|
Alerstam, T., 2006. Strategies for the transition to breeding in time-selected migration. Ardea 94, 347–357.
|
Alerstam, T., Hedenström, A., Åkesson, S., 2003. Long-distance migration: evolution and determinants. Oikos 103, 247–260. .
|
|
Alerstam, T., Lindström, Å., 1990. In: Gwinner, E. (Ed.), Optimal bird migration: the relative importance of time, energy, and safety, Bird Migration. Springer, Berlin, pp. 331–351.
|
|
Ao, P., Wang, X., Meng, F., Batbayar, N., Moriguchi, S., Shimada, T., et al., 2020. Migration routes and conservation status of the Whooper Swan Cygnus cygnus in East Asia. Wildfowl 6, 43–72.
|
Baker, A.J., González, P.M., Piersma, T., Niles, L.J., do Nascimento Ide, L., Atkinson, P.W., et al., 2004. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proc. Biol. Sci. 271, 875–882. .
|
Barton, K., Barton, M.K., 2015. Package “Mumin”. R package version 1.18. .
|
Bates, D., Maechler, M., Bolker, B., Walker, S., 2014. lme4: linear mixed-effects models using Eigen and S4_. R package. version 1.1-7. .
|
Bauer, S., Madsen, J., Klaassen, M., 2006. Intake rates, stochasticity, or onset of spring: what aspects of food availability affect spring migration patterns in Pink-footed Geese Anser brachyrhynchus? Ardea 94, 555–566. .
|
Becker, P.H., Dittmann, T., Ludwigs, J.D., Limmer, B., Ludwig, S.C., Bauch, C., et al., 2008. Timing of initial arrival at the breeding site predicts age at first reproduction in a long-lived migratory bird. Proc. Natl. Acad. Sci. USA 105, 12349–12352. .
|
|
Beekman, J.H., Nolet, B.A., Marcel, K., 2002. Skipping swans: fuelling rates and wind conditions determine differential use of migratory stopover sites of Bewick's Swans Cygnus bewickii. Ardea 90, 437–460.
|
Benhamou, S., 2004. How to reliably estimate the tortuosity of an animal's path: straightness? J. Theor. Biol. 229, 209–220. .
|
|
Boiko, D., Kamp-Persson, H., Morkŭnas, J., 2014. Breeding Whooper Swans Cygnus cygnus in the baltic states, 1973–2013: result of a 207 recolonization. Wildfowl 64, 207–216.
|
Bromley, R.G., Jarvis, R.L., 1993. The energetics of migration and reproduction of Dusky Canada Geese. Condor 95, 193–210. .
|
Bruderer, B., Salewski, V., 2009. Lower annual fecundity in long-distance migrants than in less migratory birds of temperate Europe. J. Ornithol. 150, 281–286. .
|
Bêty, J., Giroux, J.F., Gauthier, G., 2004. Individual variation in timing of migration: causes and reproductive consequences in greater snow geese ( Anser caerulescens atlanticus). Behav. Ecol. Sociobiol. 57, 1–8. .
|
|
Burnham, K.P., Anderson, D.R., 2002. Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach, second ed. Springer-Verlag, New York.
|
Bustnes, J.O., Moe, B., Helberg, M., Phillips, R.A., 2013. Rapid long-distance migration in Norwegian Lesser Black-backed Gulls Larus fuscus fuscus along their eastern flyway. Ibis 155, 402–406. .
|
Champely, S., 2018. PairedData: paired data analysis. R package version 1.1.1. .
|
Choi, J., Kim, J.Y., Do, Y., Joo, G.J., 2018. Population trends of wintering whooper swan ( Cygnus cygnus) in South Korea: data from the winter water-bird census program. Korean J. Ecol. Environ. 51, 365–372. .
|
Deng, X., Zhao, Q., Fang, L., Xu, Z., Wang, X., He, H., et al., 2019. Spring migration duration exceeds that of autumn migration in far East Asian greater White-fronted geese ( Anser albifrons). Avian Res. 10, 19.
|
|
Ely, C.R., Douglas, D.C., Fowler, A.C., Babcock, C.A., Derksen, D.V., Takekawa, J.Y., 1997. Migration behavior of Tundra Swan form the Yukon-Kuskokwim Delta, Alaska. Wilson Bull. 109, 679–692.
|
Ely, C.R., Meixell, B.W., 2016. Demographic outcomes of diverse migration strategies assessed in a metapopulation of tundra swans. Mov. Ecol. 4, 10. .
|
|
Fox, J., Weisberg, S., 2011. An R Companion to Applied Regression. Sage, Thousand Oaks, CA.
|
Fransson, T., 1995. Timing and speed of migration in North and West European populations of Sylvia warblers. J. Avian Biol. 26, 39–48. .
|
Higuchi, H., Sato, F., Matsui, S., Soma, M., Kanmuri, N., 1991. Satellite tracking of the migration routes of Whistling Swans Cygnus columbianus. J. Yamashina Inst. Ornithol. 23, 6–12. .
|
|
Kamiya, K., Ozaki, K., 2002. Satellite tracking of Bewick's Swan migration from Lake Nakaumi, Japan. Waterbirds 25, 128–131.
|
|
Kear, J., 2005. Ducks, Geese and Swans: Species Accounts (Cairina to Mergus), vol. 2. Oxford University Press, Oxford.
|
Klaassen, R.H., Alerstam, T., Carlsson, P., Fox, J.W., Lindström, A., 2011. Great flights by great snipes: long and fast non-stop migration over benign habitats. Biol. Lett. 7, 833–835. .
|
Klaassen, R.H.G., Ens, B.J., Shamoun-Baranes, J., Exo, K.M., Bairlein, F., 2012. Migration strategy of a flight generalist, the Lesser black-backed Gull Larus fuscus. Behav. Ecol. 23, 58–68. .
|
Kokko, H., 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. .
|
Kölzsch, A., Müskens, G.J.D.M., Kruckenberg, H., Glazov, P., Weinzierl, R., Nolet, B.A., et al., 2016. Towards a new understanding of migration timing: slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 125, 1496–1507. .
|
|
Kutner, M.H., Nachtsheim, C.J., Neter, J., Li, W., 2004. Applied Linear Statistical Models, fifth ed. McGraw-Hill, Chicago. Irwin.
|
Li, H., Fang, L., Wang, X., Yi, K., Cao, L., Fox, A.D., 2020. Does snowmelt constrain spring migration progression in sympatric wintering Arctic-nesting geese? Results from a Far East Asia telemetry study. Ibis 162, 548–555. .
|
Li, S., Meng, W., Liu, D., Yang, Q., Chen, L., Dai, Q., et al., 2018. Migratory Whooper Swans Cygnus cygnus transmit H5N1 virus between China and Mongolia: combination evidence from satellite tracking and Phylogenetics analysis. Sci. Rep. 8, 7049. .
|
Limiñana, R., Soutullo, A., Urios, V., Reig-Ferrer, A., 2012. Migration and wintering areas of adult Montagu's Harriers ( Circus pygargus) breeding in Spain. J. Ornithol. 153, 85–93. .
|
Loria, D.E., Moore, F.R., 1990. Energy demands of migration on red-eyed vireos, Vireo olivaceus. Behav. Ecol. 1, 24–35. .
|
|
Mabey, S.E., McCann, J., Niles, L.J., Bartlett, C., Kerlinger, P., 1993. The Migratory Songbird Coastal Corridor Final Report. Virginia Department of Environmental Quality Coastal Resources Management Program, Richmond, VA. Report No. NA90AA-HCZ839.
|
Martin, T.E., Karr, J.R., 1990. Behavioral plasticity of foraging maneuvers of migratory warblers: multiple selection periods for niches. Stud. Avian Biol. 13, 353–359.
|
|
Mathiasson, S., 2013. Eurasian Whooper swan Cygnus cygnus migration with particular reference to birds wintering in southern Sweden. Wildfowl 1, 201–208.
|
McNamara, J.M., Welham, R.K., Houston, A.I., 1998. The timing of migration within the context of an annual routine. J. Avian Biol. 29, 416–423. .
|
Mellone, U., De La Puente, J., López-López, P., Limiñana, R., Bermejo, A., Urios, V., 2015. Seasonal differences in migration patterns of a soaring bird in relation to environmental conditions: a multi-scale approach. Behav. Ecol. Sociobiol. 69, 75–82. .
|
|
Moore, F.R., 1992. Ecophysiological and behavioral response to energy demand during migration. Acta Congr. Int. Onthil. 20, 753–760.
|
|
Moore, F.R., Smith, R.J., Sandberg, R., 2005. In: Greenberg, R., Marra, P. (Eds.), Stopover ecology of intercontinental migrants: en route problems and consequences for reproductive performance, Birds of Two Worlds: the Ecology and Evolution of Migration. Johns Hopkins Press, Baltimore, pp. 251–261.
|
|
Mosbech, A., Gilchrist, G., Merkel, F., Sonne, C., Flagstad, A., Nyegaard, H., 2006. Year-round movements of northern Common Eiders Somateria mollissima borealis breeding in Arctic Canada and West Greenland followed by satellite telemetry. Ardea 94, 651–665.
|
|
National Institute of Biological Resources, 2021. 2020–2021 Winter Water-Bird Census of Korea. National Institute of Biological Resources, Republic of Korea (in Korean).
|
Nilsson, C., Klaassen, R.H., Alerstam, T., 2013. Differences in speed and duration of bird migration between spring and autumn. Am. Nat. 181, 837–845. .
|
|
Nolet, B.A., 2006. Speed of spring migration of Tundra Swans Cygnus columbianus in accordance with income or capital breeding strategy? Ardea 94, 579–591.
|
Nowak, E., Berthold, P., Querner, U., 1990. Satellite tracking of migrating Bewick's swans. A European pilot study. Naturwissenschaften 77, 549–550. .
|
Nuijten, R.J.M., Kölzsch, A., Van Gils, J.A., Hoye, B.J., Oosterbeek, K., De Vries, P.P., et al., 2014. The exception to the rule: retreating ice front makes Bewick's swans Cygnus columbianus bewickii migrate slower in spring than in autumn. J. Avian Biol. 45, 113–122. .
|
Peterson, B.G., Carl, P., 2014. PerformanceAnalytics: econometric tools for performance and risk analysis. .
|
Petrie, S.A., Wilcox, K.L., 2003. Migration chronology of eastern-population tundra swans. Can. J. Zool. 81, 861–870. .
|
|
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
|
Rahn, H., Ar, A., 1974. The avian egg: incubation time and water loss. Condor 76, 147–152. .
|
|
Rees, E.C., Cao, L., Clausen, P., Coleman, J.T., Cornely, J., Einarsson, O., et al., 2019. Conservation status of the world's swan populations, Cygnus sp. and Coscoroba sp. : a review of current trends and gaps in knowledge. Wildfowl 5, 35–72.
|
Roques, S., Henry, P.-Y., Guyot, G., Bargain, B., Cam, E., Pradel, R., 2022. When to depart from a stopover site? Time since arrival matters more than current weather conditions. Ornithology 139. ukab057.
|
Sanz, J.J., Potti, J., Moreno, J., Merino, S., Frias, O., 2003. Climate change and fitness components of a migratory bird breeding in the Mediterranean region. Global Change Biol. 9, 461–472. .
|
Shamoun-Baranes, J., Baharad, A., Alpert, P., Berthold, P., Yom-Tov, Y., Dvir, Y., et al., 2003. The effect of wind, season and latitude on the migration speed of white storks Ciconia ciconia, along the eastern migration route. J. Avian Biol. 34, 97–104. .
|
Shimada, T., Yamaguchi, N.M., Hijikata, N., Hiraoka, E., Hupp, J.W., Flint, P.L., et al., 2014. Satellite tracking of migrating Whooper Swans Cygnus cygnus wintering in Japan. Ornithol. Sci. 13, 67–75. .
|
Van Noordwijk, A.J.V., McCleery, R.H., Perrins, C.M., 1995. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458. .
|
Wetlands International, 2022. Water-Bird Population Estimates. . Accessed 20 Jul 2022.
|
Yohannes, E., Biebach, H., Nikolaus, G., Pearson, D.J., 2009. Migration speeds among eleven species of long-distance migrating passerines across Europe, the desert and eastern Africa. J. Avian Biol. 40, 126–134. .
|



 DownLoad:
DownLoad:
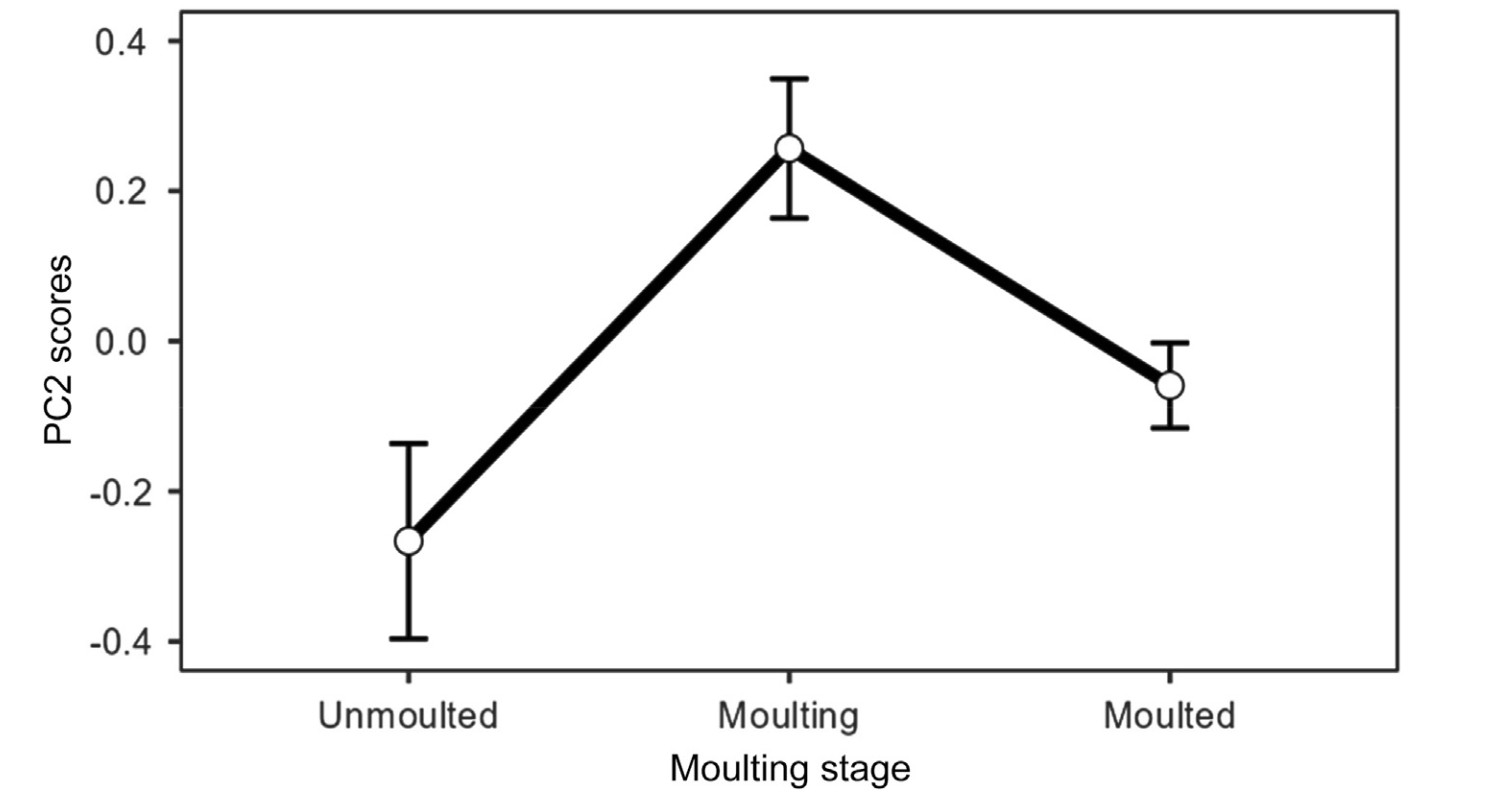
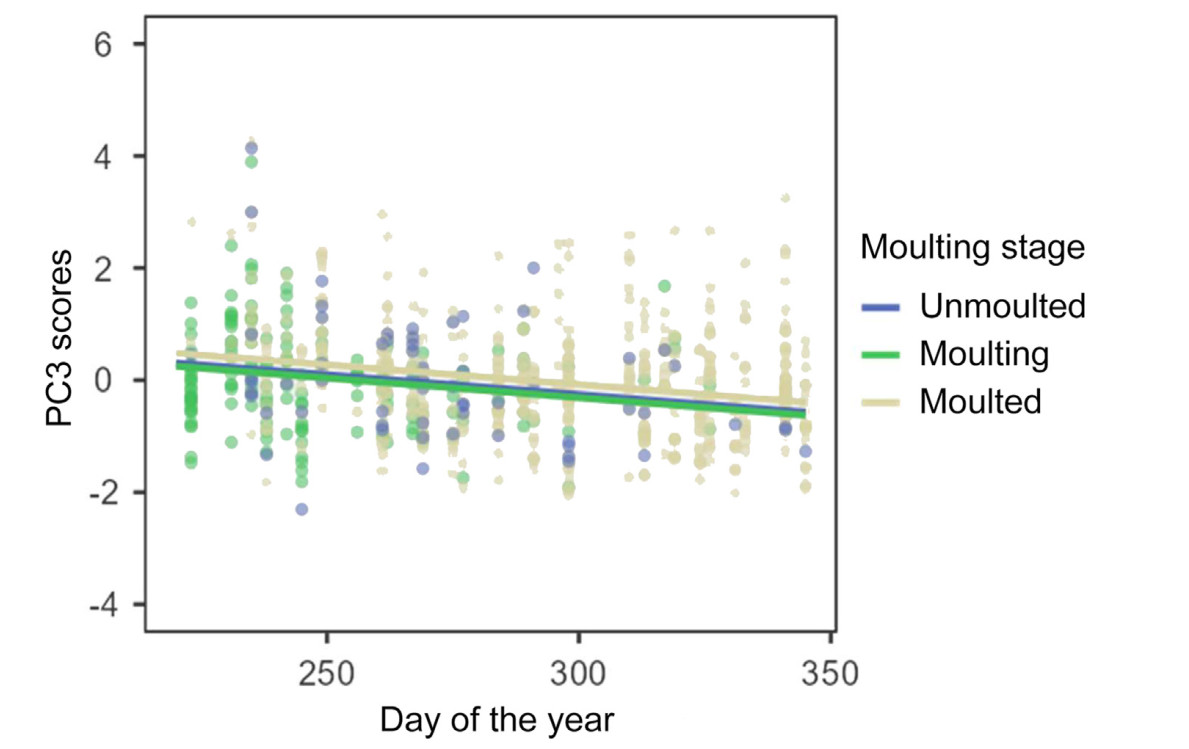
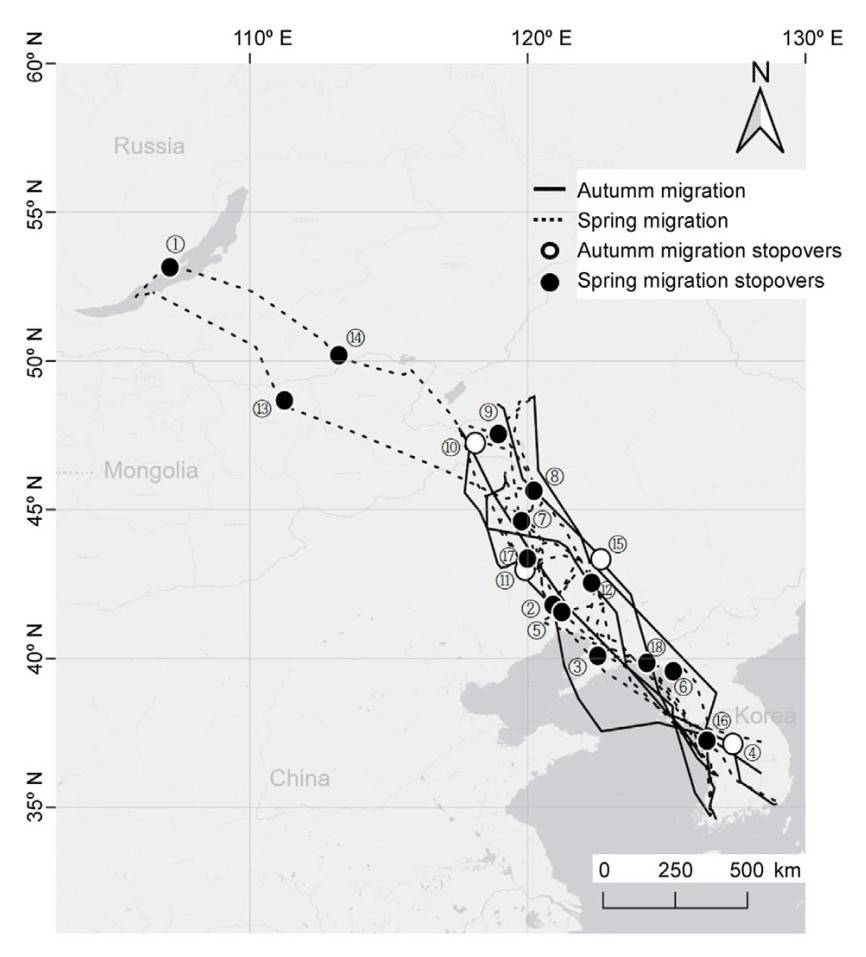





 Email Alerts
Email Alerts RSS Feeds
RSS Feeds