| Citation: | Huan Xia, Cecilia Nilsson, Kasper Thorup, Chenxi Jia, Fumin Lei. 2023: Non-breeding movements of the Black-tailed Gull (Larus crassirostris). Avian Research, 14(1): 100103. DOI: 10.1016/j.avrs.2023.100103 |
With the continued development of tracking technology and increasing interest in animal movement, our understanding of migration behavior has become more comprehensive. However, there are still many species that have not been well studied, particularly sea birds. Here, we present the first year-round Global Positioning System (GPS) tracking data of the Black-tailed Gull (Larus crassirostris) at the population level. We used solar-powered GPS-Global System for Mobile communication (GSM) loggers to successfully track 30 individuals breeding at Xingrentuo Islet, Liaoning Province, China, for 1–3 years. Except for one individual who roamed in the far north of the Yellow Sea during non-breeding period, all others did a directed southward migration. Migration routes and wintering sites differed among migrating gulls and between years for the birds tracked for two or more years. Additionally, during wintering, the migrating gulls were more likely to travel over a large body of water and shift sites, and some trajectories were quite complex, which was probably closely related to what we observed in the field about their boat-chasing behavior. Compared to wintering movements, the post-breeding movements ranged over a smaller area. Specifically, almost all of them had a long post-breeding period near the breeding islet (≥120 days, <220 km from the breeding islet), and 80% of the gulls who were tracked more than one year had at least one faithful post-breeding site. Compared to the post-breeding period, only approximately half of the migrating gulls had a pre-breeding period that was shorter (3–20 days) and closer to the breeding islet (≤80 km). Migration distance varied among migrating gulls (range 209–2405 km) and the gulls moved least distance during post-breeding period. Furthermore, we found that the southward movement of the migrating gulls occurred when the temperature near the breeding islet dropped; specifically, the gulls directly migrated southward away from the post-breeding site. Our results suggest that the Black-tailed Gull has a long post-breeding period but a short pre-breeding period near the breeding islet and high diversity of their migrating patterns (in especial migration routes and wintering sites).
As tracking technology evolves, an increasing number of migratory patterns have been described and researched (Newton, 2008), and the understanding of migration behavior has become more detailed (e.g., Hallworth and Marra, 2015). Current tracking technologies allow us to track migrants throughout the annual cycle. In particular, knowledge on movements during the non-breeding period would help us to understand how the trade-offs and effects of organismal and environmental factors (Drent, 2006). From a life-history perspective, these trade-offs and effects may underlie variation in migration strategies (Studds et al., 2008; Newton, 2011; Baert et al., 2018). The advance of tracking technologies has also triggered great progress in seabird migration ecology, which has lagged behind the research of terrestrial species for a long time (Frederiksen et al., 2016). Previous studies have focused mainly on the movements of wide-ranging and long-distance migrating seabirds (Shaffer et al., 2006; González-Solís et al., 2007; Egevang et al., 2010; Weimerskirch et al., 2015; Fayet et al., 2017). For many short-distance migrating species, such as the Black-tailed Gull (Larus crassirostris), the movements are unclear.
The Black-tailed Gull is a medium-sized seabird, distributed mainly in East Asian waters. The gulls breed mainly in China, eastern Russia, Japan, and Korea (Wang et al., 1991; Zhang et al., 2000; Lee et al., 2006; Kazama et al., 2008; Burger et al., 2020) and winter mainly in the northeast China Sea, Korea Strait, the Sea of Japan, south of the Chinese coast, Taiwan of China, and the Japanese Pacific coast (Burger et al., 2020). The global population of the Black-tailed Gull has been estimated at 1,100,000 (Langendoen et al., 2021). They are quite common in the coastal area of East Asia. Nevertheless, this population has received little attention or research, and only a few studies have been reported. Except for one study that reported year-round activities (Kazama et al., 2013), others focused mainly on reproductive behavior (e.g., Wang et al., 1991; Zhang et al., 2000; Lee et al., 2006; Kazama et al., 2008; Mizota, 2009). Thus, little is known about the migration behavior, of the gulls especially when and where they go throughout their annual cycle.
Here, we present the first GPS tracking data from 30 Black-tailed Gulls tracked for 1–3 years to study their non-breeding movements among individuals at the population level and the same individuals across years. Specifically, we focused on movement and repeatability of the post-breeding, wintering and pre-breeding periods. Additionally, we hypothesized that weather conditions could have a major impact on southward movement (Vähätalo et al., 2004; Newton, 2008). Hence, we investigated the potential effect of temperature on the departure date for wintering. Furthermore, combined with our field observations and GPS data analysis, we discuss the effects of human activities on the behavior of Black-tailed Gulls during non-breeding period.
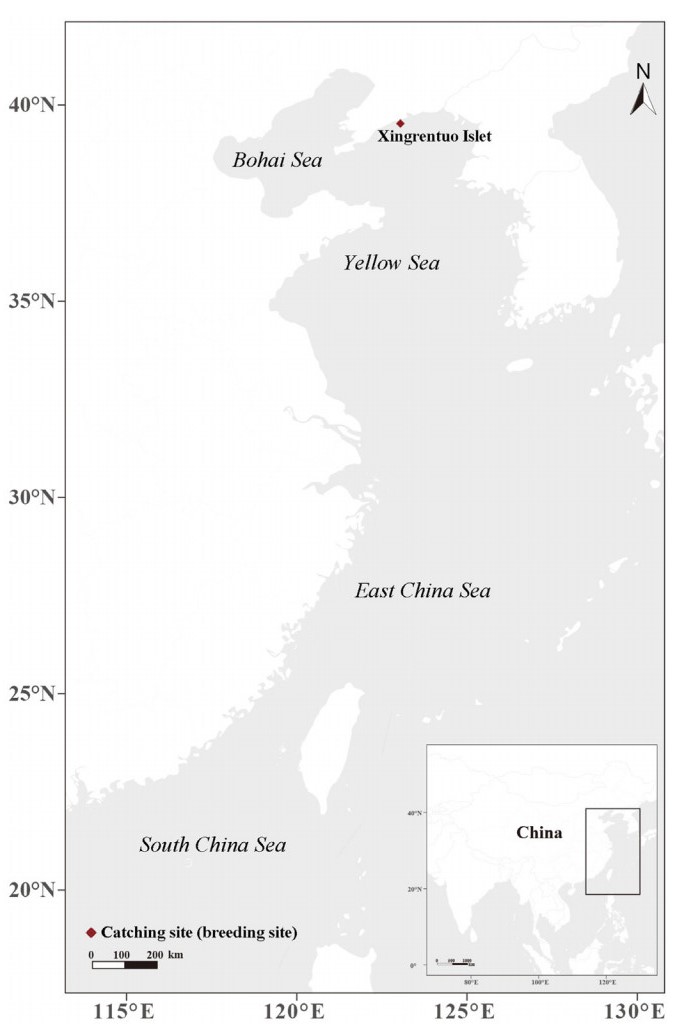
During the 13th–15th of June 2017 and 7th–11th of June 2018, 44 adult Black-tailed Gulls were caught near nests in their breeding area (Xingrentuo Islet, Liaoning Province, China; 39.528° N, 123.043° E; Fig. 1) using string noose traps. We set a string noose (approximately 10 m) on the rock where Black-tailed Gulls often stopped, and then once a gull entered, we pulled the noose around its leg to catch it (Lu and Zheng, 2003). They were fitted with solar-powered GPS-GSM loggers (Hunan Global Messenger Technology Co., Ltd., Changsha City, China). The loggers were mounted as backpacks (Dwyer, 1972) using a harness of Teflon ribbon threaded with a nylon string. All gulls were measured and weighed. In this study, we only analyzed the 30 (68%) gulls that were successfully tracked for more than half a year and had clearly started the wintering period. For these gulls, four types of GPS-GSM loggers were used. Twenty-one gulls were fitted with 12-g loggers, 6 with 9-g loggers, 2 with 17-g loggers and one with a 17.5-g logger (Appendix Table S1). The weight of the logger corresponds to 2–3.5% (less than 4%; Higuchi et al., 1996; Kanai et al., 1997; Higuchi et al., 1998) of the bird’s body weight (mean 501 ± 68 g, range 395–650 g). On a full charge, GPS measurements were generally taken from once every hour up to once every several hours, with longer time intervals between measurements in the smaller loggers than in the larger ones. When the gulls were over the open sea with a poor GSM signal, the GPS measurements were stored temporarily in the loggers until the GSM signal improved and the positions could be transmitted. The measuring frequency was lowest during this period. Although the loggers include a range of sensors, in this study, we used only the location data (longitude and latitude).
In our study, a gull’s tracking year was defined as one year from the start tracking date. We defined each tracking year of a gull as a “bird-year” following Shamoun-Baranes et al. (2017).
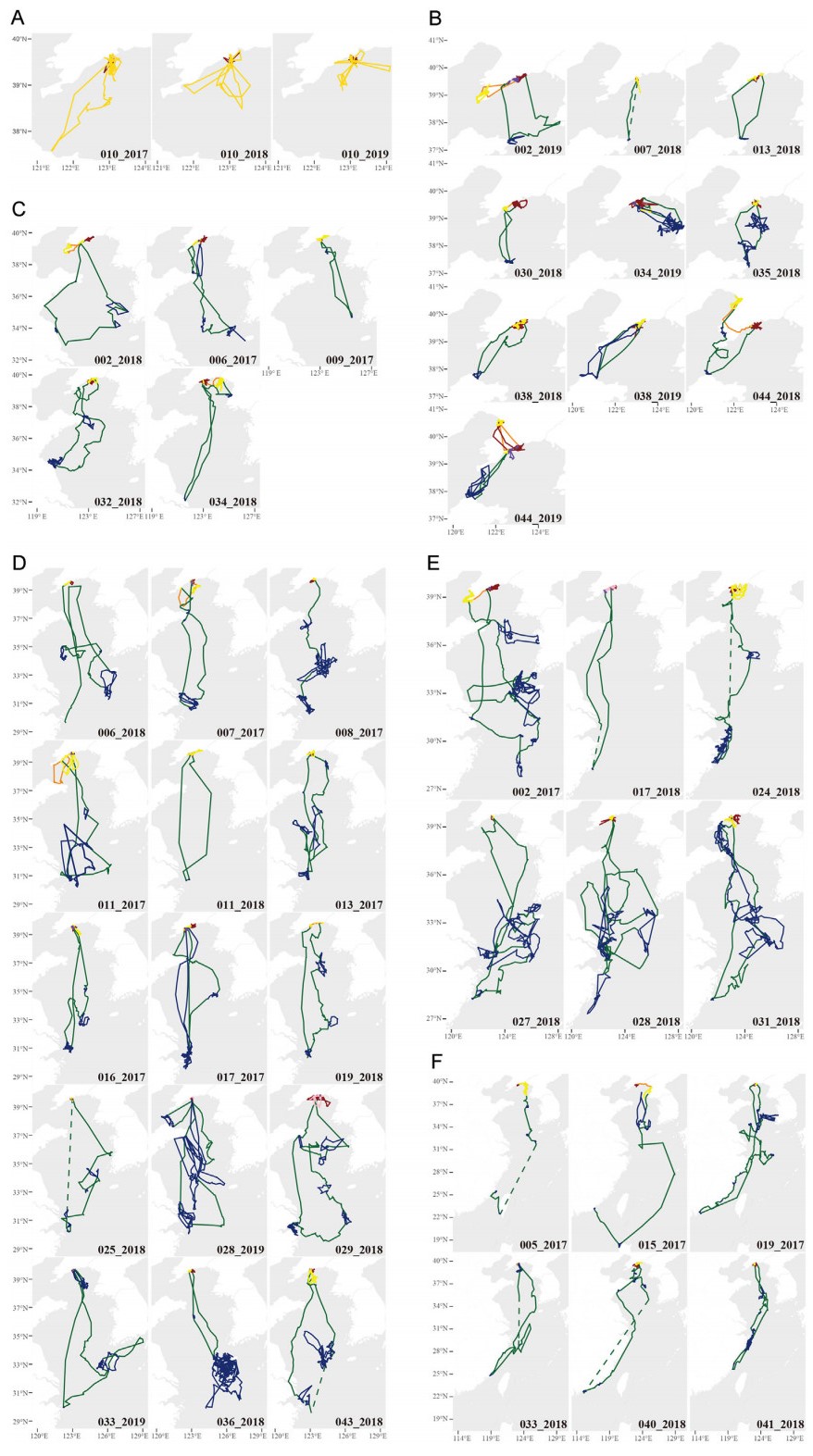
The GPS data of each bird-year were split into different periods within a gull’s annual cycle (a detailed example is presented in Appendix Fig. S1). First, we distinguished between “sedentary” periods and directed travel. In contrast to, for example, lesser Black-backed Gulls (Larus fuscus) (Klaassen et al., 2012), the Black-tailed Gulls did not strictly use the same night roost. We considered locations as being part of a “sedentary” period if the individual returned to a certain geographical range within a certain period of time. After testing multiple individuals, we decided to use a time window of 8 days (192 h) and a geographical buffer range of 15 km, as visual inspections showed that this reasonably well separated more sedentary periods from directed travel periods. We implemented this algorithm in R 3.5.3 (R Core Team, 2019). After automatic division into periods, manual correction of some outliers was needed. The core script of the algorithm is shown in Appendix. Second, we categorized the sedentary periods into breeding, post-breeding, wintering and pre-breeding periods. All the gulls bred at Xingrentuo Islet (0.012 km2). Thus, the breeding periods were easy to define, as this was also the first and last sites visited each bird-year. However, for some bird-years, we found that they stayed at this first site until November or December, suggesting that there were periods other than breeding that were indistinguishable by the algorithm. We measured the distance from the breeding islet for all positions at the first site and found that these gulls stopped returning to the breeding islet at some point between June and August. Other gulls left the first site (breeding area) between June and August, and the next few sites were also not far from the breeding area. We believe that this was the post-breeding period of the gulls (Burger et al., 2020), and these post-breeding sites were less than 220 km from the breeding islet (Appendix Table S2). The start of post-breeding was defined as the first point when they left the breeding islet and never came back. Similarly, some of the gulls had a pre-breeding period before they returned to the breeding islet, which was included in the last site identified by the algorithm. Thus, the end of the pre-breeding was defined as the first point when they arrived at the breeding islet. However, for one bird, 010, it just represented the start of non-breeding and the end of non-breeding was the first point that returned to the breeding islet in spring because it was difficult to distinguish different sites during non-breeding and the gull lacked distinctive features of migration. Birds 017_2018, 028_2019 and 029_2018 did not normally appear around the breeding islet during breeding, and it is difficult to distinguish breeding and post-breeding. Hence, we defined these periods as pre-wintering. Bird 028_2019 did not normally return to the breeding islet after winter, and it is difficult to distinguish pre-breeding and breeding. Thus, we defined these periods as summer. The remaining sites were mostly visited during the winter season (December–February), and only a few sites visited out of the winter season but were south of the first wintering sites of the gulls. Thus, these sites were all identified as wintering sites. As a result, we obtained 45 bird-years (13 of the 30 individuals were tracked over a year; see also the tracking summary section). Because bird 010 (Group 0, including 3 bird-years) did not perform direction migration movement, we obtained 42 clearly migrating bird-years except for this bird.
For the migrating bird-years, we calculated the migration distance (the maximum great circle (orthodromic) distance between the breeding islet and a position in the wintering period). We grouped these individual bird-years into five groups based on migration distance (Appendix Fig. S2). Group 1 wintered less than 500 km away from the breeding islet in the Yellow Sea north of the Shandong Peninsula. Group 2 wintered between 500 and 900 km away from the breeding islet in the southern part of the Yellow Sea. Group 3 wintered between 900 and 1200 km away from the breeding islet in the northwesternmost part of the East China Sea. Group 4 wintered 1200–1500 km away from the breeding islet, further south along the coast in the East China Sea. Group 5 wintered more than 1500 km away from the breeding islet, around Taiwan or farther along the coast. We also wanted to understand the relationship between total annual distance moved and migration distance. Different sampling rates would, however, lead to deviation in the distance estimation (Ryan et al., 2004; Mills et al., 2006; Shamoun-Baranes et al., 2012), so we subsampled the data. The maximum time interval of the raw data was 12 h, and the proportion of points with a 12-h interval was relatively large, so data were subsampled on a 12-h interval. Data were subsampled by starting from the first data point and searching for the point closest to midnight/noon and then repeating this process iteratively. Then, we summed the great circle distance between successive subsampled points in a bird-year to obtain the total distance moved annually. The proportion of effective points (resampling points/2 × 365) was calculated, and the individuals with a continuous long period of missing data (≥7 days) were eliminated. The distance moved was corrected by calculating the total distance moved divided by the proportion of effective points. The corrected total distance moved was used to explore the relationship between the total annual distance moved and the migration distance. To understand individual spatial repeatability, the distance between sites in the same periods of different years of the same individual were calculated. The distance threshold is consistent with the algorithm parameter in this study (15 km).
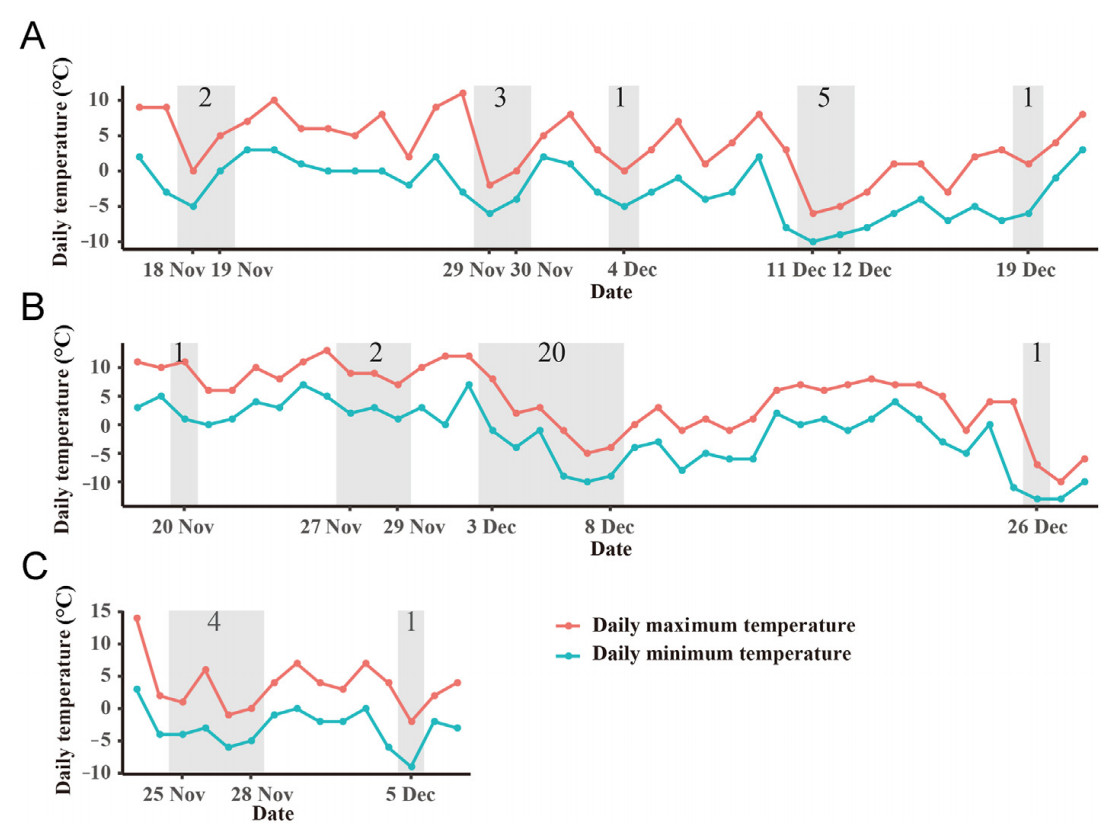
To explore the relationship between temperature and departure date for wintering, we used point-biserial correlation to compare the daily maximum and minimum temperatures of Dalian, Liaoning Province, China (38.92° N, 121.62° E) on the dates Black-tailed Gulls departed for wintering and the temperatures on the other dates in the range of departure dates among individuals. We used the temperature of Dalian for analysis because the gulls spent the post-breeding period in the Bohai Sea and the north Yellow Sea (37.54°–41.51° N, 120.47°–125.61° E; Appendix Fig. S1), and the temperature change here is consistent with the Dalian area in autumn and winter (Liu and Wu, 2017).
Of the 30 individuals and 45 bird-years, 2 birds were tracked for 3 years from 2017 to 2019, and 11 birds were tracked for 2 years, 2017–2018. We recorded 38 complete migrations. In four bird-years, transmission stopped during the wintering period (exact date see Appendix Table S1). Bird 010 did not do a directed migration for the three tracking years; rather, after breeding, the gull roamed in the far north of the Yellow Sea, returning to the breeding area at the start of the next breeding season (Group 0; Fig. 2). The other 29 birds (42 bird-years) made directed migrations southward over a variety of distances and routes (Group 1–5; Fig. 2, Appendix Fig. S2). Of the 42 migrating bird-years, 10 were assigned to Group 1 (24%), 5 to Group 2 (12%), 15 to Group 3 (36%), 6 to Group 4 (14%) and 6 to Group 5 (14%) (Appendix Table S2).
The average migration distance (bird-year) in each group was 259 ± 27 km (mean ± standard deviation (SD)), 716 ± 102 km, 1049 ± 48 km, 1284 ± 53 km, and 1946 ± 294 km (Appendix Table S2). Among individuals, the minimum migration distance was 209 km (Group 1), and the maximum was 2405 km (Group 5) (Appendix Table S2). The corrected total distance moved was significantly and positively correlated with migration distance (R2 = 0.622, p = 7.79e–06, n = 23). Exploring the cumulative distance traveled over time revealed similar patterns between individuals within the groups (Appendix Fig. S3). Additionally, the gulls traveled least distance during post-breeding period (Appendix Figs. S3 and S4).
For bird 010 (Group 0), the gull returned to the breeding islet between the 22nd of February and the 21st of March and left between the 1st and the 26th of July (Fig. 3). The other gulls (Group 1–5) had a post-breeding period prior to wintering and started this period between 22nd of June and 7th of August (Fig. 3). The migrating gulls ended the post-breeding period and started wintering between the 18th of November and the 26th of December (Fig. 3). They ended wintering between the 11th of February and the 15th of April, which was a larger spread of dates than for the previous periods (Fig. 3). Then, 48% of migrating gulls (Fig. 2; Appendix Table S3) started pre-breeding between 11th of February and 4th of April and ended between 27th of February and 16th of April (Fig. 3). Another 52% of the migrating gulls returned to the breeding islet directly after wintering (Fig. 2; Appendix Table S3). Despite the wide range of start and end dates of each period, Black-tailed Gulls were in the post-breeding period throughout September, October and most of August and in the wintering period throughout January (Fig. 3).
Despite the variation in the exact starting date of the wintering period among individuals, the gulls departed to wintering sites when the temperature started to drop or the temperature dropped to a lower level (Fig. 4). Concretely, for both the daily maximum temperature and the daily minimum temperature, the temperature on the departure day was significantly lower than the temperature on other days in the range of departure dates among gulls (point-biserial correlation: p = 0.001 and 0.002, respectively).
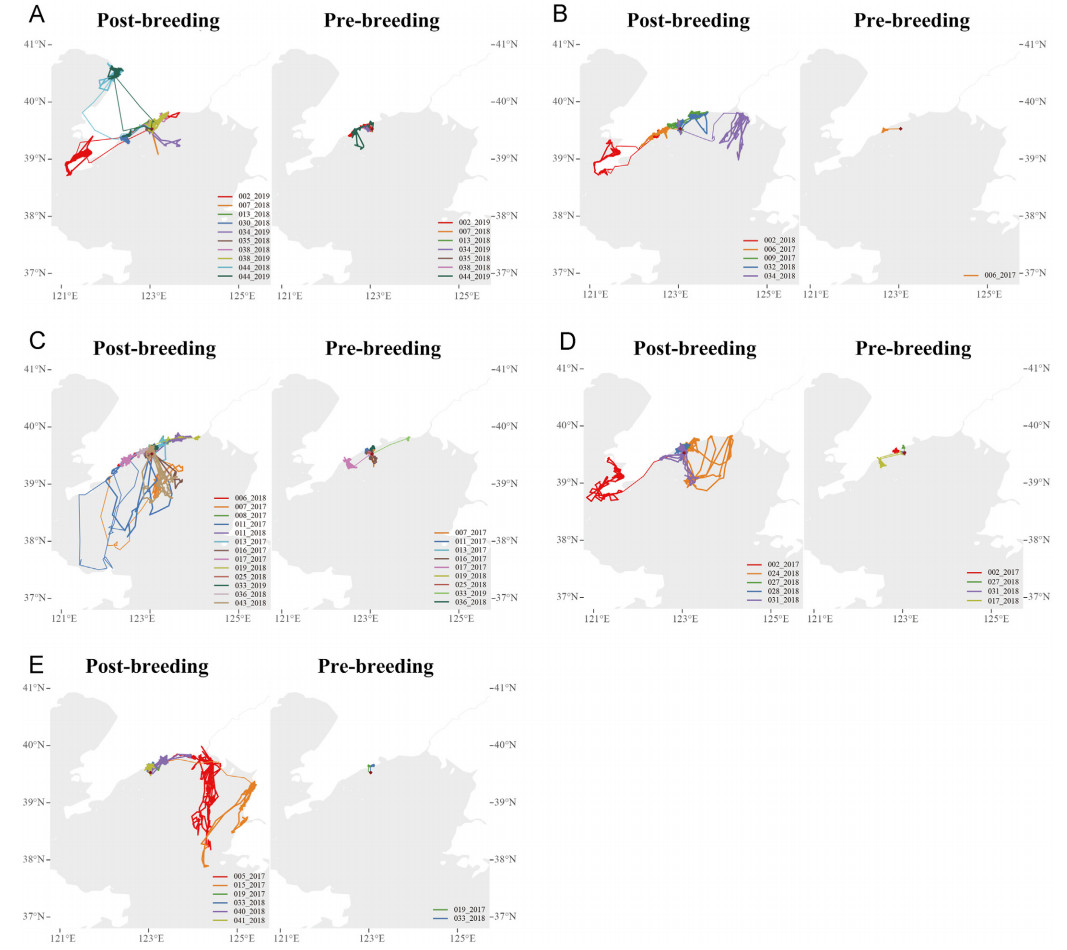
Of the 42 migrating bird-years, only 3 bird-years were unable to identify and distinguish the post-breeding period because they did not normally appear around the breeding islet during the breeding season. All other migrating bird-years (93%) performed a long post-breeding period (no less than 120 days; Appendix Table S3). During the post-breeding period, the gulls traveled and stopped along coasts frequently and entirely over the sea occasionally (Fig. 5; Appendix Fig. S5). While the gulls did not return to the breeding islet, the post-breeding sites were less than 220 km from the breeding islet (Fig. 5; Appendix Fig. S5; Table S2). Almost half of the gulls (48%) had a pre-breeding period (3–20 days; Fig. 5; Appendix Fig. S5; Table S3). The gulls traveled and stopped along coasts as well as entirely over the sea in pre-breeding period (Fig. 5; Appendix Fig. S5). Similar to the post-breeding sites, the pre-breeding sites were also less than 220 km from the breeding islet and even closer (≤80 km; Fig. 5; Appendix Fig. S5; Table S2).
Overall, individuals who started the post-breeding period early would also depart early for wintering (R2 = 0.153, p = 0.01, n = 38; Appendix Fig. S6). The duration of the post-breeding period was uncorrelated with migration distance (p = 0.62; Appendix Fig. S6). No correlations were detected between the start and end dates of the post-breeding period and the migration distance (p > 0.05 for two correlation tests; Appendix Fig. S6). Thus, birds that migrated farther away from the breeding area did not start the post-breeding period earlier or depart earlier to wintering. The gulls who started the pre-breeding period early also arrived early at the breeding islet (R2 = 0.822, p = 8.89e–08, n = 19; Appendix Fig. S7). The gulls that migrated farther away from the breeding islet started the pre-breeding period later and arrived later at the breeding islet (R2 = 0.444, p = 0.002, n = 19; R2 = 0.249, p = 0.03, n = 20; Appendix Fig. S7). However, the duration of the pre-breeding period was uncorrelated with migration distance (p = 0.22; Appendix Fig. S7).
The Black-tailed Gulls regularly migrated over water as well as along coasts (Fig. 2). Travels along coasts were sometimes necessary to reach coastal wintering sites (Fig. 2; Appendix Table S4). There were great differences in migration routes, distances, and the number and location of wintering sites (Fig. 2; Appendix Table S4). Even within groups, the diversity of migration routes and the location of wintering sites were high (Fig. 2). The number of wintering sites had a positive relationship with migration distance (R2 = 0.639, p < 0.0001, n = 33).
There was an enormous variation in the timing and duration of wintering sites (Appendix Fig. S8). Gulls that migrated farther away from the breeding islet had a longer wintering period (R2 = 0.307, p = 0.0006, n = 35; Appendix Fig. S8) and ended wintering later (R2 = 0.498, p < 0.0001, n = 36; Appendix Fig. S8). However, gulls that ended wintering late did not start later for wintering (p > 0.05; Appendix Fig. S8).
In the analysis concerning repeatability, we excluded bird 017_2018 and 028_2019 as we were unable to identify their post-breeding period (see section post-breeding and pre-breeding movements). Most gulls (80%) were faithful to their first post-breeding site (<15 km). Except for gulls 013 and 019, this site was also the one where the gulls stayed the longest (Appendix Fig. S9, Table S5). Although for bird 002, the distance between 2017 and 2019 was slightly greater than the distance threshold (15 km), the distance between two consecutive years (2017 and 2018, 2018 and 2019) was less than the threshold (Appendix Table S5). In addition, bird 002 used the same second post-breeding site in 2019 as in the previous year (Appendix Table S5).
For 11 individuals tracked for two years, only three (013, 017 and 033) had a pre-breeding period for two tracking years, and one (038) did not have a pre-breeding period for two tracking years (Appendix Table S6). In addition, bird 002, who was tracked for three years, also had a pre-breeding period for two tracking years (002_2017, 002_2019; Appendix Table S6). For these four individuals, half were faithful to their pre-breeding sites (<15 km; Appendix Fig. S9, Table S6).
The diversities of the number and location of wintering sites were high; only four individuals (bird 007, 011, 038 and 044; 33%) had one faithful wintering site each (<15 km), and this site was also the one where they stayed the longest (except 007_2017, second longest; Appendix Fig. S10, Table S7). Only three individuals (birds 011, 038 and 044; 25%) selected similar migration routes and reached similar maximum distances each during the two tracking years (Appendix Fig. S10, Table S7). The migration distance of bird 002 decreased each year over the three tracking years (Appendix Fig. S10). Similarly, 6 other birds (total 58%) migrated closer to the breeding area for the second tracking year, but two birds (17%) did the contrary (Appendix Fig. S10).
Almost all of the gulls that made a directed migration had a definite post-breeding period, and they spent 4–5 months in the post-breeding period, which is longer than many other species that have a post-breeding period; for instance, the Ivory Gull (Pagophila eburnea) (for 2–3 months; Gilg et al., 2010). Although the post-breeding period is long, the gulls did not travel much distance (Appendix Fig. S3, Fig. S4). Burger et al. (2020) reported that Black-tailed Gulls spread to food-rich areas during post-breeding which means less competition. The post-breeding period is an important energy storage period for migration (Rubolini et al., 2002). This, in turn, could be one of the reasons they move less during the post-breeding period. In addition, our results show that the distance from the post-breeding sites to the breeding islet is never more than 220 km, suggesting the possibility of high food availability near the breeding islet according to the report of Burger et al. (2020). Eighty percent of the individuals with two or more years of tracking data used at least one same post-breeding site as in the previous year. Bird 002 in particular, the gull explored a new site in the second tracking year in addition to the first site and went to both of these sites first in the third tracking year, and all of the other gulls were faithful to the first or the only post-breeding site. Therefore, gulls tend to preferentially select previously-used sites, possibly due to the high cost of shifting sites (Arlt and Pärt, 2008). Nevertheless, some gulls would explore new sites, and prospecting is a common behavior during the post-breeding period (Vega et al., 2016; Ciaglo et al., 2021) to look for potential future post-breeding sites (such as bird 002 in this study), potential future mates (Betts et al., 2008) or suitable wintering sites (Hake et al., 2001). Taken together, the gulls had a long post-breeding period near the breeding islet probably because of abundant food and familiar surroundings, which gives them enough time to molt (Marchant and Higgins, 1990; Arlt and Pärt, 2008; Fromant et al., 2020) and store energy for winter.
The patterns of pre-breeding are similar to the patterns of post-breeding, and the two periods share some sites (Fig. 5; Appendix Table S4). All these findings suggest that pre-breeding and post-breeding have some similar roles, for instance, improving body condition (Rosana et al., 2013). Although there are some similarities between pre-breeding and post-breeding, the pre-breeding period is unique. Only approximately half of gulls have a pre-breeding period that is closer to the breeding islet and relatively simple (Fig. 5) and shorter in duration (3–20 days). It is partly similar in some migratory species; for example, 42% of the Black-legged Kittiwake (Rlssa tridactyla) have a long-distance pre-breeding period that lasts approximately 14 days (Bogdanova et al., 2011). Unlike Black-tailed Gulls, all Eleonora’s Falcons (Falco eleonorae) have a pre-breeding period and are far away from the colonies (Mellone et al., 2013). However, Eleonora’s Falcons (Mellone et al., 2013; Kassara et al., 2022) and Black-tailed Gulls (in this study) both show high spatial fidelity.
Migration routes and wintering sites differed greatly among individuals even though these gulls all breed at the same small islet (Fig. 2). Even for the same individuals with two or more years of tracking data, only 33 percent of individuals just used one same wintering site as in the previous year, and only 25 percent of individuals selected similar migration routes. That is, the fidelity of the wintering sites for Black-tailed Gulls is relatively low, unlike Ring-billed Gull (Larus delawarensis) and Herring Gull (Larus argentatus) (Clark et al., 2016). Nevertheless, there are three wintering sites (Shengshan, Zhejiang Province, China, 11 times; Chengshan, Shandong Province, China, 8 times; Gageo Reef, 5 times) where Black-tailed Gulls appear frequently. The diversity and complexity in routes stand in contrast to many seabirds that tend to migrate with similar routes and congregate at a few important sites, such as the Arctic Tern (Sterna paradisaea) (Egevang et al., 2010) and Sooty Shearwater (Puffinus griseus) (Shaffer et al., 2006). Similar to the lesser Black-backed Gull (Klaassen et al., 2012), Black-tailed Gull is not restricted to migrate over land or over water, but they are more likely to travel over water, and many of them spend their wintering period almost entirely at sea (Fig. 2). From our observations in the field, Black-tailed Gulls have a habit of following boats and eating trash thrown from boats, which is a possible reason for the high complexity of wintering routes. Unusually, the annual cycle of the migrating gulls only included breeding, post-breeding, wintering and pre-breeding periods without distinct spring and autumn travel periods (Fig. 3), which is similar to the periods in the study of Black-tailed Gulls by Kazama et al. (2013). Given the above information, we believe that Black-tailed Gulls are loyal to their breeding area but not to the wintering sites, and they spend the winter mainly over a large body of water and always shift wintering sites.
The gulls were grouped by migration distance from the breeding islet, which is a common way to classify migratory strategies (Alerstam et al., 2003). Our findings show that the gulls who migrate farther away from the breeding islet spend more time on the wintering period and visit more wintering sites (Appendix Fig. S8), also invest more in movement across the annual cycle (Appendix Fig. S3). This behavior is different from some migratory species, for example, the lesser Black-backed Gull, which balances investment in movement across the annual cycle among the different migration distances (Shamoun-Baranes et al., 2017). Our results show that the migration distance among individuals varied greatly; the shortest was only 209 km, and the farthest was 2405 km, also indicating that the wintering movement patterns and migration strategies of Black-tailed Gulls are highly variable.
The post-breeding period and wintering period differ significantly in the number and range of sites, the spatial fidelity of the sites and the duration of the periods. The reasons for this are unclear. However, among individuals, although it was different for the exact ending date of the post-breeding period, the gulls started wintering when the temperature started to drop or the temperature dropped to a lower level (Fig. 4). Thus, temperature directly affects the southward migration of Black-tailed Gulls, similar to many species, such as the Pink-footed Goose (Anser brachyrhynchus) (Bauer et al., 2008). Migratory behavior is closely related to the trade-off in benefits from the local environment and a distant environment to maximize fitness (Newton, 2008; Bauer and Hoye, 2014; Weimerskirch et al., 2015). The high correlation between the date when the gulls depart from post-breeding sites to wintering sites and the temperature supports this idea to some extent. This correlation also aligns with multiple field observations, where we observed that Black-tailed Gulls did not start to migrate as many migratory species (for instance, Herring Gulls Larus argentatus; Anderson et al., 2020) did from the breeding islet immediately after breeding. Instead, they roamed slowly around, passively moving southward as the temperature dropped, as confirmed by our GPS data (a long post-breeding period close to the breeding islet and then migrating southward directly). All these results indicate that environmental change could have a large impact on Black-tailed Gull migration. In addition to the observation of boat-chasing behavior, we also observe that Black-tailed Gulls often forage in aquaculture pens during breeding and wintering, suggesting that human activity may also be a factor in their migration. It has been suggested that most bird species have the potential to migrate and that whether they do or not would depend on environment conditions (Salewski and Bruderer, 2007). In this study, we found that not all the gulls migrating, or bird 010 did not migrate southward, and partial migration offers one possibility for this view. Thus, if human activity could provide adequate food resources coupled with climate change, such as the Blackcap (Sylvia atricapilla) who established high-latitude wintering areas in Britain and Ireland (van Doren et al., 2021), it is reasonable to speculate that Black-tailed Gulls would alter their migration behavior and migration distances due to environmental changes and human activities in the future. The result that more than half of the gulls migrated shorter year by year supports this idea to some extent.
It would be interesting to perform further study combining field work, tracking and other technologies to understand the behavior, strategies, motivation, and even evolution of Black-tailed Gull migration. For instance, past studies showed that spatial segregation during the non-breeding period can lead to genetic differentiation (Friesen et al., 2007; Rayner et al., 2011; Weimerskirch et al., 2015), and in our study, we showed that the arrival date at the breeding islet is positively correlated with the migration distance in Black-tailed Gulls. This correlation could potentially affect mating time, so whether this would cause genetic divergence among Black-tailed Gulls that migrate different distances?
In summary, this study identified non-breeding movements at the population level of Black-tailed Gulls by GPS tracking for the first time. First, all except one bird migrated, but the annual cycle included only four periods (breeding, post-breeding, wintering and pre-breeding). Second, we found that the migrating gulls had a long post-breeding period (4–5 months) near the breeding islet (<220 km), and for those who tracked more than one year, eighty percent of their first post-breeding sites were faithful. Third, 48% of the migrating gulls had a short (3–20 days) pre-breeding period, which was closer to the breeding islet (≤80 km) than the post-breeding period. Fourth, the migration routes and strategies of the gulls are complex and varied. Although there were three wintering sites with frequent occurrences of the gulls, the migrating gulls were not very loyal to their wintering sites, and they traveled mainly over a large body of water and always shifted wintering sites during the wintering period. Finally, combined with field observations and our GPS data analysis, we observed that as the temperature dropped, the gulls ended post-breeding and moved southward, and they liked to chase boats and forage in aquaculture pens.
HX, FL, CJ and KT conceived the research project. HX, KT and CN designed the methodology. HX and CJ collected the data. HX analyzed the data and led the paper writing with contributions from all authors. All authors read and approved the final manuscript.
The field work was performed by experienced researchers according to the guidelines of the Animal Ethics Committee of Institute of Zoology, Chinese Academy of Sciences, and approved by the local forestry department.
The authors declared that they have no conflicts of interest to this work.
We thank Libo Zhou, Xiaobing Li, Zhiyong Jiang, and Zuohua Yin for their assistance with the field work. We thank Katherine R. S. Snell for her technical support of the R script and valuable discussions regarding raw GPS data. We thank two anonymous reviewers for their valuable comments to improve the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100103.
|
Anderson, C.M., Gilchrist, H.G., Ronconi, R.A., Shlepr, K.R., Clark, D.E., Fifield, D.A., et al., 2020. Both short and long distance migrants use energy-minimizing migration strategies in North American herring gulls. Mov. Ecol. 15, 26.
|
|
Burger, J., Gochfeld, M., Kirwan, G.M., Garcia, E.F.J., 2020. Black-tailed gull (Larus
crassirostris), version 1.0. In: Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, NY.
|
|
Drent, R.H., 2006. The timing of bird's breeding season: the Perrins hypothesis revisited, especially for migrants. Ardea 94, 305-322.
|
|
Kanai, Y., Sato, F., Ueta, M., Minton, J., Higuchi, H., Soma, M., et al., 1997. The migration routes and important restsites of Whooper Swans satellite-tracked from northern Japan. STRIX 15, 1-13.
|
|
Kazama, K., Tomita, N., Ito, M., Niizuma, Y., Takagi, M., Watanuki, Y., 2008. Responses in breeding behaviour of the Black-tailed Gull (Larus crassirostris) to different marine environments. In: Okada, H., Mawatari, S.F., Suzuki, N., Gautam, P. (Eds.), The Origin and Evolution of Natural Diversity. Hokkaido University, Sapporo, pp. 215-220.
|
|
Langendoen, T., Mundkur, T., Nagy, S., 2021. Flyway Trend Analyses Based on Data From the Asian Waterbird Census From the Period of 1987-2020. Wetlands International, Wageningen.
|
|
Liu, Y., Wu, H.D., 2017. Sea ice in the Bohai Sea and the northern Yellow Sea. Mar. Forecasts 34, 94-101 (in Chinese).
|
|
Marchant, S., Higgins, P.J., 1990. Handbook of Australian, New Zealand and Antarctic Birds. Oxford University Press, Oxford.
|
|
Newton, I., 2008. The Migration Ecology of Birds. Academic Press, London.
|
|
Ryan, P.G., Petersen, S.L., Peters, G., Grémillet, D., 2004. GPS tracking a marine predator: the effects of precision, resolution and sampling rate on foraging tracks of African penguins. Mar. Biol. 145, 215-223.
|
|
Vega, M.L., Willemoes, M., Thomson, R.L., Tolvanen, J., Rutila, J., Samaš, P., et al., 2016. First-time migration in juvenile Common Cuckoos documented by satellite tracking. PLoS One 11, 12.
|
|
Wang, L., Han, J.K., Huang, M.P., 1991. The breeding ecology of Black-tailed Gulls (Larus crassiostris). Chin. J. Wildl. 3, 29-30 (in Chinese).
|
|
Zhang, S.W., Fan, Q.D., Zhao, F., Sun, W.L., Li, W.Q., Zhong, H.B., 2000. Observation on the breeding ecology of black-tailed gull (Larus crassiostris). J. Shandong For. Sci. Tech. 4, 14-16 (in Chinese).
|