| Citation: | Takehiko Shimizu, Masayuki Senzaki, Yuichiro Fujioka, Satoquo Seino. 2023: Relative importance of tidal flats and artificial habitats for two spoonbill species and related interspecific differences. Avian Research, 14(1): 100107. DOI: 10.1016/j.avrs.2023.100107 |
Artificial/seminatural environments, such as aquacultural ponds, saltpans, and croplands, have recently been acknowledged as important habitats for coastal waterbirds. Although coastal waterbirds tend to use artificial habitats around tidal flats as roosting sites during high-tide, it remains unclear whether the importance of surrounding habitats relative to tidal flats varies among landscape types, seasons, species, or tidal conditions. The Black-faced Spoonbill (Platalea minor) and Eurasian Spoonbill (P. leucorodia) are two closely related sympatric species in East Asia with narrow and wide distribution ranges and habitat requirements, respectively. We therefore expect that both species will use surrounding artificial habitats across seasons at high tides, but Black-faced Spoonbills will use them less frequently than Eurasian Spoonbills. Here, we address these hypotheses in the Imazu tidal flat and its surrounding environments in southern Japan. We investigated the habitat use and behavioral patterns of both species through route and behavioral surveys during the fall migration and wintering seasons in 2021. We found that both species used surrounding habitats including artificial ones more frequently than the tidal flat regardless of the tidal condition or season, but spoonbills used these habitats more frequently in winter than in autumn. We also found that Eurasian Spoonbills foraged in surrounding artificial habitats more frequently than Black-faced Spoonbills. These results not only demonstrate how coastal waterbirds exploit surrounding habitats relative to tidal flats but also suggest that the importance of surrounding habitats varies among species and seasons. Our study thus emphasizes that valuing and managing surrounding habitats in addition to tidal flats are key to conserving globally declining waterbirds.
Contrary to mature old-growth forests, which host increasing numbers of cavities as large trees age, decay, and die (Wesołowski, 2007; Wiebe, 2011), there is a reduced availability of natural cavities in managed forests. This scarcity of cavities in managed forests, especially with fast-growing tree species, is largely due to the removal of snags and decaying trees, and the prevalence of small young trees that are felled before they can develop hollows (Camprodon et al., 2008; Eyre et al., 2010; Politi et al., 2010).
Among birds, cavity-nesters are the most vulnerable to the shortage of cavities in managed forests, in particular secondary cavity-nesting birds which are not able to excavate their own holes and rely on those already created by primary cavity-nesters or tree senescence (Newton, 1994). Since approximately 18% of all bird species in the world nest in tree hollows (van der Hoek et al., 2017), maintaining the structure and function of the cavity-nesting community is essential to sustain bird diversity. Providing nest-boxes as surrogate nesting cavities can be of great importance for increasing the breeding density of cavity-nesters in forest habitats (Camprodon et al., 2008; Mänd et al., 2009; Miller, 2010). Furthermore, because many cavity-nesters are important insectivores, nest-box provisioning can reinforce pest biological control (Rey Benayas et al., 2017; García et al., 2021).
Eucalypts (Eucalyptus, L’Hér. 1789) are amongst the most widely planted tree species around the world, being the most important source of wood for the pulp and paper industry (Brockerhoff et al., 2013). This fast-growing tree is typically planted in monocultures with up to 1700 trees/ha which are very susceptible to attacks of insect pests (Paine et al., 2011; Hurley et al., 2016). Concomitantly, eucalypt stands are a poor habitat for breeding birds (Calviño-Cancela, 2013; da Silva et al., 2019; Goded et al., 2019) given their little structural and compositional diversity, and the lack of tree hollows due to their management as a short rotation coppice system (10–12 years average cutting cycle in Europe; Tomé et al., 2021). Therefore, in view of the hypothesis that nest-box provisioning can increase pest regulation by insectivorous birds in eucalypt plantations, the present study was set to assess and optimise nest-box occupancy by insectivores in this habitat. As different species prefer certain characteristics of nest-boxes over others (Lambrechts et al., 2010; Møller et al., 2014), we followed nest-box design recommendations targeting occupancy by the Great Tit (Parus major) (Sorace and Carere, 1996; Browne, 2006; Bueno-Enciso et al., 2016). The Great Tit is a secondary cavity-nesting passerine, widespread in a range of habitats throughout Europe and Asia, that breeds readily in nest-boxes (del Hoyo et al., 2016). Besides the importance of Great Tit to insect pest control in various agricultural and forestry systems (e.g., Sanz, 2001; Rey Benayas et al., 2017; García et al., 2021), a recent study found evidence that it preys on eucalypt pests of major concern (da Silva et al., 2022). Although, and even if eucalypt plantations spread throughout 20 million ha worldwide (Brockerhoff et al., 2013), the implementation of nest-boxes in eucalypt stands was only investigated in two occasions, one in central Portugal (da Silva et al., 2012) and another in northern Spain (de la Hera et al., 2013). In both studies, the Great Tit was the most common species occupying nest-boxes, with an occupancy percentage that varied between 7% and 11%. In the study of de la Hera et al. (2013), nest-box occupancy (by all bird species) showed a significant positive correlation with understory development, which is somewhat contrary to results obtained for other species that prefer nest-boxes with increased nest openness (Kiss et al., 2017; Zhang et al., 2021) or little surrounding vegetation (Navara and Anderson, 2011). Other studies showed that nest-box occupancy by Great Tits can be influenced by terrain slope (Briggs and Mainwaring, 2022), nest-box orientation (Goodenough et al., 2008) and illumination (Podkowa and Surmacki, 2017), as well as by human disturbance associated with traffic noise (Halfwerk et al., 2016) and recreational infrastructures (Remacha and Delgado, 2009). However, such studies were conducted either in deciduous or mixed woodlands and differences are expected regarding other habitats, such as eucalypt plantations (Mänd et al., 2005, 2009; da Silva et al., 2012). Therefore, the factors influencing nest-box selection by Great Tits still need further study, in particular because habitat-specific effects remain largely overlooked.
The present study aimed to understand what fine, local- and landscape-level attributes influence nest-box selection by Great Tits in eucalypt plantations. In our three-year nest-box addition experiment in central Portugal, nest-boxes had the same physical characteristics, so it was expected that Great Tits would select them based on external criteria, such as topography, nest-box positioning, vegetation structure and composition, and landscape variables. By finding the best predictors of nest-box occupancy, this work furthers our understanding of the factors influencing nest-box selection by Great Tits and refines nest-box placement in eucalypt stands with a view on their effectiveness as an ecological intensification tool of wide applicability.
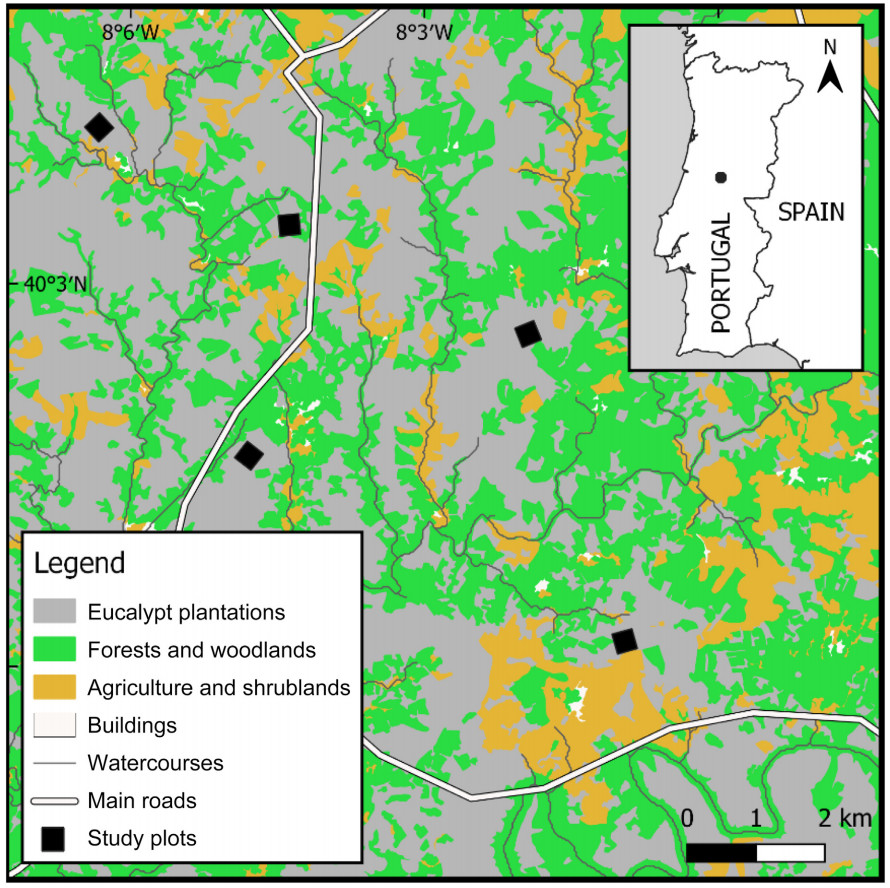
Fieldwork was carried out from 2020 to 2022 in central Portugal (centroid at 40°1ʹ50.88ʺ N, 8°3ʹ37.08ʺ W; Fig. 1). Study area is part of a mountainous region (450–750 m a.s.l.), dominated by eucalypt stands, interspersed by shrublands, Maritime Pine (Pinus pinaster) plantations, olive groves, and small villages with subsistence farming and abandoned lands. It is traversed by small and medium watercourses with riparian forest along waterways composed of a mixture of native, e.g., Chestnut (Castanea sativa), Cork Oak (Quercus suber) and Common Oak (Quercus robur), and exotic vegetation, e.g., Mimosa (Acacia dealbata) and Blackwood Acacia (Acacia melanoxylon). The region is characterized by a temperate climate with dry summers (Peel et al., 2007) — the mean monthly temperature year-round varies between 7.7 and 20.6 ℃, and the mean accumulated annual precipitation varies between 461.1 and 1101.4 mm (IPMA, 2019).
Five plots with 300 m × 300 m were established in eucalypt plantations with approximately 1200 trees/ha (Fig. 1). The minimum distance between neighbouring plots was 2.8 km and a 50-m buffer dominated by eucalypt trees was safeguarded around each plot. In January 2020, 3 months before this study began, 16 new nest-boxes were installed at 100-m intervals in each of the 5 plots (totalling 80 nest-boxes). They were attached to the trunk of eucalypt trees with their bottom standing horizontally at a variable height (ranging from 178 to 264 cm), allowing for comfortable monitoring. Nest-boxes were made of raw pine wood, 1.5 cm thick, with 15 cm × 15 cm × 20 cm (length × width × height) and a round nest opening with a diameter of 34 mm in the front panel. The entrance hole was suitable to allow Great Tits to enter, although smaller species could also enter. Each year, nest-boxes were emptied in February before the breeding season started, and from late March to late July were inspected for their occupancy and determination of the occupant species on a weekly basis by opening the roof. A nest-box was confirmed to be occupied if any egg had been laid in it.
A total of 15 parameters was collected for each nest-box, including attributes for topography, nest-box positioning, vegetation and landscape structure and composition (Table 1). This list covered parameters whose importance for Great Tit nesting had been suggested in previous studies (Goodenough et al., 2008; Remacha and Delgado, 2009; Halfwerk et al., 2016; Podkowa and Surmacki, 2017; Briggs and Mainwaring, 2022), which we adapted here to our study system.
| Nest-site attributes | Mean ± SD (in scale variables) | |
| Topography | ||
| 1 | Altitude | 569 ± 73 m |
| 2 | Slope | 28.4 ± 12.9° |
| 3 | Aspect (quadrants) | N (27%), E (9%), S (14%), W (50%) |
| Nest-box placement | ||
| 4 | Nest-box hanging height | 225.3 ± 18.4 cm |
| 5 | Direction of nest-box opening (quadrants) | N (33%), E (15%), S (17%), W (35%) |
| Vegetation structure and composition | ||
| 6 | Percent canopy cover by eucalypts | 42.6 ± 17.5% |
| 7 | Percent canopy cover by other trees | 2.8 ± 6.8% |
| 8 | Percent understory cover | 42.6 ± 26.7% |
| 9 | Canopy height | 1223.8 ± 343.6 cm |
| 10 | Understory height | 151.3 ± 63.5 cm |
| Landscape structure and composition | ||
| 11 | Distance from track | 23.9 ± 20.0 m |
| 12 | Distance from watercourse | 441.1 ± 270.9 m |
| 13 | Distance from adjacent habitat | 115.3 ± 76.5 m |
| 14 | Adjacent habitat type | forests and woodlands (67%), agriculture and shrublands (33%) |
| 15 | Adjacent habitat size | 126.7 ± 209.0 ha |
| Because adjacent habitat type is a descriptive variable, instead the percentage of records for each category is indicated. Also, for interpretation purposes, aspect and direction of nest-box opening were categorized as north (315–45°), east (45–134°), south (135–224°) and west (225–314°), with due north as 0°, and the correspondent percentage of records is indicated for each category. | ||
Within a 25-m radius around each nest-box, we recorded: (1) altitude (m); (2) degree of inclination of the slope (°); (3) aspect (°), corresponding to compass orientation of slope with respect to due north and transformed using the cosine function; (4) nest-box hanging height (cm), measured from the bottom of the nest-box to the ground; (5) direction of nest-box opening (°), corresponding to the facing orientation of the entrance hole with respect to due north and transformed using the cosine function; (6) percent canopy cover by eucalypt trees (%), estimated as the overstory density in percent of eucalypts; (7) percent canopy cover by trees of other species (%), estimated as the overstory density in percent of trees other than eucalypts, such as Mimosa, Maritime Pine, Common Oak and Cork Oak; (8) percent understory cover (%), estimated as the percentage of the ground surface covered by shrubs; (9) maximum canopy height (m); (10) maximum understory height (cm); and (11) distance from track (m), estimated distance to the nearest track or paved roadway. The diameter of the nest-hanging tree and understory species richness were also recorded but were discarded from the analyses due to their significant positive correlation, respectively, with the heights of the canopy and the understory. All measurements and estimates were taken from a single trained observer (P.B.L.) in February 2020, and no relevant changes in measured parameters occurred throughout this study, except from vegetation-related variables (e.g., eucalypt growth) which proceeded similarly in every site.
Furthermore, we used QGIS version 3.10 software (QGIS Development Team, 2020) to extract (12) distance from the nearest watercourse (m) and (13) distance from the nearest adjacent habitat (m), both calculated using the ‘NNJoin’ plugin version 3.1.3; and (14) closest adjacent habitat type, a descriptive variable divided into ‘forests and woodlands’ and ‘agriculture and shrublands’, and (15) closest adjacent habitat size (ha), both obtained from the cartography of land use and occupation of mainland Portugal for 2018, downloaded from the website of the Direção-Geral do Território (http://mapas.dgterritorio.pt/DGT-ATOM-download/COS_Final/COS2018_v1/COS2018_v1.zip).
All data exploration and modelling were performed with software R version 4.1.0 (R Core Team, 2021).
The Great Tit occupancy records were used to create a binomial variable (0 – not occupied, 1 – occupied) for all nest-boxes monitored from 2020 to 2022, totalling 240 observations (80 nest-boxes × 3 years). This was our response variable, and all models were computed assuming a binomial distribution and logit link-function.
Data were first fitted to a generalised linear model (‘glm’ procedure in the R base package; R Core Team, 2021) to assess the effects of ‘year’ and ‘plot’, as well as the interaction term. Analysis of variance and Tukey's differences for each stratum were calculated with the ‘aov’ and ‘TukeyHSD’ procedures, respectively, in the R base package (R Core Team, 2021).
Then, because previous studies suggest that birds assess nest site attractiveness using breeding evidence left by former residents or themselves the previous year (Ekner-Grzyb et al., 2014; Podkowa and Surmacki, 2017), we tested for the influence of bird nest-box occupancy from previous year. For this purpose, a simple univariate generalised linear mixed effects model (‘glmer’ procedure in package ‘lme4’; Bates et al., 2015) was calculated with the binomial explanatory variable ‘previous year nest-box occupancy’ (0 – not occupied by Great Tit or any other species in the previous year, 1 – occupied by Great Tit or any other species in the previous year), while ‘year’ and ‘plot’ were included as random factors to account, respectively, for temporal and spatial autocorrelation in the data set.
Finally, a complex global generalised linear mixed-effects model was built to analyse the combined influence of nest-site attributes on Great Tit occupancy, including ‘year’, ‘plot’ and ‘previous year nest-box occupancy’ as random factors. Variance-inflation factors were calculated using the ‘vif’ procedure in the package ‘car’ (Fox and Weisberg, 2019) and the absence of multicollinearity between the 15 explanatory variables was confirmed by checking that the values were <2.5 in the global model. Competing models were ranked according to their AICc, and model parameters were estimated for models within a ΔAICc <2 units of the best model with the lowest AICc (Grueber et al., 2011). The selection of multiple models was carried out using the procedures ‘dredge’, ‘get.models’ and ‘avg.models’ in the package ‘MuMIn’ (Barton, 2020). All model predictors were scaled and centred, using ‘standardize’ procedure in the package ‘arm’ (Gelman and Su, 2020), to allow comparing their relative effects (Schielzeth, 2010). The models included in the calculations of the full model-averaged coefficients are listed as Supplementary material (Appendix Table S1).
Of the total of 80 nest-boxes installed, 4 nest-boxes were occupied in the 3 years, 31 were occupied for 2 years, 27 were occupied for only one year, and 18 were never occupied. Great Tits did not occupy 26 nest-boxes, although 8 of them were occupied by Crested Tits (Lophophanes cristatus) or Blue Tits (Cyanistes caeruleus). In the second and third year, respectively, 4 and 1 nest-boxes had two consecutive nesting events of Great Tit which were considered as a single occupancy in the analyses. Overall, Great Tits made up 86% of the occupancy records, while Crested Tits and Blue Tits had 10% and 4% of the occupancies, respectively.
The percentages of nest-box occupancy by Great Tits in the first, second, and third years were 15%, 45% and 49%, respectively, and there were significant differences in annual occupancy numbers (F = 11.75, p < 0.001), particularly between the first and the following two years. No significant differences were found among plots (F = 1.08, p = 0.369), nor interaction between year and plot (F = 0.606, p = 0.772).
According to the mixed-effects model, the influence of nest-box occupancy in the previous year on the occupancy by Great Tits was not significant (F = 1.94, p = 0.052), although in 56% of the cases Great Tits occupied a nest-box that was occupied in the previous year.
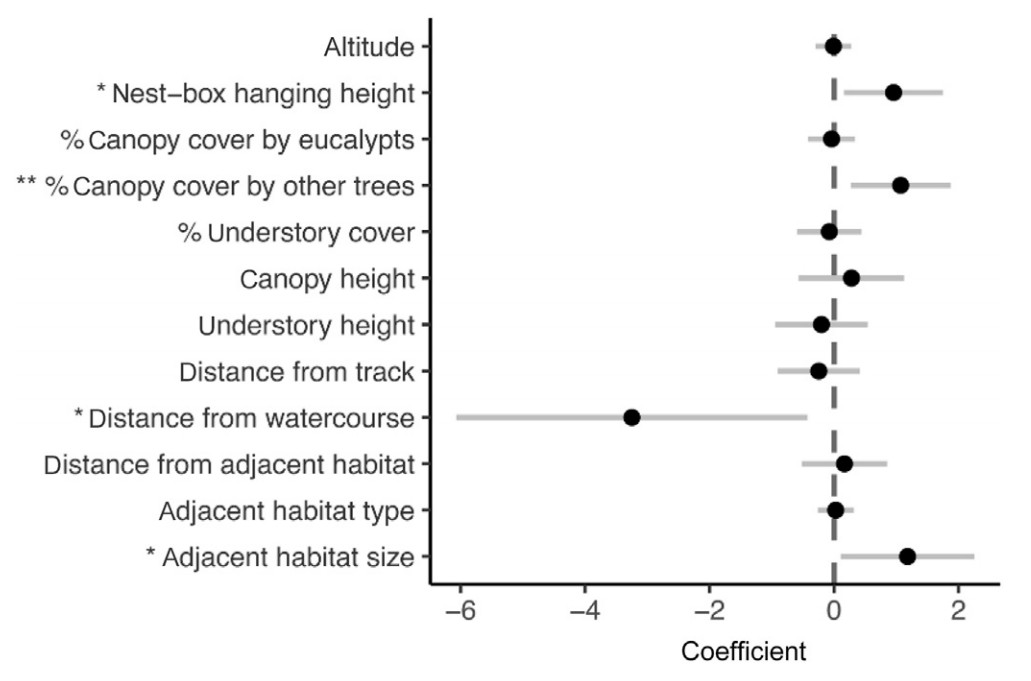
The best models for the occupancy of nest-boxes by Great Tits combined 12 explanatory variables, from the 15 explanatory variables used in the global model, and did not include slope, aspect, and direction of nest-box opening (Fig. 2; Appendix Table S1). According to the full model-averaged coefficients, Great Tit occupancy showed a significant positive correlation with the height of the nest-boxes (z = 2.33, p = 0.020; Fig. 2), percent canopy cover by trees other than eucalypts (z = 2.59, p = 0.010; Fig. 2), and the size of adjacent habitat (z = 2.17, p = 0.030; Fig. 2), while it demonstrated a significant negative correlation with distance from the nearest watercourse (z = 2.17, p = 0.030; Fig. 2).
In this study we used nest-boxes suitable for Great Tits, all identical (Sorace and Carere, 1996; Browne, 2006; Bueno-Enciso et al., 2016), so that breeding individuals would select nest-sites on the basis of criteria other than the box itself. Although our nest-boxes did not impede smaller species from using them, occupancy by Crested and Blue Tits was considerably lower given their lower abundance in eucalypt plantations (Pina, 1989; da Silva et al., 2012) and probable competitive exclusion by dominant Great Tits (Kempenaers and Dhondt, 1991; Aitken and Martin, 2008). Nonetheless, nest-box availability was not a limiting factor for breeding Great Tits in our study since, even if annual occupancy records significantly increased from the first year to the following years, less than half of the boxes were occupied annually. Moreover, nest-boxes were used similarly among plots and without significant effect of having been occupied in the previous year, contrarily to other works (Ekner-Grzyb et al., 2014; Podkowa and Surmacki, 2017), maybe due to the high availability of empty boxes.
Overall, individuals selected nest-boxes non-randomly with respect to nest-site attributes in eucalypt plantations. The measured nest-site attributes comprised a total of 15 variables that represented (1) topography (altitude, slope, aspect), (2) nest-box positioning on eucalypt trees (nest-box hanging height, direction of nest-box opening), (3) vegetation cover around the nest-boxes (percent canopy cover by eucalypts, percent canopy cover by other trees, percent understory cover, canopy height, understory height), and (4) landscape context (distance from track, distance from watercourse, distance from adjacent habitat, adjacent habitat type, adjacent habitat size). Except for topographic parameters, all evaluated categories had a significant influence on Great Tit preferences.
The right placement of nest-boxes can improve occupancy rates (Citta and Lindberg, 2007; Goodenough et al., 2008; Zhang et al., 2021). Although our nest-boxes were hung to eucalypt trees no higher than 3 m from the ground to facilitate safe monitoring, Great Tits selected those that were placed higher. Selection of higher nest-boxes has been observed for passerines in other forest habitats (Serrano-Davies et al., 2017; Zhang et al., 2021) and may be advantageous as it reduces detection and access by ground-dwelling predators (Nilsson, 1984; Newton, 1994). On the other hand, the orientation of nest-boxes (here determined by the direction of the opening and by aspect) did not influence their selection by Great Tits. Orientation can affect the microclimate of the nest-boxes due to differences in solar radiation (Wiebe, 2001; Podkowa and Surmacki, 2017), primarily because east-facing nest-boxes are warmed earlier in the morning and avoid overheating in the afternoon, and, in the northern hemisphere, those facing south receive the greatest amount of sunlight. Birds can show a preference for nest-boxes with a certain orientation, but disparities have been reported according to bird species (Goodenough et al., 2008), breeding phenology (Ardia et al., 2006), climatic region (Charter et al., 2010; Rodríguez et al., 2011; Monti et al., 2019), and latitude (Navara and Anderson, 2011; Zhang et al., 2021). However, direct solar radiation should be crucial for nest-box orientation to influence occupancy (Charter et al., 2010; Rodríguez et al., 2011), and canopy shading might mediate any microclimatic effect arising from nest-box orientation in dense eucalypt plantations as those in our study.
Considering vegetation characteristics, neither tree canopy structure (percent canopy cover by eucalypts and canopy height) nor understory structure (percent understory cover and understory height) had an influence on nest-box occupancy. However, there was an obvious effect of tree composition, as nest-box occupancy increased significantly with the proportion of trees other than eucalypts in the nest-box surrounding. In homogeneous monospecific eucalypt stands, the occurrence of different tree species around a nest-box should reflect a greater availability of food resources for the birds breeding in that territory (Both and Visser, 2003). For instance, oaks are important host-plants for caterpillars, which are the main component of Great Tit nestling diet (Cholewa and Wesołowski, 2011; Ceia et al., 2016). Also, habitat preferences for sites dominated by oaks have previously been observed in other Great Tit populations as a result of better foraging conditions (Mänd et al., 2005).
Similarly, at the landscape-scale, riparian areas and other habitats in the vicinity of eucalypt stands may offer additional foraging opportunities for Great Tits (Hsu et al., 2010). This probably explains why nest-boxes were more frequently occupied in the proximity of watercourses and large patches of adjacent habitats, irrespective of whether these were tree habitats (forests and woodlands) or not (agriculture and shrublands). Our results also suggested that no particular threat was perceived by Great Tits nesting close to tracks or paved roadways in the area. Although predator density may increase along tracks and roads (Sinclair et al., 2005), those in our study area were narrow and seldom transited, and due to their low contrast with the surrounding habitat did not markedly produce edge effects (Remacha and Delgado, 2009; see, however, Briggs and Mainwaring, 2022).
Overall, our study suggests that disentangling the significant factors for nest-box placement and spatial distribution at both local and landscape scales is important to meet species-specific requirements in a given habitat. Eucalypt plantations are important forestry systems in Europe (Brus et al., 2019) but despite being generally considered a poor habitat for wildlife (e.g., Law et al., 2017; da Silva et al., 2019), measures to make this habitat more wildlife-friendly are still lacking. The provision of nesting resources is certainly an important measure to benefit birds, in particular secondary cavity-nesters, since old trees comprising natural hollows are absent from eucalypt stands, and young eucalypt trees are devoid of cavities. To our knowledge, the implementation of nest-boxes was investigated twice in eucalypt stands (da Silva et al., 2012; de la Hera et al., 2013) and nest-box occupancy was mainly attributed to Great Tits in both cases. As in our study, these two previous studies used around one-hundred nest-boxes which had the same approximate dimensions as ours and were hung on eucalypt trees at about 3.0–3.5 m from the ground, but their maximum occupancy percentages (<11%) were substantially lower than that recorded in our study (49%). Given that the structure and composition of the stands were analogous in the three studies, differences in occupancy percentages were probably related to the fact that nest-boxes were set further from each other in our study (100-m intervals) than in the other two studies, where nest-boxes were set at 40-m intervals (da Silva et al., 2012) and 50-m intervals (de la Hera et al., 2013). Taking this into account, we recommend that the distance between nest-boxes and their density be further investigated in eucalypt plantations to maximize the outcomes of nest-box interventions. Experiments that vary the size of nest-box entrances to exclude larger species from interfering with the nesting attempts of smaller species should also be performed to evaluate species-specific nest-site preferences, though potential interactions with heterospecific and conspecific competitors must be considered (Minot and Perrins, 1986). Even if nest-box availability was not a limiting factor and the number of unused nest-boxes decreased during the period of this study, we recommend that reproductive parameters are analysed in future studies to confirm that population density in eucalypt plantations is not increasing to an extent that density-dependent effects, including competition among breeding pairs, become deleterious (Wilkin et al., 2006; Mänd et al., 2009).
Ricardo S. Ceia: Methodology, Formal analysis, Writing – Original Draft; Pedro B. Lopes: Investigation; Luís P. da Silva: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition. All authors read and approved the final manuscript.
All fieldwork was done under the required legal permits of Portugal, including bird sampling in the nest-boxes (ringing permits No. 183/2020, No. 184/2021, and No. 196/2022 from the Portuguese Institute for Nature Conservation and Forests).
The authors declare that they have no competing interests.
The project is co-financed by Fundação para a Ciência e a Tecnologia (FCT) and the European Regional Development Fund (FEDER) through Portugal 2020 Competitiveness and Internationalization Operational Programme (POCI), reference POCI-01-0145-FEDER-030250 and PTDC/ASP-SIL/30250/2017–TOPDEVIL. The work was carried out at the R&D Unit Centre for Functional Ecology–Science for People and the Planet (CFE), with reference UIDB/04004/2020, financed by FCT/MCTES through national funds (PIDDAC). FCT/MCTES also funded L.P.S. with contract CEECIND/02064/2017.
All scripts and input information for data analyses are available in GitHub (https://github.com/ricardoceia/Nest-box-selection-in-eucalypt-stands). Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2023.100098.
| Nest-site attributes | Mean ± SD (in scale variables) | |
| Topography | ||
| 1 | Altitude | 569 ± 73 m |
| 2 | Slope | 28.4 ± 12.9° |
| 3 | Aspect (quadrants) | N (27%), E (9%), S (14%), W (50%) |
| Nest-box placement | ||
| 4 | Nest-box hanging height | 225.3 ± 18.4 cm |
| 5 | Direction of nest-box opening (quadrants) | N (33%), E (15%), S (17%), W (35%) |
| Vegetation structure and composition | ||
| 6 | Percent canopy cover by eucalypts | 42.6 ± 17.5% |
| 7 | Percent canopy cover by other trees | 2.8 ± 6.8% |
| 8 | Percent understory cover | 42.6 ± 26.7% |
| 9 | Canopy height | 1223.8 ± 343.6 cm |
| 10 | Understory height | 151.3 ± 63.5 cm |
| Landscape structure and composition | ||
| 11 | Distance from track | 23.9 ± 20.0 m |
| 12 | Distance from watercourse | 441.1 ± 270.9 m |
| 13 | Distance from adjacent habitat | 115.3 ± 76.5 m |
| 14 | Adjacent habitat type | forests and woodlands (67%), agriculture and shrublands (33%) |
| 15 | Adjacent habitat size | 126.7 ± 209.0 ha |
| Because adjacent habitat type is a descriptive variable, instead the percentage of records for each category is indicated. Also, for interpretation purposes, aspect and direction of nest-box opening were categorized as north (315–45°), east (45–134°), south (135–224°) and west (225–314°), with due north as 0°, and the correspondent percentage of records is indicated for each category. | ||