| Citation: | Ruiping Xu, Canwen Yu, Liyao Mao, Mengchen Jiang, Luyao Gao, Ming Li, Jinsong Liu. 2022: Antioxidant defense mechanisms and fatty acid catabolism in Red-billed Leiothrix (Leiothrix lutea) exposed to high temperatures. Avian Research, 13(1): 100013. DOI: 10.1016/j.avrs.2022.100013 |
Extreme hot weather is occurring more frequently due to global warming, posing a significant threat to species survival. Birds in particular are more likely to overheat in hot weather because they have a higher body temperature. This study used a heat stress model to investigate the antioxidant defense mechanisms and changes in fatty acid catabolism in Red-billed Leiothrix (Leiothrix lutea) to gain an understanding of how birds adapt to high temperatures. The birds were divided into five groups: a control group (30 ℃ for 0 days), 1 D group (40 ℃ for 1 day), 3 D group (40 ℃ for 3 days), 14 D group (40 ℃ for 14 days) and recovery group (40 ℃ for 14 days, then 30 ℃ for 14 days). Our results indicated that when Red-billed Leiothrix are subjected to heat stress, malondialdehyde (MDA) content in the liver significantly increased, as did the enzyme activities of catalase (CAT), glutathione–SH–peroxidase (GSH-PX) and total antioxidant capacity (T-AOC) in the liver. Furthermore, there was a significant increase in heat shock protein 70 (HSP70) expression in the liver, while avian uncoupling protein (avUCP) expression in muscle was significantly reduced. Additionally, there was a significant reduction in fatty acid catabolism enzyme activity such as 3-hydroxyacyl-CoAdehydrogenase (HOAD) activity in the heart, and carnitine palmitoyl transferase 1 (CPT-1) and citrate synthase (CS) activity in the heart and liver. Furthermore, fatty acid translocase (FAT/CD36) in the heart, heart-type fatty acid binding protein (H-FABP) and fatty acid binding protein (FABP-pm) in the liver and heart were also significantly decreased. These changes reverted after treatment, but not to the same level as the control group. Our results indicated that when Red-billed Leiothrix are exposed to heat stress their internal antioxidant defense system is activated to counteract the damage caused by high temperatures. However, even with high antioxidant levels, prolonged high temperature exposure still caused some degree of oxidative damage possibly requiring a longer recovery time. Additionally, Red-billed Leiothrix may be able to resist heat stress by reducing fatty acid transport and catabolism.
Global warming is a threat to all forms of life on earth (Warren et al., 2018), and the frequency and severity of heatwaves is predicted to intensify in the coming decades (Stillman, 2019). Intense heat waves have already been shown to exceed species’ physiological heat tolerance limits, resulting in mass die-off events (McKechnie et al., 2012; Diffenbaugh and Field, 2013). Avian species are particularly vulnerable to heat, as they have the highest body temperature and metabolic rates of all endothermic vertebrates (Nawab et al., 2018; Riddell et al., 2021). These characteristics suggest that birds living in hot climates are subjected to immense environmental and endogenous heat loads. Heat stress occurs in homeothermic animals when the body cannot get rid of excess heat (Hulbert et al., 2007; Akbarian et al., 2016).
Heat stress contributes to oxidative stress at the cellular level by affecting mitochondrial function (Farag and Alagawany, 2018; Castiglione et al., 2020). As ambient temperature increases, animal metabolism, and hence the oxidative phosphorylation process of mitochondria accelerates, resulting in an increase in reactive oxygen species (ROS) over time (Akbarian et al., 2016). Normal cellular metabolism produces small amounts of free radicals and ROS (Beckman and Ames, 1998), while high levels of ROS are thought to raise mortality rates by increasing oxidative damage to macromolecules such as malondialdehyde (MDA) (Matés et al., 1999; Musatov et al., 2002; Zhou et al., 2015; Rehaman et al., 2017). Because ROS are constantly produced during aerobic respiration, organisms utilize a sophisticated suite of antioxidants and have evolved antioxidant scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione–SH–peroxidase (GSH-PX), which convert ROS into less reactive species (Surai et al., 2019; Jimenez et al., 2019). Therefore, the wild birds may improve their survival chances by up-regulating their antioxidant defense against oxidative stress under high temperatures.
Excess ROS levels in animals are buffered or prevented by cellular adaptations, preserving mitochondrial function and allowing tissues to adapt to heat stress (Del Vesco and Gasparino, 2013). For example, heat shock proteins (HSPs) are commonly used as a stress-sensitive molecular marker to analyze the response of organisms to environmental changes, especially high temperature (Feder and Hofmann, 1999). Their biological role is to protect essential cellular components from damage and maintain normal cell survival (Shan et al., 2020). In addition, uncoupling proteins (UCPs) are a transporter family of proteins found in the mitochondrial inner membrane that uncouple respiration from ATP synthesis by dissipating the mitochondrial proton gradient (Palmieri, 1994). Mammalian studies have found that when UCPs enter the mitochondrial membrane and are activated by fatty acids they dissipate the membrane proton gradient and reduce superoxide production (Berry et al., 2018). In birds, avUCP is thought to be involved in reducing mitochondrial oxidative stress levels through a mechanism similar to that of mammalian UCPs (Sotome et al., 2021). However, it is unclear if HSPs and avUCP play the important role in resistance to oxidative stress in birds exposed to high temperatures.
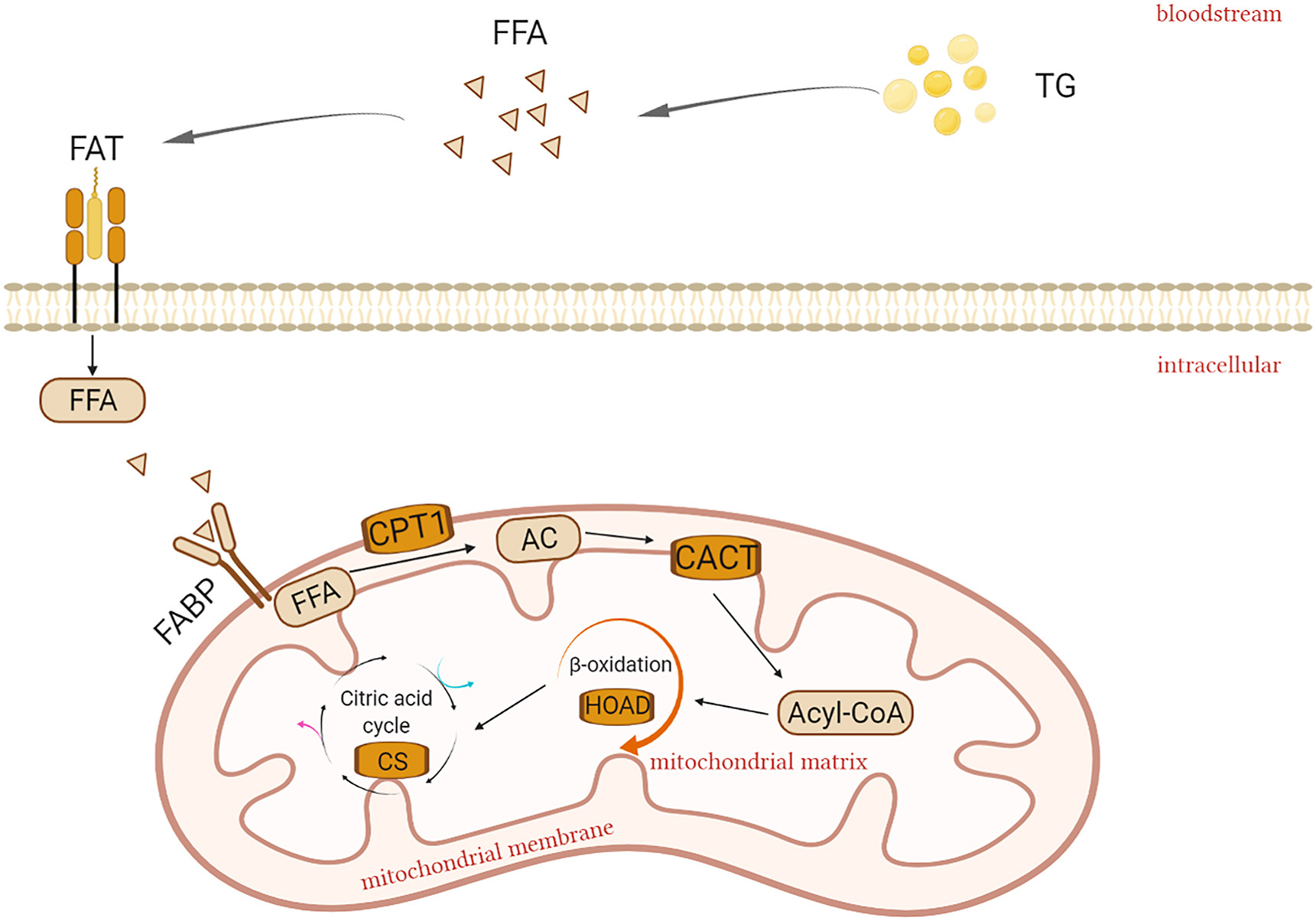
Lipid metabolism has been shown to be affected by heat stress (Mujahid et al., 2007), and ambient temperature-induced heat stress has been shown to reduce fat oxidation in different species (Vandana et al., 2021). Several studies have demonstrated reduced fatty acid catabolism during heat stress in rodents (Sanders et al., 2009), pigs (Pearce et al., 2013) and dairy cows (Shwartz et al., 2009). Reduced fatty acid catabolism appears to be an adaptation to limit heat generation in heat-stressed animals (Belhadj et al., 2016). Fatty acid transporters are thought to be key regulators of fatty acid uptake, determining the total rate of fatty acid entry into tissue cells, and therefore to some extent, influencing the mitochondrial β-oxidation (Heather et al., 2011). In general, the expression and content of fatty acid transporters are proportional to the catabolic intensity of fatty acids (Coburn et al., 2000). For example, studies in rats found that a reduction in fatty acid oxidation in the heart tissue was accompanied by downregulation of cardiac fatty acid transporters (Heather et al., 2006). The transport and catabolic processes of fatty acids are shown in Fig. 1. So how do small birds adjust fatty acid transport and catabolism to improve survival at high temperatures? Are there similarities between their metabolic regulation strategies and those of mammals?
Our research focuses on the Red-billed Leiothrix (Leiothrix lutea), a bird species native to Asia and widely distributed throughout central China, south of Xizang (Tibet) and the Yangtze River (Cui et al., 2019). This insectivorous species has a relatively low BMR, high body temperature and a narrow thermal neutral zone (TNZ) (Cui et al., 2019). Such physiological traits and feeding habits may limit its distribution to a narrow ecological zone where the climate is relatively warm and food resources abundant. Previous research has shown that when Red-billed Leiothrix were subjected to acute heat stress, their antioxidant capacity decreased and lipid peroxides increased, suggesting they can generate an oxidative stress response (Liu et al., 2015). However, it is unknown whether Red-billed Leiothrix can improve their antioxidant defense functions and adapt to high temperature stress over an extended period. Furthermore, it is unclear whether fatty acid transport and catabolism in Red-billed Leiothrix will be inhibited at high temperatures to avoid overheating. In order to answer the above two questions, we carried out this study. We hypothesized that Red-billed Leiothrix can actively enhance their antioxidant defenses and reduce fatty acid catabolism to prevent overheating in high temperatures and thus maintain survival.
We used mist nets to capture Red-billed Leiothrix in Wenzhou city, Zhejiang Province (27°29ʹ N, 120°51ʹ E, 14 m in elevation), China. The annual average temperature in Wenzhou is 17.9 ℃, and the average temperature in summer is 22–28 ℃ but temperatures up to 39 ℃ in summer have been recorded (Zheng et al., 2008).
Experiments were carried out in October 2017. Animals were transported to our laboratory and caged individually for four weeks (50 cm × 30 cm × 20 cm) under natural ambient temperature 30 ℃ and a 12:12-h light-dark photoperiod (lights on at 06:00) to adapt to the laboratory environment. Following the four weeks acclimatization period, 35 birds were randomly assigned to one of five groups: (1) control group (30 ℃, for 0 days); (2) 1 D group (40 ℃, for 1 day); (3) 3 D group (40 ℃, for 3 days); (4) 14 D group (40 ℃, for 14 days); (5) recovery group (40 ℃ for 14 days, then 30 ℃ for 14 days). The number of days is the length of time which they were at that temperature before being euthanized for data collection. Among them, data from the control, 14 D, and recovery groups were selected for body mass and fatty acid transport experiments. Data from all five groups were used for antioxidant defense experiments. At least six independent samples were selected from each group. Food and water were supplied ad libitum. All experimental procedures were approved by Wenzhou University's Animal Care and Use Committee.
Birds were euthanized by cervical bleeding. The liver was then quickly removed and stored in liquid nitrogen. Tissues were homogenized in ice-cold 0.9% NaCl solution, centrifuged at 3000 g for 15 min and the resultant supernatant saved for further analysis (Chen et al., 2014; Zhou et al., 2015). Malondialdehyde Kit (A003-1-2; Nanjing Jiancheng Bioengineering Institute, China) was selected to determine the MDA level as a marker of lipid peroxidation. MDA levels were analyzed as described by Mihara and Uchiyama (1978). Briefly, 0.5 mL of 10% homogenate was transferred into a 10 mL centrifuge tube, and 3 mL of 1% phosphoric acid and 1 mL of 0.6% TBA solution were added. The mixture was heated for 45 min on a boiling water bath. Once cool, 4 mL of n-butanol was added and mixed vigorously producing a pink complex (Mihara and Uchiyama, 1978). The OD value at 532 nm was measured immediately using a spectrophotometer and producing an absorption peak at 532 nm. Levels of tissue MDA were expressed as nmol/mg protein.
Commercial kits (Nanjing Jiancheng Bioengineering Institute, China) were used according to the manufacturer's instructions, to measure the activity of antioxidant enzymes, including SOD (A001-3-2), CAT (A007-1-1), GSH-PX (A005-1-2), and total antioxidant capacity (T-AOC) (A015-1-2). These particular assay kits were selected because of their high degree of sensitivity, specificity and inter-assay and intra-assay precision (Chen et al., 2014). Enzyme activity was defined as follows: one unit of SOD activity equaled the amount of enzyme that caused 50% inhibition of superoxide radical produced by the reaction between xanthine and xanthine oxidase at 37 ℃; one unit of CAT activity was the decomposition of 1 μmol H2O2 per min; one unit of GSH-PX activity was the amount of enzyme that oxidized 1 mmol/L of GSH in the reaction system at 37 ℃ per min; and one unit of T-AOC was the extent to which optical density increased by 0.01 per mg protein per min (Chen et al., 2014). Protein concentration was determined by the method described in the commercial kit (Nanjing Jiancheng Bioengineering Institute), using bicinchoninic acid (BCA) (A045-4-2) as a standard.
To determine the oxidative capacity of pectoralis muscles, liver and heart, three groups were selected in the experimental group to determine the maximal enzyme activities of carnitine palmitoyl transferase (CPT), 3-hydroxyacyl-CoAdehydrogenase (HOAD), and citrate synthase (CS). The CPT activity was assayed as Price et al. (2010). The reaction medium contained 50 mmol/L Tris buffer (pH 8.0), 5 mmol/L carnitine, 0.15 mmol/L 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), 0.035 mmol/L palmitoyl CoA, and 10 μL of homogenate diluted 1:10. Activity was calculated by measuring the change in absorbance at 412 nm, over 2 min after the addition of carnitine. The HOAD activity was assayed in pectoralis muscle according to Guglielmo (2010). The reaction medium contained 50 mmol/L imidazole buffer (pH 7.4), 0.2 mmol/L NADH, 1 mmol/L EDTA, 0.1 mmol/L acetoacetyl-CoA, and 10 μL of homogenate diluted 1:10. Absorbance was taken at 340 nm. For CS assays, we followed Banergee and Chaturvedi (2016). In brief, the reaction medium was prepared by adding 50 mmol/L Tris buffer (pH 8.0), 0.5 mmol/L oxaloacetic acid, 0.15 mmol/L DTNB, 0.3 mmol/L acetyl-CoA, and 10 μL of homogenate diluted 1:10. The increase in absorbance was measured at 412 nm. The liver and heart are treated the same way.
To quantify the heat shock protein 70 (HSP70), avian uncoupling proteins (avUCP), fatty acid translocase (FAT/CD36), fatty acid binding protein (FABP-pm), and heart-type fatty acid binding protein (H-FABP) mRNA expression of birds, total RNA was prepared from liver, pectoralis muscles and heart using TRIzol Reagent (TAKARA, Dalian, China) (Zhou et al., 2015). The whole process of the experiment was completed under the RNase-free condition. Following the methods previously used in Li et al. (2017), RNase-free water was used to resuspend the dried RNA pellets and the concentration and purity of total RNA was determined spectrophotometrically at 260/280 nm. First-strand cDNA was synthesized from 2 μg of total RNA using oligo (dT)15 primers and M-MLV Reverse Transcriptase according to the manufacturer's instructions (TAKARA, Dalian, China). Synthesized cDNA was diluted five times and stored at – 80 ℃ before use (Li et al., 2017). The qPCR was performed using Roche Light Cycler 480 real-time qPCR system (Forrentrasse CH-6343 Rotkreuz, Switzerland) (Tan et al., 2016). The sequence of primers used for the reaction is shown in Table 1. Reactions were performed in a 20 μL reaction mixture containing 10 μL of 2 × SYBR Premix EX Tag TM (TAKARA, Dalian, China), 2 μL of diluted cDNA, 0.4 μL of the forward and reverse primers, and 7.2 μL of PCR-grade water (Li et al., 2017). The resulting cDNA was divided into packages. An initial polymerase activation step at 95 ℃ for 60 s was followed by 40 amplification cycles (95 ℃ for 5 s, 55 ℃ for 30 s and 72 ℃ for 30 s) after which the reaction was terminated by the built-in melt curve (Zhou et al., 2015). The 2-△△Ct method was used to determine the relative quantity of mRNA expression based on the Ct values of the PCR products, which were expressed as the fold-change relative to the β-actin gene (Li et al., 2014; Osakabe et al., 2017).
| Genes | Accession number | Primer sequence (5′–3′) | Product length (bp) |
| HSP70 | XM_013807608.1 | Forward: CACGGCTGCTGCTATT | 178 |
| Reverse: TACGGTGTCAAAGTCCTC | |||
| avUCP | XM_019135200.1 | Forward: CGGTGGATGTGGTGAAGACG | 144 |
| Reverse: AGGAAGGASGGGACGAAGC | |||
| FAT/CD36 | XM_014883464.1 | Forward: CATACTGGGAAGGCCACTGT | 169 |
| Reverse: CTGTATCCGTGCAGAAGCAA | |||
| FABP-pm | XM_005517927.2 | Forward: GTGGAAGGAGTTGGCAGCTA | 128 |
| Reverse: GTGGAAGGAGTTGGCAGCTA | |||
| H-FABP | XM_015648987.1 | Forward: AAGACCCAGAGCACCTTCAA | 146 |
| Reverse: AACAGCGATGTCTCCTTCC | |||
| β-Actin | XM_014261104.1 | Forward: TGCGTGACATCAAGGAGAAG | 157 |
| Reverse: TGCCAGGGTACATTGTGGTA |
We used SPSS (version 20.0) for all our statistical analyses. We tested variables for normality using the Kolmogorov-Smirnov test, and non-normal data was log-transformed before being analyzed. A one-way ANOVA was used to compare data between groups and a Tukey's HSD post hoc test was used to detect significant differences. P-values < 0.05 were considered statistically significant.
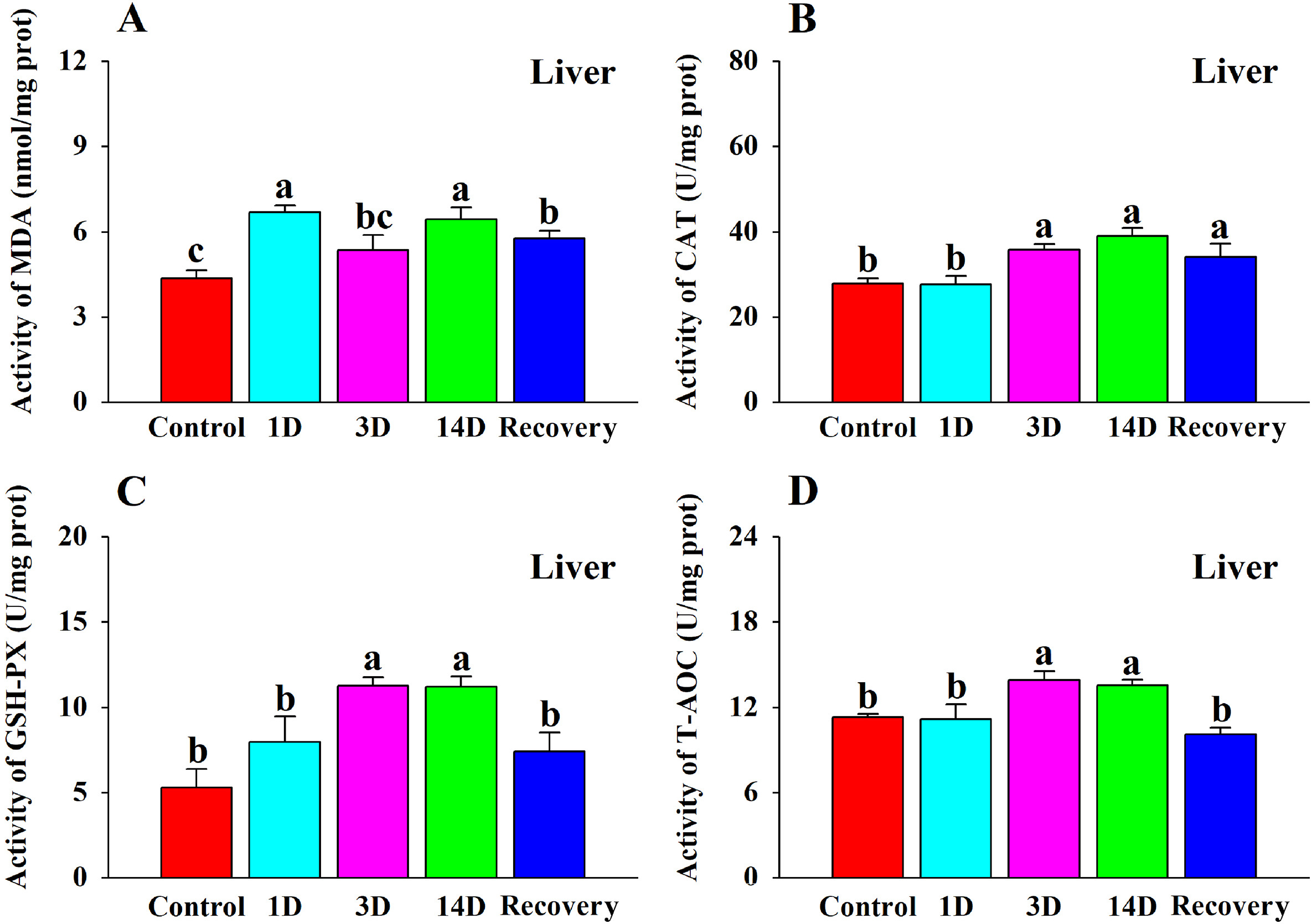
There was a significant change in MDA content following heat stress and recovery (Table 2; Fig. 2A). The 1 D and 14 D groups had significantly higher MDA levels compared to the control group. However, there was no significant difference in MDA content between the 3 D group and the control group. The MDA content in the recovery group declined but was still significantly higher than the control group.
| Type of variable | Variable | Tissue | df | F | P |
| Enzyme activity | MDA | Liver | 4, 30 | 19.963 | <0.001 |
| SOD | Liver | 4, 30 | 1.247 | 0.313 | |
| CAT | Liver | 4, 30 | 6.948 | 0.001 | |
| GSH-PX | Liver | 4, 30 | 6.803 | <0.001 | |
| T-AOC | Liver | 4, 30 | 6.835 | <0.001 | |
| CPT-1 | Liver | 2, 29 | 6.935 | 0.003 | |
| Heart | 2, 29 | 18.873 | <0.001 | ||
| Muscle | 2, 29 | 2.206 | 0.128 | ||
| CS | Liver | 2, 29 | 4.424 | 0.021 | |
| Heart | 2, 29 | 16.699 | <0.001 | ||
| Muscle | 2, 29 | 1.151 | 0.330 | ||
| HOAD | Liver | 2, 29 | 2.216 | 0.127 | |
| Heart | 2, 29 | 35.176 | <0.001 | ||
| Muscle | 2, 29 | 0.933 | 0.445 | ||
| Expression of mRNA | HSP70 | Liver | 4, 30 | 4.939 | 0.004 |
| avUCP | Muscle | 4, 30 | 19.963 | <0.001 | |
| FAT/CD36 | Liver | 2, 29 | 0.994 | 0.383 | |
| Heart | 2, 29 | 4.516 | 0.020 | ||
| Muscle | 2, 29 | 0.732 | 0.490 | ||
| FABP-pm | Liver | 2, 29 | 4.054 | 0.028 | |
| Heart | 2, 29 | 7.008 | 0.003 | ||
| Muscle | 2, 29 | 0.232 | 0.794 | ||
| H-FABP | Heart | 2, 29 | 3.804 | 0.035 |
Except SOD (Table 2), CAT, GSH-PX, and T-AOC all increased significantly following heat stress (Table 2; Fig. 2B, C, D). CAT activity was significantly higher in the 3 D, 14 D and recovery groups compared to the control group as heat stress time increased. GSH-PX activity was significantly higher in the 3 D and 14 D groups compared with the control group. There was no significant difference between the 1 D group and the control group. The level of T-AOC in the 3 D and 14 D groups was significantly higher than that in the control group. There was no significant difference in T-AOC activity between the recovery group and the control group.
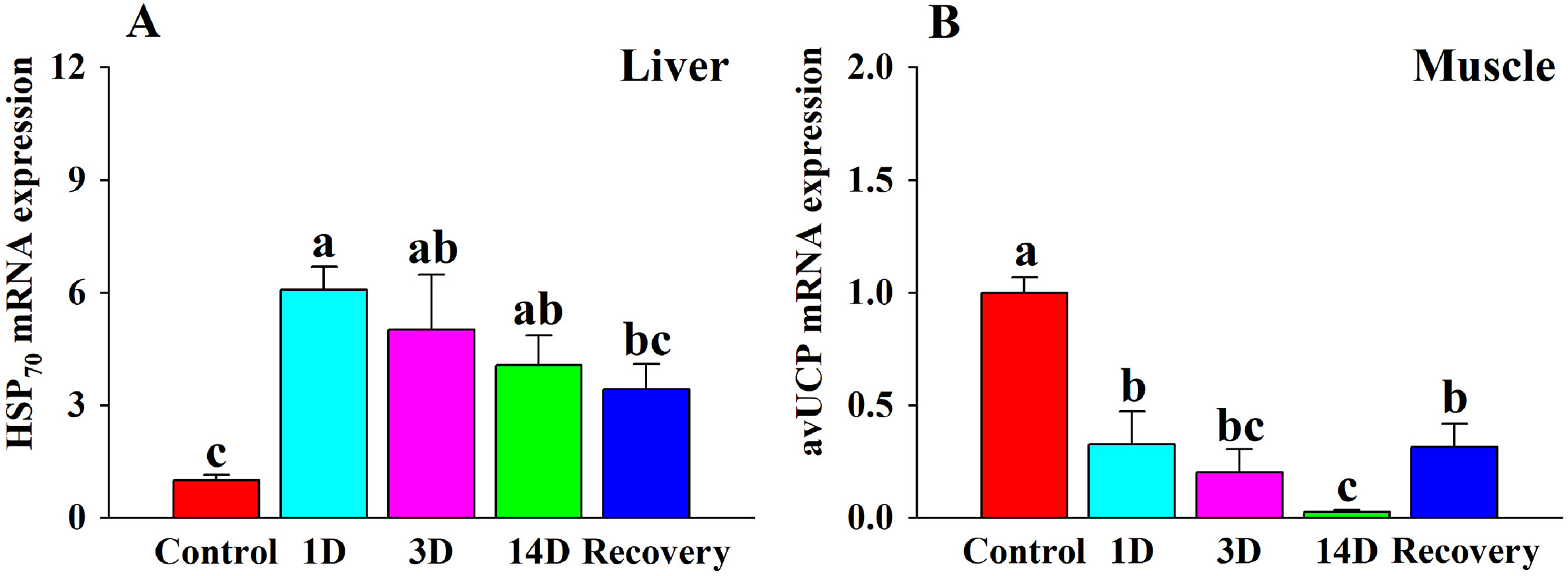
The expression of HSP70 mRNA during heat stress was significantly higher in the 1 D, 3 D and 14 D groups compared to the control group (Table 2; Fig. 3A). The expression of avUCP was significantly lower in the 1 D, 3 D, 14 D and recovery groups (Table 2; Fig. 3B) compared with the control group.
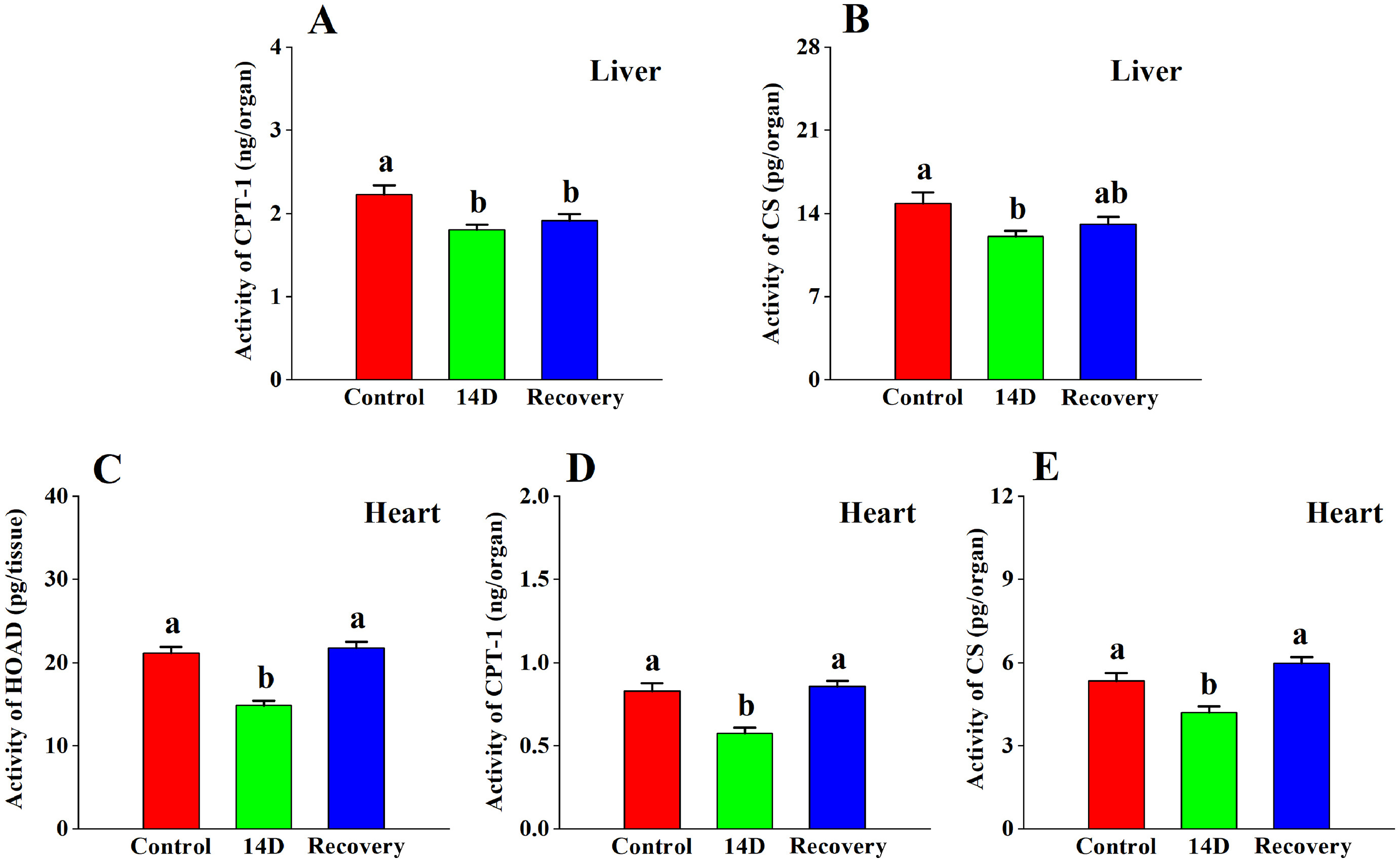
CPT-1 (Table 2; Fig. 4A) and CS (Table 2; Fig. 4B) enzyme activity in the liver changed significantly, but there was no significant change in HOAD (Table 2) with heat stress. The enzyme activity of CPT-1 in the 14 D group and the recovery group were significantly lower than that in the control group. After 14 days of heat treatment, the enzyme activity of CS was significantly lower compared to the control group, but there was no significant difference between the recovery group and the control group.
Enzyme activity of HOAD, CPT-1, and CS in the heart tissue showed significant changes (Table 2; Fig. 4C, D, E). Enzyme activity of HOAD, CPT-1 and CS in 14 D group decreased compared to the control group. There was no significant difference in HOAD, CPT-1 and CS activity between the recovery group and the control group.
No significant change in enzyme activity in muscle was seen for HOAD, CPT-1, and CS (Table 2).
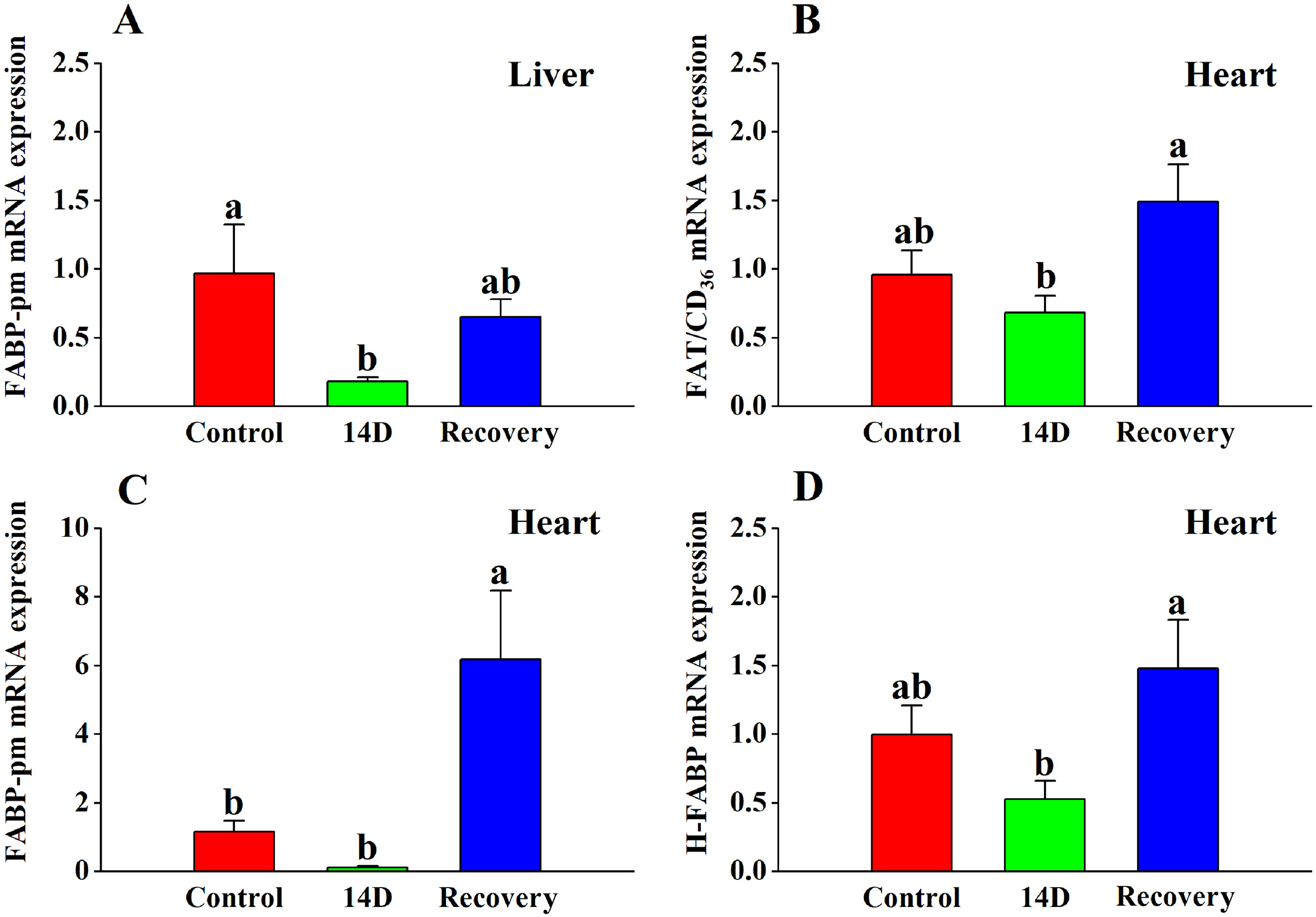
There was no significant change in FAT/CD36 mRNA expression in the liver (Table 2), however the 14 D group showed a decreasing trend. FABP-pm expression was significantly (Table 2; Fig. 5A) lower than the control group. No significant differences were found between the recovery group and the control group, or between the 14 D group and the recovery group.
There was a significant overall change in the expression levels of FAT/CD36, FABP-pm and H-FABP mRNA in the heart (Table 2; Fig. 5B, C, D). FAT/CD36 and H-FABP were significantly higher in the recovery group compared to the 14 D group. There was no significant difference in FAT/CD36 and H-FABP between the 14 D group and control group. The recovery group showed an increasing trend compared to the control group, but the difference was not significant. FABP-pm expression decreased after heat treatment but was not significantly lower in any of the groups compared to the control group. FABP-pm expression in the recovery group was significantly higher than the control group and 14 D group.
The expression of FAT/CD36 and FABP-pm mRNA in muscle did not change significantly (Table 2).
The heat has been shown to affect a wide variety of morphological, physiological and behavioral functions in birds (Cooper et al., 2020; Freeman et al., 2020). In the present study of Red-billed Leiothrix, we found that the heat had significant effects on the levels of oxidative stress and antioxidant defense, all of which increased significantly in birds acclimated to a high temperature. These birds also underwent a significant increase fatty acid transport and catabolism in liver and heart following high temperature acclimation.
In our study, we found heat stress causes oxidative stress in Red-billed Leiothrix and triggers a positive antioxidant enzyme system response. MDA content increased significantly after 1 D of heat stress treatment, suggesting that heat stress caused oxidative damage. From 3 D to 14 D, the activity of antioxidant enzymes (T-AOC, CAT and GSH-PX) increased significantly, indicating that from day 3 individuals can actively mobilize antioxidant enzymes to remove ROS, thus maintaining an oxidation-antioxidant equilibrium. Our MDA results indicated no significant oxidative damage after three days of heat stress treatment, suggesting that an increase in antioxidant enzymes may prevent oxidative stress caused by heat stress in the short term. In contrast to our findings, Ismail et al. (2013) found that broiler chickens exposed to high ambient temperature (39 ± 2 ℃) had increased lipid peroxidation, decreased SOD and CAT activity, and lower glutathione transferase (GST) serum levels. Similarly, Zeng et al. (2014) observed Pekin ducks exposed to 39 ± 0.5 ℃ for 1 h had lower SOD and CAT activity and decreased total antioxidant capacity (T-AOC) in the liver. These findings suggest that Red-billed Leiothrix may have a greater capacity to withstand heat stress than other domestic birds. However, MDA levels in Red-billed Leiothrix were still significantly higher at 14 D compared to the control group, indicating that while antioxidant levels increased to repair oxidative damage, elevated antioxidant enzyme activity could not repair the damage accumulated over long-term exposure to high temperature. Furthermore, following recovery at a thermoneutral temperature for 14 D MDA content remained significantly higher than that of the control group while most enzyme activity returned to the control level, although CAT activity remained significantly higher than that of the control group. These results suggest that despite ongoing repair, heat-induced oxidative damage persists long-term.
In the event of stress, the expression of HSPs can increase by 15% or more (El Golli-Bennour and Bacha, 2011). Consistent with this, we found a rapid increase in HSP70 expression in Red-billed Leiothrix following heat treatment. After 1 D of heat treatment HSP70 mRNA expression was five times higher compared to control levels and did not return to the same level as the control for 14 days. Consistent with our findings, Hutter et al. (1996) and Yu et al. (2008) respectively found that HSP60 and HSP70 mRNA expression in heart tissue of rats and broiler chicks was significantly upregulated after 2 h in heat stress. Increased HSP70 synthesis can decrease stress-induced DNA, protein and lipid damage, enhance cell recovery, and provide effective immune protection (Mayer and Gierasch, 2019). Our results suggest that HSP70 expression in the liver of Red-billed Leiothrix is heat stress sensitive and maintains a good response to heat stimulation throughout the experimental period (14 days). This may be an effective adaptive mechanism enabling Red-billed Leiothrix to withstand high temperature environments (Pamplona and Costantini, 2011).
UCPs were first implicated in the negative regulation of mitochondrial ROS production by (Nègre-Salvayre et al., 1997). In terms of negative regulation, some researchers have demonstrated that up-regulation of avUCP expression in skeletal muscle can reduce ROS synthesis during chronic heat stress in quails and broilers (Akbarian et al., 2016; Hu et al., 2019). In contrast, other studies found that synthesis of avUCP protein was downregulated in heat-stressed broilers, indicating that acute heat stress stimulates mitochondrial superoxide production in muscle, possibly via downregulation of avUCP (Mujahid et al., 2006). avUCP expression in our study was significantly lower after 1 day of heat stress and remained significantly lower up to 14 days and into the recovery phase compared to control levels. This shows that heat stress does not activate the avUCP upregulation mechanism to resist oxidative stress in Red-billed Leiothrix, but rather it inhibits the expression of avUCP because of oxidative stress. This in turn may lead to excessive production of ROS, resulting in oxidative stress and elevated levels of MDA.
Studies have shown that the enzyme activities of HOAD and CS in the heart of pika at low altitude with warm temperature were lower than that at high altitude, which implied that the activities of fatty acid oxidase might decrease when the energy requirements were reduced in one tissue (Sheafor, 2003). Similarly, we found significantly lower enzyme activities of HOAD and CS in the liver, as well as HOAD, CPT-1 and CS in the heart, than those in the control group, suggesting that Red-billed Leiothrix resist heat stress by reducing fatty acid catabolism in the liver and heart to decrease the supply of metabolic substrates. It was interesting that compared with muscle tissue, heart and liver tissue showed more sensitive responsiveness to heat stimulation in Red-billed Leiothrix. About that, studies in human medicine have shown that the heart is more likely to choose fatty acids as substrates than other metabolic substrates (such as carbohydrates, ketone bodies, and amino acids) (Gibb and Hill, 2018; Tran and Wang, 2019). Fang et al. (2014) reported that in birds, the liver is the chief organ responsible for fatty acid formation. Therefore, it is not difficult to understand that no differences were measured in enzyme activity of muscle tissue in our study. Another interesting phenomenon was that HOAD and CS enzyme activities and CPT-1 mRNA levels in muscle of broilers were significantly decreased after 14 days of heat stress (Azad et al., 2010; Lu et al., 2017). Therefore, we speculated that fatty acid oxidation levels in poultry muscle appear to be more susceptible to heat stress than in small wild birds.
A number of transport proteins are involved in fatty acid uptake, including FAT/CD36, FABP-pm and H-FABP (Coort et al., 2004). In study of White-throated Sparrows (Zonotrichia albicollis) (McFarlan et al., 2009) and mice (Putri et al., 2015), increased FAT/CD36 and FABP-pm mRNA levels can improve their capacity in transport of fatty acids into tissue and can therefore supply the oxidative machinery with a greater influx of exogenous fatty acids when energy demand increases due to migration or cold temperature. Contrary to the above findings, the mRNA expression levels of FABP-pm (in the liver and heart), FAT/CD36 (in the heart) and H-FABP (in the heart) decreased significantly after 14 days of heat stress in Red-billed Leiothrix. Our findings suggested that the uptake of free fatty acids by the liver and heart was reduced in Red-billed Leiothrix when exposed to high temperatures because of lower levels of mitochondrial oxidation at high temperatures (Jørgensen et al., 2021). And we also found that the absence of significant changes in fatty acid transporters in muscle is consistent with the absence of changes in muscle fatty acid catabolism. This indicates that the fatty acid catabolism pathway in muscles did not respond significantly to heat stress. It seems that the effect of heat stress on fatty acid catabolism and transport in Red-billed Leiothrix was tissue specific. Although we did not measure the content of free fatty acids in tissues, we hypothesize that reduced fatty acid transport might lead to a reduction in free fatty acid content in liver and heart, which in turn leads to a reduction in fatty acid catabolism.
When exposed to heat stress, Red-billed Leiothrix suffer oxidative stress and actively mobilize their antioxidant defense system to avoid oxidative damage. However, despite the Red-billed Leiothrix exhibiting a greater antioxidant capacity than that seen in poultry under similar heat stress conditions of up to 14 days, it was still in a state of oxidative damage following treatment recovery. Our results suggest that Red-billed Leiothrix are able to resist heat stress by reducing fatty acid transport and catabolism. Similar to mammals, a reduction in fatty acid transport and catabolism may be an adaptive mechanism to limit heat production in heat-stressed small birds. In conclusion, by increasing their antioxidant defense mechanisms and decreasing fatty acid catabolism, Red-billed Leiothrix may be able to survive prolonged high temperatures.
JL and ML provided the research idea and designed the experiments. RX, CY, MJ and LG performed experiments; RX and CY analyzed data; RX and LM interpreted results of experiments; LM prepared Figs; RX and CY drafted manuscript; JL and ML edited and revised manuscript. All authors assume responsibility for the content of the paper. All authors read and approved the final manuscript.
No conflicts of interest, financial or otherwise, are declared by the authors.
We would like to thank the reviewers for their important suggestions for the publication of this paper and for their guidance to improve the author's academic level. We also thank Dr Katrina Seelye nee Hale of Biological Science Editing, New Zealand for revising this manuscript. This study was financially supported by grants from the National Natural Science Foundation of China (No. 31971420; 32171497).
|
Banerjee, S., Chaturvedi, C.M., 2016. Migratory preparation associated alterations in pectoralis muscle biochemistry and proteome in Palearctic-Indian emberizid migratory finch, red-headed bunting, Emberiza bruniceps. Comp. Biochem. Physiol. D 17, 9–25
|
|
Cooper CE, Hurley LL, Deviche P. Physiological responses of wild zebra finches (Taeniopygia guttata) to heatwaves. J Exp Biol. 2020;223:1-20
|
|
Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243-82
|
|
Liu M, Wang XL, Yuan SB. Study on heat stress and anti-stress effect of chitosan on the Red-billed Leiothrixs (Leiothrix lutea). J China West Norm U (Nat Sci Ed). 2015;36:1-6
|
|
Rehaman Z-U, Chand N, Khan S. Evaluating the immune response and antioxidant potential in four broiler strains under chronic high ambient temperature. Pak J Zool. 2017;49:2087-91
|
|
Sanders SR, Cole LC, Flann KL. Effects of acute heat stress on skeletal muscle gene expression associated with energy metabolism in rats. FASEB. 2009;23:589
|
| Genes | Accession number | Primer sequence (5′–3′) | Product length (bp) |
| HSP70 | XM_013807608.1 | Forward: CACGGCTGCTGCTATT | 178 |
| Reverse: TACGGTGTCAAAGTCCTC | |||
| avUCP | XM_019135200.1 | Forward: CGGTGGATGTGGTGAAGACG | 144 |
| Reverse: AGGAAGGASGGGACGAAGC | |||
| FAT/CD36 | XM_014883464.1 | Forward: CATACTGGGAAGGCCACTGT | 169 |
| Reverse: CTGTATCCGTGCAGAAGCAA | |||
| FABP-pm | XM_005517927.2 | Forward: GTGGAAGGAGTTGGCAGCTA | 128 |
| Reverse: GTGGAAGGAGTTGGCAGCTA | |||
| H-FABP | XM_015648987.1 | Forward: AAGACCCAGAGCACCTTCAA | 146 |
| Reverse: AACAGCGATGTCTCCTTCC | |||
| β-Actin | XM_014261104.1 | Forward: TGCGTGACATCAAGGAGAAG | 157 |
| Reverse: TGCCAGGGTACATTGTGGTA |
| Type of variable | Variable | Tissue | df | F | P |
| Enzyme activity | MDA | Liver | 4, 30 | 19.963 | <0.001 |
| SOD | Liver | 4, 30 | 1.247 | 0.313 | |
| CAT | Liver | 4, 30 | 6.948 | 0.001 | |
| GSH-PX | Liver | 4, 30 | 6.803 | <0.001 | |
| T-AOC | Liver | 4, 30 | 6.835 | <0.001 | |
| CPT-1 | Liver | 2, 29 | 6.935 | 0.003 | |
| Heart | 2, 29 | 18.873 | <0.001 | ||
| Muscle | 2, 29 | 2.206 | 0.128 | ||
| CS | Liver | 2, 29 | 4.424 | 0.021 | |
| Heart | 2, 29 | 16.699 | <0.001 | ||
| Muscle | 2, 29 | 1.151 | 0.330 | ||
| HOAD | Liver | 2, 29 | 2.216 | 0.127 | |
| Heart | 2, 29 | 35.176 | <0.001 | ||
| Muscle | 2, 29 | 0.933 | 0.445 | ||
| Expression of mRNA | HSP70 | Liver | 4, 30 | 4.939 | 0.004 |
| avUCP | Muscle | 4, 30 | 19.963 | <0.001 | |
| FAT/CD36 | Liver | 2, 29 | 0.994 | 0.383 | |
| Heart | 2, 29 | 4.516 | 0.020 | ||
| Muscle | 2, 29 | 0.732 | 0.490 | ||
| FABP-pm | Liver | 2, 29 | 4.054 | 0.028 | |
| Heart | 2, 29 | 7.008 | 0.003 | ||
| Muscle | 2, 29 | 0.232 | 0.794 | ||
| H-FABP | Heart | 2, 29 | 3.804 | 0.035 |