DAD, DDD conceptualized the study. DAD, DDD collected the data and conveyed the field survey. DAD, DDD and GSP run the analysis. DAD wrote the manuscript. DDD, GSP, SAS contributed critically to the drafts. All authors read and approved the final manuscript.
Permits for exemption from the prohibitions related to the Eastern Imperial Eagle imposed by Art. 38 of the Biological Diversity Act, namely capturing for tagging, disturbance during the breeding season, rescuing individuals from abandoned broods and collecting found dead individuals, as well as keeping and transporting the latter in order to implement relevant scientific surveys, were provided by the Ministry of Environment and Water of Bulgaria, No. 203/25.05.2009; No. 408/12.05.2011.
The authors declare that they have no competing interests.
Many volunteers assisted with the monitoring and conservation efforts, and we are grateful for the assistance of Vera Dyulgerska, Svetoslav Spasov, Edita Difova, Ivaylo Angelov, Nikolai Terziev, Tzeno Petrov, Petar Iankov, Vladimir Trifonov, Nedko Nedyalkov, Atanas Demerdzhiev, Krasimira Demerdzhieva, Georgi Georgiev, Dimitar Plachiysky, Georgi Gerdzhikov, Vladimir Dobrev, Vanyo Angelov, Alexander Georgiev, Vasilena Georgieva. We express our gratitude to Miguel Ferrer, Todd Katzner, Marton Horvath, Volen Arkumarev and Gary Ritchison for their valuable comments and suggestions, which significantly improved the quality of the early version of the manuscript. We are grateful to the two anonymous reviewers for their valuable comments and recommendations on the manuscript. This work was funded by the LIFE Program of the European Union under the project 'Restoration and sustainable management of Imperial Eagle's foraging habitats in key Natura 2000 sites in Bulgaria' LIFE14 NAT/BG/001119.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100030.
| Citation: | Dimitar Demerdzhiev, Dobromir Dobrev, Georgi Popgeorgiev, Stoycho Stoychev. 2022: Landscape alteration affects the demography of an endangered avian predator by reducing the habitat quality. Avian Research, 13(1): 100030. DOI: 10.1016/j.avrs.2022.100030 |
Habitat transformation is identified as major threat to biodiversity loss globally, affecting threatened raptors. Changes in land use can alter the abundance and distribution of birds of prey by affecting habitat availability and quality. In this study, we used multivariate analyses to investigate the effect of habitat alteration on the demography of a declining Eastern Imperial Eagle (Aquila heliaca), making assumptions for future population trajectories. We used the Generalized Linear Mixed Models with Poisson distribution and Log link function, searching a relationship between the demographic parameters and the landscape structure and possible effects of fragmentation. In our study area, habitat change affected dramatically permanent grasslands, shrinking their availability. As we expected, the share of grasslands in eagles' territories significantly affected occupancy rate, but not productivity and breeding frequency. We found that occupancy rate decreased significantly, while productivity and breeding frequency showed no trend. Modeling the effect of habitat alteration on Eastern Imperial Eagle demography, we found out that territory quality was a more powerful factor driving the response of a top predator to the alteration of favorite foraging habitats. Only the habitat quality in source territories had a significant positive effect on eagle productivity. We found that simple rules to predict adverse agricultural impact on territory abandonment or breeding productivity of a top predator were not reliable. Our results could be used for planning conservation of other large territorial raptors, facing the same type of threat.
Changes in land use can alter the abundance and distribution of species by affecting habitat availability and quality. Habitat alteration and loss are among the major threats to biodiversity on a global scale (Donald et al., 2013). Some landscapes are changing rapidly, mostly due to agricultural expansion and intensification (Foley et al., 2005) and these changes may lead to habitat loss (Newbold et al., 2015) and fragmentation (Jongman, 2002; Jaeger et al., 2008). Globally, agriculture is the main cause of habitat loss and the most common threat to threatened species of raptors (McClure et al., 2018). However, raptors show a different response with respect to agriculture depending on the behavior and ecology of the species as well as the land use pattern and intensity. Although some species of raptors may be positively influenced by the increased agricultural land use resulting in increased breeding performance, perhaps linked to increases in prey availability or abundance of suitable nest sites (Cardador et al., 2011; Sternalski et al., 2013; Coates et al., 2014; Murgatroyd et al., 2016), the effects for most species include reduced breeding productivity, adult survival, or territory occupancy rate (Arroyo et al., 2002; Demerdzhiev et al., 2014a; Väli et al., 2017).
Grasslands were listed as the second most commonly required habitat type for raptors globally (McClure et al., 2018), offering foraging and breeding habitats for many species. Until the beginning of the 21st century, semi-natural grasslands were still common in Bulgaria, but their area has declined significantly (Meshinev et al., 2005), primarily due to the conversion of grasslands into arable land.
Eastern Imperial Eagles (Aquila heliaca, hereafter EIE) are long-lived, territorial raptors that breed from central and southern Europe to western and central Asia (del Hoyo et al., 1994). These eagles inhabit mostly steppes and open forest-steppe areas, river valleys, and agricultural land with solitary trees or groups of trees, but also breed in woodlands (Ferguson-Lees and Christie, 2001). The demography of the EIE and factors that influence it have rarely been investigated in different parts of its range (Bragin, 2000; Katzner et al., 2005; Horváth et al., 2010, 2014). Moreover, there is no research demonstrating the link between the species demography and changes in land use due to agricultural intensification. Although the EIE population is considered stable and increasing in some areas of Europe, Kazakhstan, and Russia (Demerdzhiev et al., 2011; Karyakin, 2018), the species is classified as globally vulnerable (VU) with a declining global population (BirdLife International, 2020). Since 2000, increased conservation efforts have led to a gradual increase in their population in Bulgaria, a process clearly evident until around 2014 (Demerdzhiev et al., 2014b, 2015), followed by a noticeably slower population growth (Demerdzhiev et al., 2018). The country's accession to the EU entailed conversion of grasslands of utmost significance to the species into arable land, which calls for a detailed assessment of the impact on the eagle demography.
We assessed the loss of different types of grasslands, being the most important foraging habitats for the EIE, searching for possible impacts on the eagle demography. We made the following prediction: loss of grasslands at different scales affected the demographic parameters in different ways because territories in areas with greater alteration were more often abandoned than those undergoing less change. If EIE were influenced by grassland transformation, we predicted that productivity and breeding frequency would be lower in territories with a greater loss of grassland than territories of low level of modification. We also suggest that the habitat alteration affected the territory quality. Understanding how eagles respond, in general, to habitat loss will be important knowledge for the conservation of other large territorial raptors that face the same type of threat. Finally, we used our demographic data from three studied periods, source-sink dynamics, and threshold of alteration to construct a simple model of the long-term effect of agricultural transformation on eagle demography.
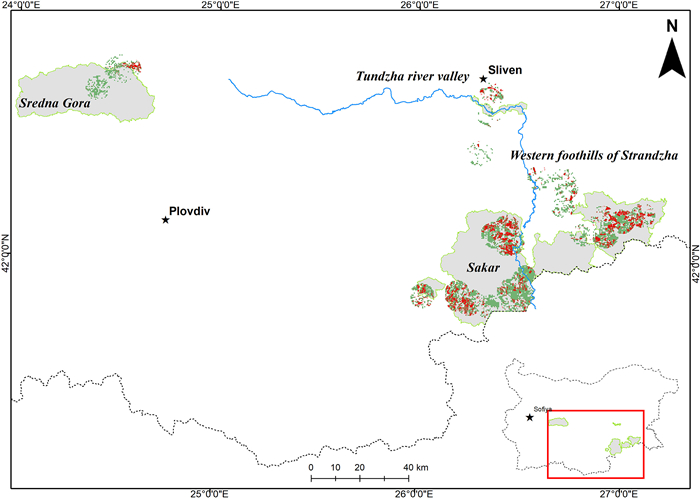
The study covered 24 breeding territories occupied by EIE pairs in Bulgaria (Fig. 1). Most were located in the southeastern part of the country, but three territories were located in central mountain areas. Twenty breeding territories were located in seven Special Protected areas (SPAs) and four are found beyond the borders of the NATURA 2000 Network. The southeastern part of the country includes hilly and low mountain areas located along the Bulgarian-Turkish border. Territories in central Bulgaria were located in the Sredna Gora Mnt. In the eponymous SPA. Forests of European Beech (Fagus sylvatica), Sessile Oak (Quercus petraea), and Hornbeam (Carpinus betulus) prevail in the vegetation of this mountain region (Galabov, 1982). The southeastern parts are characterized by continental-Mediterranean climate with particularly dry summers. The woodlands are of limited distribution, including mixed deciduous forests of Turkey Oak (Quercus cerris), Hungarian Oak (Quercus farnetto), Downy Oak (Quercus pubescens), and Oriental Hornbeam (Carpinus orientalis) (Bondev, 1991). Large parts of the region are occupied by agricultural areas, mainly sown with wheat, sunflower, and rapeseed, or pastures and meadows. Open areas are overgrown with shrubs of Jerusalem Thorn (Paliurus spina-christi), and combined with xerothermic grass communities dominated by Yellow Bluestem (Dichantieta ischaemi), Bulbous Bluegrass (Poaeta bulbosae and Poaeta concinnae), Scented Grass (Chrysopogoneta grylli), and Ephemera (Ephemereta) (Bondev, 1991).
Surveys were conducted between 2001 and 2020, divided into three periods of almost equal duration. The first period included the years between 2001 and 2006, prior to Bulgaria's accession to the EU. This period was characterized by extensive agriculture and animal husbandry, with large areas of arable land, cultivated during the socialist regime, overgrown or transformed into semi-natural grasslands. The second period from 2007 to 2013, related to the country's accession to the EU, was characterized by intensification of agriculture through subsidies. During that period, extensive ploughing of natural and semi-natural grasslands and conversion to arable lands was recorded. The third period, from 2014 to 2020, included the last programming period of the European Commission's CAP (Common Agriculture Policy). It was characterized by a new loss of various types of grasslands, financially stimulated by subsidies, but the cumulative effect of the habitat changes accumulated in the previous period should also be taken into account. We grouped 24 studied eagles' territories in four regions: Sakar Mnt. (N = 11), western foothills of Strandzha Mnt. (N = 5), Tundzha river valley (N = 5), and Sredna Gora Mnt. (N = 3). The minimum distance between pairs in different neighboring regions was > 25 km.
During the breeding season (February–August), we monitored the eagles' territories two or three times per month until young fledged. For further details on the field methodology applied in this survey, see Demerdzhiev et al. (2014b, 2015). To evaluate the effect of intraspecific competition on the eagles' demography, we measured the nearest neighbor distance (NND), a surrogate variable for breeding density. For each territory, demographic parameters were recorded every year, including occupancy, incubation and number of fledglings. For all of the three studied periods, we considered the averaged values of the demographic parameters. Occupancy rate (OR) was calculated as the number of years that a territory was occupied in a given period, divided by the number of years monitored (Sarà and Di Vittorio, 2003). A territory was considered occupied if an EIE pair with a nest and breeding behavior was present during the breeding season (February–August) (see Katzner et al., 2005; Demerdzhiev et al., 2015). If a pair was not seen during at least four visits in a year and there was no sign of breeding activity, the territory was considered unoccupied. Breeding frequency (BF) was estimated as the proportion of years in which the pairs incubated. Productivity of territory (P) was evaluated as the number of fledglings divided by the number of breeding attempts in a period.
In some cases, birds in immature plumage occupied a territory occasionally, with the pair falling apart the following year (Demerdzhiev, 2011). To avoid the effect of this phenomenon on the results, we only analyzed the territories occupied for at least two consecutive years. To eliminate the bias due to non-natural factors, we excluded from the analysis eight cases when breeding was compromised due to nest robbing (Whitfield et al., 2007).
Assessment of habitat quality is a problematic process because it is also related to the individual quality of the birds, both phenomena being linked and difficult to distinguish from each other (Sergio et al., 2009; Reynolds et al., 2019). Since long-lived raptors with strong site fidelity are faithful to their territories even if better territories exist, occupancy measures of habitat quality may be ambiguous (Sergio and Newton, 2003). With raptors, low-quality territories are more frequently occupied by immature breeders, because high-quality individuals, such as adults, usually settle in territories offering high-quality conditions (Steenhof et al., 1983; Newton, 1991; Ferrer et al., 2006). Thus, to evaluate territory quality, we used presence of immature breeders as a measure of their quality. Immature breeders in raptor species tend to occupy territories with low quality and are used as a surrogate measurement of this phenomenon (Newton, 1979; Margalida et al., 2008). We considered territories, occupied by adult individuals more than 60% of the time as high-quality territories, and those with less than 60% as low-quality territories (Sarà and Di Vittorio, 2003).
The location of nest sites (N = 42) and the preferences of breeding territories (N = 24) were analyzed through Arc GIS 10.4.1 (ESRI, 2016). The analysis was based on a Corine Land Cover layer of Bulgaria valid for 2006, as well as an orthophoto layer, valid for that same year (http://212.122.182.101/MRRB/). In both cases, a buffer of 5-km radius (NND = 9060.2 m ± 5559.1 SD) around each nest was used. This is the measurement approach used to identify the width of the minimal hypothetical territory of different raptor species, representing half of the distance between neighboring breeding pairs (González et al., 1992; Liberatori and Penteriani, 2001; Penteriani et al., 2001, 2003). The area of 78.5 km2 was considered an average hypothetical territory occupied by an EIE pair in Bulgaria (Demerdzhiev, 2011). However, taking into account the lack of published telemetric data on the size of the breeding territories of EIE, we accepted that the size of the territory we proposed was close to that actually used by the eagles. Thus, the territory boundaries were conditionally set as a 5 km buffer around occupied nest. When running the habitat analysis, we related the territory to the nest occupied for the longest period of time to avoid overlapping of habitat layers and pseudoreplication. Whenever a pair changed its nest ≤1 km from the previous one, we considered this is the same territory (Demerdzhiev, 2011). With > 1 km changes, we assumed that EIE had occupied a new territory (N = 1). Data modeling was done in an Arc GIS environment. Remote verification of the habitats was carried out during the first period of the study through ortho-photography covering the country's territory (http://212.122.182.101/MRRB/). Changes in land-use patterns were verified through visits to all polygons in 2012 and 2013 to detect any differences compared to the baseline. All polygons were visited again in 2019 and 2020 to record changes that occurred after 2013. Thus, for each territory, changes that occurred during the survey periods were recorded. For field verification, we used 1:25, 000 scale maps of the regions, as well as GPS, to mark each polygon where changes occurred. The habitat categorization was based on the Corine Land Cover classes. To evaluate the effect of habitat change, we considered a territory as greatly affected if grassland types decreased by more than 12.7%, and, respectively, as slightly affected if the decrease was below this value, compared to the baseline. We took this value as a threshold because this corresponded to the average percentage of grassland habitat loss for all studied territories.
For the purpose of statistical processing of data, the habitat categories with no biological sense for feeding grounds of EIE (such as: infrastructure, water bodies, forest, etc.) were not included in the analysis. Since EIE is strongly associated with different types of grasslands, for fragmentation analysis this habitat was subject to further categorization: permanent grasslands without shrubs, grasslands with shrubs up to 30%, grasslands with shrubs over 31%. This distinction was made using ortho-photo pictures and calculation of the proportion of shrubs in the pastures for each polygon. Thus, initially we analyzed 25 different variables related to foraging habitats, competition, and age of the territory holders (Appendix Table S1). For each of surveyed territory, we calculated the diversity index (H') for the habitats using the original land cover types over the different periods of the study (Shannon and Weaver, 1949). Analysis of the individual fragments of the habitats was run through the application Patch Analyst for ArcGIS 10.4.1. (Appendix Table S1).
As a first step to reduce number of predictors we ran Principal Component Analysis (PCA) at two scales: first, within a 1 km radius around nests as a core area, intensively defended by the eagles, where the birds spend most of their time (Demerdzhiev, 2011); and second, within a 5 km radius, described as the home range. All variables that showed factor scores > 0.6 for PCA 1 and PCA 2 were used in the subsequent analyses (Appendix Table S2). After that we used Spearman correlation coefficient (r) to explore the inter-correlations of the significant variables. When the coefficient of a pair of correlated variables was > 0.6, one of the variables was removed and the other considered to be most important was kept in the subsequent analysis (Green, 1979). The decision as to which of the correlating indicators should remain and which should be removed was made on the basis of their factor score in the PCA, as the indicator with the higher score was kept (Appendix Table S3).
Finally, we used the Generalized Linear Mixed Models (GLMM) with Poisson distribution and Log link function, searching a relationship between the demographic parameters and the landscape structure and possible effects of fragmentation. We ran our GLMMs again at two scales: first, within a 1 km radius and second, within a 5 km radius around the nests. Our response variables were demographic parameters (OR, P, BF) and our categorical factors were period, intensity of effect of habitat change, and territory quality. After PCA and Spearman correlation we used 7 predictors in 1 km scale and 4 predictors in 5 km scale (Appendix Table S3). "Eagle territory" was included in the models as a random effect to account for pseudo-replication. We used Akaike Information Criterion corrected for small sample sizes (AICc) for model selection and chose the models with the lowest AICc value from the set of our candidate models. All models with an AICc value < 2 from the model with the lowest AICc (AICcmin) were considered best models (ΔAICc = AICi – AICcmin) (Burnham and Anderson, 2002). The relative importance of each model was estimated through the weight of AICc (w), so that all the weights for all models added up to 1. We also used explanatory parameter estimates with a standard error and a probability value (P) with regard to the explanatory factors.
All data were analyzed using Statistica for Windows, Release 10 (StatSoft Inc., 2011), R v.2.15.2 (R Core Team, 2012) and Past Version 3.14 (Hammer et al., 2001). The data were analyzed for normal distribution through the Kolmogorov–Smirnov test and log-transformed with function log (x + 1). Results with P ≤ 0.05 were considered significant. Values were provided as means ± SE.
Land-use patterns in EIE territories were subject to a significant transformation over time. While arable land was significantly increasing (F = 5.2, df = 2, P = 0.01), all categories of grassland types suffered from reduction, a process extremely severe for permanent grasslands (F = 12.9, df = 2, P < 0.001). In the regional context, loss of permanent grasslands was established in the Sakar Mnt. (F = 13.1, df = 2, P < 0.001), Tundzha river valley (F = 4.6, df = 2, P = 0.04) and the western foothills of the Strandzha Mnt. (F = 4.3, df = 2, P = 0.04), whereas arable lands increased significantly only in Sakar Mnt. (F = 8.2, df = 2, P = 0.001; Fig. 2).
The average occupancy rate demonstrated gradual decrease (F = 4.01, df = 2, P = 0.03), while the average productivity (F = 1.23, df = 2, P = 0.3) and breeding frequency (F = 0.9, df = 2, P = 0.41) indicated no change over the periods (Fig. 3).
As we predicted, the amount of grassland in home ranges had a significant effect on occupancy rate (Kendall Tau = 0.17, P = 0.05) but not on productivity (Kendall Tau = 0.09, P = 0.29), and breeding frequency (Kendall Tau = 0.1, P = 0.24; Fig. 4). Examining of individual territories, we found out that nine of the twenty-four studied territories reduced their occupancy rate in the past 13 years as the loss of grassland types varied between 0.16% and 21.26%. However, five of these nine territories were classified as high-quality class. On the other side, four of the home ranges improved their occupancy rate over the years despite their loss of grasslands (between 2.73% and 21.77). Moreover, in five of the good-quality ranges the occupancy rate had not changed although their grassland cover suffered from alteration of more than 20–35%.
Our "Global model" confirmed the significant impact of the territory quality factor on the demography of the EIE. Only "high-quality classes" had a significantly positive effect on the productivity, both at "core area" (β = 0.83, Wald. Stat. = 6.52, P = 0.01) and "home range" scale (β = 0.83, Wald. Stat. = 8.61, P = 0.01). This factor determines the first ranked models for all of the studied demographic parameters (OR, P, BF), both at 1 km scale and for home range (Appendix Table S4). It should be noted that factors NFGr and high-quality territories include the second ranked models for productivity (ΔAIC = 1.18, w = 0.20) and occupancy rate (ΔAIC = 1.04, w = 0.12) at home range scale.
We found that land-use patterns of our study area changed significantly after the country's accession to the EU. Spectacular loss of different types of grasslands, especially permanent pastures, was recorded in the first programming period for Bulgaria (2007–2013), following the implementation of CAP. This occurred throughout most of the country (Dobrev et al., 2014), particularly affecting the Sakar Mnt., the adjacent Dervent Heights, and the western foothills of the Strandzha Mnt., where most of the EIE population in Bulgaria is concentrated and where most of the areas are source territories (Demerdzhiev et al., 2014b). This resulted in a change in the land-use pattern expressed as widespread conversion of grasslands into cropland and establishment of large arable monoculture fields mainly sown with cereals, sunflower, and oil rape. Hence, in the 1990s, a significant part of the arable land in the country was abandoned. Therefore, by 2007 (the year of Bulgaria's accession to the EU), only 58.4% of the arable land in Bulgaria was cultivated (Agrostatistics, 2011). After Bulgaria's accession to the EU and the related pre-accession funding, a gradual process of partial restoration of livestock breeding began in the semi-mountainous regions. Thus, formerly arable lands that were subsequently abandoned were converted into semi-natural permanent grasslands. However, the accession to the EU and the implementation of the CAP and the Single Area Payment Scheme (SAPS) in particular, led to widespread plowing of these semi-natural permanent grasslands and their conversion back to arable areas (Dobrev et al., 2014).
We found significant changes in occupancy rate of territories gradually decreasing over the years. Territory abandonment occurs when successful breeding is no longer possible, because pairs with low breeding success are more likely to abandon their territory after the habitat alteration, since they are closer to the threshold at which successful breeding is impossible (Whitfield et al., 2007). Habitat deterioration had a more profound effect on the occupancy of poor territories than those of good quality (Ferrer and Donàzar, 1996). In this regard, habitat alteration increased the eagles' selectivity for territories and this led to the increased occupancy rate of high-quality ranges and, at the same time, a reduction of the occupancy rate of low-quality territories, resulting in the complete abandonment of some of them. Mean productivity and breeding frequency of eagles showed no trend. When a territory persisted temporally because of the abundance and availability of prey, and suitable breeding sites, regardless of some habitat alterations, eagles would tend to occupy it until marginal changes occur. Thus, their productivity and breeding frequency would remain unchanged in the long run.
Our expectation of a strong influence of grassland vegetation on territory occupation found clear support. Being the main foraging habitat, more grassland in a territory means more opportunities for hunting and vice versa, less grassland in the territory supposes fewer feeding chances, as these circumstances drive the eagles' choice when selecting a territory. Different grassland landscapes are rich in various reptiles, rodents, and other medium-size preys, offering good potential for foraging and prey deliveries, hence the attracted eagles start breeding and raise more progeny. Reflecting the findings of Demerdzhiev et al. (2019) for another large predator such as the Lesser Spotted Eagle (Clanga pomarina), we found a positive effect of the presence of grasslands in the home range on the territory occupation.
In direct contrast to our prediction, the loss of grasslands in some territories did not notably impact eagle demography. Then the question is how much habitat will be enough in a given territory? Why are some territories still frequently occupied and with high productivity, even though a significant part of the grassland has been destroyed, while other territories, transformed to a small extent, suffer a severe decline of the breeding parameters and/or are rarely occupied? We found that the response of individual EIE to habitat alteration was highly variable, with some pairs abandoning territories when the grassland cover in the territory had shrunk by less than 5%, whereas others showed enhanced breeding productivity even when more than a third of their optimal foraging grounds were devastated by agricultural intensification. Landscape attributes that influence the occurrence of species and benefit the breeding parameters included type and quality of habitat, fragment shape and size, and presence of suitable foraging and nesting sites. The influential factor was not only grassland cover, but also configuration and diversity of foraging sites related to prey abundance and availability. In different territories, eagles use resources from different parts of the area, so grasslands transformed in different patterns could affect the capacity of the species to adapt to the novel landscape in different ways. Home range size varies with prey density (Fernández et al., 2009) and is determined by the number of feeding places used and the distances between them (Newton et al., 1979) to maximize the fitness of the individual (Patterson, 1980) and minimize the energy requirements for food supply (Gill and Wolf, 1975) or/and the costs for defense against competitors (Krebs, 1971).
Our simple models of the effect of habitat alteration implemented at different scale (core area of the territory and home range) demonstrated that habitat quality of the territories shaped eagle demography. The average model for productivity confirmed that high-quality habitats had strong positive influence on the breeding success. The number of grasslands fragments in the territory affect the productivity and the occupancy rate of eagles. The fragmentation of grassland habitats leads to the isolation of the fragments, changes the landscape structure, hence the behavior, availability, and abundance of the eagle's prey, which also results in a shift in the species foraging behavior and the habitat utilization within the territory. Then, these changes influence the response of the eagle as well as its ability to survive in a modified landscape. Probably, the EIE persists in the fragmented landscape by incorporating multiple fragments in its territory or daily foraging movements. Such behavior is known for its sister species Spanish Imperial Eagle (Aquila adalberti), which utilizes certain areas less intensively and others more intensively (Fernández et al., 2009). It was recorded that this eagle hunted intensively in small-sized areas (200–800 ha) scattered across the entire territory.
One of our general hypotheses expressed in the assumption that habitat alteration affects territory quality, so source territories that have undergone a significant change become sink areas, whereas those transformed to a lower degree continue to be of good quality, found partial confirmation. We suggest that population-based transformation of high-quality habitats will entail several consequences in the future similar to other studies (Ferrer and Donàzar, 1996). First, the continued loss of high-quality habitats will probably result in the formation of an ecological trap, because in this scenario the rapid environmental change in high-quality habitats, already significantly deteriorated, pushes eagles to prefer to settle in poor-quality territories (Bender et al., 1998). If good breeding sites in the source areas are rare and poor breeding sites in the sink territories are common, most of the population may reside in the sink areas (Pulliam, 1988). On the other side, because the species is characterized by high natal philopatry (Rudnick et al., 2005; Vili et al., 2013), eagles that fledged in former source territories may return to these areas and attempt to occupy the already transformed and unsuitable sites. In this case, they prefer falsely attractive habitats and, in general, avoid high-quality, but less-attractive habitats (Schlaepfer et al., 2002; Battin, 2004). Thus, the deteriorated habitats and the reduced food availability therein will result in lower productivity and higher mortality rate of the eagles they harbor. So, although these territories will be of high occupancy rate, they will in fact function as sink areas. In this scenario, the source-sink dynamics will be gradually shifted towards higher numbers of eagles breeding in sink territories characterized by lower productivity, high turnover rate, and increased mortality. Subsequently, the population growth will gradually slow down, reaching a plateau until the depletion of the floater resource and/or the inflow of immigrants, because the sink territories will not have the capacity to produce enough offspring. Since a sink population depends on immigration of individuals from the source part of the population, a severe violation of the source dynamics may cause a rapid decline of the whole population (Balbontín et al., 2003).
Second, the degradation or destruction of the source habitat will, in turn, impact the sink or trap populations, potentially over large distances (Tittler et al., 2006). Habitat deterioration will affect other important demographic components of the population dynamics such as dispersal and survival of the floaters section of the population. The deterioration of habitats in source territories and temporary settlement areas will result in avoidance by floaters and decline of floaters' persistence in the population. In the long run, this process could possibly re-direct the "inflow of floaters" towards neighboring sub-populations, thus increasing the emigration rate. Last, but not least, it is known that quality differs between individuals occupying different sites (Sergio et al., 2009; Reynolds et al., 2019), leading to long-term fitness consequences. Eagles in human-altered territories may produce nestlings of the lowest quality, characterized by poor condition or immunodeficiency (Sternalski et al., 2010, 2012a, b; Ferrer and Morandini, 2017), potentially suffering higher mortality rates during the dispersal period. Also, what we have not assessed is how changes in habitat affect the eagle's prey and what its foraging response will be, as well as what impact it will have on the fitness and the condition of the juveniles and on the energy costs and behavior of adults. However, little is known about these circumstances that need further investigation. Thus, all these stochastic processes occurring simultaneously and affecting both the breeding section of the population and the "available reserve of floaters" mark out the uncertain future of the EIE population in southeast Europe.
Agricultural change led to habitat loss for raptor species similar to EIE (González et al., 1990; Real and Mañosa, 1997; Pedrini and Sergio, 2001; Pongrácz and Szitta, 2015). Since Bulgaria's accession to the EU, two processes have been running simultaneously – ploughing of grasslands and devastation of grassland habitats with shrub vegetation, due to the SAPS applied within NATURA 2000 sites. Grassland habitats with shrubs are subject to alteration, with shrub vegetation being either burnt down or destroyed by shredders and bulldozers. This leads to direct extermination of a number of animals such as Northern White-breasted Hedgehog (Erinaceus roumanicus), Tortoises (Testudo graeca and Testudo hermani), and reptiles, all being important prey for EIE and other large top-predators (Demerdzhiev et al., 2022). However, the efforts invested in the conservation of the species in the past decades were aimed at increasing the demographic parameters such as breeding success and survival of adults and floaters (Demerdzhiev et al., 2014b, 2015). On the other hand, in many areas the process of habitat alteration rapidly increased, which requires prompt and adequate management decisions to stop the habitat loss immediately and restore the deteriorated habitats in the near future.
We found that in the last decade the land-use patterns of EIE were subject to a significant transformation expressed by an increase in the share of arable land and shrinking of different grassland types. The presence of grassland in eagle territories had a significant positive effect on the occupancy rate. However, the average occupancy rate decreased, while productivity and breeding frequency showed no trend. Our results should be interpreted with caution because of the small sample size and the fact that the effect of habitat alteration on the species may take long to become fully evident, especially for long-lived species such as EIE. We found out that territory quality was a more powerful factor affecting eagle demography, so high-quality habitats had a significant positive effect on the productivity.
As with other top predators (Whitfield et al., 2007), the response of EIE to habitat transformation is more complex and we should pay attention to the individual differences between territories and pairs of eagles, taking into account the source-sink dynamics of the population and the long-term changes in the land-use pattern. Using a universal set of criteria to predict when a given extent of grassland transformation would result in territory abandonment is a problematic and uncertain process.