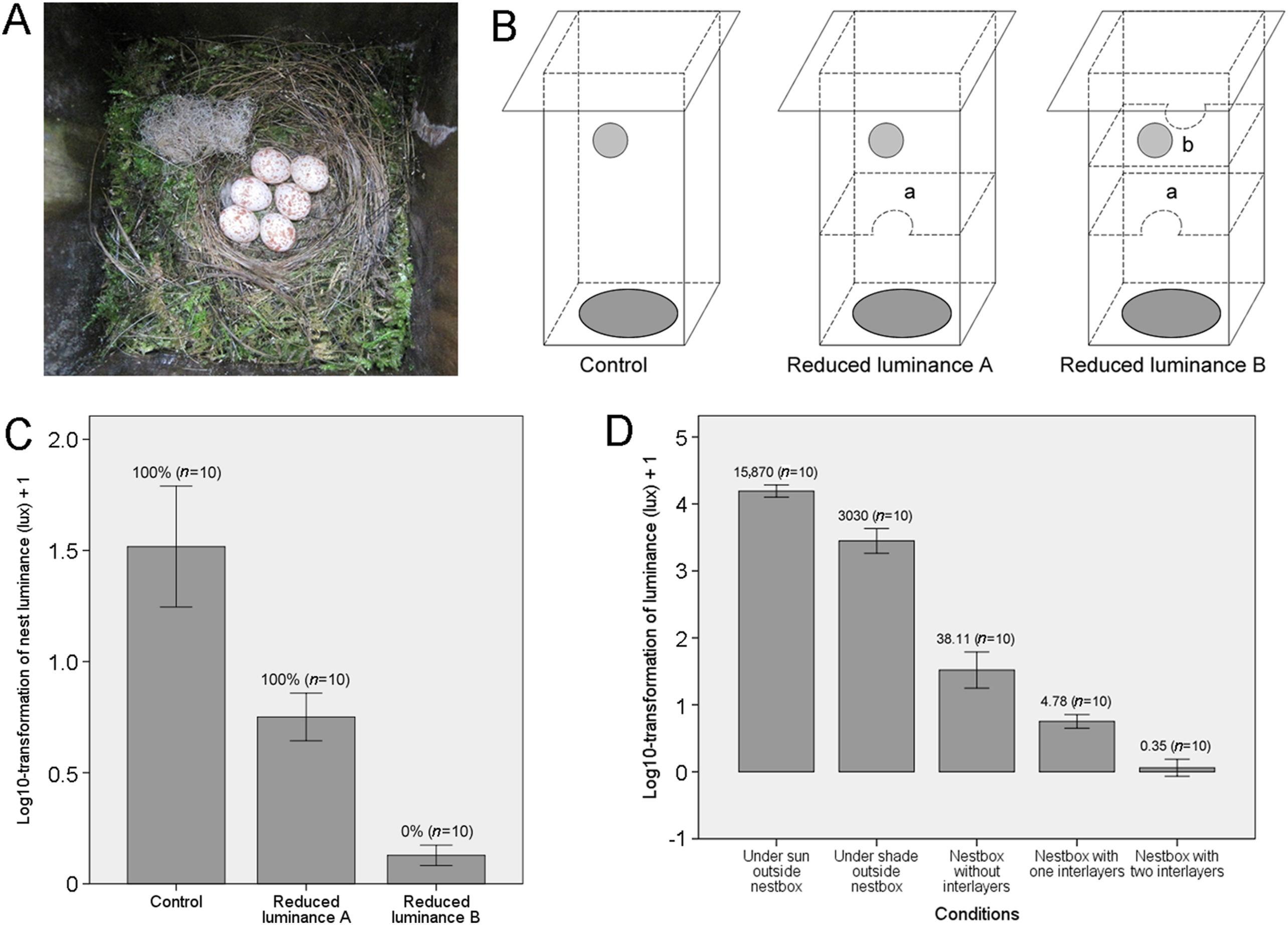
| Citation: | Canchao Yang, Anders Pape Møller, Wei Liang. 2022: Light matters: Nest illumination alters egg rejection behavior in a cavity-nesting bird. Avian Research, 13(1): 100016. DOI: 10.1016/j.avrs.2022.100016 |
Egg discrimination by cavity-nesting birds that build nests under dim light conditions was presumed to depend on nest luminance, although this hypothesis has rarely been tested. Tests of egg discrimination ability by cavity-nesting tits under dim light conditions may reveal the selection pressure from brood parasitism that they encounter under natural interactions. We manipulated the intensity of luminance of nests of the Green-backed Tit (Parus monticolus), a potential cuckoo host that possesses a strong discrimination ability of non-mimetic foreign eggs. We performed experiments to test their egg discrimination ability under different light conditions. Our results showed that Green-backed Tits discriminate against non-mimetic foreign eggs under normal light conditions in nest boxes, and this ability persisted at nest luminance as low as 4.78 ± 1.31 lux that is several times lower than normal luminance (38.11 ± 24.02 lux). However, egg discrimination by Green-backed Tits disappeared when nest luminance was reduced to a minimum of 0.35 ± 0.15 lux. The latter value represents total darkness for humans. The present study shows that nest luminance plays a key role in egg discrimination by Green-backed Tits that build nests under dim light conditions. This study provides strong experimental evidence for nest illumination altering egg rejection behavior in cavity-nesting birds.
In the well-known and intricate arms race between parasitic cuckoos and their hosts, the high fitness costs of parasitism (Lyu and Liang, 2021) has selected for several methods of anti-parasitic behavior (Rothstein and Robinson, 1998; Soler, 2017). Furthermore, egg discrimination is an effective defence against parasitism if hosts have evolved recognition and the ability to reject cuckoo eggs (Davies, 2000). Most host species adopt egg coloration as a clue to recognition and rejection (e.g., Spottiswoode and Stevens, 2010; Yang et al., 2010; Hanley et al., 2017, 2019; Luro et al., 2018), except for a few cases of egg coloration based on egg size or shape (Marchetti, 2000; Stokke et al., 2010).
Dim light conditions may constrain both the evolution of cavity-nesting and nest sites selection by cavity-nesting birds because some nest cavities may be too dark to be usable while some species may not have sufficient visual sensitivity to utilize them (Maziarz and Wesołowski, 2014). In that case, egg discrimination was presumed to depend on nest luminance (Avilés et al., 2008). Theoretical physiological models also predict a higher relative relevance of achromatic over chromatic discrimination mechanisms at dim light conditions (Vorobyev and Osorio, 1998). In addition, the nest illumination hypothesis states that detection of color differences is worse under dim light (Cherry and Bennett, 2001; Honza et al., 2014). However, empirical studies have rarely tested these hypotheses in cavity-nesting birds (but see Peer et al., 2006; Avilés et al., 2011; Medina and Langmore, 2019). One notable cuckoo-host system is brood parasitism of the Redstart (Phoenicurus phoenicurus). Redstarts are the only regular cuckoo host that breeds in cavities; other regular cuckoo hosts are open-cup nesting species where the cuckoo lays directly into the nest cup (Thomson et al., 2016). This may be due to the fact that many cavity-nesting birds are not used in egg discrimination experiments because they are not utilized by brood parasites such as Common Cuckoos (Cuculus canorus) (Davies, 2000). These patterns are clear in Europe because the cuckoos are generally too large to access the host clutches by small entrances, but they become more complex in Asia because of a high diversity of Asian cuckoo species that vary from large (e.g., Large Hawk Cuckoo Hierococcyx sparverioides, egg size: 27.0–30.4 × 20.6–21.8 mm) over medium (e.g., Himalayan Cuckoo C. saturatus, egg size: 20.6–23.4 × 13.5–16.1 mm) to small brood parasite eggs (e.g., Asian Emerald Cuckoo Chrysococcyx maculatus, egg size: 17.0–17.2 × 12.4–12.8 mm) (Yang et al., 2012). Cuckoos that lay small eggs are also small in body size, which favors them to access the host clutches in cavities for parasitism. Therefore, cavity-nesting birds in Asia cannot escape from cuckoo parasitism by building nests in holes. Although cavity-nesting tits (Paridae) have been regarded as a text-book example of unsuitable cuckoo hosts (Davies, 2000; but see Grim et al., 2014), recent studies in Europe and Asia have shown that cavity-nesting birds in Asia, including Great Tit (Parus major) and Green-backed Tit (P. monticolus), have strong discrimination ability to reject non-mimetic eggs (Liang et al., 2016; Yang et al., 2019; Liu et al., 2020). Subsequently, reviews and cross-foster experiments by Grim et al. (2014) confirmed that Paridae tits are very successful at raising cuckoo chicks and even better than those raised by common hosts. The context in Asia hence provides an optimal opportunity to study egg discrimination related to dim light conditions of host nests. The frequency of cuckoo parasitism in natural nests of Paridae tits remains a mystery. Their current parasitism rate has been considerably underestimated due to widespread use of artificial nest boxes with small entrances. Therefore, testing the egg discrimination of Paridae tits under dim light conditions allows quantification of the selection pressure due to brood parasitism that they encounter in nature. Here we manipulated the luminance intensity of Green-backed Tit nests, in which we subsequently performed parasitism experiments to test their egg discrimination ability. The aim of this study was to elucidate the effect of light conditions on egg discrimination, and this study thus tests whether potential cuckoo hosts can discriminate against cuckoo eggs.
This study was carried out from April to August 2009 in Kuankuoshui Nature Reserve (28°10ʹ N, 107°10ʹ E), a subtropical forest area in Southwest China, where multiple cuckoo species varying in size from large to small coexist and breed sympatrically (Yang et al., 2010, 2019). The Green-backed Tit is a sister species of Great Tit that is distributed and breeds from Southwest China to Nepal and on Taiwan Island of China (MacKinnon and Phillipps, 1999). It looks very similar to the Great Tit with minor differences in wing and plumage color (Yang et al., 2019). To the best of our knowledge, the Green-backed Tit has not been found to be utilized by any parasite species.
We provided 100 nest boxes for the Green-backed Tit during the breeding season (April to August) and checked them regularly to confirm nest building in progress. For interlayer manipulation, we used a relative deep nest box (made of 2 cm wideness pine, 35 cm × 11 cm × 15 cm in size, with a hole entrance of ca 4.0 cm in diameter; see Yang et al., 2019 for more details). The hole entrance provided the only source of light. Parasitism experiments were conducted on the day following clutch completion, or during the early incubation period (Fig. 1A), by randomly painting one egg blue (i.e., non-mimetic to tit eggs that are white with spots, see Liang et al., 2016). Following the parasitism experiment, active nests were randomly treated with the following procedure: (1) reduced luminance A (n = 10), in which an interlayer with entrance was fitted into the nest boxes to reduce nest luminance, in this case the host nests were located below one interlayer and on the bottom of nest boxes (Fig. 1B); (2) reduced luminance B (n = 10), in which two interlayers with entrance were fitted into the nest boxes to further reduce nest luminance, in this case the host nests were located below two interlayers and on the bottom of nest boxes (Fig. 1B); and (3) luminance control (n = 10), in which no interlayer was fitted into the nest boxes. The interlayer was added after clutch completion to avoid nest desertion and ensure that the host nests were built at the bottom of nest boxes. The second interlayer did not obstruct egg removal. No desertion was detected after interlayer manipulation. Nests with artificial parasitism were monitored for six days on a daily basis and classified into (1) acceptance if foreign eggs were incubated or kept warm, or (2) rejection if foreign eggs were ejected, buried or deserted. Additionally, control nests in which clutches were visited without manipulation were also conducted to quantify any disturbance effect. No rejection or desertion behavior was detected in this control group (n = 10). The clutch size was 7.13 ± 1.49 (mean ± SD, range: 4–10, n = 40), with no significant differences between the experimental and control groups (F = 0.180, df = 3, P = 0.909, ANOVA). Luminance was measured by using an ST-80C illuminometer (lux as unit; Photoelectric Instrument Factory of Beijing Normal University, China). Although nest illuminance is impossible to be invariant in a day, we chose the time at noon (11:30–12:30) for luminance measurement so that they are comparable between experimental groups.
Statistical analysis was performed using SPSS v.20.0 for Windows (IBM, Armonk, NY, USA). Either χ2 test or Fisher's exact test was used to compare the rejection rate of foreign eggs while Welch's ANOVA was used for comparison of luminance. All values presented as means ± SD.
For reduced luminance A, light intensity in nest boxes was reduced to 4.78 ± 1.31 lux whilst for reduced luminance B, this value was reduced to 0.35 ± 0.15 lux. These values were significantly lower than the luminance of control nest boxes (i.e., luminance control; 38.11 ± 24.02 lux; F2,12.2 = 65.35, P < 0.000001, Welch's ANOVA).
Parasitism experiments showed that Green-backed Tits reject 100% of foreign eggs in both normal luminance (n = 10) and reduced luminance A (n = 10) in nest boxes (Fig. 1C). Furthermore, all cases of rejection were by ejection. However, for reduced luminance B, light intensity was reduced to near zero lux at which the host accepted all foreign eggs (n = 10). A likelihood ratio test showed that χ2 = 38.19, df = 2, P < 0.0001. However, since rejection rate of foreign eggs was either 100% or 0%, χ2 test or Fisher's exact test was suspect because more than 20% of cells had expected counts less than five. A Kendall rank order correlation showed z = −0.87, P < 0.0001.
Cavity- or dome-nesting birds are presumed to rely strongly on nest luminance to identify parasitic eggs because birds are known to use brightness in discriminatory contexts, including egg discrimination (Avilés et al., 2008). This hypothesis of luminance-based egg discrimination was tested using different approaches in recent years. Langmore et al. (2009) combined a phylogenetic analysis and a model of avian visual processing to test this hypothesis, explaining why some bronze-cuckoo species (Chalcites spp.) and their hosts have pursued an alternative coevolutionary trajectory, resulting in the evolution of cryptic cuckoo eggs. Antonov et al. (2011) quantified host-model egg similarity and compared egg rejection predicted by a discrimination visual model with the observed rejection pattern in Nightingales (Luscinia megarhynchos). They found that blue and white eggs, which had poorer achromatic matching with host eggs, suffered a higher rejection rate than black and green eggs, which had better achromatic matching. Furthermore, greener and bluer eggs were less likely to be rejected than eggs that are brown (Dainson et al., 2017; Hanley et al., 2017). Here we used model egg of only one color to avoid any potential effects caused by color difference. According to our results, Green-backed Tits have a strong discrimination ability of non-mimetic foreign eggs under normal light conditions of nest boxes. This ability persisted when nest luminance was reduced to 4.78 ± 1.31 lux that is several times lower than control luminance (38.11 ± 24.02 lux). However, the egg discrimination of Green-backed Tits disappeared when nest luminance was reduced to 0.35 ± 0.15 lux, which is total darkness according to human vision.
Our findings do not correspond with previous studies of the Great Reed Warbler (Acrocephalus arundinaceus) (Honza et al., 2011) and the European Magpie (Pica pica) (Avilés et al., 2015), which found no correlation between increased ambient light levels and egg rejection rate and, the Red Bishop (Euplectex orix) (Honza et al., 2014) and Yellow-rumped Thornbill (Acanthiza chrsyorrhoa) (Medina and Langmore, 2019). These species have high variation in luminosity within nests, which is not related to their egg rejection ability. Although Green-backed Tits lost their egg discrimination ability under near zero luminance, we argue that they possess a strong ability to recognize and reject foreign eggs under dim light conditions. This can be illustrated by comparing the luminance among different conditions shown in Fig. 1D. The luminance under sun or shade can reach 20,000 or 5000 lux, respectively. The normal luminance of nest boxes was only 38.11 lux on average and the reduced luminance B was more than 100 times lower than that (Fig. 1D). Therefore, light conditions did not constrain the ability of egg discrimination for Green-backed Tits unless luminance almost reached zero. Previous studies have found that the luminance of nest site selected by cavity-nesting birds such as Great Tit and Marsh Tit (Poecile palustris) can be extremely low (Wesołowski and Maziarz, 2012; Maziarz et al., 2015; Podkowa and Surmacki, 2017). This implies that such situation may also exist in the selection of natural nest-site by Green-backed Tits. Avilés et al. (2011) conducted egg discrimination experiments and found that light intensity did not limit egg detectability in Spotless Starlings (Sturnus unicolor). Here we have shown that detectability can be considerably reduced only if light intensity was reduced to near zero lux. This indicates that the risk of cuckoo parasitism for tits may be considerably underestimated because most studies of tits have used artificial nest boxes with small entrances that are much smaller than those in natural cavities (Grim et al., 2014). Furthermore, unlike situation in Europe, cavity-nesting birds in Asia cannot escape from parasitism (Yang et al., 2012) even if they build nests in very small holes because cuckoos that are small in body size and lay small eggs are abundant in Asia. Additionally, nest light environment may be related to parasitism risk that brighter nests suffered a higher risk of parasitism (Muñoz et al., 2007). Paridae tits may prefer to breed in holes with small entrances as an anti-parasitism or anti-predatory adaptation against cuckoos, predators or both. These alternatives need more investigation. Light conditions may vary extensively among natural cavities with different entrance sizes with smaller entrances creating the most reduced light conditions (Maziarz and Wesołowski, 2014). Although some previous studies have shown that egg discrimination is light-dependent (e.g., Antonov et al., 2011; Avilés et al., 2011), Green-backed Tits were able to recognize foreign eggs even under very dark conditions. UV (ultraviolet) was proposed to play a functional role in egg detectability under dim light condition (Avilés et al., 2006), and this was supported by some empirical studies (Honza and Polaćiková, 2008; Yang et al., 2013; but see Underwood and Sealy, 2008; Węgrzyn et al., 2011). Therefore, it is possible that the Green-backed Tits used UV, or other factors, e.g. olfaction (Golüke et al., 2016), to detect and reject foreign eggs under such light condition. The persistence of acute discrimination ability of foreign eggs under dim light conditions in Green-backed Tits provides evidence that they are currently under selection from brood parasitism in nature. However, this hypothesis requires further tests. Finally, the Green-backed Tits rejected poor mimetic and highly mimetic eggs by different frequency (Yang et al., 2019), therefore, the egg mimicry may interact with light condition to affect egg recognition that higher mimetic eggs may be harder to detect under dim light condition. Further studies are needed to confirm such assumption.
In this experimental study, we have shown that cavity-nesting Green-backed Tits have a strong discrimination ability against non-mimetic foreign eggs under normal light conditions in nest boxes. Such egg rejection persisted when nest luminance was reduced to the level that is several times lower than normal luminance. However, the egg discrimination of Green-backed Tits disappeared when nest luminance was reduced to the level (0.35 ± 0.15 lux), which is total darkness according to human vision. Therefore, our study provides strong experimental evidence that nest light condition can alter egg rejection behavior in cavity-nesting birds.
WL and CY designed the study; CY performed field experiments; CY carried out statistical analyses and wrote the first draft of the manuscript, and WL and APM helped improve the manuscript. All authors read and approved the final submission.
The data that supports the findings of this study are available from the corresponding author on reasonable request.
The experiments reported here comply with the current laws of China. Fieldwork was carried out under permission from Kuankuoshui National Nature Reserve. Experimental procedures were in agreement with the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University (permit no. HNECEE-2011-001).
The authors declare that they have no competing interests.
We would like to thank Kuankuoshui National Nature Reserve for their help and cooperation, and Yan Cai for her assistance during fieldwork. This work was supported by the Hainan Provincial Natural Science Foundation of China (No. 320CXTD437 and 2019RC189 to CY) and the National Natural Science Foundation of China (Nos. 31772453 and 31970427 to WL).
|
Davies, N.B., 2000. Cuckoos, Cowbirds and Other Cheats. T & AD Poyser, London.
|
|
Grim T, Samas P, Prochazka P, Rutila J. Are tits really unsuitable hosts for the common cuckoo? Ornis Fennica. 2014; 91: 166−177
|
|
Medina I, Langmore NE. Nest illumination and the evolution of egg rejection in hosts ofbrood parasites. Auk. 2019; 136: 1−6
|
|
Rothstein, S.I., Robinson, S.K., 1998. Parasitic Birds and Their Hosts: Studies in Coevolution. Oxford Univ Press, New York.
|
|
Soler, M., 2017. Avian Brood Parasitism: Behaviour, Ecology, Evolution and Coevolution. Springer International Publishing AG, Chambridge.
|