Adhikari, P., Kim, B.J., Hong, S.H., Lee, D.H., 2022. Climate change induced habitat expansion of nutria ( Myocastor coypus) in South Korea. Sci. Rep. 12, 3300.
|
Allouche, O., Tsoar, A., Kadmon, R., 2006. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43, 1223–1232.
|
Araújo, M.B., New, M., 2007. Ensemble forecasting of species distributions. Trends Ecol. Evol. 22, 42–47.
|
Awan, M.N., Saqib, Z., Buner, F., Lee, D.C., Pervez, A., 2021. Using ensemble modeling to predict breeding habitat of the red-listed Western Tragopan ( Tragopan melanocephalus) in the Western Himalayas of Pakistan. Glob. Ecol. Conserv. 31, e01864.
|
Baral, B., Bhandari, S., Koirala, S., Bhandari, P., Magar, G., Basnet, D.R., et al., 2020. First distributional record of the lesser adjutant Leptoptilos javanicus Horsfield, 1821 (Ciconiiformes: Ciconiidae) from Sindhuli district, Nepal. J. Threat. Taxa 12, 17028–17031.
|
|
Baral, H.S., Inskipp, C., 2005. Important bird areas in Nepal: key sites for conservation. In: Bird Conservation Nepal and BirdLife International (Kathmandu and Cambridge).
|
Baral, S., Kunwar, A., Adhikari, D., Kandel, K., Mandal, D.N., Thapa, A., et al., 2023. The potential distribution of the yellow monitor, Varanus flavescens (Hardwick & Gray) under multiple climate, land cover and dispersal scenarios in Nepal. Wildl. Res. .
|
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W., Courchamp, F., 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377.
|
Bhattarai, B.P., Adhikari, J.N., Rijal, M., 2021. Nesting habitat selection and challenges of conservation of the vulnerable Lesser Adjutant Leptoptilos javanicus (Horsfield, 1821) in the Chitwan National Park, Nepal. Ornis Hung. 29, 33–46.
|
BirdLife International, 2021. Species factsheet: Leptoptilos javanicus. . (Accessed 3 August 2021).
|
Boria, R.A., Olson, L.E., Goodman, S.M., Anderson, R.P., 2014. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77.
|
Bradley, A.P., 1997. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recogn. 30, 1145–1159.
|
Brambilla, M., Rubolini, D., Appukuttan, O., Calvi, G., Karger, D.N., Kmecl, P., et al., 2022. Identifying climate refugia for high-elevation alpine birds under current climate warming predictions. Global Change Biol. 28, 4276–4291.
|
Chapagain, D., Dhaubanjar, S., Bharati, L., 2021. Unpacking future climate extremes and their sectoral implications in western Nepal. Clim. Change 168, 8.
|
Chhetri, B., Badola, H.K., Barat, S., 2021. Modelling climate change impacts on distribution of Himalayan pheasants. Ecol. Indicat. 123, 107368.
|
Chhogyel, N., Kumar, L., 2018. Climate change and potential impacts on agriculture in Bhutan: a discussion of pertinent issues. Agric. Food Secur. 7, 79.
|
Coad, L., Watson, J.E.M., Geldmann, J., Burgess, N.D., Leverington, F., Hockings, M., et al., 2019. Widespread shortfalls in protected area resourcing undermine efforts to conserve biodiversity. Front. Ecol. Environ. 17, 259–264.
|
Coetzee, B.W.T., Gaston, K.J., Chown, S.L., 2014. Local scale comparisons of biodiversity as a test for global protected area ecological performance: a meta-analysis. PLoS One 9, e105824.
|
Condro, A.A., Syartinilia Higuchi, H., Mulyani, Y.A., Raffiudin, R., Rusniarsyah, L., Setiawan, Y., et al., 2022. Climate change leads to range contraction for Japanese population of the Oriental Honey-Buzzards: implications for future conservation strategies. Glob. Ecol. Conserv. 34, e02044.
|
D'Amour, C.B., Reitsma, F., Baiocchi, G., Barthel, S., Güneralp, B., Erb, K.H., et al., 2017. Future urban land expansion and implications for global croplands. Proc. Nat. Acad. Sci. USA 114, 8939–8944.
|
|
DNPWC, BCN, 2022. Birds of Nepal: an Official Checklist. Department of National Parks and Wildlife Conservation and Bird Conservation Nepal, Kathmandu, Nepal.
|
Elith, J., Leathwick, J.R., 2009. Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697.
|
|
Elliott, A., Garcia, E.F.J., Boesman, P.F.D., 2020. Lesser adjutant (Leptoptilos javanicus), version 1.0. In: del Hoyo, J., Elliott, A., Saragata, J., Christie, D.A., de Juana, E. (Eds.), Birds of the World. Cornell Lab of Ornithology, Ithaca, NY.
|
Fick, S.E., Hijmans, R.J., 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315.
|
Fois, M., Cuena-Lombraña, A., Fenu, G., Cogoni, D., Bacchetta, G., 2016. The reliability of conservation status assessments at regional level: past, present and future perspectives on Gentiana lutea L. ssp. lutea in Sardinia. J. Nat. Conserv. 33, 1–9.
|
Fonseca, C.R., Venticinque, E.M., 2018. Biodiversity conservation gaps in Brazil: a role for systematic conservation planning. Perspect. Ecol. Conserv. 16, 61–67.
|
Fusco, J., Walker, E., Papaïx, J., Debolini, M., Bondeau, A., Barnagaud, J.Y., 2021. Land use changes threaten bird taxonomic and functional diversity across the Mediterranean Basin: a spatial analysis to prioritize monitoring for conservation. Front. Ecol. Evol. 9, 612356.
|
Garcia, R.A., Cabeza, M., Rahbek, C., Araújo, M.B., 2014. Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579.
|
Ghimire, P., 2019. A review of studies on climate change in Nepal. Geogr. Base 6, 11–20.
|
Ghosh-Harihar, M., An, R., Athreya, R., Borthakur, U., Chanchani, P., Chetry, D., et al., 2019. Protected areas and biodiversity conservation in India. Biol. Conserv. 237, 114–124.
|
Gula, J., Sundar, K.S.G., Willows-Munro, S., Downs, C.T., 2023. The state of stork research globally: a systematic review. Biol. Conserv. 280, 109969.
|
|
Gulati, H., Rana, S., 2021. Nest characteristics and breeding success of Sarus Crane, Antigone antigone (Linnaeus, 1758) (Aves: Gruidae) in different habitats at Dhanauri Wetland, Uttar Pradesh, India. Record Zool. Surv. India 121, 205–210.
|
Hamal, K., Sharma, S., Baniya, B., Khadka, N., Zhou, X., 2020. Inter-annual variability of winter precipitation over Nepal coupled with ocean-atmospheric patterns during 1987-2015. Front. Earth Sci. 8, 161.
|
Hausfather, Z., 2018. Explainer: how 'Shared Socioeconomic Pathways' explore future climate change. .
|
Hijmans, R.J., 2022. raster: geographic data analysis and modeling. R package version 3.5-29. .
|
Hijmans, R.J., Cameron, S.E., Parra, J.L., Jones, P.G., Jarvis, A., 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978.
|
Hilaluddin Shah, J.N., Shawl, T.A., 2003. Nest site selection and breeding success by Cattle Egret and Little Egret in Amroha, Uttar Pradesh, India. Waterbirds 26, 444–448.
|
|
Inskipp, C., Baral, H.S., Phuyal, S., Bhatt, T., Khatiwada, M., Inskipp, T., et al., 2016. The Status of Nepal's Birds: the National Red List Series. Zoological Society of London, United Kingdom.
|
|
Jetz, W., Wilcove, D.S., Dobson, A.P., 2007. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, 1211–1219.
|
|
Kar, T., Debata, S., Mundkur, T., 2021. Population, breeding phenology, and factors affecting breeding success of River Tern (Sterna aurantia) in Eastern India. Waterbirds 44, 308–316.
|
Karki, R., Gurung, A., 2012. An overview of climate change and its impact on agriculture: a review from least developing country, Nepal. Int. J. Ecosyst. 2, 19–24.
|
Karki, R., ul Hasson, S., Schickhoff, U., Scholten, T., Böhner, J., 2017. Rising precipitation extremes across Nepal. Climate 5, 4.
|
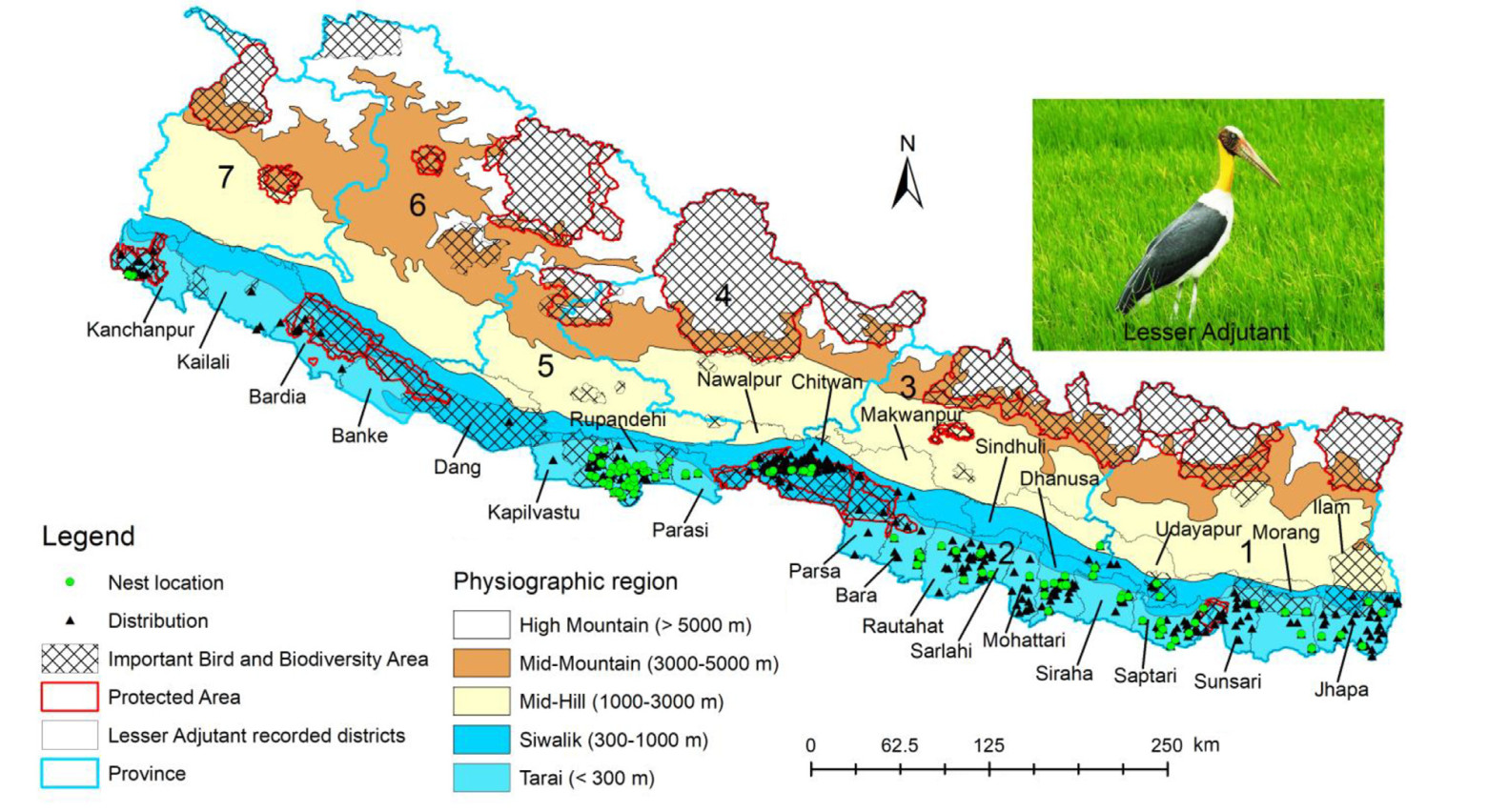
Karki, S., Thapa, T.B., 2013. Population status, nesting habitat selection and conservation threats of lesser adjutant stork ( Leptoptilos javanicus) in the eastern lowlands of Nepal. Conserv. Sci. 1, 27–35.
|
Kattel, G.R., 2022. Climate warming in the Himalayas threatens biodiversity, ecosystem functioning and ecosystem services in the 21st century: is there a better solution? Biodivers. Conserv. 31, 2017–2044.
|
Katuwal, H.B., Rai, J., Tomlinson, K., Rimal, B., Sharma, H.P., Baral, H.S., et al., 2022a. Seasonal variation and crop diversity shape the composition of bird communities in agricultural landscapes in Nepal. Agric. Ecosyst. Environ. 333, 107973.
|
|
Katuwal, H.B., Sundar, K.S.G., Zhang, M., Rimal, B., Baral, H.S., Sharma, H.P., et al., 2022b. Factors affecting the breeding ecology of the globally threatened Lesser Adjutant Leptoptilos javanicus in agricultural landscapes of Nepal. Avian Conserv. Ecol. 17, 15.
|
Kearney, S.G., Adams, V.M., Fuller, R.A., Possingham, H.P., Watson, J.E.M., 2020. Estimating the benefit of well-managed protected areas for threatened species conservation. Oryx 54, 276–284.
|
|
Khadka, K.K., Pandey, R., 2014. Changes in the distribution of Lesser Adjutant Storks (Leptoptilos javanicus) in South and Southeast Asia: a plausible evidnce of global climate change and land-use change effect. Int. J. Zool. Res. 10, 9–14.
|
|
Koju, R., Maharjan, B., Gosai, K.R., Kittur, S., Sundar, K.S.G., 2019. Ciconiiformes nesting on trees in cereal-dominated farmlands: importance of scattered trees for heronries in lowland Nepal. Waterbirds 42, 355–365.
|
Lamsal, P., Kumar, L., Atreya, K., Pant, K.P., 2017. Vulnerability and impacts of climate change on forest and freshwater wetland ecosystems in Nepal: a review. Ambio 46, 915–930.
|
Langham, G.M., Schuetz, J.G., Distler, T., Soykan, C.U., Wilsey, C., 2015. Conservation status of North American birds in the face of future climate change. PLoS One 10, e0135350.
|
Li, M., Zhou, H., Bai, J., Zhang, T., Liu, Y., Ran, J., 2022. Distribution of breeding population and predicting future habitat under climate change of Black-Necked Crane ( Grus nigricollis Przevalski, 1876) in Shaluli Mountains. Animals 12, 2594.
|
Li, X., Chen, G., Liu, X., Liang, X., Wang, S., Chen, Y., et al., 2017. A new global land-use and land-cover change product at a 1-km resolution for 2010 to 2100 based on human-environment interactions. Ann. Assoc. Am. Geogr. 107, 1040–1059.
|
Liu, C., Berry, P.M., Dawson, T.P., Pearson, R.G., 2005. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28, 385–393.
|
Liu, C.Y., Gélin, U., He, R.C., Li, H., Quan, R.C., 2021. Flexible breeding performance under unstable climatic conditions in a tropical passerine in Southwest China. Zool. Res. 42, 221–226.
|
Lobo, J.M., Jiménez-valverde, A., Real, R., 2008. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 17, 145–151.
|
|
LRMP, 1986. Land Resources Mapping Project. Kenting Earth Sciences, HMGN and Government of Canada, Land Resources Mapping Project, Kathmandu.
|
Marmion, M., Parviainen, M., Luoto, M., Heikkinen, R.K., Thuiller, W., 2009. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 15, 59–69.
|
Mishra, V., Kumar, D., Ganguly, A.R., Sanjay, J., Mujumdar, M., Krishnan, R., et al., 2014. Reliability of regional and global climate models to simulate precipitation extremes over India. J. Geophys. Res. Atmos. 119, 9301–9323.
|
Mitra, A., Chatterjee, C., Mandal, F.B., 2011. Synthetic chemical pesticides and their effects on birds. Res. J. Environ. Toxicol. 5, 81–96.
|
Moradi, S., Sheykhi Ilanloo, S., Kafash, A., Yousefi, M., 2019. Identifying high-priority conservation areas for avian biodiversity using species distribution modeling. Ecol. Indicat. 97, 159–164.
|
Naimi, B., Araújo, M.B., 2016. Sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39, 368–375.
|
Naimi, B., Hamm, N.A.S., Groen, T.A., Skidmore, A.K., Toxopeus, A.G., 2014. Where is positional uncertainty a problem for species distribution modelling? Ecography 37, 191–203.
|
Oppel, S., Meirinho, A., Ramírez, I., Gardner, B., O'Connell, A.F., Miller, P.I., et al., 2012. Comparison of five modelling techniques to predict the spatial distribution and abundance of seabirds. Biol. Conserv. 156, 94–104.
|
Pang, R.H., Yu, T.L., Busam, M., 2019. Low breeding success of the little egret ( Egretta garzetta) near residential areas and in colonies exposed to gales: a comparison of colony in Sichuan, Southwest China, with literature. Anim. Cell. Syst. 23, 235–240.
|
Pant, G., Maraseni, T., Apan, A., Allen, B.L., 2021. Predicted declines in suitable habitat for greater one-horned rhinoceros ( Rhinoceros unicornis) under future climate and land use change scenarios. Ecol. Evol. 11, 18288–18304.
|
R Core Team, 2021. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. .
|
|
Ramamohan, H., Rao, K.K., 2017. Breeding Success and Mortality Rates in the Spot-Billed Pelican (Pelecanus philippensis), at Telineelapuram Bird Protected Areas, (An IBA Site; in 229) Srikakulam District, Andra Pradesh, India, vol. 143. Indian For, pp. 1064–1070.
|
Riahi, K., van Vuuren, D.P., Kriegler, E., Edmonds, J., O'Neill, B.C., Fujimori, S., et al., 2017. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Global Environ. Change 42, 153–168.
|
Rimal, B., Sloan, S., Keshtkar, H., Sharma, R., Rijal, S., Shrestha, U.B., 2020. Patterns of historical and future urban expansion in Nepal. Rem. Sens. 12, 628.
|
|
Riordan, E.C., Montalvo, A.M., Beyers, J.L., 2018. Using species distribution models with climate change scenarios to aid ecological restoration decisionmaking for southern California shrublands. In: Research Paper—Pacific Southwest Research Station. USDA Forest Service. PSW-RP-270.
|
Sagi, O., Rokach, L., 2018. Ensemble learning: a survey. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 8, e1249.
|
Santangeli, A., Lehikoinen, A., Bock, A., Peltonen-Sainio, P., Jauhiainen, L., Girardello, M., et al., 2018. Stronger response of farmland birds than farmers to climate change leads to the emergence of an ecological trap. Biol. Conserv. 217, 166–172.
|
Sharma, H.P., Rimal, B., Zhang, M., Sharma, S., Poudyal, L.P., Maharjan, S., et al., 2020. Potential distribution of the critically endangered Chinese pangolin ( Manis pentadactyla) in different land covers of Nepal: implications for conservation. Sustainability 12, 1282.
|
Sharma, S., 2006. Population status and distribution of Lesser Adjutant ( Leptoptilos javanicus) in far-western lowland Nepal. Tigerpaper 33, 9–11.
|
Sundar, K.S.G., 2011. Agricultural intensification, rainfall patterns, and large waterbird breeding success in the extensively cultivated landscape of Uttar Pradesh, India. Biol. Conserv. 144, 3055–3063.
|
|
Sundar, K.S.G., Koju, R., Maharjan, B., Marcot Bruce, G., Kittur, S., Gosai, K.R., 2019. First assessment of factors affecting the breeding success of two stork species in lowland Nepal using Bayesian network models. Wildfowl 69, 45–69.
|
Sundar, K.S.G., Maharjan, B., Koju, R., Kittur, S., Gosai, K.R., 2016. Factors affecting provisioning times of two stork species in lowland Nepal. Waterbirds 39, 365–374.
|
Sundar, K.S.G., Yaseen, M., Kathju, K., 2018. The role of artificial habitats and rainfall patterns in the unseasonal nesting of Sarus Cranes ( Antigone antigone) in South Asia. Waterbirds 41, 80–86.
|
Tatebe, H., Ogura, T., Nitta, T., Komuro, Y., Ogochi, K., Takemura, T., et al., 2019. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. (GMD) 12, 2727–2765.
|
Tehrani, N., Naimi, B., Jaboyedoff, M., 2020. Toward community predictions: multi-scale modelling of mountain breeding birds' habitat suitability, landscape preferences, and environmental drivers. Ecol. Evol. 10, 5544–5557.
|
Thuiller, W., Lafourcade, B., Engler, R., Araújo, M.B., 2009. BIOMOD: a platform for ensemble forecasting of species distributions. Ecography 32, 369–373.
|
|
Trautmann, S., 2018. Climate change impacts on bird species. In: Tietze, D.T. (Ed.), Bird Species, How They Arise, Modify and Vanish. Springer Nature, Switzerland, pp. 217–234.
|
|
Wagle, Y., Bhattarai, B.P., Adhikari, J.N., 2022. Factors influencing distribution and habitat utilisation of Leptoptilos javanicus in and around Barandabhar Corridor Forest, Chitwan, Nepal. Nat. Conserv. Res. 7, 19–26.
|
Walther, G., Post, E., Convey, P., Menzel, A., Parmesank, C., Beebee, T.J.C., et al., 2002. Ecological response to recent climate cnahge. Nature 416, 389–395.
|
Wiens, J.J., 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14, e2001104.
|
Yang, B., Qin, S., Xu, W., Busch, J., Yang, X., Gu, X., et al., 2020. Gap analysis of Giant Panda conservation as an example for planning China's National Park System. Curr. Biol. 30, 1287–1291.
|



 DownLoad:
DownLoad:






 Email Alerts
Email Alerts RSS Feeds
RSS Feeds