| Citation: | Yujie XuanYuan, Ran Chen, Jieheng Xu, Jiacheng Zhou, Ming Li, Jinsong Liu. 2023: Seasonal acclimatization and temperature acclimation in small passerine birds is achieved via metabolic adjustments. Avian Research, 14(1): 100084. DOI: 10.1016/j.avrs.2023.100084 |
Temperature and other environmental factors play an integral role in the metabolic adjustments of animals and drive a series of morphological, physiological, and behavioral adaptions essential to survival. However, it is not clear how the capacity of an organism for temperature acclimation translates into seasonal acclimatization to maintain survival. Basal metabolic rate (BMR), evaporative water loss (EWL), and energy budget were measured in the Chinese Hwamei (Garrulax canorus) following winter and summer acclimatization, and in those acclimatized to 15 ℃ (cold) and 35 ℃ (warm) under laboratory conditions for 28 days. In addition to the above indicators, internal organ masses, as well as state 4 respiration and cytochrome c oxidase (COX) activity were also measured for the liver, skeletal muscle, heart, and kidney. Both winter-acclimatized and cold-acclimated birds exhibited significantly higher BMR, EWL, and energy budget, as well as organ masses, state 4 respiration, and COX activity compared with the summer-acclimatized and warm-acclimated birds. This indicated that the Chinese Hwamei could adapt to seasonal or just temperature changes through some physiological and biochemical thermogenic adjustments, which would be beneficial to cope with natural environmental changes. A general linear model showed that body mass, BMR, GEI, state 4 respiration in the liver and kidney, and COX activity in the skeletal muscle, liver, and kidney were significantly affected by temperature and acclimation. A positive correlation was observed between BMR and each of the other parameters (body mass, EWL, energy budget, heart dry mass, kidney dry mass, state 4 respiration) in the muscle, heart, and kidney and also between BMR and COX activity in the muscle and kidney. The results suggested that similar to seasonal acclimatization, Chinese Hwameis subjected to temperature acclimation also exhibited significant differences in metabolism-related physiological and biochemical parameters, depending on the temperature. The data also supported the prediction that metabolic adjustment might be the primary means by which small birds meet the energetic challenges triggered by cold conditions.
Physiological ecology seeks to understand physiological processes that respond to environmental stress and influence species distribution (Feder et al., 1987; Schmidt-Nielsen, 1997; Swanson, 2010). Phenotypic flexibility refers to phenotypic changes that are reversible, temporary, and repeatable (Piersma and Drent, 2003). An important component of thermoregulation in birds is the phenotypic flexibility in the metabolic adjustments in response to changing seasonal energy demands (McKechnie et al., 2007). These seasonal metabolic adjustments typically include increases in both basal metabolic rate (BMR) and summit metabolic rate in cold climates (McKechnie, 2008; Swanson, 2010). BMR refers to the rate of energy transformation of an animal at the resting and fasting states and in the thermal neutral zones (TNZ; McKechnie, 2008; Zheng et al., 2008a, 2014a; McNab, 2009; Swanson et al., 2017). Its application as an energy consumption parameter has received considerable attention from physiologists and comparative physiologists (Zheng et al., 2008b, 2014b; Swanson, 2010; Bushuev et al., 2018). Many small winter-active birds that inhabit temperate latitudes exhibit increased BMR in winter compared with summer (Bush et al., 2008; Zheng et al., 2008b, 2014a, 2014b; Swanson et al., 2014; Li et al., 2017). Ambient temperature is considered to be one of the most significant environmental factors influencing the seasonal metabolism of animals and is the driving force for a number of morphological, physiological, and behavioral adaptations (Williams and Tieleman, 2000; Zhou et al., 2016; Cui et al., 2019; Li et al., 2020). There have been numerous studies demonstrating that cold-acclimated birds exhibit a higher BMR compared with warm-acclimated ones (Williams and Tieleman, 2000; Liu and Li, 2006; Zheng et al., 2013a; Zhou et al., 2016).
Changes in organ mass may have important effects on BMR for birds (Swanson, 2010). For example, the mass and size of certain metabolically active organs and tissues such as the skeletal muscle, liver, heart, kidney, and digestive organs may influence the level of BMR (Piersma et al., 1996; Williams and Tieleman, 2000; Vézina et al., 2006; Zheng et al., 2014b). An increase in muscle/organ size will lead to an increase in metabolic activity, while a decrease will have the opposite effect. These changes in organ mass are thought to be adaptations to various energy demands and the increases in organ mass are associated with increased metabolic activity, resulting in increased BMR (Williams and Tieleman, 2000; Swanson, 2010; Zheng et al., 2014b; Zhou et al., 2016). Furthermore, changes in the activity of the enzymes responsible for decomposition processes and/or the changes in the transport capacity of metabolic substrates can affect the metabolic intensity in organs, and hence, the metabolic rate (Zheng et al., 2008b, 2014a; Liknes and Swanson, 2011; Swanson et al., 2017). Finally, at the cellular level, cytochrome c oxidase (COX) activity and mitochondrial state 4 respiration are often utilized as enzymatic markers of BMR (Zheng et al., 2008b, 2013b; Hu et al., 2017; Cui et al., 2019). COX is found in the mitochondria of eukaryotic cells, and it participates in energy production for the cells mainly through oxidative phosphorylation, while the rate of state 4 respiration reflects the oxidative phosphorylation capacity of the birds.
Resident birds also cope with fluctuations in water availability. Evaporative water loss (EWL) is the primary means by which animals disperse heat, and it may be subject to comparable selective pressures such as energy consumption (Tieleman and Williams, 2002; Tieleman et al., 2003; Clement et al., 2012). EWL is extensively applied to interspecific or intraspecific studies in birds under different environmental conditions. For instance, research about energy and water budgets have indicated that birds in arid regions display lower BMR and EWL than birds in the mesic climatic region (Tieleman et al., 2003; Sabat et al., 2006). Several studies have shown that heat and water crises in arid regions are stressful and favor organisms showing low BMR because they have lower endogenous thermogenesis and, therefore, have less need for evaporative cooling (Tieleman and Williams, 2000; Maldonado et al., 2009).
Seasonal acclimatization patterns appear to be more variable in birds from subtropical/tropical regions compared with those from higher latitudes where winters are colder (McKechnie et al., 2015). Previous studies have found that Chinese Hwameis (Garrulax canorus) feed mainly on insects and they have a relatively low BMR compared with the BMR predicted from their body mass (Liu et al., 2005; Wu et al., 2015). Additionally, as observed in other temperate passerines, Chinese Hwameis also have high thermal conductance, narrow thermal neutral zone and high evaporative water production/metabolic water production ratio (Xia et al., 2013). These physiological characteristics and feeding habits may restrict their distribution to a natural habitat characterized by a relatively warm and moist climate with abundant food resources (Xia et al., 2013; Wu et al., 2015). However, few studies have simultaneously quantified the physiological responses of Chinese Hwameis to a stimulus in both seasonal acclimatization and laboratory acclimation settings. Thus, it is often unclear how closely the laboratory findings correspond to the physiological responses of the individual birds in the wild (Maldonado et al., 2009; Xia et al., 2015). In this study, we continued to investigate the seasonal and temperature phenotypic flexibility of the Chinese Hwamei, which was previously found to exhibit seasonal and temperature-driven variation in BMR (Wu et al., 2015; Zhao et al., 2015; Zhou et al., 2016; Wang et al., 2019). We evaluated the effects of season and temperature on basal thermogenic properties at multiple organization levels, including BMR, EWL, energy budget, nutritional and organ masses, state 4 respiration and COX activity in the skeletal muscle, liver, heart and kidney. Our objective was to compare the metabolic adjustments in Chinese Hwameis arising from the seasonal (summer/winter) acclimatization and the acclimation to different temperatures (cold/warm) in order to determine whether seasonal acclimatization could be mediated by acclimation to temperatures. Temperature is an important factor of seasonal changes and based on our previous studies and those of others, we predicted that metabolic changes in Chinese Hwameis exposed to temperature acclimation in the laboratory might be similar to metabolic changes in the same species exposed to seasonal acclimatization.
This study was carried out in Wenzhou, Zhejiang Province (27°29′ N, 120°51′ E), China, a city with a subtropical monsoon climate and more rainfall in spring and summer (Zheng et al., 2008b; Wu et al., 2015; Zhou et al., 2016). Mean daily maximum and minimum temperatures range from 34 ℃ to 25 ℃ in July, and from 15 ℃ to 8 ℃ in January.
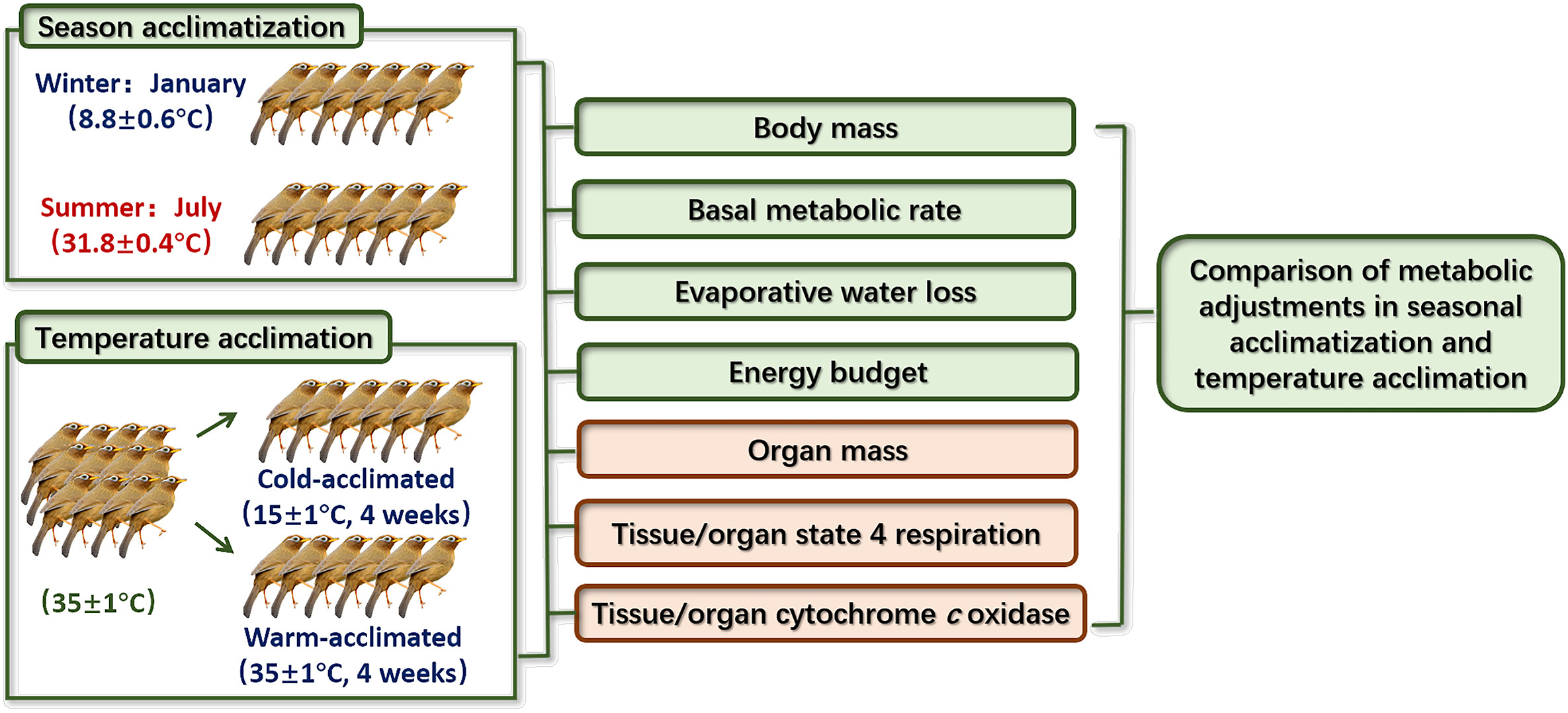
The Chinese Hwamei (Garrulax canorus; Passeriformes, Leiothrichidae) is distributed mainly in central and southern China, as well as in northern and central Vietnam (Li et al., 2006). It mainly lives in the low mountains, hills and plains at the foot of mountains and bushes below an altitude of 1500 m, but it also lives in forest margins, farmland, wilderness, small trees, bamboo forests and gardens near villages and towns (Li et al., 2006). The Chinese Hwamei is an omnivorous species, but its diet predominantly consists of insects throughout the year, especially during the breeding season. During the non-breeding season, the diet consists of fruits of various plants, weed seeds or tender vegetables (Li et al., 2006). The experimental design is shown in Fig. 1.
Using mist nets, six adult birds were captured in January (winter birds), while another six were captured in July (summer birds) in 2017. Following capture, the body mass of each bird was measured with an electronic balance (Sartorius BT25S, Germany). After that, the captured birds were placed into 50 cm × 30 cm × 20 cm cages in the laboratory and were kept outdoors for one day under natural photoperiod and temperature for physiological measurement (Zheng et al., 2008b; Wu et al., 2015; Wang et al., 2019). Food (Jiangsu XieTong Bioengineering Co., China) and water were provided ad libitum (Zhou et al., 2016). During the experiment, the average field temperature was 31.8 ± 0.4 ℃ in summer and 8.8 ± 0.6 ℃ in winter.
Twelve adult birds were captured for the temperature acclimation experiments in July 2017. The birds were housed in individual cages (50 cm × 30 cm × 20 cm) inside the laboratory under a constant temperature of 35 ± 1 ℃ and a 12L: 12D photoperiod with lights on at 06:00 a.m. (Zhou et al., 2016). The captured birds were acclimatized to these laboratory conditions for two weeks. Food and water were provided ad libitum. After acclimation, each bird was assigned one of two groups: one group was kept in a room maintained at 35 ± 1 ℃ ("warm-acclimated group", n = 6) whereas the group was kept in another room maintained at 15 ± 1 ℃ ("cold-acclimated group", n = 6; Barceló et al., 2009) for four weeks. A temperature of 35 ℃ is equivalent to the average maximum daily temperature that birds in the wild are exposed to in summer. Considering that our previous studies have found that half of the birds died a few days after acclimation at 10 ℃ (mean daily temperatures in winter), in this study the birds were acclimated to 15 ℃ instead to ensure the survival of all birds used in the experiment (Zhou et al., 2016). Previous research has shown that the Chinese Hwamei consumes nearly 63% more oxygen at 15 ℃ than at 35 ℃, as shown by the increase in BMR (Zhou et al., 2016). Thus, we were confident that the 15 ℃ and 35 ℃ temperature settings would present the birds with contrasting thermoregulatory demands (Maldonado et al., 2009).
The oxygen consumption rate was quantified using an open circuit respirometry system (TSE, Germany), following the methods previously described by Zhou et al. (2016) and Wen et al. (2019), with minor modifications. Briefly, the air was pumped through the metabolic chamber (where the birds were resting) housed inside an incubator with the temperature set to 30 ± 0.5 ℃, which is within the thermal neutral zone of the Chinese Hwamei (Wu et al., 2015). Dry air was used as a control, and air scrubbed of H2O was pumped through the metabolic chamber at a rate of 1000 mL/min. After drying, the outgoing air was passed through an oxygen analyzer at a flow rate of 300 mL/min (Wu et al., 2015; Wen et al., 2019). The data were recorded and analyzed by an analog-to-digital converter and standard software analysis. Oxygen consumption rates were measured in the thermal neutral zone (at 30 ± 0.5 ℃), as previously described by Wu et al. (2015) and Zhou et al. (2016). The lowest rate (over 10 min) was used to quantify the BMR (Zhao et al., 2015; Wen et al., 2019). After the experiment, the body temperature was recorded using a thermocouple inserted in the cloaca, and the output was digitized using an Oakton thermocouple meter (Eutech Instruments, Singapore) (Zhou et al., 2016). The body mass of each bird was recorded before and after the experiment to determine the mean body mass for each group. All measurements of gas exchange were obtained during the rest phase of the birds' circadian cycles (between 20:00 p.m. and 04:00 a.m.) in a dark chamber. Food was removed 4 h before each measurement to minimize the heat increment associated with feeding. Oxygen consumption measurements were initiated when the birds were observed to perch peacefully in the chamber, and each bird was placed in the metabolic chamber for at least 2 h (Zhou et al., 2016; Wang et al., 2019).
Evaporative water loss (EWL) was measured after the determination of metabolic rates. The water content of the air was determined before the experiment. A 'U' tube (containing silica gel) was connected in series behind the respiratory chamber and weighed (±0.1 mg; Zhu et al., 2008, 2010). The amount of EWL from each bird was absorbed by silica gel and could be measured by reweighing the U tube at the end of the experiment. The duration of the experiment was 1 h (Xia et al., 2013). If the birds defecated during the experiment, the data were excluded from the relevant analyses. The experiment was then continued until the individual did not eliminate feces for 1 h. EWL was calculated using the same formula as described by Xia et al. (2013):
|
EWL(mg/h)=(Tubeweightafterexperiment−tubeweightbeforeexperiment)/time |
During the four weeks of temperature acclimation, residual food and feces were removed and discarded. Residual food and feces were then collected for the three days that immediately followed the end of the acclimation period. For seasonal acclimatization, food residues and feces were collected for three days after the metabolic trials. Food residues and feces were collected from each cage, separated, and dried at 60 ℃ to obtain a constant mass. A C2000 oxygen bomb calorimeter (IKA, Germany) was used to determine the gross energy content of the food and feces. GEI and DEI were calculated according to the following formula (Wu et al., 2014):
|
GEI(kJ/day)=Foodintake(g/day)×drymattercontentofthediet(%)×energycontentoffood(kJ/g); |
|
DEI(kJ/day)=GEI−[drymassoffeces(g/day)×energycontentoffeces(kJ/g)] |
Birds were euthanized, and their skeletal muscles, liver, heart, and kidneys were extracted and weighed (Cui et al., 2019; Wang et al., 2019). State 4 respiration was measured for the muscle, liver, heart, and kidneys using a Clark electrode at 30 ℃ and 1.96 mL of respiration medium (Hansatech Instruments, UK; DW-1) containing succinate as the substrate (Zheng et al., 2008b, 2014a; Zhou et al., 2016; Hu et al., 2017; Tang et al., 2022). COX activity in the muscle, liver, heart, and kidneys was measured polarographically using a Clark electrode at 30 ℃ and in the presence of cytochrome c as described by Zhou et al. (2016). Both results were represented as whole organ activity expressed in mmol O2/(min·organ) (Wang et al., 2019; Mao et al., 2019). The remaining organ parts were dried for two days at 65 ℃ to a constant mass and then weighed (Cui et al., 2019).
Data were expressed as mean ± SEM and data analysis was conducted using the SPSS 20.0 statistics software. Variables were tested for normality using the Kolmogorov-Smirnov test. BMR, EWL, GEI, and DEI were analyzed with body mass as a covariate. Tissue/organ masses (muscle, liver, kidney, and heart) were analyzed with the difference between body mass and organ mass as a covariate to avoid statistical problems with part-whole correlations (Christians, 1999). We used one-way AN(C)OVAs to test the differences in body mass and BMR between the two temperature-acclimated groups of birds prior to the experiment. In order to test for differences between season-acclimatization and temperature-acclimation, we tested the effects of temperature, acclimation and their interaction in a linear regression framework (two-way AN(C)OVA), where the temperature was either cold (winter acclimatization and 15 ℃ acclimation) or warm (summer acclimatization and 35 ℃ acclimation) and acclimation being yes (15 ℃ acclimation and 35 ℃ acclimation) or no (winter acclimatization and summer acclimatization). P < 0.05 was regarded as statistically significant.
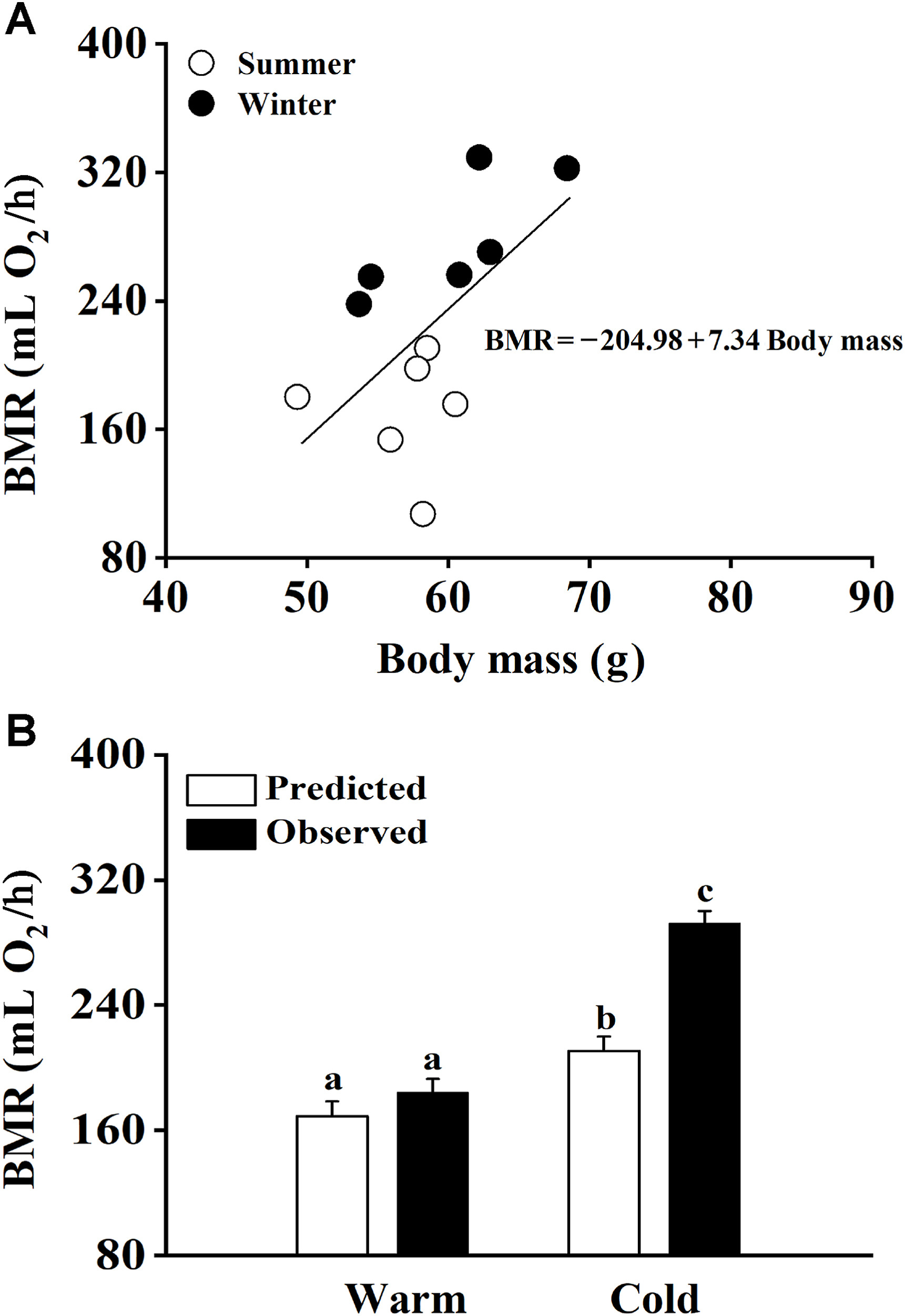
Body mass was significantly affected by both temperature (F1, 20 = 8.087, PT = 0.010; Fig. 2A) and acclimation (F1, 20 = 8.260, PA = 0.009; Fig. 2A), but not by the interaction between temperature and acclimation (F1, 20 = 0.355, PT × PA = 0.558; Fig. 2A). No difference was found in body mass between the two temperature-acclimated groups of birds prior to the experiment (F1, 11 = 0.925, P = 0.359). The body masses of the cold groups (winter-acclimatized and cold-acclimated birds) were heavier compared with those of the warm groups (summer-acclimatized and warm-acclimated birds; Fig. 2A). The non-acclimated groups (winter- and summer-acclimatized birds) exhibited heavier body masses than the acclimated groups (warm-and cold-acclimated birds; Fig. 2A). These results suggested that seasonal acclimatization and temperature acclimation had a similar effect on bird body mass. However, body temperature was not significantly affected by temperature (F1, 20 = 0.020, PT = 0.888; Fig. 2B), acclimation (F1, 20 = 2.947, PA = 0.101; Fig. 2B) and the interaction between temperature and acclimation (F1, 20 = 1.783, PT × PA = 0.197; Fig. 2B).
BMR was analyzed with body mass as a covariate. BMR was significantly affected by both temperature (F1, 19 = 48.324, PT < 0.001; Fig. 2C) and acclimation (F1, 19 = 4.954, PA = 0.038; Fig. 2C) but not by the interaction between temperature and acclimation (F1, 19 = 0.076, PT × PA = 0.786; Fig. 2C). There was also no significant difference in the BMR for the temperature-acclimated birds prior to the experiment (F1, 9 = 0.060, P = 0.812). The cold groups exhibited higher BMRs than the warm groups (Fig. 2C). The temperature-acclimated groups showed higher BMRs than the non-acclimated groups (Fig. 2C). EWL was significantly affected by temperature (F1, 19 = 10.182, PT = 0.005; Fig. 2D), but not by acclimation (F1, 19 = 0.947, PA = 0.343; Fig. 2D) or the interaction between temperature and acclimation (F1, 19 = 0.337, PT × PA = 0.569; Fig. 2D). The cold groups exhibited higher EWLs than the warm groups (Fig. 2D). These results suggested that both seasonal acclimatization and temperature acclimation had the same effect on BMR and EWL as far as the Chinese Hwamei is concerned.
GEI was significantly affected by temperature (F1, 19 = 80.915, PT < 0.001; Fig. 2E), acclimation (F1, 19 = 6.655, PA = 0.018; Fig. 2E) and the interaction between temperature and acclimation (F1, 19 = 0.735, PT × PA = 0.042; Fig. 2E). GEI was even higher in the cold groups than in the warm groups (Fig. 2E). The temperature-acclimated groups showed a higher GEI than the non-acclimated groups (Fig. 2E). The results suggested that both seasonal acclimatization and temperature acclimation exerted a similar effect on GEI. DEI was significantly affected by temperature (F1, 19 = 84.130, PT < 0.001; Fig. 2F) and the interaction between temperature and acclimation (F1, 19 = 5.538, PT × PA = 0.030; Fig. 2F), but not by acclimation (F1, 19 = 0.642, PA = 0.433; Fig. 2F). The cold groups showed a higher DEI than the warm groups (Fig. 2F), suggesting that the effect of temperature acclimation on DEI may be weaker than the effect of seasonal acclimatization (Fig. 2F).
Skeletal muscle mass was significantly affected by acclimation (F1, 19 = 18.652, PA < 0.001; Fig. 3A), but not by temperature (F1, 19 = 0.007, PT = 0.936; Fig. 3A) or the interaction between temperature and acclimation (F1, 19 = 0.596, PT × PA = 0.450; Fig. 3A). The non-acclimated birds exhibited a heavier skeletal muscle mass compared with the acclimated birds (Fig. 3A). Liver mass was not significantly affected by temperature (F1, 19 = 3.355, PT = 0.083; Fig. 3B), acclimation (F1, 19 = 2.250, PA = 0.150; Fig. 3B), or the interaction between temperature and acclimation (F1, 19 = 0.599, PT × PA = 0.448; Fig. 3B). Heart mass was significantly affected by temperature (F1, 19 = 13.456, PT = 0.002; Fig. 3C) and the interaction between temperature and acclimation (F1, 19 = 4.624, PT × PA = 0.045; Fig. 3C), but not by acclimation (F1, 19 = 1.653, PA = 0.214; Fig. 3C). Birds in the cold groups had a heavier heart mass than those in the warm groups (Fig. 3C). Thus, the effect of seasonal acclimatization on heart mass may be stronger than the effect of temperature acclimation (Fig. 3C). Kidney mass was significantly affected by temperature (F1, 19 = 20.360, PT < 0.001; Fig. 3D), but not by acclimation (F1, 19 = 2.054, PA = 0.168; Fig. 3D) or the interaction between temperature and acclimation (F1, 19 = 0.002, PT × PA = 0.963; Fig. 3D). Birds in the cold groups had a heavier kidney mass than those in the warm groups (Fig. 3D).
State 4 respiration was significantly affected by temperature in the skeletal muscle (F1, 20 = 15.984, PT = 0.001; Fig. 4A), liver (F1, 20 = 29.810, PT < 0.001; Fig. 4B), heart (F1, 20 = 14.775, PT = 0.001; Fig. 4C) and kidney (F1, 20 = 38.512, PT < 0.001; Fig. 4D). State 4 respiration was also affected by acclimation in the liver (F1, 20 = 33.327, PA < 0.001; Fig. 4B) and kidney (F1, 20 = 4.656, PA = 0.043; Fig. 4D). For the birds in the cold groups, the skeletal muscle, liver, heart and kidney all had significantly higher state 4 respiration compared with those in the warm groups (Fig. 4). Birds in the non-acclimated groups showed significantly higher state 4 respiration in the liver and kidney compared with those in the acclimated groups (Fig. 4B and D). Furthermore, the interaction between temperature and acclimation also exerted a significant effect on state 4 respiration in the liver (F1, 20 = 14.415, PT × PA = 0.001; Fig. 4B) and kidney (F1, 20 = 6.772, PT × PA = 0.017; Fig. 4D). COX activity was significantly affected by temperature in the skeletal muscle (F1, 20 = 18.738, PT < 0.001; Fig. 5A) and liver (F1, 20 = 15.932, PT = 0.001; Fig. 5B). COX activity was also significantly affected by acclimation in the skeletal muscle (F1, 20 = 24.430, PA < 0.001; Fig. 5A) and liver (F1, 20 = 72.298, PA < 0.001; Fig. 5B). In addition, the interaction between temperature and acclimation showed no significant effect on COX activity in the skeletal muscle (F1, 20 = 0.989, PT × PA = 0.332; Fig. 5A) and liver (F1, 20 = 2.644, PT × PA = 0.120; Fig. 5B). Kidney COX activity was significantly affected by temperature (F1, 20 = 28.039, PT < 0.001; Fig. 5D), acclimation (F1, 20 = 34.498, PA < 0.001; Fig. 5D) and the interaction between temperature and acclimation (F1, 20 = 5.358, PT × PA = 0.031; Fig. 5D). The skeletal muscle, liver and kidney of the birds in the cold groups had significantly higher COX activity compared with those in the warm groups (Fig. 5A, B and 5D). The skeletal muscle, liver and kidney of the birds in the non-acclimated groups had significantly higher COX activity compared with the acclimated groups (Fig. 5A, B and 5D). However, heart COX activity was not affected by temperature (F1, 20 = 3.749, PT = 0.067; Fig. 5C), acclimation (F1, 20 = 0.775, PA = 0.389; Fig. 5C) and the interaction between temperature and acclimation (F1, 20 = 0.557, PT × PA = 0.464; Fig. 5C). These results implied that seasonal acclimatization and temperature acclimation had similar effects on state 4 respiration and COX activity.
Small bird species from temperate climates have developed several adaptations to cope with cold stress during winter. These include adjustments in BMR (Piersma et al., 1996; Wiersma et al., 2007), energy budget (Zhou et al., 2016; Wang et al., 2019), and enzymatic activity (Zheng et al., 2008b; Hu et al., 2017). According to our data, Chinese Hwameis were found to exhibit temperature- and season-induced changes in BMR and EWL, with BMR and EWL being higher in cold conditions and during winter, and lower in warm conditions and during summer. For this species of birds, GEI, DEI, organ mass, state 4 respiration, and COX were higher when the birds were acclimated to a cold temperature or acclimatized to winter compared with those that were acclimated to a warm temperature or acclimatized to summer. Some of the effects of temperature that we observed could have arisen by chance because of the small sample size.
Many birds exhibit considerable phenotypic flexibility in order to meet their energy requirements, including the adjustment of BMR during seasonal acclimatization (McKechnie, 2008; Swanson et al., 2017). For example, Zheng et al. (2008b) found that the BMR of Eurasian Tree Sparrows (Passer montanus) in winter and autumn was significantly higher than in spring and summer. Similar increases in BMR associated with seasonal acclimatization have also been observed in Common Redpolls (Acanthis flammea), White-breasted Nuthatchs (Sitta carolinensis), Downy Woodpeckers (Picoides pubescens), White-crowned Sparrows (Zonotrichia leucophrys) and Chinese Bulbuls (Pycnonotus sinensis; Dawson and Carey, 1976; Southwick, 1980; Liknes and Swanson, 1996; Zheng et al., 2008a). In the case of the Chinese Hwamei, the data revealed a higher BMR for the winter-acclimatized birds than for the summer-acclimatized birds, consistent with previous research showing that seasonal acclimatization can alter the BMR of the Chinese Hwamei (Wu et al., 2015; Zhao et al., 2015; Wang et al., 2019). However, the phenotypic adjustment as exemplified by BMR is often not correlated with seasonal climates in subtropical or tropical birds, including Rufous-crowned Sparrow (Zonotrichia capensis) and Australian Owlet-nightjar (Aegotheles cristatus; Doucette and Geiser, 2008; Maldonado et al., 2009). Swanson (2010) suggested that higher latitudes with cold winters incur a much greater thermoregulatory demand for birds in winter than in summer, thereby exhibiting a common pattern of seasonal metabolic variation, while subtropical or tropical birds are more variable in their responses to temperature than birds from higher latitudes (Londoño et al., 2015). These data might suggest that thermogenic demand is a common modulator of BMR for small birds in different environments (van de Ven et al., 2013a, 2013b; Zheng et al., 2014a; Noakes et al., 2017; Swanson et al., 2017; Noakes and McKechnie, 2020a; Oswald et al., 2021). Cold-acclimated birds also show increased BMR compared with warm-acclimated birds (Swanson, 2010; Swanson et al., 2017). This is evident in a number of bird species, for example, Rufous-collared Sparrow (Maldonado et al., 2009), Laughing Dove (Streptopela senegalensis; McKechnie et al., 2007), and Red-billed Leiothrix (Leiothrix lutea; Cui et al., 2019). Our investigation of Chinese Hwameis also revealed a higher BMR for the cold-acclimated birds compared with the warm-acclimated ones, consistent with a previous study of temperature-acclimated Chinese Hwameis (Zhou et al., 2016).
Total cutaneous water loss, respiratory water loss or EWL is usually used as a parameter in water balance studies (Tieleman et al., 2004; Williams et al., 2012; Xia et al., 2013). In small bird species, EWL accounts for more than five times the water loss through feces and urine (Muñoz-García and Williams, 2007). For example, Wang et al. (2019) have observed seasonal variation in EWL for Chinese Hwameis, with the birds caught in winter showing 50% higher EWL than those caught in summer. These authors also found a positive correlation between BMR and EWL. Additionally, Lin et al. (2014) found a significantly higher EWL in cold-acclimated (10 ℃) Chinese Bulbuls than in their warm-acclimated (30 ℃) counterparts. In this study, we found that EWL was higher in both winter-acclimatized and cold-acclimated Chinese Hwameis compared with the summer-acclimatized and warm-acclimated birds. Williams and Tieleman (2000) proposed that increased metabolic rates would lead to an increase in respiratory frequency and consequently higher EWL. Our observation regarding the winter and cold-acclimated birds displaying a greater BMR than the summer and warm-acclimated birds would support the result that higher metabolic rates would lead to a higher EWL (Williams, 1999; Tieleman and Williams, 2002; Xia et al., 2015; Wang et al., 2019). An increase in BMR under cold conditions can also be compensated for by adjustments in GEI and DEI (Zhou et al., 2016; Mao et al., 2019). GEI and DEI were found to be significantly higher in the winter-acclimatized and cold-acclimated Chinese Hwameis, consistent with the phenomenon of cold-induced energy adaptation documented for other small birds (Salvante et al., 2010; Syafwan et al., 2012; Wu et al., 2014). Wu et al. (2014) found that GEI and DEI in Chinese Bulbuls are significantly higher in winter. Cui et al. (2019) also showed that Red-billed Leiothrixs kept under cold conditions need more dietary intake compared with those living in warm conditions. Our data indicated a remarkable rise in BMR within the range of winter and cold-acclimated birds. Moreover, the GEI and DEI of these groups of birds were significantly higher and positively correlated with BMR. These findings would add to the growing body of evidence that adaptation to cold temperatures can take place both morphologically and physiologically. Several studies have reported reversible changes in internal organs in response to variations in energy requirement (Daan et al., 1990; Piersma and Lindstrom, 1997). Increased energy demands, for example, have been associated with organ enlargements that contribute significantly to BMR (Daan et al., 1990; Clapham, 2012). Consistent with these reported findings, our data showed that during winter, the Chinese Hwamei exhibited a significant increase in heart dry mass compared with summer. Furthermore, an increase in kidney dry mass and kidney wet mass for both winter and the cold-acclimated birds compared with summer and the warm-acclimated birds. However, no differences in the skeletal and liver masses were detected between winter- and summer-acclimatized or between cold- and warm-acclimated birds. Thus, adjustments of organ mass would not entirely explain the variation in BMR. Additional variables such as the specific metabolic activity of the internal organs may need to be considered when accounting for the variation in BMR in future studies (Maldonado et al., 2009; Zheng et al., 2014b; Zhou et al., 2016). Regulating the activity of catabolic enzymes and transporters involved in substrate delivery pathways may be part of the process of adjusting the ability of the tissues and/or organs to carry out aerobic metabolism at the cellular level (Marsh et al., 1990; Zhou et al., 2016., Cui et al., 2019). For example, Li et al. (2017) found state 4 respiration and COX activity in the heart and skeletal muscles of Silky Starlings (Sturnus sericeus) are significantly higher in winter than in summer and both parameters are positively correlated with BMR. Red-billed Leiothrixs kept under a cold temperature also showed improved state 4 respiration and COX activity in several organs, including the liver, kidneys, muscle and heart compared with individuals kept under a warm temperature, indicating that an improved ability of the metabolically active organs may have a significant influence on the thermoregulation of leiothrixs during exposure to cold conditions (Cui et al., 2019). Winter and cold-acclimated Chinese Hwameis exhibited higher BMRs as well as increased COX activity and state 4 respiration in the liver, kidney, and skeletal muscle relative to those of the summer and warm-acclimated birds. This finding was in agreement with previous observations that under seasonal acclimatization and temperature acclimation, Chinese Hwameis can alter state 4 respiration and COX activity of the liver, kidney, and skeletal muscle, as well as BMR (Wu et al., 2015; Zhou et al., 2016; Wang et al., 2019). Adjustments in cellular aerobic capacities and enzymatic changes might, therefore, only partially explain the variation in BMR.
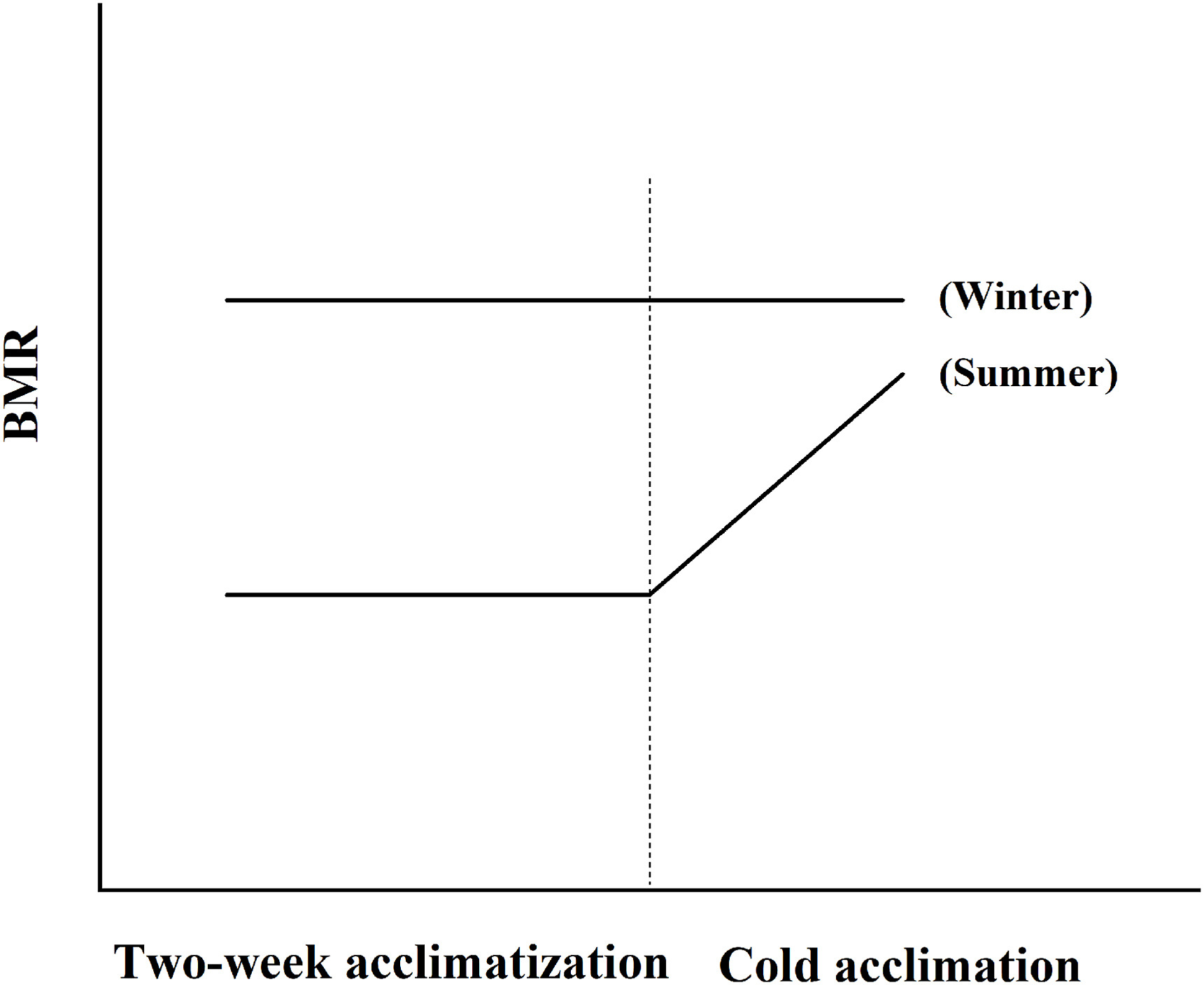
Birds inhabiting seasonal environments often display adjustments in BMR (Swanson, 2010; Zheng et al., 2014a). There has been a growing body of evidence that morphological and physiological indicators can respond quickly to low environmental temperatures. Some studies have investigated the response of seasonal variations in BMR to ambient temperature, but the results of these studies have been inconsistent (Maldonado et al., 2009; Noakes et al., 2017). It appears that BMR might show different patterns during seasonal adjustments, depending on the latitude of the location where the bird is found. For example, Zheng et al. (2008b, 2014a) found that Eurasian Tree Sparrows and Chinese Bulbuls display variation in BMR between summer and winter. However, Maldonado et al. (2009) who studied the Rufous-collared Sparrows from Quebrada de la Plata in Chile reported no variation in BMR between summer and winter. These authors also suggested that wild birds have more options available for behavioral thermoregulation than laboratory-acclimated birds. Our results have shown that both seasonal acclimatization and temperature acclimation may cause changes in the BMR of the Chinese Hwamei, however, the fluctuations in BMR between the seasonal acclimatized birds and the laboratory-acclimated birds were different. Laboratory birds have to fully acclimate to lower temperatures, whereas wild birds can avoid this because they can behaviorally escape from some of the cold conditions. In this study, we have designed a model to describe the correlation between body mass and BMR in seasonal acclimatization and used this model to predict the BMR for laboratory-acclimated birds. The body mass of the temperature-acclimated birds was used to predict the value of BMR based on the formula of seasonal acclimatization. No difference between the predicted and the measured BMR was found during warm acclimation, but a significant difference was observed between the predicted and measured BMR values during cold acclimation. The model showed that birds that underwent cold acclimation had a higher BMR than those that went through winter acclimatization (Fig. 6). However, according to the ANCOVA model which examined the interaction between seasonal acclimatization and temperature acclimation (Fig. 2C), winter acclimatization basically yielded the same effect as cold acclimation, implying a lack of interaction between temperature and acclimation. Similar to the result obtained with our model, Swanson and Olmstead (1999) previously concluded that changes in BMR in response to the changes in exposure conditions can be better predicted under short-term temperature acclimation than long-term temperature acclimation. Thus, our finding could be considered further evidence for the rapid response of morphological and physiological indicators to low environmental temperatures. This could imply that small bird species would benefit from the capacity to readjust their physiological indicators rapidly in response to sudden changes in ambient temperatures.
Seasonal acclimatization can influence the flexibility in the BMR of the birds at the time of capture. The sensitivity of the Chinese Hwamei to cold temperatures varies according to season, and its ability to adapt to cold conditions is influenced by fluctuations in temperature (Wu et al., 2015; Wang et al., 2019). With Chinese Hwameis, our data showed that individuals acclimated to cold (15 ℃) during July had a significantly higher BMR compared with the summer birds. However, those that were acclimated to 15 ℃ during January showed no significant difference in BMR compared with the winter birds (winter: 230.00 ± 7.09 mL O2/h; cold acclimation: 237.75 ± 8.64 mL O2/h; unpublished data). The lack of effect from cold acclimation on the winter-acclimatized birds was consistent with the finding reported by Swanson et al. (2020). The acclimation capacity of a bird is influenced by its recent acclimatization history. During the seasonal acclimatization experiment, the average field temperature was 31.8 ± 0.4 ℃ in summer and 8.8 ± 0.6 ℃ in winter. The differences between daily minimum and maximum ambient temperatures varied from 6 to 11 ℃ in summer, and from 7 to 15 ℃ in winter. The temperature differences were much less than the 20 ℃ difference between the temperatures of the warm and cold acclimations (Maldonado et al., 2009; Wu et al., 2015). Thus, the low BMR obtained for the seasonally acclimatized birds could be explained by the small fluctuations in daily temperatures. In nature, an adaption to cold is complicated by temperature fluctuations and gradual decreases in mean daily temperatures. Therefore, a model of cold acclimation for winter may differ from that for summer (Fig. 7). Additionally, the low BMR of seasonally acclimatized birds could also be due to behavioral thermoregulation options that were not available to the laboratory-acclimated birds. Furthermore, during seasonal acclimatization, an insulative change (increased plumage or subcutaneous fat) or a shift in the TNZ may be an efficient energy adaptation, despite the fact they are relatively slow to develop (Weathers, 1997; Bush et al., 2008). Swanson (1991) found higher fat scores and plumage masses in Dark-eyed Juncos (Junco hyemalis) individuals acclimatized to winter compared with those acclimatized to summer, although increments in insulation did not offset the increased thermogenic requirements for passerines (Dawson and Marsh, 1983). Insulation in small birds is increased in winter and winter-acclimatized Chinese Hwameis, and conservation is further enhanced by an ability to decrease thermal conductance with decreasing ambient temperatures (Wu et al., 2015). However, seasonal variation in insulation in passerines is not always associated with seasonal changes in cold tolerance (Swanson, 1991). Some birds display a flexible TNZ, such as the Common Redpolls (West, 1972) and Rock Kestrels (Falco rupicolis; Bush et al., 2008). For the Chinese Hwamei, its TNZ is 29–33 ℃ in summer, and 28–35 ℃ in winter (Wu et al., 2015). Thus, a wide TNZ appears to be an adaptive strategy to cold winter temperatures. Finally, the fluctuations in food availability may give rise to the differences in the magnitude of BMR changes between seasonal acclimatization and cold acclimation during the milder winters at lower latitudes (Smit and McKechnie, 2010; Noakes and McKechnie, 2020b). The Chinese Hwamei eats insects throughout the year, especially during the breeding season. However, it also eats fruits of various plants or tender vegetables in the non-breeding season (Li et al., 2006). Winter is associated with a pronounced decrease in food availability but without significant increases in thermoregulatory demands in subtropical regions (Smit and McKechnie, 2010). Variations in environmental variables (e.g., diet composition and food availability) under seasonal acclimatization can influence the metabolic rate and energy intake of Chinese Hwameis. However, these variables were kept constant in the temperature acclimation experiments, and this could be a possible explanation for the differences caused by fluctuations in food availability.
In conclusion, this study has demonstrated the presence of a systemic response in the Chinese Hwamei to seasonal acclimatization and temperature acclimation, and such a response seemed to involve elevated body mass, EWL, metabolic rate, energy budget, and enhanced enzymatic activity in the organs. These findings would support the hypothesis proposed by McKechnie (2008) and Swanson et al. (2017) that physiological plasticity in metabolic adjustments is a general characteristic of bird adaptation in response to cold environments.
ML and JL conceived the study and designed the experiments. YX, RC, JX and JZ collected the data. YX, RC and JZ analyzed the data. YX, RC and ML wrote the manuscript. YX, ML and JL interpreted data and revised the manuscript. All authors read and approved the final manuscript.
All experimental procedures were approved by the Animal Care and Use Committee of the Wenzhou University.
The authors declare that they have no competing interests.
We thank Dr. Katrina Seelye nee (Hale of Biological Science Editing, New Zealand) and Dr. Alan K. Chang (Wenzhou University) for revising this manuscript and the Animal Physiological Ecology Group, Institute of applied ecology of Wenzhou University, for their helpful suggestions. This study was financially supported by grants from the National Natural Science Foundation of China (No. 31971420 and No. 32171497).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100084.
|
Daan, S., Masman, D., Groenewold, A., 1990. Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am. J. Physiol. Reg. I. 259, 333-340.
|
|
Dawson, W.R., Marsh, R.L., Yacoe, M. EYacoe, M.E., 1983. Metabolic adjustments of small passerine birds for migration and cold. Am. J. Physiol. 245, 755-767.
|
|
Feder, M.E., Bennett, A.F., Burggren, W.W., Huey, R.B., 1987. New Directions in Ecological Physiology. Cambridge University Press, Cambridge.
|
|
Hu, S.N., Zhu, Y.Y., Lin, L., Zheng, W.H., Liu, J.S., 2017. Temperature and photoperiod as environmental cues affect body mass and thermoregulation in Chinese bulbuls (Pycnonotus sinensis). J. Exp. Biol. 220, 844-855.
|
|
Lin, L., Cao, M.T., Hu, Y.L., Huang, L.L., Li, Z., Liu, J.S., 2014. Effect of environmental temperature on thermogenesis and evaporative water loss in Chinese bulbuls (Pycnonotus sinensis). Acta Ecol. Sin. 34, 564-571.
|
|
Liu, J.S., Wang, D.H., Sun, R.Y., 2005. Climatic adaptations in metabolism of four species of small birds in China. Acta Zool. Sin. 51, 24-30.
|
|
Liu, J.S., Li, M., 2006. Phenotypic flexibility of metabolic rate and organ masses among tree sparrows Passer montanus in seasonal acclimatization. Acta Zool. Sin. 52, 469-477.
|
|
Marsh, R.L., Dawson, W.R., Camilliere, J.J., Olson, J.M., 1990. Regulation of glycolysis in the pectoralis muscles of seasonally acclimatized American goldfinches exposed to cold. Am. J. Physiol. 258, 711-717.
|
|
Noakes, M.J., McKechnie, A.E., 2020a. Phenotypic flexibility of metabolic rate and evaporative water loss does not vary across a climatic gradient in an Afrotropical passerine bird. J. Exp. Biol. 223, jeb220137.
|
|
Schmidt-Nielsen, K., 1997. Animal Physiology: Adaptation and Environment. Cambridge University Press, Cambridge.
|
|
Southwick, E.E., 1980. Seasonal thermoregulatory adjustments in white-crowned sparrows. Auk 97, 76-85.
|
|
Swanson, D.L., 2010. Seasonal metabolic variation in birds: functional and mechanistic correlates. In: Thompson, C.F. (Ed.), Current Ornithology, 17. Springer, Berlin, pp. 75–129.
|
|
Wang, Y., Shan, S.S., Zhang, H.D., Dong, B.B., Zheng, W.H., Liu, J.S., 2019. Physiological and biochemical thermoregulatory responses in male Chinese Hwameis to seasonal acclimatization: phenotypic flexibility in a small passerine. Zool. Stud. 58, 6.
|
|
Xia, S.S., Yang, F., Wang, R.M., Zheng, W.H., Zhao, Z.J., Liu, J.S., 2015. Physiological responses in Chinese bulbuls to seasonal acclimatization and temperature acclimation. Acta Ecol. Sin. 35, 2349-2359.
|
|
Zheng, W.H., Lin, L., Liu, J.S., Pan, H., Cao, M.T., Hu, Y.L., 2013a. Physiological and biochemical thermoregulatory responses of Chinese bulbuls Pycnonotus sinensis to warm temperature: phenotypic flexibility in a small passerine. J. Therm. Biol. 38, 483-490.
|
|
Zheng, W.H., Lin, L., Liu, J.S., Xu, X.J., Li, M., 2013b. Geographic variation in basal thermogenesis in little buntings: relationship to cellular thermogenesis and thyroid hormone concentrations. Comp. Biochem. Phys. A 164, 240-246.
|
|
Zhou, L.M., Xia, S.S., Chen, Q., Wang, R.M., Zheng, W.H., Liu, J.S., 2016. Phenotypic flexibility of thermogenesis in the Hwamei (Garrulax canorus): responses to cold acclimation. Am. J. Physiol. Reg. I. 310, 330-336.
|