| Citation: | Lubna ALI, Noor-un-nisa, Syed Shahid SHAUKAT, Rafia Rehana GHAZI. 2011: Sex and age classes of prey items (rats/mice) in the diet of the Barn Owl (Tyto alba) in Sindh, Pakistan. Avian Research, 2(2): 79-86. DOI: 10.5122/cbirds.2011.0012 |
Barn Owl (Tyto alba) pellets were collected from nine locations and two districts of Sindh, Pakistan and 937 prey items were recovered from 619 pellets. Rats/mice (59.6%) were the most dominant food items consumed by the Barn Owl. Shrews (22.3%), bats (1.3%), birds (12.0%), insects (1.3%), frogs (2.2%) and plant materials (1.3%) were found in their diet as well. Study of the pelvic girdle bones of rats/mice, used only for sexing, proved to be a useful device in population dynamics. In the pelvic bone, pelvic symphysis is found only in female rats/mice developed as a result of sex hormones that occur during gestation. Among the diet of rats/mice, males were found to be significantly dominant. Tooth wear patterns on the occlusal surfaces of molariform teeth of the rats/mice were found to provide an effective criterion for establishing age classes of rats/mice. In the present study, adult rats/mice were found to be dominant over sub-adults and old adults. ANOVA showed significant differences in the number of rats/mice and shrews (prey items) and the other prey items/plant materials in the diet of Barn Owls in the district Thatta and district Karachi. Chi-square test disclosed non-significant differences in age and sex categories.
Most predators prefer some species of prey over others to take on in their diet, a condition that may have an important effect on the amount of prey as well as on community structures (Sih et al., 1985; Kuno, 1987; Dickman et al., 1991). Owl pellets provide information about small vertebrate fauna, mainly mammals of a particular area, because the structure of the prey community near the nest is reflected in owl pellets (Twente and Baker, 1951; Smith et al., 1972; Seçkin and Coşkun, 2005, 2006). Predators may also differentially consume different sex and age groups of rats and mice within populations of prey species, with minor effects on prey population dynamics in any area (Longland and Jenkins, 1987; Dickman et al., 1991). Alvarez-Castaňda et al. (2004) stated that owls prey mainly on small rodents of 7–25 g, catch and consume the requisite amount of prey to cover their necessary energy needs and probably do not have any preference for either large or small prey. However, Yom-Tov and Wool (1997) reported that Barn Owls (Tyto alba) do not hunt on some species preferentially, although the contents of the pellets may be biased towards larger prey. There are no previous studies on the diet of Barn Owls from Sindh Province, Pakistan except that by Khan et al. (1994). The present study aims to determine the age and sex categories of rodents that are the most preferred food items in the diet of Barn Owls from Sindh, Pakistan.
Geographically, Sindh is the third largest province of Pakistan, stretching about 579 km from north to south and 442 km (extreme) or 281 km (average) from east to west, with an area of 140915 km2 of Pakistani territory. Sindh is bounded by the Thar Desert to the east, the Kirthar Mountains to the west and the Arabian Sea in the south. In the center is a fertile plain around the Indus River. The study was conducted during 2007 to 2010 in the Karachi and Thatta districts, Sindh Province. The Karachi district lies at 24°86′0″ north latitude, 67°01′0″ east longitude and the Thatta district, at 24°30′0″ north latitude and 67°50′0″ east longitude, is located in lower Sindh Province.
A total of 619 pellets of Barn Owls were collected from nine different locations and the two districts. Of the total, 198 pellets were collected from six different locations in the Thatta district, i.e., M.K. Soomro Goth (L1), Ghulamaullah (L2), Pirpatho (L3), Jatti (L4), Warshah (L5) and Bello (L6) and 421 pellets were collected from three locations in the Karachi district, i.e., Port Qasim (L7), Baili (L8) and Malir Agricultural Farms (L9).
Pellets were dissected using standard techniques (Dickman et al., 1991; Yom-Tov and Wool, 1997; Seçkin and Coşkun, 2005, 2006). Each pellet was analyzed in the laboratory and initially the color, shape, weight, length and width were recorded. Each pellet was soaked in tap water for 1 to 2 hours for an analysis of its content. The content of the pellet was separated and sorted carefully for different prey items, such as bones, skulls, vertebrae, lower jaws, feathers, hairs and insects remnants. All remnants were packed up in different marked polythene bags.
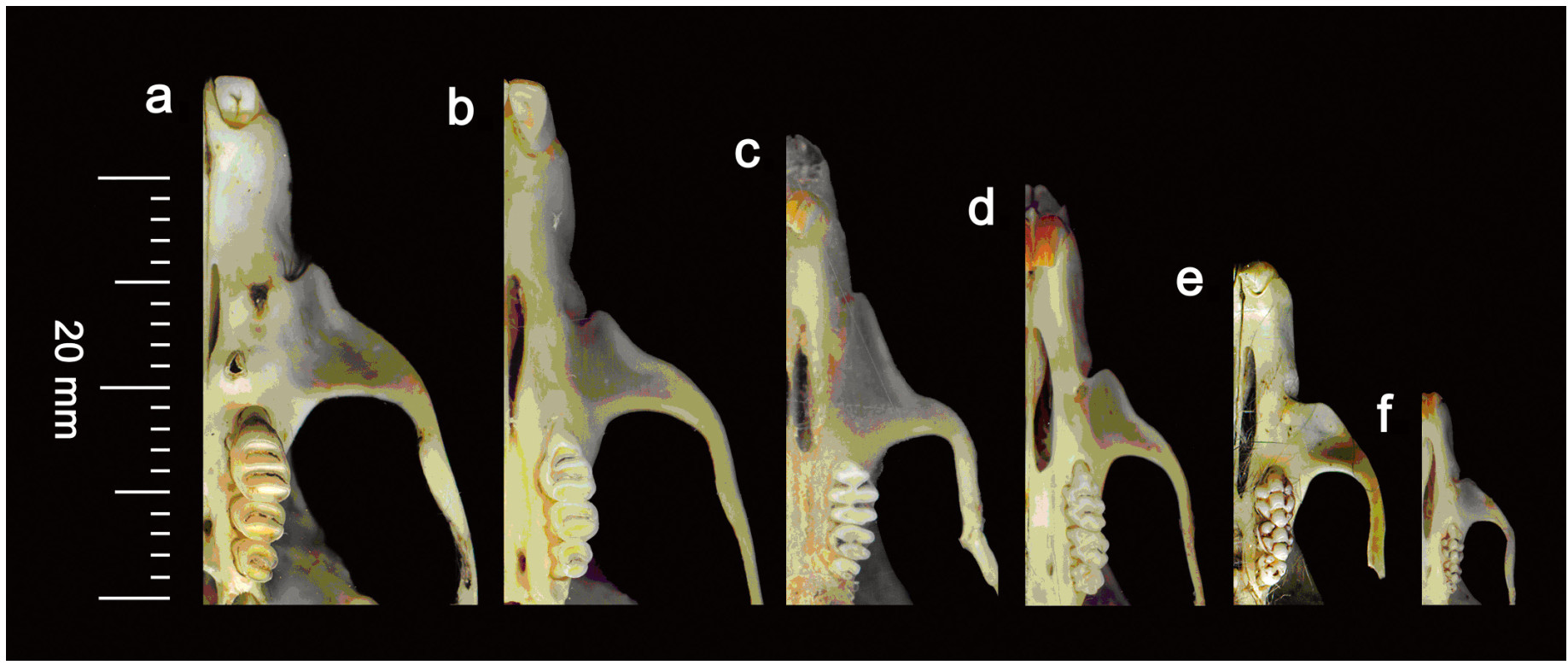
Skulls, lower jaws and other bones were examined carefully to determine whether they belonged to small mammals (including bats), birds, amphibians, insects and plant materials (Seçkin and Coşkun, 2005, 2006; Shehab and Al Charabi, 2006). In the case of mammals the skulls and teeth were used to identify the species of rats/mice (Fig. 1). To facilitate identification, reference skulls and teeth of rats/mice, known to be present in the study area, were available in the laboratory for comparison from reference literature (Greene, 1935; Roberts, 1977, 2005) and the reference collection of the Vertebrate Pest Control Institute, SARC, PARC, Karachi University, Pakistan.
Many methods were used for determining the age of rats/mice in the world, such as weight differences, bacular characteristics, cranial dimensions and ossification of ballae, in order to establish age groups in various kinds of animals. However, the pattern of molar eruption and the rate of tooth wear were found much less variable than that of body growth; thus the tooth pattern technique is more useful than other techniques for identification of age group of rats and mice prey (Iqbal, 1975; Zaman, 1975; Mushtaq-ul-Hassan et al., 2007). In this study three age categories, i.e., sub-adult, adult and old were established on the basis of the tooth wear pattern of rats and mice.
Many techniques were used for identification of rodent sexual dimorphism, using the remains of prey in the pellets of owl. Sexual dimorphism or sexes of rats and mice are identified in the diet of this Barn Owl, usually from structural differences of pelvic bones (Dunmire, 1955). The pelvic girdle bone shape is different between the sexes. The pubic symphysis area is found only in females, because female rodents show separation of the pubis bone; the pubic symphysis region occurs during gestation and is caused by sex hormones (Hall and Newton, 1946; Hall, 1947). The pelvic bone shape is clearly different in both sexes of rats and mice. The pelvic area of both sexes show the ileum, pubis, ischium and acetabulum, but the pubic symphysis shows only in females (Fig. 2), owing to modifications of the female pelvic to facilitate the separation of the pelvic bones. Barn Owls consume 1 to 5 prey per day (Shehab and Al Charabi, 2006) depending on availability and size of prey species. Generally, 1 to 4 prey items are found in pellets (Seçkin and Coşkun, 2006), but at times skulls may be absent from the pellet, in which case the species cannot be identified with certainty from pelvic bones alone. However, on the basis of pelvic bones, a distinction can be made between the sexes.
Data regarding frequency of occurrence, percentage of prey species (Table 1), frequency, means, standard deviations and standard errors of the age categories of rats/mice in Thatta (Table 2) and Karachi (Table 3) in the pellets of Barn Owls were estimated following the method established by Zar (1996) and analyzed using Excel version 2003. Frequency, percentage of frequency, means, standard deviations and standard errors of different species of rats/mice sex categories were collected or calculated from the pellets of Barn Owls at all study areas (Table 4). ANOVA were used with the rats/mice and shrews (prey items) and the other prey items / plant materials separately. Chi-square tests were used to test for differences in sex ratios and age ratios of rats/mice, with Minitab versions 11 and 12.
| Prey items | Thatta district | Karachi district | Total | |||||
| N | %N | N | %N | N | %N | |||
| Rats/mice | 384 | 64.9 | 182 | 51.0 | 566 | 59.6 | ||
| Bandicota bengalensis | 54 | 9.1 | 9 | 2.5 | 63 | 6.6 | ||
| Nesokia indica | 28 | 4.7 | 23 | 6.4 | 51 | 5.4 | ||
| Millardia meltada | 98 | 16.6 | 28 | 7.8 | 126 | 13.3 | ||
| Mus musculus | 78 | 13.2 | 38 | 10.6 | 116 | 12.2 | ||
| Rattus rattus | 20 | 3.4 | 0 | 0.0 | 20 | 2.1 | ||
| Tatera indica | 71 | 12.0 | 57 | 16.0 | 128 | 13.5 | ||
| Unidentified rat species | 35 | 5.9 | 27 | 7.6 | 62 | 6.5 | ||
| Shrews | 134 | 22.6 | 78 | 21.8 | 212 | 22.3 | ||
| Suncus stoliczkanus | 2 | 0.3 | 0 | 0.0 | 2 | 0.2 | ||
| Suncus murinus | 130 | 22.0 | 76 | 21.3 | 206 | 21.7 | ||
| Unidentified shrew species | 2 | 0.3 | 2 | 0.6 | 4 | 0.4 | ||
| Bats | ||||||||
| Trophozous sp. | 4 | 0.7 | 8 | 2.2 | 12 | 1.3 | ||
| Bird species | 53 | 9.0 | 61 | 17.1 | 114 | 12.0 | ||
| Insect remains | 8 | 1.4 | 4 | 1.1 | 12 | 1.3 | ||
| Frogs | 5 | 0.8 | 16 | 4.5 | 21 | 2.2 | ||
| Plant materials | 4 | 0.7 | 8 | 2.2 | 12 | 1.3 | ||
| Total | 592 | 100 | 357 | 100 | 949 | 100 | ||
| Rats/mice species | Age categories | Study site | Total | Mean ± SD | SE mean | |||||
| L1 | L2 | L3 | L4 | L5 | L6 | |||||
| Bandicota bengalensis | Sub-adult | 1 | 1 | 3 | 0 | 0 | 1 | 6 | 1.0 ± 1.1 | 0.5 |
| Adult | 1 | 2 | 10 | 3 | 0 | 7 | 23 | 2.5 ± 3.9 | 1.6 | |
| Old | 1 | 5 | 13 | 1 | 0 | 5 | 25 | 4.2 ± 4.8 | 2.0 | |
| Nesoki indica | Sub-adult | 4 | 2 | 0 | 0 | 0 | 0 | 6 | 0.5 ± 0.8 | 0.3 |
| Adult | 0 | 6 | 6 | 3 | 0 | 1 | 16 | 2.7 ± 2.8 | 1.2 | |
| Old | 1 | 3 | 10 | 0 | 0 | 1 | 15 | 2.5 ± 3.8 | 1.6 | |
| Millardia meltada | Sub-Adult | 0 | 5 | 7 | 0 | 5 | 0 | 17 | 2.8 ± 3.2 | 1.3 |
| Adult | 0 | 13 | 11 | 1 | 23 | 5 | 53 | 8.8 ± 8.7 | 3.5 | |
| Old | 2 | 9 | 8 | 1 | 6 | 4 | 30 | 5.0 ± 3.2 | 1.3 | |
| Mus musculus | Sub-adult | 1 | 3 | 10 | 2 | 0 | 3 | 19 | 3.4 ± 3.4 | 1.4 |
| Adult | 6 | 12 | 14 | 3 | 8 | 3 | 46 | 8.2 ± 5.5 | 2.2 | |
| Old | 1 | 2 | 3 | 2 | 1 | 0 | 14 | 1.5 ± 1.1 | 0.4 | |
| Rattus rattus | Sub-adult | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 0.5 ± 0.6 | 0.2 |
| Adult | 0 | 7 | 3 | 2 | 0 | 0 | 12 | 2.0 ± 2.8 | 1.1 | |
| Old | 0 | 1 | 3 | 0 | 0 | 1 | 5 | 8.3 ± 1.2 | 0.4 | |
| Tatera indica | Sub-adult | 0 | 1 | 1 | 0 | 1 | 3 | 6 | 1.0 ± 1.1 | 0.5 |
| Adult | 0 | 15 | 14 | 2 | 1 | 13 | 45 | 7.0 ± 7.2 | 2.9 | |
| Old | 0 | 6 | 9 | 0 | 2 | 7 | 24 | 4.0 ± 3.9 | 1.6 | |
| Unidentified rats/mice | Sub-adult | 0 | 1 | 3 | 0 | 1 | 0 | 5 | 0.8 ± 1.2 | 0.5 |
| Adult | 0 | 8 | 5 | 0 | 5 | 7 | 2 | 4.2 ± 3.4 | 1.4 | |
| Old | 0 | 0 | 4 | 1 | 0 | 0 | 5 | 0.8 ± 1.6 | 0.7 | |
| Rats/mice species | Age categories | Study site | Total | Mean ± SD | SE mean | ||
| L7 | L8 | L9 | |||||
| Bandicota bengalensis | Sub-adult | 2 | 1 | 1 | 4 | 1.3 ± 0.7 | 0.3 |
| Adult | 6 | 2 | 2 | 10 | 3.3 ± 2.3 | 1.3 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Nesoki indica | Sub-adult | 0 | 0 | 4 | 4 | 1.3 ± 2.3 | 1.3 |
| Adult | 9 | 4 | 3 | 16 | 5.3 ± 3.2 | 1.9 | |
| Old | 2 | 2 | 2 | 6 | 2.0 ± 0.0 | 0.0 | |
| Millardia meltada | Sub-adult | 3 | 1 | 1 | 5 | 1.7 ± 1.2 | 0.6 |
| Adult | 8 | 5 | 9 | 22 | 7.3 ± 2.1 | 1.2 | |
| Old | 0 | 0 | 3 | 3 | 1.0 ± 1.7 | 1.0 | |
| Mus musculus | Sub-adult | 10 | 0 | 4 | 14 | 4.7 ± 5.0 | 2.9 |
| Adult | 13 | 0 | 9 | 22 | 7.3 ± 6.7 | 3.8 | |
| Old | 1 | 0 | 4 | 5 | 1.7 ± 2.1 | 1.2 | |
| Rattus rattus | Sub-Adult | 0 | 1 | 5 | 6 | 2.0 ± 2.7 | 1.5 |
| Adult | 0 | 1 | 6 | 7 | 2.3 ± 3.2 | 1.9 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Tatera indica | Sub-adult | 6 | 0 | 2 | 8 | 2.7 ± 3.1 | 1.8 |
| Adult | 27 | 4 | 14 | 45 | 15 ± 11.5 | 6.7 | |
| Old | 8 | 2 | 6 | 16 | 5.3 ± 3.1 | 1.8 | |
| Unidentified rats | Sub-adult | 0 | 2 | 1 | 3 | 1.0 ± 1.0 | 0.6 |
| Adult | 7 | 4 | 13 | 24 | 8.0 ± 4.6 | 2.7 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Locations | N | Male rodents | Female rodents | |||
| %(n) | Mean ± SD | %(n) | Mean ± SD | |||
| Thatta district | ||||||
| L1 | 20 | 46.1(6) | 0.30 ± 0.80 | 53.9(7) | 0.35 ± 0.93 | |
| L2 | 79 | 57.6(49) | 0.62 ± 0.87 | 42.4(36) | 0.46 ± 0.62 | |
| L3 | 161 | 56.0(51) | 0.32 ± 0.69 | 44.0(40) | 0.25 ± 0.56 | |
| L4 | 33 | 50.0(12) | 0.36 ± 0.65 | 50.0(12) | 0.36 ± 0.60 | |
| L5 | 61 | 54.7(26) | 0.46 ± 0.67 | 45.3(24) | 0.39 ± 0.56 | |
| L6 | 67 | 48.9(22) | 0.33 ± 0.59 | 51.1(23) | 0.34 ± 0.57 | |
| Total | 421 | 54.3(169) | 0.40 ± 0.72 | 45.7(142) | 0.34 ± 0.60 | |
| Karachi district | ||||||
| L7 | 62 | 56.1(55) | 0.89 ± 0.73 | 43.9(43) | 0.69 ± 0.69 | |
| L8 | 24 | 75.0(18) | 0.75 ± 0.85 | 25.0(6) | 0.25 ± 0.44 | |
| L9 | 112 | 51.9(28) | 0.25 ± 0.65 | 48.1(26) | 0.23 ± 0.52 | |
| Total | 198 | 57.4(101) | 0.51 ± 0.76 | 42.6(75) | 0.38 ± 0.61 | |
For our analyses we used all 619 regurgitated Barn Owl pellets, collected from the six different locations in the Thatta district and the three locations in the Karachi district. The most dominant food, rats and mice (59.6%) were founded in the diet of the Barn Owl, while shrews (22.3%), bats (1.3%), birds (12.0%), insects (1.3%), frogs (2.2%) and plant materials (1.3%) were also found (Table 1). Among rats and mice, the most common species consumed were Tatera indica (13.5%), Millardia meltada (13.3%), Mus musculus (12.2%), Bandicota bengalensis (6.6%), Nesokia indica (5.4%), Rattus rattus (2.1%) and some unidentified rat species (6.5%). Among Suncus spp. the second most dominant food items in the diet of the Barn Owl were Suncus murinus (21.7%), Suncus stoliczkanus (0.2%) and some unidentified shrew species (0.4%).
The results of analyses of variance of our completely randomized design (CRD) of the prey species rats/mice and shrews, with respect to pellets of Barn Owls in the Thatta and Karachi districts showed highly significant differences (F = 31.26, df = 9 and F = 20.46, df = 7; p < 0.001 respectively) while the other prey items/plant materials also showed significant differences (F = 20.46, df = 9 and F = 105.07, df = 7; p < 0.001 respectively).
The average frequencies, means, standard deviations (SD), standard errors (SE) of age categories of different rats/mice species found in Thatta and Karachi districts are shown in Tables 2 and 3. Chi-square tests for differences in age ratios of rats/mice calculated for the two districts were found to be non-significant (χ2 = 9.82, df = 10 and χ2 = 2.48, df = 4; ns. respectively).
An average frequency n = 169 (55.3%) and 142 (45.7%) of males and females of different rats/mice species was found in the Thatta district and average frequencies of 101 (57.4%) and 75 (42.6%) in Karachi (Table 4). Chi-square tests for differences in sex ratios between male and female rats/mice in both districts were found to be non-significant (χ2 = 1.56, df = 5 and χ2 = 3.78, df = 2; ns. respectively).
Pellet analysis was the most suitable system for our study because predators and prey could be easily observed and the remains of prey recovered from pellets and placed into sex and size categories. Jorgensen et al. (1998) believed that Barn Owls have a hunting area preference, so it might use a suburban area for nesting and fly to an adequate hunting area. Clarke (1983) reported that due to the presence of light, owls are more effective predators. Brown et al. (1988) performed an experiment with Barn Owls, which showed that Chaetodipus shifted activity away from risky open microhabitats in the presence of light, but the analysis by Alvarez-Castaňda et al. (2004) showed that the Barn Owl is an opportunistic species, as in other areas. Our own analysis also shows that the Barn Owl is an opportunistic prey species, corroborating the findings of Alvarez-Castaňda et al. (2004).
Small mammals were the most commonly recovered prey in Barn Owl pellets (Dickman, 1991; Ingels, 1995; Huebschman et al., 2000; Alvarez-Castaňda et al., 2004; Alivizatos et al., 2005; Shehab and Al Charabi, 2006). Small mammals were found to be the main food items in pellets of Barn Owl diets in Barazil (Escarlate-Tavares and Pessôa, 2005), in Greece (Glue, 1971; Campbell et al., 1987; Yom-Tov and Wool, 1997; Goutner and Alivizatos, 2003; Alivizatos et al., 2005), in northern Syria (Shehab and Al Charabi, 2006), in the Negev Desert (Tores and Yom-Tov, 2003), in the Nigerian savanna (Lekunze et al., 2001) and in central Punjab (Mushtaq-ul-Hassan et al., 1999, 2004).
In the present study, 566 rats/mice skulls were found in all pellets of Barn Owls, belonging to three different orders and four families from nine different locations in the Thatta and Karachi districts of Sindh Province, Pakistan. Six rats/mice spp., two shrews spp. and one bat sp. were found in the diet of Barn Owls. In the Nigerian savanna, six small mammalian species were recorded (Lekunze et al., 2001). In Nebraska, 19 identifiable species and 21 genera, comprising mammalian prey in the diet of owls were found (Huebschman et al., 2000).
Mushtaq-ul-Hassan et al. (2007) studied the age categories of M. meltada from the pellets of Short-eared Owls (Asio flammeus). Among them, 9.9% were immature, 50.8% were sub-adults, 24.9% were adults, 11.0% were middle-age adults and 3.3% were old adults and Mushtaq-ul-Hassan et al. (2007) also identified M. musculus species in Barn Owl pellets, of which 12.8% were sub-adults, 66.0% adults and 21.2% old adults. The mean diet of Short-eared Owls and Barn Owls consisted largely of sub-adults of M. meltada and adults of M. musculus. In our present study, we enumerated the size of all rats/mice in the diet of the Barn Owl, which revealed that more adult rats/mice were consumed than sub-adults and old rats/mice. In our study, we found that sub-adults accounted for 13.16%, adults for 56.71% and old rats and mice for 30.13% in the Thatta district and in the Karachi district sub-adults accounted for 17.74%, adults for 68.82% and old rats and mice for 13.44%.
Predators consume often males/old rats and hunt selectively within a population (Morse, 1980). According to Marti and Hogue (1979) and Morris (1979) smaller female/juvenile rats and mice are usually consumed. Normally, males are more vulnerable to predation because of their larger size, conspicuous coloration, or because movements or sexual behavior attract the attention of predators (Tuttle and Ryan, 1981; Gwynne, 1987). Generally, female rodents have a separation of their pubic bones; their pubic symphysis region occurs during gestation and is directly caused by sex hormones (Hall and Newton, 1946; Hall, 1947; Dunmire, 1955). This interpubic separation is characteristic of all the forms studied by us. The pelvic shape is clearly different between the sexes of both rats and mice of all species. Pellet analysis is the most convenient system for study because predators and prey can be easily observed and the remains of prey can be recovered from pellets and placed into sex and size categories. In our present study, we enumerated the size and sex of all rats/mice in the diet of the Barn Owl and have shown that Barn Owls select especially male rats and mice from prey populations.
|
Alivizatos H, Goutner V, Zogaris S. 2005. Contribution to the study of four owl species (Aves, Strigiformes) from mainland and island areas of Greece. Belg J Zool, 135: 109–118.
|
|
Escarlate-Tavares F, Pessoa LM. 2005. Bats (Chiroptera, Mammalia) in Barn Owl (Tyto alba) pellets in northern pantanal, Mato Grosso, Brazil. Mastozool Neotrop Mendoza, 12(1): 61–67.
|
|
Glue DE. 1971. Avian predator pellet analysis and the mammalogist. Mammal Rev, 21: 200–210.
|
|
Goutner V, Alivizatos H. 2003. Diet of the barn owl (Tyto alba) and little owl (Athene noctua) in wetlands of northeastern Greece. Belg J Zool, 113: 15–22.
|
|
Greene EC. 1935. Anatomy of the Rat. Manufactured by the Haddan Craftsmen, INC. CamDen, New Jersey, U.S.A.
|
|
Hall K. 1947. The effects of pregnancy and relaxin on the histology of the pubic symphysis in the mouse. J Endocrinol, 5: 174–182.
|
|
Huebschman JJ, Freeman PW, Genoways HH, Gubanyi JA. 2000. Observations on small mammals recovered from owl pellets from Nebraska. Prairie Nat, 32(4): 209–215.
|
|
Ingels C. 1995. Summary of California studies analyzing the diet of barn owls. Sustain Agr, 7(2): 14–16.
|
|
Iqbal HM. 1975. Evaluation of the degree of tooth wear and morphometric changes as a criterion of determining age groups in soft-furred field rat (Millardia meltada). M. Sc. Thesis. Department of Zoology, University of Agriculture, Lyallpur, Pakistan, pp 60.
|
|
Jorgensen EE, Sell SM, Demarais S. 1998. Barn owl prey use in Chihuahuan Desert Foothills. Southwest Nat, 43(1): 53–56.
|
|
Khan HA, Beg MA, Khan AA. 1994. Food of Barn Owl (Tyto alba) in Lower Sindh. Pak J Agr Sci, 31(3): 233–235.
|
|
Kuno E. 1987. Principles of predator-prey interaction in the oretical, experimental and natural population systems. Adv Ecol Res, 66: 1426–1438.
|
|
Lekunze LM, Ezealor AU, Aken'Ova T. 2001. Prey groups in the pellets of the barn owl Tyto alba (Scopoli) inthe Nigerian savanna. Afr J Ecol, 39: 38–44.
|
|
Marti CD, Hogue JG. 1979. Selection of prey size in Screech Owls. Auk, 96: 319–327.
|
|
Morse DH. 1980. Behavioral Mechanisms in Ecology. Harvard University Press, Cambridge, MA, U.S.A.
|
|
Mushtaq-ul-Hassan M, Ghazi RR, Noor-un-Nisa. 2007. Food preference of the Short-Eared Owl (Asio flammeus) and Barn Owl (Tyto alba) at Usta Muhammad, Balochistan, Pakistan. Turk J Zool, 31: 91–94.
|
|
Mushtaq-ul-Hassan M, Mahmood-ul-Hassan M, Beg MA, Khan AA. 1999. Reproduction and abundance of the house shrew suncus murinus in the villages and houses of central Punjab (Pakistan). Pak J Zool, 29: 199–202.
|
|
Mushtaq-ul-Hassan M, Raza MN, Shahazadi B, Ali A. 2004. The diet of barn owl (Tyto alba) from canal bank, canal rest house and graveyard of Gojra. J Res Sci, 15(3): 291–296.
|
|
Roberts TJ. 1997. The Mammals of Pakistan. Vol. 1. Oxford University Press, Oxford, U.K.
|
|
Roberts TJ. 2005. Field guide to the Small Mammals of Pakistan. Oxford University Press, Oxford. pp 280.
|
|
Seçkin S, Coşkun Y. 2006. Mammalian Remains in the Pellets of Long-eared Owls (Asio otus) in Diyarbakir Province. Turkey. Turk J Zool, 30: 271–278.
|
|
Shehab AH, Al Charabi SM. 2006. Food of the Barn Owl Tyto alba in the Yahmool Area, Northern Syria. Turk J Zool, 30: 175–179.
|
|
Smith DG, Wilson CR, Frost HH. 1972. Seasonal habits of barn owls in Utah. Great Basin Nat, 32: 229–234.
|
|
Tores M, Yom-Tov Y. 2003. The Diet of the Barn Owl (Tyto alba) in the Negev Desert. Isr J Zool, 49: 233–236.
|
|
Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the neotropics. Science, 214: 677–678.
|
|
Twente JW, Baker RH. 1951. New records of mammals from barn owl pellets. J Mammal, 32: 120–121.
|
|
Yom-Tov Y, Wool D. 1997. Do the contents of barn owl pellets accurately represent the proportion of prey species in the field? Condor, 99: 972–976.
|
|
Zaman R. 1975. Age determination, age structure, sex ratio and age specific reproduction in Tatera indica (Hardwicke, 1807) population in the Punjab. Thesis, University of Agriculture, Faisalabad, Pakistan.
|
|
Zar JH. 1996. Biostatistical Analysis. 3rd edition. Prentice Hall, Upper Saddle River, New Jersey, USA, pp 662.
|
| Julia Zurdo, Paula Gómez-López, Adrián Barrero, Daniel Bustillo-de la Rosa, Julia Gómez-Catasús, Margarita Reverter, Cristian Pérez-Granados, Manuel B. Morales, Juan Traba. 2023: Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines. Avian Research, 14(1): 100151. DOI: 10.1016/j.avrs.2023.100151 | |
| Tatyana Kovinka, Alexander Sharikov, Tatyana Massalskaya, Sergey Volkov. 2023: Structure and heterogeneity of habitat determine diet of predators despite prey abundance: Similar response in Long-eared, Short-eared Owls and Common Kestrels. Avian Research, 14(1): 100072. DOI: 10.1016/j.avrs.2022.100072 | |
| Chun Ting Chung, Hok Sze Wong, Man Long Kwok, Qi Meng, King Ming Chan. 2021: Dietary analysis of the House Swift (Apus nipalensis) in Hong Kong using prey DNA in faecal samples. Avian Research, 12(1): 5. DOI: 10.1186/s40657-021-00242-z | |
| Charlotte E. Hacker, Brandon D. Hoenig, Liji Wu, Wei Cong, Wei Cong, Jingjing Yu, Yunchuan Dai, Ye Li, Jia Li, Yadong Xue, Yu Zhang, Yunrui Ji, Hanning Cao, Diqiang Li, Yuguang Zhang, Jan E. Janecka. 2021: Use of DNA metabarcoding of bird pellets in understanding raptor diet on the Qinghai-Tibetan Plateau of China. Avian Research, 12(1): 42. DOI: 10.1186/s40657-021-00276-3 | |
| Audrey A. Sanchez, Andrew W. Bartlow, Allison M. Chan, Jeanne M. Fair, Aaron A. Skinner, Kelly Hutchins, Maria A. Musgrave, Emily M. Phillips, Brent E. Thompson, Charles D. Hathcock. 2021: A field guide for aging passerine nestlings using growth data and predictive modeling. Avian Research, 12(1): 28. DOI: 10.1186/s40657-021-00258-5 | |
| Dong Dong, Xinping Ye, Zhong Lin, Xia Li, Min Li, Huaqiang Wang, Xiaoping Yu. 2018: Effects of breeding success, age and sex on breeding dispersal of a reintroduced population of the Crested Ibis (Nipponia nippon) in Ningshan County, China. Avian Research, 9(1): 40. DOI: 10.1186/s40657-018-0132-7 | |
| Satish Pande, Reuven Yosef, Federico Morelli, Rajkumar Pawar, Ram Mone. 2018: Diet and habitat affinities in six raptor species in India. Avian Research, 9(1): 36. DOI: 10.1186/s40657-018-0129-2 | |
| Zhifeng Ding, Fang Ji, Qiuli Huang, Longwu Wang, Aiwu Jiang, Chunlan Zhang, Yongjun Feng, Yuan Tian, Huijian Hu, Wei Liang. 2017: Brood sex ratio in the Yellow-bellied Prinia (Prinia flaviventris). Avian Research, 8(1): 15. DOI: 10.1186/s40657-017-0074-5 | |
| Kazuto KAWAKAMI, Masaki FUJITA, Motohiro HASEGAWA, Hiroshi MAKIHARA. 2011: Dietary characteristics of the Malayan Night Heron (Gorsachius melanolophus) in the Yaeyama Islands, southern Japan. Avian Research, 2(2): 87-93. DOI: 10.5122/cbirds.2011.0015 | |
| Ning WANG, Jianqiang LI, Yingying LIU, Zhengwang ZHANG. 2010: Improvement on molecular sex identification primers for Passeriform bird species. Avian Research, 1(1): 65-69. DOI: 10.5122/cbirds.2009.0009 |
| Prey items | Thatta district | Karachi district | Total | |||||
| N | %N | N | %N | N | %N | |||
| Rats/mice | 384 | 64.9 | 182 | 51.0 | 566 | 59.6 | ||
| Bandicota bengalensis | 54 | 9.1 | 9 | 2.5 | 63 | 6.6 | ||
| Nesokia indica | 28 | 4.7 | 23 | 6.4 | 51 | 5.4 | ||
| Millardia meltada | 98 | 16.6 | 28 | 7.8 | 126 | 13.3 | ||
| Mus musculus | 78 | 13.2 | 38 | 10.6 | 116 | 12.2 | ||
| Rattus rattus | 20 | 3.4 | 0 | 0.0 | 20 | 2.1 | ||
| Tatera indica | 71 | 12.0 | 57 | 16.0 | 128 | 13.5 | ||
| Unidentified rat species | 35 | 5.9 | 27 | 7.6 | 62 | 6.5 | ||
| Shrews | 134 | 22.6 | 78 | 21.8 | 212 | 22.3 | ||
| Suncus stoliczkanus | 2 | 0.3 | 0 | 0.0 | 2 | 0.2 | ||
| Suncus murinus | 130 | 22.0 | 76 | 21.3 | 206 | 21.7 | ||
| Unidentified shrew species | 2 | 0.3 | 2 | 0.6 | 4 | 0.4 | ||
| Bats | ||||||||
| Trophozous sp. | 4 | 0.7 | 8 | 2.2 | 12 | 1.3 | ||
| Bird species | 53 | 9.0 | 61 | 17.1 | 114 | 12.0 | ||
| Insect remains | 8 | 1.4 | 4 | 1.1 | 12 | 1.3 | ||
| Frogs | 5 | 0.8 | 16 | 4.5 | 21 | 2.2 | ||
| Plant materials | 4 | 0.7 | 8 | 2.2 | 12 | 1.3 | ||
| Total | 592 | 100 | 357 | 100 | 949 | 100 | ||
| Rats/mice species | Age categories | Study site | Total | Mean ± SD | SE mean | |||||
| L1 | L2 | L3 | L4 | L5 | L6 | |||||
| Bandicota bengalensis | Sub-adult | 1 | 1 | 3 | 0 | 0 | 1 | 6 | 1.0 ± 1.1 | 0.5 |
| Adult | 1 | 2 | 10 | 3 | 0 | 7 | 23 | 2.5 ± 3.9 | 1.6 | |
| Old | 1 | 5 | 13 | 1 | 0 | 5 | 25 | 4.2 ± 4.8 | 2.0 | |
| Nesoki indica | Sub-adult | 4 | 2 | 0 | 0 | 0 | 0 | 6 | 0.5 ± 0.8 | 0.3 |
| Adult | 0 | 6 | 6 | 3 | 0 | 1 | 16 | 2.7 ± 2.8 | 1.2 | |
| Old | 1 | 3 | 10 | 0 | 0 | 1 | 15 | 2.5 ± 3.8 | 1.6 | |
| Millardia meltada | Sub-Adult | 0 | 5 | 7 | 0 | 5 | 0 | 17 | 2.8 ± 3.2 | 1.3 |
| Adult | 0 | 13 | 11 | 1 | 23 | 5 | 53 | 8.8 ± 8.7 | 3.5 | |
| Old | 2 | 9 | 8 | 1 | 6 | 4 | 30 | 5.0 ± 3.2 | 1.3 | |
| Mus musculus | Sub-adult | 1 | 3 | 10 | 2 | 0 | 3 | 19 | 3.4 ± 3.4 | 1.4 |
| Adult | 6 | 12 | 14 | 3 | 8 | 3 | 46 | 8.2 ± 5.5 | 2.2 | |
| Old | 1 | 2 | 3 | 2 | 1 | 0 | 14 | 1.5 ± 1.1 | 0.4 | |
| Rattus rattus | Sub-adult | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 0.5 ± 0.6 | 0.2 |
| Adult | 0 | 7 | 3 | 2 | 0 | 0 | 12 | 2.0 ± 2.8 | 1.1 | |
| Old | 0 | 1 | 3 | 0 | 0 | 1 | 5 | 8.3 ± 1.2 | 0.4 | |
| Tatera indica | Sub-adult | 0 | 1 | 1 | 0 | 1 | 3 | 6 | 1.0 ± 1.1 | 0.5 |
| Adult | 0 | 15 | 14 | 2 | 1 | 13 | 45 | 7.0 ± 7.2 | 2.9 | |
| Old | 0 | 6 | 9 | 0 | 2 | 7 | 24 | 4.0 ± 3.9 | 1.6 | |
| Unidentified rats/mice | Sub-adult | 0 | 1 | 3 | 0 | 1 | 0 | 5 | 0.8 ± 1.2 | 0.5 |
| Adult | 0 | 8 | 5 | 0 | 5 | 7 | 2 | 4.2 ± 3.4 | 1.4 | |
| Old | 0 | 0 | 4 | 1 | 0 | 0 | 5 | 0.8 ± 1.6 | 0.7 | |
| Rats/mice species | Age categories | Study site | Total | Mean ± SD | SE mean | ||
| L7 | L8 | L9 | |||||
| Bandicota bengalensis | Sub-adult | 2 | 1 | 1 | 4 | 1.3 ± 0.7 | 0.3 |
| Adult | 6 | 2 | 2 | 10 | 3.3 ± 2.3 | 1.3 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Nesoki indica | Sub-adult | 0 | 0 | 4 | 4 | 1.3 ± 2.3 | 1.3 |
| Adult | 9 | 4 | 3 | 16 | 5.3 ± 3.2 | 1.9 | |
| Old | 2 | 2 | 2 | 6 | 2.0 ± 0.0 | 0.0 | |
| Millardia meltada | Sub-adult | 3 | 1 | 1 | 5 | 1.7 ± 1.2 | 0.6 |
| Adult | 8 | 5 | 9 | 22 | 7.3 ± 2.1 | 1.2 | |
| Old | 0 | 0 | 3 | 3 | 1.0 ± 1.7 | 1.0 | |
| Mus musculus | Sub-adult | 10 | 0 | 4 | 14 | 4.7 ± 5.0 | 2.9 |
| Adult | 13 | 0 | 9 | 22 | 7.3 ± 6.7 | 3.8 | |
| Old | 1 | 0 | 4 | 5 | 1.7 ± 2.1 | 1.2 | |
| Rattus rattus | Sub-Adult | 0 | 1 | 5 | 6 | 2.0 ± 2.7 | 1.5 |
| Adult | 0 | 1 | 6 | 7 | 2.3 ± 3.2 | 1.9 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Tatera indica | Sub-adult | 6 | 0 | 2 | 8 | 2.7 ± 3.1 | 1.8 |
| Adult | 27 | 4 | 14 | 45 | 15 ± 11.5 | 6.7 | |
| Old | 8 | 2 | 6 | 16 | 5.3 ± 3.1 | 1.8 | |
| Unidentified rats | Sub-adult | 0 | 2 | 1 | 3 | 1.0 ± 1.0 | 0.6 |
| Adult | 7 | 4 | 13 | 24 | 8.0 ± 4.6 | 2.7 | |
| Old | 0 | 0 | 0 | 0 | – | 0.0 | |
| Locations | N | Male rodents | Female rodents | |||
| %(n) | Mean ± SD | %(n) | Mean ± SD | |||
| Thatta district | ||||||
| L1 | 20 | 46.1(6) | 0.30 ± 0.80 | 53.9(7) | 0.35 ± 0.93 | |
| L2 | 79 | 57.6(49) | 0.62 ± 0.87 | 42.4(36) | 0.46 ± 0.62 | |
| L3 | 161 | 56.0(51) | 0.32 ± 0.69 | 44.0(40) | 0.25 ± 0.56 | |
| L4 | 33 | 50.0(12) | 0.36 ± 0.65 | 50.0(12) | 0.36 ± 0.60 | |
| L5 | 61 | 54.7(26) | 0.46 ± 0.67 | 45.3(24) | 0.39 ± 0.56 | |
| L6 | 67 | 48.9(22) | 0.33 ± 0.59 | 51.1(23) | 0.34 ± 0.57 | |
| Total | 421 | 54.3(169) | 0.40 ± 0.72 | 45.7(142) | 0.34 ± 0.60 | |
| Karachi district | ||||||
| L7 | 62 | 56.1(55) | 0.89 ± 0.73 | 43.9(43) | 0.69 ± 0.69 | |
| L8 | 24 | 75.0(18) | 0.75 ± 0.85 | 25.0(6) | 0.25 ± 0.44 | |
| L9 | 112 | 51.9(28) | 0.25 ± 0.65 | 48.1(26) | 0.23 ± 0.52 | |
| Total | 198 | 57.4(101) | 0.51 ± 0.76 | 42.6(75) | 0.38 ± 0.61 | |