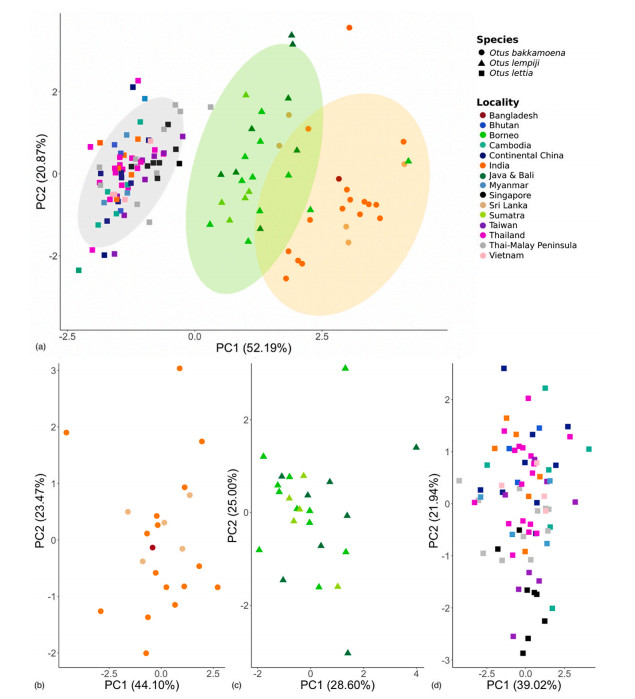
On the basis of the six vocal parameters analysed herein, we found three independent PCA clusters that correspond largely but not entirely with the existing species level division of the complex into O. bakkamoena, O. lempiji and O. lettia (Fig. 2A). The first and second principal components explained 52.2% and 20.9% of data variation, respectively (Fig. 2A). The greatest difference to the traditional taxonomic arrangement related to the taxon from the Malay Peninsula and Singapore, cnephaeus, long classified under O. lempiji, but in our analysis grouping with the continental Southeast Asian species O. lettia instead. Our bioacoustic data also revealed that the two geographically disjunct species O. bakkamoena from the Indian subcontinent and O. lempiji from the Indonesian Archipelago (Fig. 1) are more similar to each other as compared to the vocally more divergent but geographically intervening species O. lettia (Fig. 2A).
A closer bioacoustic analysis within each species cluster did not reveal additional vocal population structure in O. bakkamoena and O. lempiji (Fig. 2B and C). Within O. lettia, on the other hand, a geographical cline appeared to emerge along PC2 from the south of the range (Singapore, Thai-Malay Peninsula) towards the north (Himalayan foothills, continental China) (Fig. 2D). This result was consistent with conclusions from qualitative vocal inspections showing that the element shape of the territorial call slowly changes from a flat note in the southern Malay Peninsula to a rising inflection as one moves north (Fig. 1).
Means and standard deviations of all six vocal measurements varied among taxa (Fig. 3). The measurements between O. bakkamoena and O. lettia were mostly non-overlapping, while O. lempiji was often intermediate between the other two in vocal parameter measurements (Fig. 3). Pairwise t-tests comparing all six parameters between the taxa demonstrated significant differences (p ≤ 0.05) for most comparisons, with the exception of comparisons between O. lettia and O. lempiji in lowest frequency as well as O. bakkamoena and O. lempiji in highest and peak frequency (Appendix Table S2). However, under the Isler criterion, shown to be more discriminating than the common t-test (Isler et al., 1998), no diagnosable traits were found in any pairwise comparisons.
We provide a quantification of territorial call variation of the members of the Collared Scops Owl complex across its natural range (Fig. 1). We identified three call shapes—‘inflected’, ‘deflected’ and ‘level’ (Fig. 1)—using vocal parameters that had been used to successfully delimit other owl species (Gwee et al., 2019; Movin et al., 2022), partly in conjunction with genomic results (Gwee et al., 2017).
The three commonly accepted species-level taxa of the Collared Scops Owl complex occur along an east-west axis from the Indian subcontinent across continental Southeast Asia down to the western Indonesian archipelago (Fig. 1). We show that the two terminal species, O. bakkamoena in the west and O. lempiji in the east, are vocally closer to each other than towards the geographically intervening O. lettia (Fig. 2). In many respects, O. lempiji was intermediate between O. bakkamoena and O. lettia in measurements of six vocal parameters, with a few exceptions (Fig. 3; Appendix Table S2). We identified distinct element shapes in territorial calls diagnosable even by the human eye and ear for each species-level taxon (Fig. 1). The fact that the geographically central O. lettia, with its vigorous and often deflected call note, stood out within the complex from the two peripheral forms characterized by a more inflected call note points to a bioacoustic leapfrog pattern (Cadena and Cuervo, 2010; Rheindt et al., 2011).
In a classical study, Remsen (1984) concluded that leapfrog patterns primarily evolve in regions characterized by geographic linearity, topographic relief and high species riches. Meanwhile, bioacoustic leapfrog patterns in particular have also been detected in insular settings (Rheindt et al., 2011). Here we showcase a vocal leapfrog pattern across the continental scale in a bird group with a broad elevational tolerance, supporting the notion that this pattern may be much more pervasive in birds than currently assumed.
In owls vocalisations are innate and play an important role in mate selection; they are thus thought to be under strong selective pressure (König and Weick, 2008). Vocal differentiation can act as a mating barrier between populations, potentially leading to speciation. In Collared Scops Owl vocalizations, the geographically terminal phenotype, i.e., the inflected call note of O. lempiji and O. bakkamoena, is likely plesiomorphic (=ancestral) while the central populations (O. lettia) evolved a novel phenotype, a deflected call note, possibly promoted by character displacement near the range limits between the three evolving species to prevent hybridisation in contact zones. This observation particularly applies to the contact zone between O. lettia and O. bakkamoena, whose deflected and inflected call notes, respectively, meet quite abruptly along a narrow ecotone in the Himalayan foothills of the northern Indian subcontinent (Rasmussen and Anderton, 2005).
The contact zone between O. bakkamoena and O. lettia runs along the foothills of the Himalayas, a common suture zone between species pairs in birds, e.g., between Red (Gallus gallus) and Grey Junglefowl (G. sonneratii) (McGowan and Kirwan, 2020a, 2020b), or between Blyth's (Terpsiphone affinis) and Indian Paradise-Flycatcher (T. paradisi) (Moeliker et al., 2020; Rasmussen et al., 2022). The vocal turn-over between the two scops owls here is sharp and occurs across only a few kilometers (Rasmussen and Anderton, 2005).
In contrast, the geographic boundary between O. lettia and O. lempiji is much less well understood. Most current taxonomic treatments assign the taxon cnephaeus from the Malay Peninsula to O. lempiji, which would place the boundary between the two species somewhere along the Isthmus of Kra between the Thai-Malay Peninsula and the main Southeast Asian continent (Holt et al., 2020a, 2020b). However, our bioacoustic data place samples of cnephaeus from Singapore and the Thai-Malay Peninsula firmly within O. lettia, suggesting that the actual turnover zone is across the Singapore Straits that cut between Sumatra and the Peninsula (Figs. 1 and 2).
Qualitatively, the southern peninsular and Singaporean populations of the taxon cnephaeus have a unique vocal signature, characterized by a fairly level-pitched call, which gradually and clinally shifts into a deflected call note as one moves north along the Peninsula until one reaches populations near the Isthmus of Kra, which are the first to sound unequivocally deflected like other populations of O. lettia (Fig. 1). Conversely, when moving from the southernmost tip of the Peninsula across the Singapore Straits to Sumatra, there is a sharp turn-over from the level-pitched call of the taxon cnephaeus to the distinctly inflected call note of O. lempiji, which is reflected in the distant placement of Sumatran and Singaporean samples in the bioacoustic PCA (Fig. 2A).
The taxon cnephaeus from the Thai-Malay Peninsula and Singapore has previously been identified as distinct from other surrounding taxa (Deignan, 1950), and has even been mooted to warrant recognition at the species level due to its smaller body size, other morphological traits and its distinct level-pitched call (Wells, 1999; König and Weick, 2008). Our more comprehensive bioacoustic inquiry now reveals that this level call, however, is connected to more deflected call notes to the north through a gradual geographic cline, supporting subspecies status of cnephaeus under O. lettia, as reflected in bioacoustic PCA (Figs. 1 and 2).
Many tropical Asian bird species complexes are divided into an equatorial rainforest species centred around the western Indonesian Archipelago and a closely related monsoon-forest inhabiting species from the Asian mainland (Eaton et al., 2021). Whenever birds display such a biogeographic constellation, the Sundaic species virtually always extends north to the Thai-Malay Peninsula and reaches its northern limit at the Isthmus of Kra, where the range of the continental monsoon species takes over. Owls of the Collared Scops Owl complex were long thought to follow this geographic pattern, with cnephaeus from the Thai-Malay Peninsula invariably assigned to O. lempiji from Indonesia. However, our bioacoustics-based reassignment of cnephaeus to O. lettia suggests that these scops owls defy this biogeographic pattern, as the continental O. lettia extends south all the way to Singapore (Fig. 1).
Collared Scops Owls may not be the only owl group that departs from this biogeographic rule. In another recent taxonomic revision based on bioacoustic characters, Gwee et al. (2019) divided the Collared Owlet (Glaucidium brodiei) into two species, a continental one reaching south to the Thai-Malay Peninsula and an archipelagic one restricted to Sumatra and Borneo in the Indonesian Archipelago. A third such biogeographic arrangement is not universally accepted and relates to the Brown Wood Owl (Strix leptogrammica) from tropical and subtropical Asia, which was recently separated into a mainland species (Brown Wood Owl S. indranee) and an Indonesian archipelagic species (Sunda Wood Owl S. leptogrammica) based on pronounced differences in call notes (Eaton et al., 2021). The reasons why owls defy the usual biogeographic pattern and have Malay peninsular populations that are related to Asian mainland species rather than Indonesian archipelagic ones is puzzling and will require future inquiry.
The results of our vocal analysis of the Collared Scops Owl species complex allow for taxonomic conclusions. A genetic study detected limited divergence in this complex (Fuchs et al., 2008), which has tempered many taxonomists’ desire to divide the species complex based on bioacoustic differences that have been known for decades. However, the genetic signature was dominated by the information signal of mitochondrial DNA, which can be subject to biases, especially as relates to shallow divergences caused by genetic introgression events masking deeper genomic divergence (e.g., Rheindt and Edwards, 2011). Our analyses demonstrate deep bioacoustic differences among the three main species-level groups within the complex, albeit with a reassignment of cnephaeus from O. lempiji to O. lettia. Vocal turn-over is steep wherever the three species come close to a neighbour, and our vocal PCA dispels the notion of any bioacoustic clinality among the three species. The only vocal cline detected was within O. lettia as here circumscribed, including populations with a deflected call note over most of the range gradually turning to a more level-pitched call note on the Thai-Malay Peninsula within the range of cnephaeus. Our lack of vocal material for the taxon condorensis, confined to Con Son Island off the southern tip of Vietnam, precludes a certain assignment to either species. But given the location of this island on the Sunda Shelf at the northern limit of a former rainforest bloc of subcontinental proportions, and based on the results of recent genomic analyses shedding light on the affinities of taxa on nearby islands (Garg et al., 2022), we provisionally retain the most widely-followed treatment of condorensis as under O. lempiji.
The following is our proposed taxonomic treatment of the Collared Scops Owl (O. bakkamoena) complex. Distributions of all the taxa involved are shown in Fig. 1:
● Otus bakkamoena Pennant, 1769: Indian Scops Owl
○ O. b. bakkamoena Pennant, 1769: southern India and Sri Lanka
○ O. b. gangeticus Ticehurst, 1922: northern India along the Ganges drainage
○ O. b. marathae Ticehurst, 1922: northern peninsular India
○ O. b. deserticolor Ticehurst, 1922: arid northwestern India and Pakistan
● Otus lettia (Hodgson, 1836): Collared Scops Owl
● O. l. lettia (Hodgson, 1836): central Himalayan foothills through Myanmar to Indochina
● O. l. plumipes Hume, 1870: western Himalayan foothills
● O. l. erythrocampe (Swinhoe, 1874): southern Chinese mainland, northern Vietnam
● O. l. glabripes (Swinhoe, 1870): Taiwan Island
● O. l. umbratilis (Swinhoe, 1870): Hainan Island
● O. l. cnephaeus Deignan (1950): Thai-Malay Peninsula, Singapore
● Otus lempiji (Horsfield, 1821): Sunda Scops Owl
● O. l. lempiji (Horsfield, 1821): Java, Bali, southern Sumatra, southern Borneo
● O. l. hypnodes Deignan (1950): northern and central Sumatra
● O. l. lemurum Deignan, 1950: northern Borneo
● O. l. kangeanus Mayr, 1942: Kangean Islands
● O. l. condorensis Kloss, 1930: Con Son Island (= Condor Island, Vietnam)
Meng Yue Wu: Data curation, Methodology, Writing – original draft, Writing – review & editing. Frank E. Rheindt: Conceptualization, Methodology, Writing – review & editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are indebted to the recordists who have submitted their sound material to xeno-canto sound archive (https://www.xeno-canto.org). We thank Geraldine Lee for her preliminary exploration of the vocalizations, and Movin S/O Nyanasengeran for general assistance.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100141.
|
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R.H.B., Singmann, H., et al., 2020. lme4: linear mixed-effects models using “Eigen” and S4. R package version 1.1-23.
|
|
Blondel, J., Aronson, J., Bodiou, J.Y., Boeuf, G., 2010. The Mediterranean Region: Biological Diversity in Space and Time. Oxford University Press, Oxford.
|
|
Blondel, J., Clamens, A., Cramm, P., Gaubert, H., Isenmann, P., 1987. Population studies on tits in the Mediterranean region. Ardea 75, 21-34.
|
|
Dias, P.C., Meunier, F., Beltra, S., Cartan-Son, M., 1994. Blue tits in Mediterranean habitat mosaics. Ardea 82, 363-372.
|
|
Gezelius, L., Grahn, M., Källander, H., Karlsson, J., 1984. Habitat-related differences in clutch size of the pied flycatcher Ficedula hypoleuca. Ann. Zool. Fenn. 21, 209-212.
|
|
GRASS Development Team, 2020. Geographic Resources Analysis Support System (GRASS) Software v 7.8.2. Open Source Geospatial Foundation.
|
|
Hansson, L., Fahrig, L., Merriam, G., 1995. Mosaic landscapes and ecological processes. Chapman and Hall, London.
|
|
Moreno-Rueda, G., 2003. Seleccion de cajas-nido por aves insectivoras en Sierra Nevada. Zool. Baetica 13/14, 131-138.
|
|
Orshan, G., 1989. Plant Pheno-morphological Studies in Mediterranean Type Ecosystems. Springer Netherlands, Dordrecht.
|
|
Perrins, C.M., 1979. British Tits. Collins, Glasgow.
|
|
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., R Core Team, 2019. Nlme: linear and Nonlinear mixed effects models. R package version 3.1-141.
|
|
Pitala, N., Siitari, H., Gustafsson, L., Brommer, J.E., 2009. Ectoparasites help to maintain variation in cell-mediated immunity in the blue tit-hen flea system. Evol. Ecol. Res. 11, 79-94. https://doi.org/.
|
|
QGIS Development Team, 2020. QGIS Geographic Information System v. 3.10.5. Open Source Geospatial Foundation.
|
|
R Development Core Team, 2020. R: A language and environment for statistical computing.
|
|
Stearns, S.C., 1992. The Evolution of Life Histories. Oxford University Press, New York.
|
|
Stenning, M., 2018. The blue tit, first ed. Bloomsbury Publishing Plc, London.
|
|
Wickman, H., 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York.
|
|
Zuur, A.F., Ieno, E.N., Walker, N., Saveliev, A.A., Smith, G.M., 2009. Mixed Effects Models and Extensions in Ecology with R. Statistics for Biology and Health. Springer, New York.
|