| Citation: | Pan Chen, Yanhong Chen, Huimin Chen, Taiyu Chen, Bin Liu, Manyu Zhang, Silu Wang, Changhu Lu. 2023: Vinous-throated parrotbills breed in invasive smooth cordgrass habitat: Can native birds avoid the potential ecological trap?. Avian Research, 14(1): 100119. DOI: 10.1016/j.avrs.2023.100119 |
Native animals are facing long-term coexistence with invasive plants worldwide, the impacts of which on animal behavior remain poorly known. Potential ecological traps could threaten native birds breeding in invasive plant habitats, but behavioral strategies for birds to avoid such risks are few concerned. The invasion of Smooth Cordgrass (Spartina alterniflora) has seriously varied the vegetation landscape in the coastal wetlands of eastern China, and affected the habitat selection of native birds. Here, we investigated the nesting and breeding characteristics of a common native passerine, the Vinous-throated Parrotbill (Sinosuthora webbiana), in habitats dominated by native Common Reed (Phragmites australis) and exotic cordgrass. We found that parrotbills could complete their breeding cycle in cordgrass habitat. Most nest failure was attributed to predation in native habitat but tide inundation in cordgrass habitat. However, the nest success rate and daily survival rate (DSR) in cordgrass habitat were higher. Moreover, nest height was markedly higher in cordgrass habitat and was the most important influencing variable (positively correlated with the DSR). These results suggest that cordgrass habitat is a potential ecological trap due to the tide inundation, but some parrotbills seem to effectively avoid this risk by increasing nest height. Our study reveals that a native passerine changes its nesting behavior to accommodate invasive plant habitat and highlights that habitat changes caused by invasive plants may drive the adaptive evolution of native animal behavior. The limitation of these results must be acknowledged for the small sample size, and there is a need for a larger sample and long-term data for further verification.
Invasion by exotic species is continuously increasing worldwide and is having profound effects on native biota and ecosystems (Butchart et al., 2010; Crystal-Ornelas and Lockwood, 2020). Invasive plants are among the most pervasive invasive taxa, often strongly altering the biotic and abiotic characteristics of ecosystems and then affecting native animals (Sheehan and Ellison, 2014; Davidsdottir et al., 2016). However, most studies on invasive plants have focused on their impacts on native animals at the population or community level, e.g., biodiversity and abundance (Crystal-Ornelas and Lockwood, 2020). The impacts of invasive plants on native animal behavior and the resulting ecological consequences after their long-term coexistence are less studied (Stewart et al., 2021).
Birds are one of the most widely distributed groups of terrestrial vertebrates and occupy diverse habitat types in modern ecosystems (Oliveros et al., 2019). Some species exhibit strong behavioral flexibility and are able to respond rapidly to changes in habitat characteristics caused by factors such as human activities, urbanization, or invasive exotic plants (Jordaan et al., 2011; Ortega et al., 2014). A few native birds may choose to nest in invasive vegetation habitats and use nonnative plants as nesting materials (Aslan and Rejmanek, 2010; Smith and Finch, 2014; Mejías and Nol, 2020). However, birds sometimes face a variety of unknown risks in invasive vegetation habitats. The pioneers may fall into potential ecological traps created by invasive plants by relying on previous nesting cues and experiences (that were appropriate in their native habitats) when choosing their nest sites (Schlaepfer et al., 2005). Their outdated nesting experiences may result in a higher predation rate (Schmidt and Whelan, 1999), fewer food resources (Skorka et al., 2010), or a higher risk of natural disaster (Nordby et al., 2009), eventually leading to a decline in population viability.
Some birds employ remarkable behavioral plasticity in nesting when they face adverse environments to eliminate dilemmas (Peluc et al., 2008). For example, parental Siberian Jays (Perisoreus infaustus) adjust the security of the subsequent nesting site when the predation risk increases (Eggers et al., 2006). King Rails (Rallus elegans) increase building effort and alter nest height in response to the risk of nest inundation by tides (Clauser and McRae, 2016). After long-term coexistence, it is still unclear whether native birds that colonize invasive vegetation habitats may also adapt to the new environmental characteristics through behavioral variation to avoid potential ecological traps.
Smooth Cordgrass (Spartina alterniflora) is a globally notorious invasive plant native to the Atlantic and Gulf coasts of North America, mainly distributing in the intertidal zone and tolerating periodic tidal inundation, which was deliberately introduced along the Yellow Sea coast of China in the 1970s to improve the coastal environment, e.g., for erosion control, dike protection and soil amelioration (Gao et al., 2012; Zuo et al., 2012). However, cordgrass outcompeted and rapidly replaced native plants over the next several decades and had an important ecological effect on the native fauna, e.g., zoobenthos and birds (Li et al., 2009; Ning et al., 2021). Early studies suggested that the invasion of cordgrass has had significant negative effects on bird foraging and reproduction, with most native bird species avoiding the use of pure cordgrass habitat because of its high stem density and homogenous structure (Li et al., 2009; Gan et al., 2010). Later studies showed that several native small birds had moved to invaded habitats to breed (Nordby et al., 2009; Lampert et al., 2014; Ma et al., 2014), and this change may be related to the amount of time since cordgrass invasion. Unfamiliar environments may lead to unknown breeding risks and challenges for native birds. In San Francisco Bay, Song Sparrows (Melospiza melodia) still displayed the traditional nesting habit (favored the lower part of the dense grass) in native vegetation habitat when they moved to invasive cordgrass habitat at low elevations. As a result, the breeding success in invaded habitat was much lower than that in native habitat due to nest inundation by tides, and cordgrass habitat was also considered an ecological trap for birds (Nordby et al., 2009).
The Vinous-throated Parrotbill (Sinosuthora webbiana) is a widely distributed East Asian-endemic passerine with strong adaptability to multiple ecosystems from urban shrubs to coastal wetlands (BirdLife International, 2022). As a small omnivorous passerine, the parrotbill displays a wide range of feeding habits (feeding on, e.g., insects, fruits, seeds, and flowers) and chooses a variety of habitat types (e.g., shrubs, woodlands, forest edges, and wetlands) for nesting and reproduction (Robson, 2020). These birds are also found in many anthropogenic environments, such as parks, courtyards, campuses, and orchards (Robson, 2020). In recent years, we have observed adults and juveniles of the Vinous-throated Parrotbill in cordgrass habitat, and the population has been increasing annually (unpublished data). This small native passerine can exploit invasive vegetation habitat and may benefit from its broad diet and generalized habitat use. Meanwhile, the shrinkage of native habitat due to cordgrass invasion may drive more birds that formerly relied on native vegetation to try nesting in invaded habitat. However, the populations of Vinous-throated Parrotbill moving to cordgrass habitat inevitably face the threat of nest inundation by tides at low elevations. Given the trend in population growth, we speculate that Vinous-throated Parrotbills in cordgrass habitat alter their nesting strategy to cope with the adverse environment. In this study, we tested whether cordgrass habitat is a potential ecological trap for the Vinous-throated Parrotbill by comparing nesting and breeding characteristics in native and exotic vegetation habitats and whether this native passerine displays behavioral plasticity and can evolve new nesting habits to deal with the adverse factors in invasive vegetation habitat.
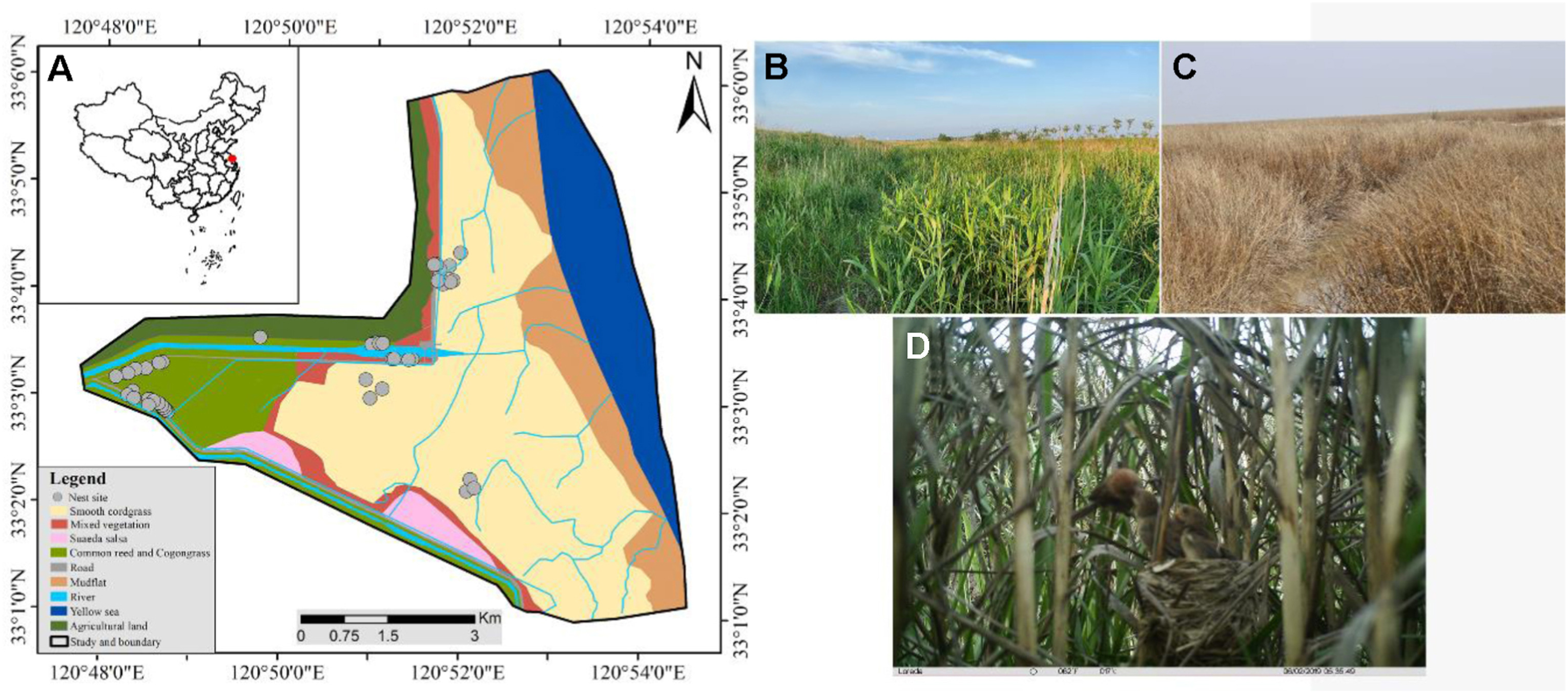
The study was conducted during the breeding season in 2018 and 2019 in the core area of Yancheng National Nature Reserve (YNNR) (32°59′–33°03′ N, 120°47′–120°53′ E), Jiangsu Province, China. The YNNR maintains the rare natural landscape of the Yellow Sea tidal flat, with low human disturbance and high biodiversity, and serves as a suitable habitat for many wetland birds. Historically, the vegetation of the reserve mainly consisted of Common Reed (Phragmites australis), Cogon Grass (Imperata cylindrica) and Weepweed (Suaeda salsa). Because of the long-term invasion of cordgrass, the vegetation landscape has been transformed into a wide band of cordgrass monoculture in the coastal zone, while the native vegetation degenerates inland along an elevational gradient (Chen et al., 2019). As a result, the native habitat area of birds has decreased annually. According to our long-term field observations, the number of native bird species using cordgrass habitat is increasing, and the Vinous-throated Parrotbill is the most common passerine during the breeding season.
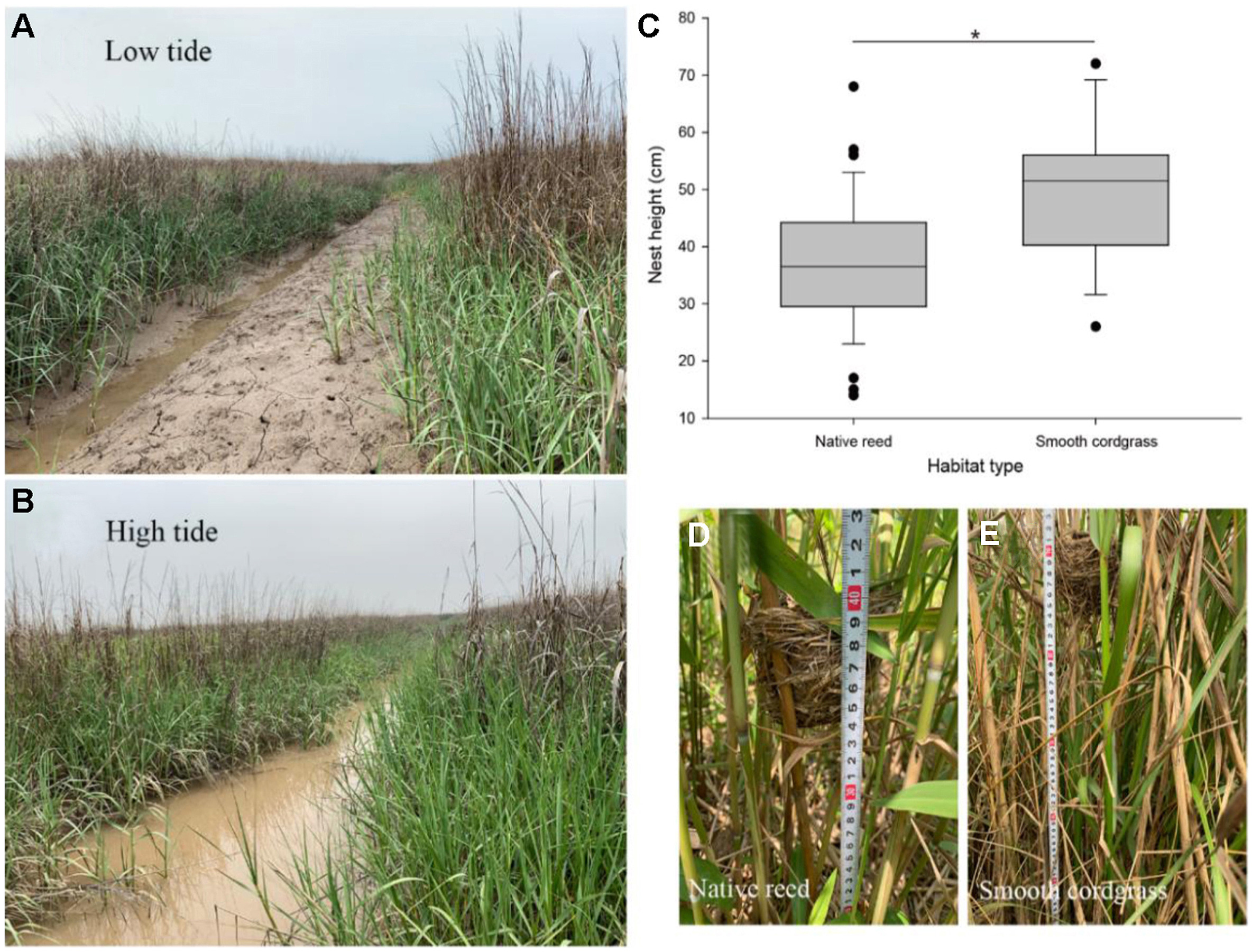
The salt and submergence tolerance of exotic cordgrass are significantly stronger than that of native reed, so it could grow in the low tide zone while reed is distributed only in the high tide zone (Yuan et al., 2017). In our study area, the sampling sites in native reed habitat were approximately 5–6 km from the coast at low tide, and the relative elevations were approximately 4–6 m, without tidal flooding. The sampling sites in exotic cordgrass habitat were approximately 2–3 km from the coast at low tide, the relative elevations were approximately 1–2 m, with periodic tidal flooding (Fig. 1A–C; Fig. 2A and B).
During the 2018 and 2019 breeding seasons (from April to July), we searched for Vinous-throated Parrotbill nests in both native reed and exotic cordgrass habitats. The survey was carried out between 7:00 and 10:00 a.m. and again from 15:00 to 18:00 p.m. on sunny days, with at least two observers. The location of nests was mainly determined by carefully inspecting the preferred nesting substrate of the Vinous-throated Parrotbill (thickets near the water) and by following adults' activities (e.g., carrying nesting material or alert chirping). Once a nest was found, we marked its location with a GPS device (GARMIN, eTrex-221x) and determined the state of the nest (nesting, incubating, nestling or inactive) by observing the freshness of the nest material and parental behavior. If a newly observed fresh empty nest did not contain at least one egg in the follow-up observation, it was considered inactive. Instead of flagging, we drew a simple map of the locations of nests based on surrounding vegetation and distant landmarks to facilitate relocation.
For inactive nests (nesting material was obviously not fresh and worn out), we immediately measured and recorded nest characteristics, namely, (1) external nest depth (cm, the vertical distance between the top and bottom of the nest), (2) nest external diameter (cm, the external diameter of the nest cup), (3) nest inner diameter (cm, the inner diameter of the nest cup), and (4) internal nest depth (cm, the depth of the internal nest cup), and nest-site characteristics, namely, (1) nest height (cm, the vertical distance from the ground to the bottom of the nest), (2) attached stem diameter (cm, the average diameter of vegetation stems attached to the nest), (3) vegetation height (cm, the average height of the plant stems supporting the nest, usually three to six stems), (4) vegetation density (stems/m2, the number of plant stems within the 1 m2 area centered on the nest), (5) litter coverage (%, the litter coverage in the 1 m2 area below the nest), (6) distance to water (m, the distance from the nest site to the nearest body of water), and (7) distance to road (m, the distance between the nest site and the nearest road with human activity). To exclude the impact of vegetation height on nest height (the stem height of cordgrass is usually lower than that of reed; Chen et al., 2019), the relative nest height (nest height/attached stem height × 100%) was also calculated. For active nests, we measured the above variables after determining nest fate (successful or failed) to avoid interfering with the parents.
We kept track of the fate of each active nest. Nest age was estimated at the first discovery and calibrated based on some previous studies on the breeding ecology of this species (one egg laid per day, 12–13 days for incubation, 12–13 days for the nestling phase and no more than 30 days per breeding attempt) (Guo et al., 2006). Each nest was visually checked every 3–5 days until it was deemed successful (at least one nestling fledged) or to have failed. The reasons for nest failure were identified based on nest state after the event, including (1) predation (eggs were broken, or the nest was empty and dry), (2) flooding (the nest was soaked and empty or washed down along with the supporting stems), and (3) abandonment (eggs were intact, and the nest was dry). In addition, we recorded detailed data related to clutch size, egg color, incubation and nestling periods for active nests through regular visual inspection.
We also selected some nests for which it was easy to obtain a satisfactory shooting angle (low concealment of the nest site) and set up infrared cameras (yianws, L710) for monitoring. To prevent the parents from abandoning the nest because of human interference, cameras were installed after incubation had begun rather than after the first discovery (DeGregorio et al., 2016). We mounted the camera on a special bamboo pole 0.8–1.2 m away from the nest and disguised it with surrounding plant leaves. During regular nest visits, we checked the shooting angle of the infrared camera and replaced the memory card and battery before they were exhausted.
We compared the nest and nest-site characteristics of the Vinous-throated Parrotbill between native reed and exotic cordgrass habitats using an independent-sample t-test. The main breeding variables (clutch size, egg color, incubation period, and nestling period) were also compared using the same method. We tested for differences in the causes of nest failure between the two types of habitats using the chi-square test. We estimated the daily survival rate (DSR) of nests in the two types of habitats and identified the best explanatory variables using the nest survival model in Program MARK (versions 9.0, CSU, Fort Collins, CO) (White and Burnham, 1999; Dinsmore et al., 2002). First, we estimated the constant DSR based on the Mayfield model and calculated the variation in the cumulative survival rate with nest age (DSRd × 100%, where d is the days of nest age) (Mayfield, 1961, 1975). Then, we established the univariate nest survival model to examine the effects of related variables on the DSR, including the nest-site variables (mentioned above) and time variables (nest age, breeding date, and year), using the design matrix tools and a logit-link function in Program MARK. Before analysis, the variables were automatically standardized, and a multicollinearity test was performed. We estimated whether there was a significant relationship between each variable and the DSR by evaluating whether the 95% confidence intervals (CIs) of beta coefficients overlapped zero and used the significantly correlated variables to construct multiple-variable models. Finally, we ranked the models based on Akaike’s information criterion with correction (AICc), which was adjusted for a small sample size. The model with the lowest AICc value was generally considered to best fit the data, and other models were also considered to have substantial support if ΔAICc ≤ 2 (Burnham and Anderson, 2002). All statistical analyses and graphing were performed in R (R Core Team, 2020) and SigmaPlot (versions 12.5, Systat Inc, San Jose, CA) software.
During the 2018–2019 breeding seasons, we found a total of 43 and 16 Vinous-throated Parrotbill nests in native reed and exotic cordgrass habitats, respectively. All nests were typical deep, cup-shaped nests, and nest materials were sourced from the vegetation (reed or cordgrass) near nest sites. There was no significant difference in any nest characteristic between the two habitats (Table 1). Regardless of reed or cordgrass habitat, Vinous-throated Parrotbills preferred to nest in dense grass near the water. Therefore, in cordgrass habitat nests were nearly always found in tidal ditches, where nests would periodically face the risk of tide inundation, the interval between high and low tides was about 6 h and the average tide height difference was about 60–80 cm in sampling sites (Fig. 2A and B). Regarding nest-site characteristics, nest height (t = −2.41, P = 0.019; Fig. 2C‒E) and vegetation height (t = −2.97, P = 0.004) were greater and litter coverage was lower (t = 7.40, P = 0.001) in cordgrass habitat (Table 1). The relative nest height (34.5 ± 2.23%) in cordgrass habitat was also significantly greater than that (29.2 ± 1.26%) in native reed habitat (t = −2.18, P = 0.034, Appendix Fig. S1). The other nest-site characteristics did not differ between the two habitats (Table 1).
| Empty Cell | Variable | Habitat type | df | t | P | |
| Common Reed (n = 43) | Smooth Cordgrass (n = 16) | |||||
| Nest characteristics | External nest depth (cm) | 8.80 ± 0.23 | 8.88 ± 0.33 | 51 | −0.17 | 0.866 |
| Nest external diameter (cm) | 7.22 ± 0.11 | 6.93 ± 0.15 | 51 | 1.47 | 0.148 | |
| Nest inner diameter (cm) | 4.97 ± 0.07 | 4.78 ± 0.11 | 51 | 1.41 | 0.164 | |
| Internal nest depth (cm) | 5.13 ± 0.06 | 5.16 ± 0.06 | 51 | −0.24 | 0.815 | |
| Nest-site characteristics | Nest height (cm) | 38.44 ± 2.45 | 49.13 ± 3.06 | 51 | −2.41 | 0.019* |
| Attached stem diameter (cm) | 0.39 ± 0.01 | 0.40 ± 0.24 | 51 | −0.57 | 0.571 | |
| Vegetation height (cm) | 122.26 ± 3.76 | 145.31 ± 7.80 | 51 | −2.97 | 0.004** | |
| Vegetation density (stems/m2) | 181.40 ± 7.17 | 182.50 ± 6.49 | 51 | −0.09 | 0.930 | |
| Litter coverage (%) | 92.00 ± 20.00 | 46.00 ± 9.00 | 51 | 7.40 | 0.001** | |
| Distance to water (m) | 6.04 ± 2.02 | 3.63 ± 1.84 | 51 | 0.73 | 0.469 | |
| Distance to road (m) | 45.61 ± 4.16 | 71.23 ± 30.10 | 51 | −1.25 | 0.218 | |
| Shown are the mean ± SE. *P < 0.05; **P < 0.01. | ||||||
We tracked the fate of 37 and 15 active Vinous-throated Parrotbill nests in native reed and exotic cordgrass habitats, respectively. All but nine nests failed, and the final nest success rates were 16.22% (six nests in native reed habitat) and 20.00% (three nests in cordgrass habitat). Through continuous monitoring, we demonstrated that the Vinous-throated Parrotbill, a native passerine, could complete its entire breeding cycle in cordgrass habitat (Fig. 1D). There was a significant difference in the fate of nests (different causes of nest failure were thought to be different fates) between the two habitats (χ2 = 10.88, P = 0.012), and the main causes of nest failure changed. The nest failures in native reed habitat were mainly due to predation, which accounted for 22 (59.46%) nest failures. Seven nests (18.92%) were abandoned by parents, but only two nests (5.41%) were flooded (the reason was rainy season precipitation rather than periodic tides) (Fig. 3). However, the nest failures in cordgrass habitat were mainly due to flooding, which accounted for six (40.00%) nest failures; four nests (26.67%) were preyed upon, and two nests (13.33%) were abandoned (Fig. 3).
According to the constant model, the DSRs of nests in the two habitats were 0.89 ± 0.02 (95% CI = 0.86 to 0.93, reed) and 0.93 ± 0.02 (95% CI = 0.88 to 0.96, cordgrass). Therefore, the nest survival rate (12.31%) in cordgrass habitat was higher than that (4.11%) in reed habitat (Fig. 4). Regarding the main breeding variables (clutch size, egg color, incubation and nestling periods), there was no significant difference between the two habitats (Appendix Fig. S2).
According to the rank of models considering all habitats, the best model contained nest height and distance to water (AIC weight = 0.865, Table 2). The other variables showed no significant correlation with the DSR due to their 95% CIs overlapping zero (Appendix Table S1). Therefore, the change in nest sites to cordgrass habitat had no significant effect on the DSR of the Vinous-throated Parrotbill. Similar results were obtained when considering only native reed habitat. The best model still included nest height and distance to water (AIC weight = 0.669, Table 3). The other variables showed no significant correlation with the DSR (Appendix Table S2). Considering only cordgrass habitat, distance to water was the most important predictor of the DSR according to the model ranking (AIC weight = 0.211, Table 4). However, its 95% CIs overlapped zero, so the relationship was not statistically significant (Appendix Table S3). The second-best univariate model included nest height (AIC weight = 0.183, Table 4), and there was a significant positive correlation with the DSR (R2 = 0.97, P < 0.001, Fig. 5). The other variables showed no significant correlations with the DSR (Appendix Table S3).
| Model | ΔAICca | wib | Kc |
| Nest height + distance to water | 0 | 0.865 | 3 |
| Nest height | 3.802 | 0.129 | 2 |
| Global model | 11.314 | 0.003 | 12 |
| Distance to water | 12.821 | 0.001 | 2 |
| Breeding date | 16.522 | 0.000 | 2 |
| Nest age | 16.864 | 0.000 | 2 |
| Vegetation height | 18.339 | 0.000 | 2 |
| Null model | 18.372 | 0.000 | 1 |
| Habitat type | 18.778 | 0.000 | 2 |
| Distance to road | 19.822 | 0.000 | 2 |
| Year | 19.972 | 0.000 | 2 |
| Litter coverage | 20.287 | 0.000 | 2 |
| Vegetation density | 20.373 | 0.000 | 2 |
| Attached stem diameter | 20.386 | 0.000 | 2 |
| a Difference between current model and best-fit model; AICc of best-fit model = 182.59; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||
| Model | ΔAICca | wib | Kc |
| Nest height + distance to water | 0 | 0.669 | 3 |
| Nest height | 1.600 | 0.300 | 2 |
| Distance to water | 8.179 | 0.011 | 2 |
| Nest age | 10.138 | 0.004 | 2 |
| Breeding date | 10.263 | 0.004 | 2 |
| Global model | 10.345 | 0.004 | 11 |
| Vegetation height | 11.579 | 0.002 | 2 |
| Null model | 11.779 | 0.002 | 1 |
| Vegetation density | 13.141 | 0.001 | 2 |
| Attached stem diameter | 13.543 | 0.001 | 2 |
| Litter coverage | 13.555 | 0.001 | 2 |
| Distance to road | 13.756 | 0.001 | 2 |
| a Difference between current model and best-fit model; AICc of best-fit model = 126.59; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||
| Model | ΔAICca | wib | Kc |
| Distance to water | 0 | 0.211 | 2 |
| Nest height | 0.289 | 0.183 | 2 |
| Null model | 1.227 | 0.114 | 1 |
| Vegetation density | 1.524 | 0.099 | 2 |
| Litter coverage | 2.167 | 0.072 | 2 |
| Year | 2.344 | 0.065 | 2 |
| Breeding date | 2.720 | 0.054 | 2 |
| Nest age | 2.813 | 0.052 | 2 |
| Attached stem diameter | 2.882 | 0.050 | 2 |
| Distance to road | 2.897 | 0.050 | 2 |
| Vegetation height | 3.180 | 0.043 | 2 |
| Global model | 6.842 | 0.007 | 11 |
| a Difference between current model and best-fit model; AICc of best-fit model = 61.78; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||
With ongoing colonization by invasive plants around the world, the cases of native birds breeding in invasive vegetation habitats are gradually increasing, especially the utilization of invasive shrub and herb habitats by native passerines (Jones and Bock, 2005; Kennedy et al., 2009; Gennet et al., 2017). In this study, we demonstrated that the Vinous-throated Parrotbill, a native passerine, could complete its entire breeding cycle in exotic cordgrass habitat. As a widely distributed East Asian-endemic passerine, the Vinous-throated Parrotbill is able to nest in a variety of habitats, and this outstanding adaptability might be an important foundation for its exploitation of exotic vegetation habitat. In addition, birds generally use vegetation structure rather than vegetation species to choose their nest locations (Karr and Roth, 1971), and rely on previous nesting cues and experiences (Schlaepfer et al., 2005). Therefore, after long-term coexistence with invasive cordgrass, some native birds inhabiting reed habitat might gradually spread to cordgrass habitat with similar vegetation structure to reed. According to our results, there was no significant difference in most nest characteristics of the Vinous-throated Parrotbill between reed and cordgrass habitats. Only the nest materials, which were sourced from vegetation near the nest site, were different. Vinous-throated Parrotbills still preferred to nest in dense vegetation near the water when they moved into cordgrass habitat, showing the same nest-site selection preference as in native reed habitat.
However, there were significant differences in some nest-site variables (vegetation height, litter coverage and nest height) between the two habitats. Vinous-throated Parrotbills preferred to nest in the lower vegetation in native reed habitat, which might be due to their smaller body size or intense interspecific competition for nest-site niches (sympatric birds, e.g., the Reed Parrotbill Paradoxornis heudei and Plain Prinia Prinia inornata). In cordgrass habitat, Vinous-throated Parrotbills preferred dried old-growth stems (with the greatest height exceeding 1.5 m) for nesting. Therefore, although the stems of cordgrass were generally lower than those of reed, the height of nesting vegetation in cordgrass habitat was greater than that in reed habitat. The litter layer in cordgrass habitat was often washed away by periodic tides, especially near the tidal ditch preferred by Vinous-throated Parrotbills, so the litter coverage was lower than that in reed habitat. These differences are caused by vegetation and environmental factors, but the difference in nest height may be the outcome of an active strategy employed by individuals.
Some studies have suggested that although some native birds choose to breed in invasive vegetation habitat, the habitat variation caused by exotic vegetation might form a potential ecological trap and lead to the decline of bird fecundity (Schmidt and Whelan, 1999; Schlaepfer et al., 2002; Remes, 2003). The main breeding risks in exotic vegetation habitats include a decrease in food resources or quality (Lloyd and Martin, 2005), an increase in the nest predation rate (Schmidt and Whelan, 1999; Borgmann and Rodewald, 2004), and a high frequency of natural disasters (Nordby et al., 2009). These factors eventually lead to a decline in fledgling quality or nest failure. In cordgrass habitat, Vinous-throated Parrotbills preferred to nest in the middle and lower parts of the dense grass beside the tidal ditch, so periodic tides would lead to the inundation of some nests at lower positions. In this study, nest predation was the main reason for nest failure in native reed habitat (accounting for approximately 60% of failures), while tide inundation was the most frequent reason in cordgrass habitat (accounting for more than 40% of failures). Our results were similar to those of the study of song sparrows in San Francisco Bay; exotic cordgrass provided a nesting habitat with less competition for native birds, but periodic tide inundation made it a potential ecological trap (Nordby et al., 2009).
Some studies also suggested that there was no significant difference in bird fecundity between native and invasive vegetation habitats, and birds might even gain additional benefits from exotic vegetation (Ruehmann et al., 2011; Miller et al., 2013; Stinson and Pejchar, 2018). A study of the Marsh Grassbird (Locustella pryeri) in Chongming Dongtan suggested that the breeding success rate of birds in cordgrass habitat did not differ from that in native vegetation habitats in northeastern China and Japan (Ma et al., 2014). In this study, the nest success rate and DSR of the Vinous-throated Parrotbill in cordgrass habitat were higher than those in native reed habitat. This indicates that cordgrass habitat can not only provide nesting sites and brooding resources for Vinous-throated Parrotbills but also may have some breeding advantages compared to native reed habitat. Some studies have suggested that invasive plants provide safer breeding shelters for birds due to their denser or unique vegetation structure (Ruehmann et al., 2011; Miller et al., 2013; Meyer et al., 2015). In south San Francisco Bay, cordgrass provided important refuge cover from predators and nesting habitat for the endangered Clapper Rail (Rallus longirostris) (Lampert et al., 2014; Overton et al., 2014). In this study, the nest predation rate of the Vinous-throated Parrotbill in cordgrass habitat was much lower than that in native reed habitat. The lower predation in cordgrass habitat might have resulted from the extremely dense and homogenous vegetation structure impairing predators' search for prey, and fewer native predators that have adapted to searching in the thick grasses. This lower predation may also indirectly compensate for the fertility loss caused by tide inundation such that the Vinous-throated Parrotbill population can breed stably in cordgrass habitat.
A few studies have demonstrated that some birds employ behavioral plasticity in nesting when facing adverse environments (Gjerdrum et al., 2005; Clauser and McRae, 2016). However, this behavioral strategy of birds in invasive vegetation habitats seems to be less studied (Stinson and Pejchar, 2018; Stewart et al., 2021). In this study, we found that nest height was the most important variable affecting the nest survival of the Vinous-throated Parrotbill. A greater nest height could effectively reduce the threat from ground predators (rodents and snakes; unpublished data) in native reed habitat. In cordgrass habitat, a greater nest height can effectively eliminate the risk of tide inundation. Interestingly, we found that both nest height and relative nest height in cordgrass habitat were significantly greater than those in native reed habitat. These results suggest that Vinous-throated Parrotbills generally increase their nest height in response to tidal stress in cordgrass habitat. We speculate that pioneer birds maintained the same nesting habits as in native reed habitat, which might have led to the failure of most nests due to tide inundation. Then, some individuals increased their nest height to avoid the risk of tide inundation, and these parent birds accumulated successful experience and gradually developed breeding fidelity in cordgrass habitat. A study in the origin area (Connecticut coast) of cordgrass suggested that Seaside Sparrows (Ammodramus maritimus) avoided especially high tide by nesting in tall vegetation (Gjerdrum et al., 2005). The findings of other studies have also suggested that increasing nest height is a common behavioral strategy displayed by birds facing flooding stress during the breeding season (Clauser and McRae, 2016; Ma et al., 2019). Alternatively, higher nests may also lead to other adverse effects, e.g., nests built near the top of relatively tall vegetation are more vulnerable to wind damage (Bowman and Woolfenden, 2002), or attract aerial predators (Millones and Frere, 2017). The average wind speed in cordgrass habitat was significantly higher than that in native habitat because the areas of cordgrass invasion were closer to the coastline (Chen et al., 2022). However, no adverse effects of strong winds on nests were observed in this study, possibly because most nests of Vinous-throated Parrotbill remained in the lower layers of vegetation, although the relative height of nests increased in cordgrass habitat (Appendix Fig. S1). The uncertain ecological consequences of higher nests may need further ongoing research.
We also compared the main breeding variables of the two habitats, including clutch size, egg color, and the incubation and nestling periods, but no significant differences were found. The egg color of the Vinous-throated Parrotbill differs between nests, i.e., white or light blue, but the eggs in a nest are generally uniform. This may be an adaptation for avoiding parasitism or the result of differences between geographically separated populations (Lee and Jablonski, 2012). In this study, no nest parasitism was detected, and the percentages of each egg color in cordgrass and native reed habitats were similar. This might indicate similar levels of genetic diversity in the populations of Vinous-throated Parrotbills between cordgrass and native reed habitats.
We conclude that the Vinous-throated Parrotbill can use exotic cordgrass habitat to complete its breeding cycle. Periodic tide inundation is the primary reason for nest failure, and cordgrass habitat can be considered a potential ecological trap for breeding birds. However, Vinous-throated Parrotbills effectively respond to this risk by increasing their nesting height. Higher nest survival may also result from better concealment provided by the vegetation structure and the lack of predators in invaded habitats, which should be considered in further studies. This study reveals that a native bird avoids a potential ecological trap in invaded habitat by employing a behavioral strategy and highlights that habitat changes caused by invasive plants may drive the adaptive evolution of the behavior of some native birds. Of course, our results may be limited by the small sample size, and there is a need for a larger samples and long-term data for further verification. In the future, we recommend conducting expansive research on ecological networks, including the predators and food resources of birds, in invaded habitats.
PC, YC and CL conceived the idea and wrote the draft editorial. HC and SW constructed the figures. PC, TC, BL, and MZ carried out the field work. PC, YC and HC shared in the writing and analyses. PC, YC and CL made modifications to the final version. All authors read and approved the final manuscript.
We obtained approval from the reserve management office before carrying out the experiment. All the field observations conducted in this study were performed in accordance with the current laws of the China and the Animal Care and Use Committee at the Anhui Normal University (Permit # 00117).
The authors declare that they have no competing interests.
We thank patrols at the YNNR for assistance and support in the field sampling.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100119.
|
Burnham, K.P., Anderson, D.R., 2002. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, New York.
|
|
Guo, Z., Chen, W., Hu, J., 2006. Analysis on nest habitation factors and chick growth of Paradoxornis webbianus. Sichuan. J. Zool. 25, 858–861. (in Chinese).
|
|
Lee, J.-W., Jablonski, P.G., 2012. Egg color polymorphism and morph-ratio variation in Korean populations of the Vinous-throated Parrotbill. Chinese Birds 3, 312–319.
|
|
Mayfield, H.F., 1961. Nesting success calculated from exposure. Wilson Bull. 73, 225–261.
|
|
Mayfield, H.F., 1975. Suggestions for calculating nest success. Wilson Bull. 87, 456–466.
|
| Empty Cell | Variable | Habitat type | df | t | P | |
| Common Reed (n = 43) | Smooth Cordgrass (n = 16) | |||||
| Nest characteristics | External nest depth (cm) | 8.80 ± 0.23 | 8.88 ± 0.33 | 51 | −0.17 | 0.866 |
| Nest external diameter (cm) | 7.22 ± 0.11 | 6.93 ± 0.15 | 51 | 1.47 | 0.148 | |
| Nest inner diameter (cm) | 4.97 ± 0.07 | 4.78 ± 0.11 | 51 | 1.41 | 0.164 | |
| Internal nest depth (cm) | 5.13 ± 0.06 | 5.16 ± 0.06 | 51 | −0.24 | 0.815 | |
| Nest-site characteristics | Nest height (cm) | 38.44 ± 2.45 | 49.13 ± 3.06 | 51 | −2.41 | 0.019* |
| Attached stem diameter (cm) | 0.39 ± 0.01 | 0.40 ± 0.24 | 51 | −0.57 | 0.571 | |
| Vegetation height (cm) | 122.26 ± 3.76 | 145.31 ± 7.80 | 51 | −2.97 | 0.004** | |
| Vegetation density (stems/m2) | 181.40 ± 7.17 | 182.50 ± 6.49 | 51 | −0.09 | 0.930 | |
| Litter coverage (%) | 92.00 ± 20.00 | 46.00 ± 9.00 | 51 | 7.40 | 0.001** | |
| Distance to water (m) | 6.04 ± 2.02 | 3.63 ± 1.84 | 51 | 0.73 | 0.469 | |
| Distance to road (m) | 45.61 ± 4.16 | 71.23 ± 30.10 | 51 | −1.25 | 0.218 | |
| Shown are the mean ± SE. *P < 0.05; **P < 0.01. | ||||||
| Model | ΔAICca | wib | Kc |
| Nest height + distance to water | 0 | 0.865 | 3 |
| Nest height | 3.802 | 0.129 | 2 |
| Global model | 11.314 | 0.003 | 12 |
| Distance to water | 12.821 | 0.001 | 2 |
| Breeding date | 16.522 | 0.000 | 2 |
| Nest age | 16.864 | 0.000 | 2 |
| Vegetation height | 18.339 | 0.000 | 2 |
| Null model | 18.372 | 0.000 | 1 |
| Habitat type | 18.778 | 0.000 | 2 |
| Distance to road | 19.822 | 0.000 | 2 |
| Year | 19.972 | 0.000 | 2 |
| Litter coverage | 20.287 | 0.000 | 2 |
| Vegetation density | 20.373 | 0.000 | 2 |
| Attached stem diameter | 20.386 | 0.000 | 2 |
| a Difference between current model and best-fit model; AICc of best-fit model = 182.59; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||
| Model | ΔAICca | wib | Kc |
| Nest height + distance to water | 0 | 0.669 | 3 |
| Nest height | 1.600 | 0.300 | 2 |
| Distance to water | 8.179 | 0.011 | 2 |
| Nest age | 10.138 | 0.004 | 2 |
| Breeding date | 10.263 | 0.004 | 2 |
| Global model | 10.345 | 0.004 | 11 |
| Vegetation height | 11.579 | 0.002 | 2 |
| Null model | 11.779 | 0.002 | 1 |
| Vegetation density | 13.141 | 0.001 | 2 |
| Attached stem diameter | 13.543 | 0.001 | 2 |
| Litter coverage | 13.555 | 0.001 | 2 |
| Distance to road | 13.756 | 0.001 | 2 |
| a Difference between current model and best-fit model; AICc of best-fit model = 126.59; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||
| Model | ΔAICca | wib | Kc |
| Distance to water | 0 | 0.211 | 2 |
| Nest height | 0.289 | 0.183 | 2 |
| Null model | 1.227 | 0.114 | 1 |
| Vegetation density | 1.524 | 0.099 | 2 |
| Litter coverage | 2.167 | 0.072 | 2 |
| Year | 2.344 | 0.065 | 2 |
| Breeding date | 2.720 | 0.054 | 2 |
| Nest age | 2.813 | 0.052 | 2 |
| Attached stem diameter | 2.882 | 0.050 | 2 |
| Distance to road | 2.897 | 0.050 | 2 |
| Vegetation height | 3.180 | 0.043 | 2 |
| Global model | 6.842 | 0.007 | 11 |
| a Difference between current model and best-fit model; AICc of best-fit model = 61.78; AICc is Akaike’s information criterion for small samples. b Akaike weights. c Number of parameters in the model. |
|||