| Citation: | Yexi Zhao, Jiayu Zhang, Zihan Li, Qinmijia Xie, Xin Deng, Chenxi Zhang, Nan Wang. 2024: Use of evergreen and deciduous plants by nocturnal-roosting birds: A case study in Beijing. Avian Research, 15(1): 100177. DOI: 10.1016/j.avrs.2024.100177 |
With continually increasing urbanization, the land cover in urban areas continues to change, resulting in the loss of biodiversity. Birds are highly sensitive to changes in habitat. Most forest birds perch on plants that provide increased safety to reduce the risk of predation, and small birds may also consider insulation when using roosting plants in winter because of cold weather. Landscaping plants thus shape the nocturnal roosting environment of urban birds, and proper planting is essential for the survival of birds at night. The use of roosting plants by urban birds should therefore be studied to provide a reference for landscaping. In the current study, we observed 1865 nocturnal roosting birds in Beijing from 2021 to 2022, with 23 species of birds from 12 families and 45 species of plants from 22 families recorded. Juniperus chinensis exhibited the highest bird rarity-weighted richness, followed by Fraxinus pennsylvanica, Phyllostachys propinqua, Pinus tabuliformis, and Ulmus pumila. The diameter at breast height, tree height, and crown width of plants used by birds was largest in summer and smallest in winter, and the perch height of birds was the highest in spring and summer and the lowest in winter. Birds used the highest proportion of deciduous plants in summer and the highest proportion of evergreen plants in winter. A significant seasonal difference in the use of evergreen and deciduous plants by small birds was noted, with a preference for deciduous plants in summer and evergreen plants in winter, while this preference was not found in large birds. These findings indicate that evergreen plants provide a vital nocturnal roosting environment for small birds in winter. To provide a better nocturnal roosting habitat for urban birds, we recommend paying attention to the combination of evergreen and deciduous plants when carrying out landscape construction.
Rapid urbanization has become one of the major concerns in conservation biology (Miller and Hobbs, 2002). The growth of the urban population drives the continued expansion of city size (Melles et al., 2003; McDonald et al., 2008), and land changes to build cities and meet urban population needs, causing the loss of biodiversity (Grimm et al., 2008; Nilon et al., 2017). Hence, biodiversity conservation is receiving increasing attention (Oke et al., 2021). Of note, determining the habitat requirements for maintaining biodiversity within urban areas is essential not only for species conservation (Clergeau et al., 1998; Sulaiman et al., 2013) but also for improving the inhabitants' quality of life (Fontana et al., 2011; Carrus et al., 2015). Furthermore, urban ecosystems are highly dynamic and can provide useful insights into the management of biodiversity in other ecosystems (Savard et al., 2000). Importantly, understanding the relationship between biodiversity and urban habitat can provide a theoretical basis for conservation actions to mitigate the negative impacts of urbanization (Clergeau et al., 1998; Miller and Hobbs, 2002).
Birds are the most conspicuous wildlife in cities and one of the most sensitive and vulnerable species to disturbance (Sulaiman et al., 2013; Shwartz et al., 2014). They serve as indicators of urban habitat quality and availability (Blair, 1999; Chace and Walsh, 2006), as they are highly sensitive to vegetation composition and structure in cities (Clergeau et al., 1998; Chace and Walsh, 2006). It comes as no surprise that rapid urbanization affects global bird diversity (Melles et al., 2003), and understanding how birds roost in cities is critical to conserving urban bird diversity (Kemp, 2004; Pei et al., 2018).
In urban ecosystems, landscaping plants are an important part of bird habitat, and increasing the number and diversity of landscaping plants can support the survival of birds and maintain bird diversity during urbanization (Emlen, 1974; Clergeau et al., 1998; Savard et al., 2000). Landscaping plants provide a range of valuable ecosystem services such as noise reduction and air filtration, as well as many benefits to wildlife, including value to birds (Bolund and Hunhammar, 1999; Chiquet et al., 2013).
Roosting sites are places where animals sleep at night (Moraes et al., 2018). Since predation at night is a major cause of animal mortality (Yorzinski and Platt, 2012), animals use suitable roosts to reduce their risk of predation (Rechtschaffen, 1998; Yorzinski and Platt, 2012). Different plants have different physical structures and provide different conditions of warmth and concealment for birds. Avian fasting endurance increases with body weight, and the survival time of large birds is longer than that of small birds in cold environments (Buttemer, 1985). Most forest birds prefer to roost on safer (safe, i.e., taller and with a larger crown width) plants (Peh and Sodhi, 2002), but small birds may also consider insulation when using roosting plants in winter. Importantly, the quality of roosting sites in urban areas made up of landscaping plants directly influences bird fitness (Yuan et al., 2012). Evergreen and deciduous plants with different heights are nocturnal roosting habitats available to urban birds. Planting the appropriate plants is therefore essential for the survival of birds at night (Watts et al., 2021). Some studies on the roosting sites of birds, such as the House Crow (Corvus splendens) (Peh and Sodhi, 2002), Dunlin (Calidris alpina) (Conklin and Colwell, 2007), Coconut Lorikeet (Trichoglossus haematodus) (Jaggard et al., 2015), White Wagtail (Motacilla alba) (Jiang et al., 2021), and Northern Bobwhite (Colinus virginianus) (Klimstra and Ziccardi, 1963), have been conducted. However, most studies have focused on readily detected communally roosting species or have used radio-telemetry to locate birds of a small number of key species at roosting sites (Mitchell and Clarke, 2019). Few studies have explored the use of roosting plants by birds in large cities.
Most of the northern regions of China are located in the temperate zone, with many deciduous and evergreen plants (Liu et al., 2003). Evergreen plants like pines, cypresses, and bamboo are commonly used in urban areas where there is a scarcity of evergreens. Beijing is a representative city of northern China and one of the largest cities in the world and has undergone significant urbanization over the past few decades (Yang et al., 2014). Between 2000 and 2019, the built-up area of Beijing increased from 488 km2 to 1469 km2 (Urban Socio-economic Surveys Group, 2001). Beijing is a key migration corridor for birds, and its urban gardens play an important role in supporting bird roosting and breeding activities (Zhao et al., 2022). University campuses and city parks are very representative garden environments in Beijing, with rich plant species and high bird diversity, making them ideal environments for studying the nocturnal roosting of urban birds. In this study, we investigated the plant use of nocturnal-roosting birds in urban environments in Beijing to provide theoretical support for the management or design of landscaping as a habitat for birds (Huang et al., 2015). Specifically, we wanted to know which landscaping plants provide suitable nocturnal-roosting environments for urban birds. To address this question, we established three objectives: (1) to examine the use of plants by nocturnal-roosting birds and to determine whether nocturnal-roosting birds have a preference for the use of deciduous and evergreen plants; (2) to investigate differences in the use of roosting plants by birds in different seasons; and (3) to identify the use of roosting plants by birds of different body weights. We tested two hypotheses: birds will prefer to use evergreen plants for nocturnal roosting in winter because they can provide good insulation for birds (hypothesis 1); there will be seasonal differences in the use of evergreen and deciduous plants for nocturnal roosting by both large and small birds (hypothesis 2).
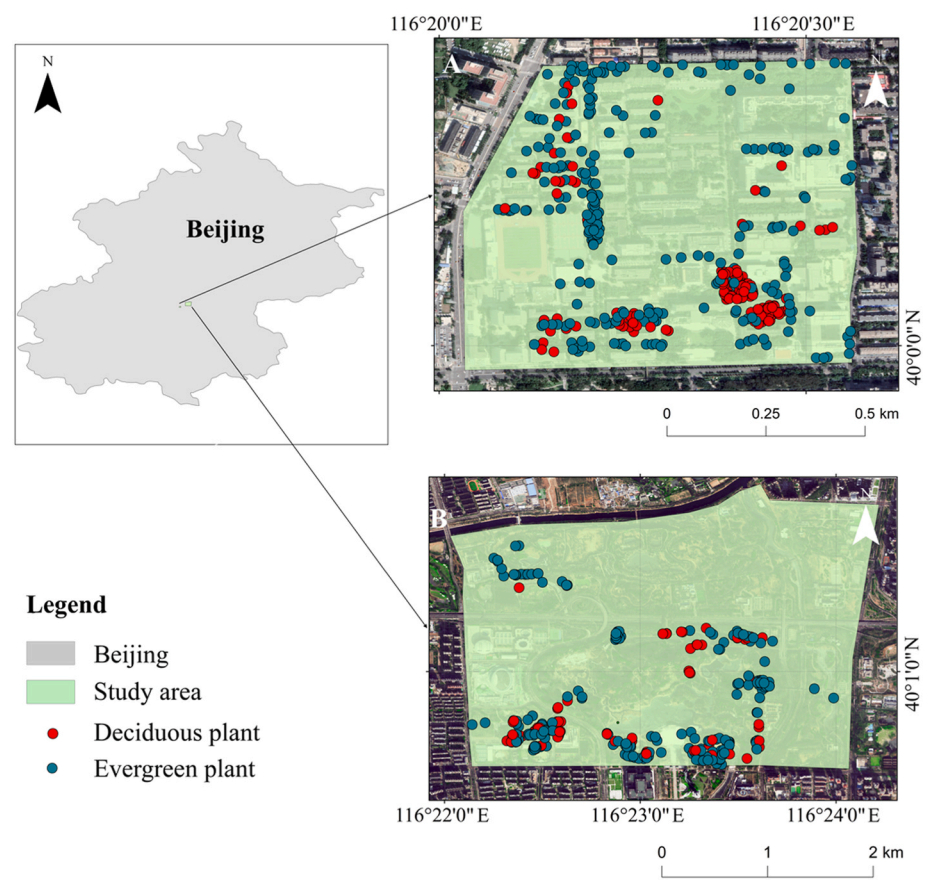
The study was based in Beijing (39°38′–41°05′ N, 115°24′–117°30′ E), China. Overall, 508 species of birds from 22 orders and 74 families have been recorded in Beijing (Zhao et al., 2021). Two sites were analyzed in the current study: Beijing Forestry University and Olympic Forest Park (Fig. 1). Beijing Forestry University is rich in plant species and has a stable plant community structure, with 247 species of plants in 72 families and 147 genera on campus, mainly warm temperate conifers and broad-leaved species (Hu et al., 2018; Chinese University iPlant Association, 2020). Evergreen plants were mainly Juniperus chinensis, Pinus tabuliformis, and Pinus bungeana. Deciduous plants were mainly Fraxinus pennsylvanica, Styphnolobium japonicum, and Malus micromalus. The survey at Beijing Forestry University was conducted during a non-vacation period, and the number of students on campus during the survey period did not change significantly among seasons. Olympic Forest Park boasts a total of 295 species of plants in 75 families and 203 genera, with more than half the number of plant species in Beijing's urban areas, making it a park with high plant diversity (Yu et al., 2012). Evergreen plants were mainly Pinus tabuliformis, Juniperus chinensis, and Platycladus orientalis. Deciduous plants were mainly Lonicera maackii, Fraxinus pennsylvanica, and Ginkgo biloba. The period of the Olympic Forest Park survey was during the COVID-19 pandemic, with less intensity and seasonal variation in human disturbance.
From April 2021 to March 2022, avian sleeping behavior was observed and recorded at the study sites. Surveys started 2 h after sunset, 2–4 h each time, 1–2 times a week. We repeated the surveys at these two survey sites in each season, and the intensity of the surveys was consistent in four seasons. The season division was set as spring (from March 1st to May 31st), summer (from June 1st to August 31st), autumn (from September 1st to November 30th) and winter (from December 1st to February 28th) (Chen et al., 2019; Yu et al., 2019). Surveys were conducted along the same transect and survey areas each season to ensure the same level of human interference on nocturnal-roosting birds among seasons. During the surveys, six individuals walked at a rate of 1 km/h along the sample line looking for nocturnal-roosting birds. Infrared thermography is an effective tool for detecting nocturnal-roosting birds (Mitchell and Clarke, 2019), and the Infiray E2N Thermal Imaging Monocular and Warsun Searchlights were used to spot birds in herbaceous and woody plants at the study sites. When we found nocturnal-roosting birds, bird species were identified using the BOSMA telescope. A Leica DISTO D3a multi-function laser distance measure and a diameter measuring tape were used to measure the perch height of birds, diameter at breast height (DBH, cm), tree height (m), crown width (m) of woody plants, and length (m), width (m), and height (m) of herbaceous plants. The latitude and longitude of the roosting plants were recorded using GPS. The species and number of plants within 20 m on both sides of the survey track were recorded. The animal use protocol of this study was reviewed and approved by the Ethics and Animal Welfare Committee (Approval No. EAWC_BJFU_2022026).
The residence type of birds in the Beijing area was divided as described elsewhere (Zheng, 2023). The birds were divided into dominant species (>10%) and non-dominant species (<10%) according to the proportion of the number of each species to the total number of birds. Published reference values (Tobias et al., 2022) were used for birds' body weight. Plants were classified into woody and herbaceous according to their growth form, and into two plant types, evergreen and deciduous, based on their leaf attributes.
The Shannon–Wiener index was used to calculate nocturnal-roosting bird diversity and roosting plant diversity, and the rarity-weighted richness (RWR) (Williams et al., 1996) to identify key plants. To prevent skewing of the results by the more numerous resident and dominant species which could overwhelm the data on the use of roosting plants by other, less numerous birds, different weights were assigned to each bird species, with birds that used fewer plant species receiving higher weights than those that used more plant species. The calculation process was divided into two steps: (1) birds' rarity was scored by the inverse of the number of species of roosting plants used by birds, with the highest rarity (1.0) for birds using only one plant and the lowest rarity (1/29 or 0.0345) for birds using the largest number of roosting plants; (2) the RWR score for each roosting plant was the sum of the individual scores of all birds using this plant. The formula is RWR =∑ni=11Ti, where Ti represents the number of plant species used by the nocturnal-roosting bird, and n represents the number of birds using the roosting plant. A chi-square test was used to analyze whether there was a significant difference in the use of evergreen and deciduous plants within 20 m on both sides of the survey track by birds. The Kolmogorov–Smirnov test for normal distribution was used to analyze the normality of DBH, tree height, crown width, and birds' perch height of woody plants, with the significance level set at 0.05. When P < 0.05, indicating a normal distribution of variables, one-way ANOVA was used for the analysis of variance. The Kruskal–Wallis test was used for the analysis of the variance of variables that were not normally distributed. We constructed generalized linear mixed models (GLMMs) in the package 'lme4' (Bates et al., 2015) to analyze the effects of season on plant type using the binomial distribution and DBH, tree height, crown width, and birds' perch height using the gamma distribution. We used bird species as a random effect to control its potential effect on the season. We performed multiple comparisons with the Tukey method with the "emmeans" function in the "emmeans" package (Russell, 2022). Spearman correlation analysis was used to analyze the correlation between bird body weight and perch height. Data analysis was performed using R software (v4.4.1) (R Core Team, 2021).
Nocturnal-roosting birds at the study sites were recorded 1865 times, including 649 times at the Olympic Forest Park and 1216 times at the Beijing Forestry University. Overall, 23 species of birds from 3 orders and 12 families were recorded, of which 20 species of birds from 3 orders and 11 families were recorded in the Olympic Forest Park, and 9 species of birds from 2 orders and 8 families were recorded in the Beijing Forestry University (Fig. 2). Overall, 45 species of landscaping plants from 34 genera and 22 families were recorded, of which 16 families, 23 genera and 27 species were recorded in the Olympic Forest Park and 19 families, 23 genera and 27 species were recorded in the Beijing Forestry University (Fig. 2). Nine species of passage migrants (number: 7.61%), eight species of residents (number: 89.01%), five species of summer visitors (number: 10.24%), two species of winter visitors (number: 0.38%), and one species of vagrant visitor (number: 0.05%) were recorded. Some of the species exhibit multiple residence types in Beijing, such as the Chinese Grosbeak (Eophona migratoria), which acts as a summer visitor and vagrant visitor. The four dominant species among the nocturnal-roosting birds were the Azure-winged Magpie (Cyanopica cyanus), Eurasian Tree Sparrow (Passer montanus), Oriental Magpie (Pica Pica), and Spotted Dove (Streptopelia chinensis), accounting for 84.72% of the total number of nocturnal-roosting birds. Nineteen non-dominant species accounted for 15.28% of the total number of nocturnal-roosting birds. The Azure-winged Magpie exhibited the largest use of roosting plant species (29 species), followed by the Eurasian Tree Sparrow (27 species), Oriental Magpie (26 species), Spotted Dove (23 species), and Chinese Blackbird (Turdus mandarinus) (12 species). Similarly, the Azure-winged Magpie showed the highest used plant diversity (Shannon–Wiener index of 2.504), followed by the Eurasian Tree Sparrow (2.341), Spotted Dove (2.282), Oriental Magpie (2.175), and Chinese Blackbird (1.619).
Of the 45 plant species recorded, 9 were evergreen plants (20.00%) and 36 were deciduous plants (80.00%). The number of evergreen plants within 20 m on both sides of the survey track was 20.84%, and the number of deciduous plants was 79.16%. There was a significant difference in the use of evergreen and deciduous plants by birds (χ2 = 567.81, df = 1, P < 0.01), with 41.34% of nocturnal-roosting birds on evergreen plants and 58.66% of birds on deciduous plants out of 1865 nocturnal-roosting birds recorded (Fig. 2). The plant that attracted the highest number of birds was J. chinensis (22.47%) (Appendix Table S1). It also attracted the highest number of bird species (13 species) and the highest bird diversity (Shannon–Wiener index of 1.662), with the highest bird RWR (5.445) (Appendix Table S1). Resident birds used significantly fewer evergreen plants than non-residents (χ2 = 82.000, df = 1, P < 0.01), and dominant species used significantly fewer evergreen plants than non-dominant species (χ2 = 157.91, df = 1, P < 0.01). The DBH (χ2 = 228.39, df = 1, P < 0.01), tree height (χ2 = 455.25, df = 1, P < 0.01), and crown width (χ2 = 676.83, df = 1, P < 0.01) of evergreen plants were significantly smaller than those of deciduous plants (Fig. 3). Furthermore, the perch height of birds during nocturnal roosting on evergreen plants was significantly lower than that on deciduous plants (χ2 = 148.41, df = 1, P < 0.01) (Fig. 3).
Juniperus chinensis was the plant with the highest bird RWR in spring and winter (1.448 and 4.445, respectively), while F. pennsylvanica was the plant with the highest bird RWR in summer and autumn (1.153 and 1.945, respectively) (Appendix Table S1).
There were significant differences in the use of plant types by birds in each season (Z = −2.289, P < 0.01), with deciduous plants comprising the highest proportion of used plants (92.44%) in summer and evergreen plants (75.19%) comprising the highest proportion of used plants in winter (Fig. 4), which is consistent with our first hypothesis. Furthermore, seasonal differences in the use of roosting plants by birds in terms of DBH (t = 7.612, P < 0.01), tree height (t = 5.116, P < 0.01), crown width (t = 5.219, P < 0.01), and perch height (t = 3.887, P < 0.01) were noted. The DBH, tree height, and crown width of plants used by birds were the highest in summer and the lowest in winter (P < 0.01), and the perch height of birds was the lowest in winter (P < 0.01) (Fig. 3).
Spearman correlation analysis revealed a significant positive correlation between bird body weight and perch height among nocturnal-roosting birds (r = 0.51, P < 0.01). Birds on deciduous plants (122.67 ± 2.40 g) weighed significantly more than those on evergreen plants (78.99 ± 1.89 g) (χ2 = 107.95, df = 1, P < 0.01). Nocturnal-roosting birds were classed as large (> 200 g) or small (< 200 g) based on their mean body weight, with significant differences between the body weight of the large (217.61 ± 0.07 g) and small (73.16 ± 1.23 g) birds (χ2 = 1001.7, df = 1, P < 0.01). There was a significant difference in the use of evergreen and deciduous plants by large birds (χ2 = 64.598, df = 1, P < 0.01), with 4.93% of large birds on evergreen plants and 95.07% of large birds on deciduous plants out of 406 large birds recorded. No significant seasonal differences in the use of evergreen and deciduous plants by large birds were apparent (Z = −0.892, P = 0.37), which is inconsistent with our second hypothesis. There was a significant difference in the use of evergreen and deciduous plants by small birds (χ2 = 951.45, df = 1, P < 0.01), with 51.47% of small birds on evergreen plants and 48.53% of small birds on deciduous plants out of 1459 small birds recorded. There was a significant seasonal difference in the use of evergreen and deciduous plants by small birds was noted (Z = −2.203, P < 0.05), with a preference for deciduous plants in summer (90.85%) and evergreen plants in winter (90.63%) (Fig. 5).
This study provides the first description of the use of evergreen and deciduous roosting plants by urban birds in Beijing. Our results show that birds use roosting plants with different characteristics (such as whether they are evergreen or deciduous, DBH, tree height, and crown width) in different seasons. There were no significant differences in the use of plant type by large birds across seasons, but significant differences were seen in small birds.
The proper allocation of landscaping plants can improve bird diversity (Fontana et al., 2011), and appropriate gardening during urbanization can help protect urban bird diversity (Pei et al., 2018). The Eurasian Tree Sparrow, Azure-winged Magpie, and Oriental Magpie are the most abundant birds in Beijing (Zhang et al., 2008; Zheng and Dong, 2019). They use the highest number and diversity of plants when they roost, reflecting their high adaptability to the urban environment (Zhang et al., 2008; Bao et al., 2019). Based on the bird RWR calculations, we concluded that J. chinensis is one of the most important plants in the nocturnal roosting environment and can provide suitable nocturnal roosts for birds. Specifically, J. chinensis also hosted the highest number and diversity of bird species. F. pennsylvanica and Ph. propinqua, which had a high RWR and hosted a high number of bird individuals and species, were equally important in the nocturnal roosting environment. The RWR of Platanus occidentalis was lower than that of the above species. However, this plant hosts a high number of individuals, species, and diversity of birds, suggesting that it is used more often by birds that have a large number. Resident and dominant species used less than 40% of the evergreen plants, while non-resident and non-dominant species used more than 70% of the evergreen plants. This indicates that evergreen plants play a crucial role in shaping the nocturnal-roosting environment of birds, as they are used by more than 40% of birds, although they represent only 20% of the total species of roosting plants. Most of the less abundant birds preferred nocturnal roosting on evergreen plants, which was also evident from their use of plants with a smaller DBH, tree height, and crown width.
According to some studies, one of the biggest mistakes in constructing bird habitats is the introduction of alien plants (Sulaiman et al., 2013), and the inappropriate introduction of alien plants is the most important factor responsible for the decline in bird numbers (Kemp, 2004). However, we showed that the proportion of alien plants used by urban birds in Beijing during winter nocturnal roosting was approximately 20% higher than that in other seasons, reaching 95% (Zhao et al., 2023). Hence, the role of alien plants in the nocturnal roosting of birds should not be ignored during landscape construction [of note, in the current study, the alien plants were classified according to Wang et al. (2012)].
Seasonal changes can impact bird behavior (Morrison et al., 1986), with significant differences in the use of roosting plants by birds during different seasons (Pan et al., 2019). Safety is the primary factor influencing a bird's use of roosting plants (Amo et al., 2011; Yuan et al., 2012). Insulation is another important factor (Buttemer, 1985; Walsberg, 1986). We observed that in summer, birds used a significantly higher percentage of deciduous plants (92.44%) than evergreen plants (7.56%). Plants with larger DBH and height help to ensure the safety of birds during night roosting (Jaggard et al., 2015). Deciduous plants have larger DBH and crown width than evergreen plants, provide diverse microhabitats with higher perches, and have larger crown density before the falling of the leaves, providing a safer habitat for different bird species (Jhenkhar et al., 2016). Since birds in the nocturnal roosting state will be less responsive to external stimuli and have a correspondingly lower response rate to predators (Webb, 1974), the safety of the nocturnal roosting environment is critical (Amo et al., 2011). Studies on the Grey-faced Buzzard (Butastur indicus) (Deng et al., 2003), Western Capercaillie (Tetrao urogallus) (Finne et al., 2000), and Wild Turkey (Meleagris gallopavo) (Phillips et al., 2011) suggest that perching on plants with large crown widths may reduce the chances of predation (Jirinec et al., 2016). Nocturnal roosting on plants with higher tree heights increases the distance between birds and pedestrians and other predators on the ground (Peh and Sodhi, 2002), which helps to protect the roosting safety of birds. Larger plants also help to protect birds from adverse weather and thus afford roost comfort (Clergeau and Quenot, 2007).
Birds prefer to use evergreen plants for nocturnal roosting in cold weather (Thompson and Fritzell, 1988), as their ability to effectively block air convection in winter slows the temperature drop rate and meets the bird's need for insulation at night (Francis, 1976; Walsberg, 1986). For instance, birds, such as the American Goldfinch (Spinus tristis) (Buttemer, 1985) and Western Capercaillie (Tetrao urogallus) (Thiel et al., 2007), favor evergreen plants for nocturnal roosting. In our study, 75.19% of the analyzed birds used evergreen plants in winter, which is generally consistent with the findings of the previous study. Birds rely on energy stored during the day to maintain normal life activities at night. However, as the ambient temperature at night is usually low and maintaining a constant body temperature requires a large amount of energy, the choice of microhabitat during nocturnal roosting is crucial for birds (Walsberg, 1986). During the period after deciduous plants drop their leaves in autumn and before they grow leaves in spring, evergreen plants, although with smaller DBH and crown width than deciduous plants, still show a high degree of depression and can provide good insulation for birds (Walsberg, 1986).
Large birds consume a lower proportion of their total energy reserves during nocturnal roosting and can tolerate cold weather for longer periods than small birds (Reinertsen and Haftorn, 1986). Energy requirements increase during winter as a consequence of the inverse relationship between metabolic rate and ambient temperature (Huertas and Díaz, 2001). Therefore, small birds are more dependent on the insulation provided by roosting plants in winter than large birds and large birds are less restricted to using plants that offer higher security. Deciduous plants provide higher perch height for birds than evergreen plants, which allows birds to avoid predators from the ground better than lower perches (Peh and Sodhi, 2002). Large birds use deciduous plants that provide higher security in all seasons, while small birds are limited by cold weather and can only use evergreen plants with better insulation for nocturnal roosting in winter (Webb and Rogers, 1988). Consequently, the weight of nocturnal-roosting birds found on evergreen plants is significantly smaller than that of birds found on deciduous plants.
We found significant differences in the types and characteristics of plants used by different birds for nocturnal roosting. We suggest improving the diversity of landscaping plants in cities, combining trees and shrubs, avoiding monoculture planting, and providing diverse nocturnal roosting environments for different birds. When carrying out landscape construction to preserve urban bird diversity, attention should be given to the combination of evergreen and deciduous plants, and increasing the planting of plants such as J. chinensis, F. pennsylvanica, Ph. propinqua, Pi. tabuliformis, and U. pumila. Evergreen plants can provide suitable nocturnal habitats for small birds in winter, non-dominant species and non-resident birds in Beijing prefer nocturnal roosts with evergreen plants. Hence, we recommend increasing the species and number of evergreen plants when carrying out landscape construction.
All procedures applied during the research followed the guidelines for animal care outlined by Chinese wildlife conservation laws. The animal use protocol of this study was reviewed and approved by the Ethics and Animal Welfare Committee (Approval No. EAWC_BJFU_2022026).
Yexi Zhao: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. Jiayu Zhang: Investigation. Zihan Li: Investigation. Qinmijia Xie: Investigation. Xin Deng: Investigation. Chenxi Zhang: Investigation. Nan Wang: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Fangyuan Hua, Liang Ma, Chunrong Mi, Zhongwen Jiang, and Kangqing Zhang for their helpful comments and suggestions on the manuscript. Furthermore, we thank Ruiying Bao, Lei Tuo, Ning Xu, and Xinshui Cui for their assistance and help with fieldwork.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100177.
|
Bao, M., Yang, S., Yang, Y., Zhou, S., Li, C., 2019. Tolerance distance of common birds to human disturbances in urban areas. J. Biol. 36, 55–59.
|
|
Deng, W.H., Gao, W., Guang, M., 2003. Nest and roost habitat characteristics of the grey-faced buzzard in northeastern China. J. Raptor Res. 37, 228–235.
|
|
Hu, N., Wang, Y.H., Li, X., 2018. Construction of campus green space from the perspective of green campus—taking Beijing Forestry University as example. Landsc. Archit. 25, 25–31.
|
|
Jhenkhar, M., Jadeyegowda, M., Kushalappa, C., Ramesh, M.N., Sathish, B.N., 2016. Bird diversity across different vegetation types in kodagu, central western ghats, India. Int. J. Zool. Res. 6, 25–36.
|
|
Jiang, X.Y., Zhang, C.J., Zhou, B., Liang, W., 2021. Sleeping in a noisy world: roosting sites of large aggregations of White Wagtails Motacilla alba in a tropical city, China. Ornithol. Sci. 20, 109–113.
|
|
Kemp, D.D., 2004. Exploring Environmental Issues: an Integrated Approach. Routledge, London.
|
|
Liu, Z.L., Ma, H.H., Dai, S.L., 2003. Representation of winter plants in the northern garden landscape. J. Hebei For. Sci. Tech. 1, 47–50.
|
|
Melles, S., Glenn, S.M., Martin, K., 2003. Urban bird diversity and landscape complexity: species–environment associations along a multiscale habitat gradient. Ecol. Soc. 7, 5.
|
|
Morrison, M., With, K.A., Timossi, I., 1986. The structure of a forest bird community during winter and summer. Wilson Bull. 98, 214–230.
|
|
Pan, Y., Gao, J.X., Zhou, K.X., An, S.Q., Yuan, B.D., 2019. Night roosting habitat selection of oriental magpie robin (Copsychus saularis) during breeding and non-breeding stages in urban green land. Chin. J. Ecol. 38, 2772–2779.
|
|
Phillips, C.E., Demaso, S.J., Kuvlesk, W.P., Brennan, L.A., Hewitt, D.G., 2011. Landscape metrics related to Rio Grande wild Turkey winter roosts in south Texas. Proc. Natl. Wild Turk. Symp. 10, 251–264.
|
|
R Core Team, 2021. R: a Language and Environment for Statistical Computing. R Foundation for Stat. Comput.
|
|
Sulaiman, S., Mohamad, N.H., Idilfitri, S., 2013. Contribution of vegetation in urban parks as habitat for selective bird community. Procedia–Soc. Behav. Sci. 85, 267–281.
|
|
Urban Socio-economic Surveys Group, 2001. China City Statistical Yearbook—2001. National Bureau of Statistics of China. China Statistics Press, Beijing.
|
|
Zhao, X.R., Zhu, L., Ye, H., Liu, M., Huang, H.C., Zhao, M., 2021. Birds of Beijing. China Forestry Publishing House, Beijing.
|
|
Zheng, G.M., 2023. A Checklist on the Classification and Distribution of the Birds of China, fourth ed. Science Press, Beijing.
|
|
Zheng, B.J., Dong, L., 2019. Study on plant landscape of Beijing urban linear parks based on bird diversity improvement. Landsc. Archit. 26, 53–57.
|