| Citation: | Martín Alejandro Colombo, Adrián Jauregui, Luciano N. Segura. 2024: Weather influenced nestling growth of an insectivorous but not a granivorous grassland passerine in Argentina. Avian Research, 15(1): 100173. DOI: 10.1016/j.avrs.2024.100173 |
Nestling growth of birds can be affected by weather fluctuations. In general, it is expected that higher temperatures favor growth by improving food availability and nestling metabolism, while rain hinders it by reducing foraging efficiency. However, most of these patterns have been described in insectivorous cavity-nesting birds in temperate forests. We tested these predictions in two neotropical grassland ground-nesting birds with contrasting nestling diets and therefore potentially different responses to weather. We measured nestlings of the Hellmayr's Pipit (Anthus hellmayri, an insectivorous passerine) and the Grassland Yellow-Finch (Sicalis luteola, which feeds its nestlings exclusively with seeds) during three breeding seasons (2017–2020) in central-eastern Argentina. We took measurements of tarsus and body mass, modeled growth curves using nonlinear mixed-effects models, and evaluated the effects of minimum daily temperature and precipitation during the growth period and the 30 days prior to hatching. For pipits (60 nestlings from 21 nests), minimum temperatures during the growth period were positively associated with tarsus and body mass asymptotes. Also, there was a positive association between precipitation during the pre-hatching period and tarsus asymptote. Conversely, none of the weather variables analyzed had significant effects on nestling growth of finches (131 nestlings from 35 nests). Dietary contrast between species may explain the different results. Arthropod activity and abundance can be affected by weather variations within the span of a breeding season, whereas seeds may depend on conditions from previous years, making the effects harder to detect. Fledglings with reduced asymptotic size can have reduced chances of survival. Hence, pipit populations could be impacted if they experience cold and dry conditions during their breeding season, which is of major relevance in the current context of climate change.
Climate is one of the major factors governing ecosystems and is currently changing more rapidly than ever before (IPCC, 2023). Animals can adapt and respond to predictable changes in weather, although they may be challenged in the current context of global climate change, with long-term consequences being hard to predict (Charmantier et al., 2008; Cohen et al., 2018). In addition to changes at the population or community level induced by global climate conditions, animals also respond individually to short-term weather fluctuations at a smaller scale (Frick et al., 2012; Mainwaring and Hartley, 2016). Their response to weather fluctuations ultimately depends on their species' life history traits and ecological role. Studying how animals respond to short-term weather fluctuations is important to understand the consequences of changes in their immediate environment and can also be helpful to predict the effects of larger scale climate changes.
Among animals, birds are a valuable group for studying the effects of weather because they are affected in different ways throughout their life cycle. Some species respond by shifting their distribution ranges (Charmantier et al., 2008) or migration patterns (Cox, 2010). In the span of a breeding season, weather can have different effects at different stages of the breeding cycle, including egg laying, incubation, early- and late-nestling periods, and after fledging (Kosicki and Indykiewicz, 2011; Facey et al., 2020). Altricial birds complete a major part of their development during the nestling stage, making this a critical period in which weather can have a significant impact. Therefore, understanding which factors affect nestling growth is important because birds that leave the nest in suboptimal condition can have reduced fitness or reproductive performance as adults (Andrew et al., 2017; Sauve et al., 2021), which can have long lasting consequences for their populations.
Low air temperatures during early stages of nestling growth can have a direct detrimental effect because of the high energetic cost of heat generation (Rodríguez and Barba, 2016). Cold temperatures can also affect young nestlings indirectly by forcing parents to spend more time brooding to keep them warm, allowing less time for food search and delivery (Öberg et al., 2015). Another important indirect effect is related to food abundance and availability, given that higher temperatures usually favor the abundance of arthropods (Rypstra, 1986; Sauve et al., 2021). Precipitation levels are also expected to play an important role in nestling growth. Abundant rainfall during the nestling stage can reduce the availability of many food items and hinder food searching by adult birds, reducing nestling provisioning rate (Mainwaring and Hartley, 2016; Cox et al., 2019). In addition, although high precipitation usually has a positive effect on overall arthropod biomass (Frampton et al., 2000; Pinheiro et al., 2002), a combination of extreme precipitation and low temperatures can have negative effects on arthropods, severely impacting nestling growth of passerines (Pérez et al., 2016).
The effects of weather on nestling growth are not broadly generalizable across habitats, regions, and species (Tuero et al., 2018; Sauve et al., 2021). Research on the subject has been conducted mostly in the northern hemisphere and is heavily biased toward a few cavity nesting species that use nest boxes (for example, Mainwaring and Hartley, 2016; Rodríguez and Barba, 2016; Cox et al., 2019; Marques-Santos and Dingemanse, 2020). Few studies have analyzed nestling growth of grassland birds (Bradbury et al., 2003; Pérez et al., 2016), which are more exposed and could be more sensitive to weather fluctuations. Moreover, little research on nestling growth has been conducted in the Neotropics, which have their own climatic conditions, seasonal regime, and projected climate change (Lopes et al., 2023).
It is also noteworthy that most studies focused on passerines that feed their nestlings with arthropods (Węgrzyn, 2013; Mainwaring and Hartley, 2016). To this day, virtually no studies have analyzed the growth of grassland birds that feed their nestlings with seeds. Given that grassland plants and arthropods may have specific responses to weather fluctuations (Barnett and Facey, 2016; Dudney et al., 2017), the effects of weather on nestling growth may vary between birds with different diets (Bradbury et al., 2003; Wheelwright et al., 2022). For instance, grass seed production is mostly determined by long term weather conditions spanning years (Dudney et al., 2017); hence, it is expected that food availability for granivorous birds is not greatly affected by short-term weather variations occurring during nestling growth. In contrast, the abundance and activity of many arthropods are affected by daily air temperature and rainfall (Bale et al., 2002; Barnett and Facey, 2016; Belitz et al., 2021). Therefore, it is expected that food availability for insectivorous birds is more responsive to short-term weather fluctuations.
For this study we assessed if variation in air temperature and precipitation influenced nestling growth of two neotropical grassland birds with contrasting nestling diets. We measured nestlings of the insectivorous Hellmayr's Pipit (Anthus hellmayri Hartert, 1909) and the granivorous Grassland Yellow-Finch (Sicalis luteola (Sparrman, 1789)) during three breeding seasons in a temperate grassland of Argentina. We investigated two temporal scales of analysis: immediate effects of conditions during the nestling stage which could affect metabolism and provisioning rate, and effects of conditions over the 30 days prior to hatching which could affect food abundance and availability. Our first prediction was that growth of both species would be favored by higher air temperatures during the nestling stage because of reduced cost of thermoregulation and more time available for adults to search for food. The second prediction was that abundant rainfall during the nestling stage would have a negative influence on growth because of reduced adult foraging efficiency. Our last prediction was that insectivorous nestlings would be more strongly affected by weather conditions at both temporal scales than granivorous nestlings, because compared to grass seeds, arthropod availability and abundance are more sensitive to weather variability.
We conducted this study on a private farm located in Punta Indio, northeast of Buenos Aires province, Argentina (35°20ʹ S, 57°11ʹ W). The farm comprises an area of ~2000 ha, mainly represented by grasslands and approximately 15% by forests. Grasslands in the farm are a natural result of its particular climate and soil conditions and are composed mainly of native species such as Nassella spp., Paspalidium spp., Leersia hexandra, and Baccharis spp., among other less abundant native and exotic grasses (Roitman and Preliasco, 2012). Most of the farm's surface is dedicated to extensive cattle-grazing.
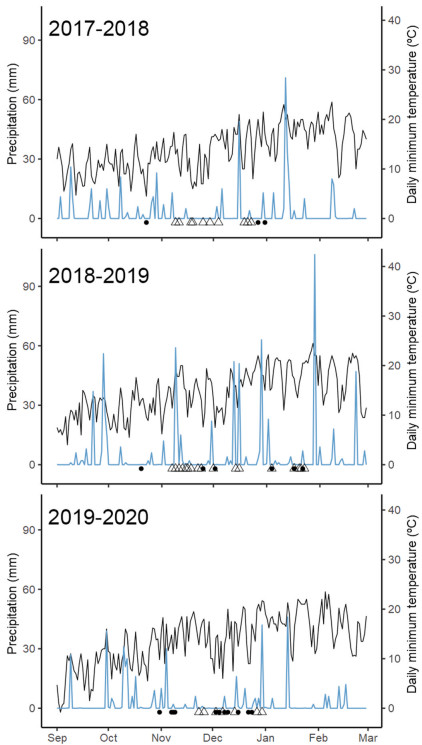
The area is located within the Flooding Pampa, a sub-region of the Pampas Grasslands characterized by frequent seasonal floods due to the poor drainage properties of the soil (Matteucci, 2012). The climate of the sub-region is temperate sub-humid, with usual rainfall peaks during winter to early spring (July–September) and late summer (end of January–February) (Matteucci, 2012). Weather data registered during the study period are presented in Fig. 1.
The Hellmayr's Pipit (hereafter 'pipit') is a small insectivorous passerine endemic to South America. It inhabits short-to medium-height grasslands in three allopatric areas from southern Brazil to southern Argentina and builds its nests on the ground (Norambuena et al., 2022; Colombo and Segura, 2023). Clutch size is typically four eggs, hatching is asynchronous (three nestlings are born the same day and one after) after 14 days of incubation, and nestlings remain in the nest for an average 11.5 days after hatching (Colombo and Segura, 2023). Both parents feed their young exclusively with insects which are captured in short races on the ground (M.A. Colombo, pers. observation). Breeding season in the study area spans from late September to mid-February (Colombo and Segura, 2023).
The Grassland Yellow-Finch (hereafter 'finch') is a granivorous passerine that inhabits medium-height and tall grasslands across Central and South America (Rising et al., 2020). It is a common species that nests directly on the ground or a few centimeters above (Freitas and Francisco, 2012). Clutch size ranges from three to six eggs, which hatch synchronously after 11 days of incubation (Salvador and Salvador, 1986; M.A. Colombo, unpubl. data). Nestlings are fed with seeds and remain in the nest for an average of 10.5 days before fledging (Salvador and Salvador, 1986; M.A. Colombo, unpubl. data). Adults collect seeds from tall and short grasses on the ground (M.A. Colombo, pers. observation). Breeding season in the study area spans from late October to early February.
We searched for pipit and finch nests from September to February during three breeding seasons (2017–2018, 2018–2019, and 2019–2020). We found nests during the incubation stage by rope-dragging or systematic walking, and during the nestling stage by observing adults delivering food (for more details on nest search and monitoring, see Colombo and Segura, 2023). We visited nests daily in the final days of incubation to obtain reliable hatching dates to estimate nestling age. For nests found after hatching (nine for pipits and six for finches), we estimated nestling age by visual cues, including: opening of eyes, feather coverage, and development of feathers. We considered hatch day as age = 0 days. During the nestling stage, we visited nests every one or two days to obtain body measurements. We marked nestlings with non-toxic permanent markers at each visit for individual identification in subsequent visits. We used vernier calipers to obtain tarsus length to the nearest 0.1 mm. We used 10, 20, or 50 g capacity Pesola spring balances to obtain nestling body mass with 0.1 g, 0.2 g, and 0.5 g precision, respectively, depending on nestling size at each visit. To avoid disturbance in the nest surroundings and reduce the scent trails near the nest that could be followed by predators, all measurements were taken at a distance of ~10 m from the nest. We avoided measuring pipits and finches after they were 10 and 8 days old, respectively, to reduce the risk of premature fledging (at those ages, nestlings reach adult tarsus length and ~95% of body mass, M.A. Colombo, Unpubl. data).
We obtained daily minimum temperatures (℃) and precipitation (mm) data from the nearest weather station (Punta Indio, SMN), located 8 km from the study site. We chose daily minimum as a proxy of temperature because the weather station does not provide 24-h data to calculate a real mean, and it showed high correlation with the daily maximum (Spearman's r = 0.69). For each nestling, we defined the 'immediate' temporal scale as the four-day period during which nestlings were two to six days old. This includes the inflection point (maximum growth rate) and the time window when adults stop brooding, leaving nestlings more exposed to weather conditions. We then calculated the mean minimum temperature and the total precipitation for the immediate growth period of each nestling. We included a second temporal scale to analyze effects of conditions over the 30 days prior to hatching (hereafter, 'pre-hatching' scale), for which we obtained the mean minimum temperature and the total precipitation.
We fitted nestling growth curves of tarsus and body mass to Richard's equations, building non-linear mixed models using the W0 parameterization suggested by Tjørve and Tjørve (2010): yt = A (1 + ((W0/A) (1–d) – 1) exp (−kt/d d)) 1/(1–d), where yt is the size at age t (in days), W0 is the size at age = 0 (i.e., size at hatching), A is the upper asymptote, k is the maximum relative growth rate (1/days unit), and d is the shape parameter, a unit-less number that represents the proportion of the asymptote at which the inflection is reached (Tjørve and Tjørve, 2010). Due to the lack of measurements at age = 0 for most nestlings, we fixed the value of W0 to the mean size at hatching (Svagelj and Quintana, 2017) (pipits: W0 tarsus = 6.80 ± 0.14 mm, W0 mass = 2.23 ± 0.41 g, n = 16 nestlings; finches: W0 tarsus = 6.49 ± 0.11 mm, W0 mass = 2.33 ± 0.05 g, n = 36 nestlings). We only used data from nestlings with at least three measurements that covered the range between two and eight days old and used age with a precision of 0.5 days. To account for the lack of independence due to repeated measurements of the same individuals from the same nests, we included the identity of each nestling and nest as random factors (Svagelj, 2019). We first created a null model to obtain the population growth curves of tarsus and body mass for both species and describe their average curve parameters. We also calculated the maximum absolute growth rate (A × k), which represents the maximum daily growth rate reached during the growing period (Tjørve and Tjørve, 2010).
We analyzed the effects of weather covariates on curve parameters A and k using two models that represented the two temporal scales (immediate and pre-hatching). Shape parameter d is typical of each species and can be considered constant (Tuero et al., 2018). We fitted growth curves of tarsus and body mass for each species including all mentioned random factors and weather variables. Given that monitoring occurred through multiple years with different overall weather conditions (see Fig. 1), we also included season as a three-level random factor, one level corresponding to each breeding season. For both scales, we centered the temperature variable by subtracting the mean from individual values to produce more biologically meaningful parameter estimates. The models of pipits' tarsus and weight as well as finches' weight showed heteroscedasticity, so we included a power variance function to allow variance to increase with the mean of fitted values.
To find the most parsimonious models, we used a stepwise procedure to obtain minimum adequate models by testing the significance of random effects for nest and season (we kept nestling identity in all models to the lack of independence of individual measurements) using likelihood-ratio tests, and of fixed effects (temperature and precipitation) using F-statistics, sequentially discarding non-significant effects (p > 0.05), refitting the model until all non-significant effects had been removed (Crawley, 2015). We followed the same procedure for both temporal scales. We performed all analyses in software R (R Core Team, 2022), using the nlme package (Pinheiro and Bates, 2022), and report results as mean ± SE.
The tarsus growth curve had an upper asymptote (A) of 25.46 ± 0.27 mm, maximum relative growth rate (k) of 0.098 ± 0.002 days−1, and shape parameter (d) of 3.50 ± 0.31 (n = 60 nestlings from 21 nests). The maximum absolute growth rate was 2.49 mm day−1. The body mass growth curve had an asymptote of 18.26 ± 0.53 g, maximum relative growth rate of 0.124 ± 0.005 days−1, and shape parameter of 2.13 ± 0.19 (n = 60 nestlings from 21 nests) (see Appendix Fig. S1). The maximum absolute growth rate was 2.24 g day−1.
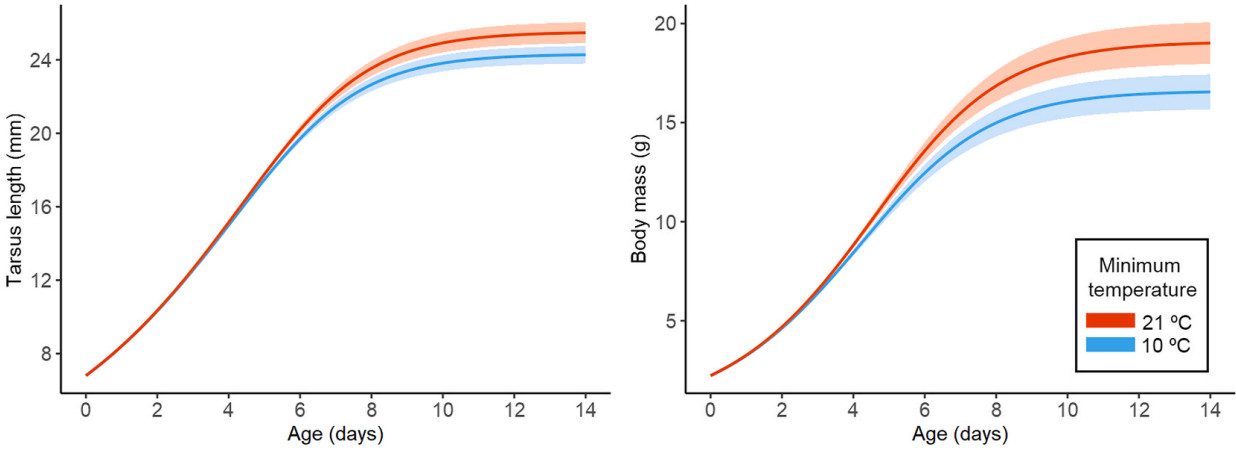
At the immediate scale, the minimum adequate models for pipits showed that mean minimum temperature had significant influence on tarsus and body mass. This variable had a positive relationship with the upper asymptote A (Table 1), suggesting that nestlings that experienced days with higher minimum temperatures fledged with a significantly longer tarsus and higher mass, compared to those that experienced lower temperatures (Table 1, Fig. 2). None of the minimum adequate models included effects of precipitation at the immediate time scale (see Appendix Tables S1–S2).
| Time scale | Measure | Parameter | Variable | Estimate ±SE | df | t | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immediate | Tarsus length | A | Intercept | 24.865±0.234 | 152 | 106.05 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T | 0.104±0.046 | 152 | 2.27 | 0.02 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.103±0.003 | 152 | 33.06 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 4.010±0.266 | 152 | 24.45 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 17.738±0.414 | 152 | 42.84 | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T | 0.215±0.086 | 152 | 2.49 | 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.129±0.005 | 152 | 21.75 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.452±0.196 | 152 | 12.51 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre-hatching | Tarsus length | A | Intercept | 24.684±0.232 | 152 | 106.34 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pp | 0.006±0.002 | 152 | 2.89 | 0.004 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.099±0.003 | 152 | 37.77 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 3.45±0.12 | 152 | 14.08 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 17.493±0.390 | 153 | 44.79 | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.131±0.006 | 153 | 22.27 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.539±0.200 | 153 | 12.67 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| We fitted mixed-effects models to a Richard's equation with the parameters A (upper asymptote), k (maximum relative growth rate), d (shape parameter), and fixed W0 (size at age=0). Immediate time scale=4 days within the growing period. Pre-hatching scale=30 days prior to hatching. In the initial model for each measure, we included precipitation (pp) and mean minimum temperature (T) as predictor variables for A and k at each different time scale, while d was considered constant within species. We only kept significant effects in the final models. SE=standard error, df=degrees of freedom, t=test statistic, p=p-value of each estimate. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
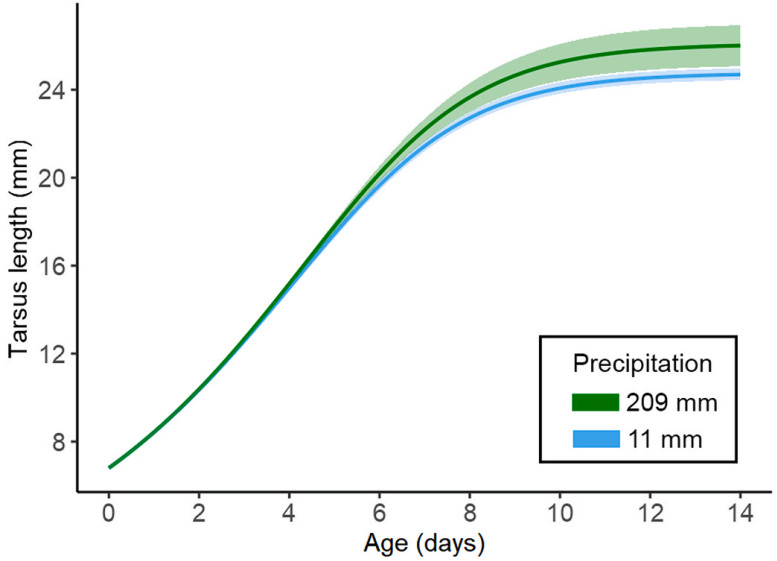
At the pre-hatching scale, the minimum adequate model included only the effects of total precipitation, which had a positive effect on the upper asymptote of the tarsus (Table 1), meaning that rainy conditions before hatching improved the size of the tarsus at fledging (Fig. 3). None of the variables at the pre-hatching scale affected the body mass curve (see Appendix Tables S1–S2).
The tarsus growth curve had an upper asymptote of 18.92 ± 0.21 mm, a maximum relative growth rate of = 0.11 ± 0.003 days−1, and a shape parameter of 5.99 ± 0.59 (n = 131 nestlings from 35 nests). The maximum absolute growth rate was 2.08 mm day−1. The body mass growth curve had an upper asymptote of 14.38 ± 0.28 g, a maximum relative growth rate of 0.12 ± 0.004 days−1, and a shape parameter of 2.54 ± 0.18 (n = 131 nestlings from 35 nests) (see Appendix Fig. S2). The maximum absolute growth rate was 1.72 g day−1. None of the minimum adequate models included effects of weather variables on any parameter at either temporal scale (Table 2, see also Appendix Tables S3–S4).
| Measure | Parameter | Variable | Estimate ±SE | df | t | p | |||||||||||||||||||||||||||||||||||
| Tarsus length | A | Intercept | 18.862±0.165 | 345 | 114.09 | – | |||||||||||||||||||||||||||||||||||
| k | Intercept | 0.116±0.004 | 345 | 31.90 | – | ||||||||||||||||||||||||||||||||||||
| d | Intercept | 6.29±0.599 | 345 | 10.52 | – | ||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 14.335±0.434 | 345 | 33.03 | – | |||||||||||||||||||||||||||||||||||
| k | Intercept | 0.123±0.005 | 345 | 25.86 | – | ||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.56±0.185 | 345 | 13.85 | – | ||||||||||||||||||||||||||||||||||||
| We fitted mixed-effects models to a Richard's equation with the parameters A (upper asymptote), k (maximum relative growth rate), d (shape parameter), and fixed W0 (size at age=0) and evaluated the effects of minimum temperature and precipitation at the immediate time scale (4 days within the growing period) and the pre-hatching scale (30 days prior to hatching) and sequentially discarded non-significant parameters. SE=standard error, df=degrees of freedom, t=test statistic, p=p-value of each estimate. | |||||||||||||||||||||||||||||||||||||||||
Our results showed that nestling growth of the insectivorous pipit improved with higher temperatures at the immediate time scale during the nestling period, and with higher precipitation over the period of 30 days prior to hatching. Conversely, none of the weather variables had a significant effect on nestling growth of the granivorous finch. These results partially support our first prediction that higher temperatures during the nestling period should favor their growth. They also support our third prediction that insectivorous nestlings should be more affected by weather variables than granivorous ones, at least at the time scales studied.
Lower minimum temperatures at the immediate time scale reduced the maximum size attained by pipits, suggesting that cold snaps during the nestling stage can have an important detrimental effect on development. Cold snaps could reduce nestling growth due to physiological constraints because nestlings growing in cold environments face a trade-off between maintaining optimal body temperature and allocating energy to develop body traits (Dawson et al., 2005). For example, Larson et al. (2015) and Rodríguez and Barba (2016) found similar results when studying nest box temperatures of Crimson Rosella (Platycercus elegans) and Great Tit (Parus major), respectively, suggesting that lower temperatures experienced by nestlings before being fully feathered have detrimental effects on their final size. Although we did not assess nest microclimatic conditions due to logistical constraints, air temperature is a good proxy of the temperature that pipit nestlings experience because their nests are open cups without much insulation (Martin et al., 2017). However, finches did not suffer similar effects of cold snaps, which leads us to believe that the physiological effect of temperature was weak (at least within the observed temperature ranges) and food availability had a more prominent role, because seed availability is unlikely to be affected by short-term temperature drops. Nonetheless, studying nest microclimate differences between species could be useful to understand to what extent air temperature outside the nest affects nestling thermoregulation.
For insectivorous birds, minimum temperatures can affect the immediate availability of their prey items, limiting their food provisioning rate. Previous studies in other species have found that availability of their preferred insects is likely to decrease in cold snaps (Arlettaz et al., 2010; Whitehouse et al., 2013; Garrett et al., 2022). In general, insects are more active during warmer days and become more abundant as the extent of the warm period increases (Williams, 1961; Briers et al., 2003). Although further research is needed to completely understand pipit nestlings' diet, we observed and documented events of feeding with caterpillars and moths (Lepidoptera), katydids and grasshoppers (Othoptera: Tettigoniidae and Acrididae), spiders (Araneae), and some small unidentified flying insects (M.A. Colombo, unpubl. data). Studying the daily abundance patterns of these arthropods and the provisioning rates of pipits in further detail will elucidate the underlying mechanism of temperature effects on growth. It is worth mentioning that daily minimum temperature tended to increase throughout the breeding season (see Fig. 1); hence pipits nesting later in the season could have higher chances of raising their nestlings under favorable temperatures. However, if temperature exceeds critical thresholds, heat stress could impair nestling growth (Cunningham et al., 2013) or even cause mortality of young nestlings with limited thermoregulation capabilities (Murphy, 1985), a scenario which is more likely to happen late in the season (end of January to February) when temperatures can surpass 37 ℃. Further research on critical temperature thresholds will be of great help in the current context of global warming.
Neither pipits nor finches were influenced by precipitation at the immediate time scale, which does not support our prediction that rainfall during the nestling stage would deter growth by reducing foraging efficiency of adult birds. Previous studies suggesting this mechanism are heavily focused on aerial insectivorous birds, which may see their flight capabilities reduced under heavy rain and encounter fewer insects flying (Cox et al., 2019; Garrett et al., 2022). Our results could be explained by the fact that pipits obtain food for their nestlings while walking on the ground and therefore are not affected by the potential difficulties of flying under the rain nor by fewer insects available in the air, while finches search for grass seeds, for which availability and abundance are not greatly affected by rainfall within a few days.
In contrast, more precipitation during the 30-day pre-hatching period was positively related to pipits' tarsus asymptote. Many studies have found that arthropod diversity and abundance is higher in wetter conditions (Poulin et al., 1992; Supriya et al., 2019). Williams (1961) showed that it is possible to correlate insect abundance with rainfall from previous months, especially during the summer season, and argued that this was due to a positive effect on abundance rather than increased activity (which is more related to daily changes in weather). Rainy seasons improved nestling growth for Scissor-tailed Flycatcher (Tyrannus forficatus) presumably due to higher arthropod abundance (Tuero et al., 2018). Although abundance patterns of grassland arthropods need to be assessed, evidence from other habitats has shown that arthropod abundance is generally positively associated with rainfall within a season (Pinheiro et al., 2002; Jahn et al., 2010). We believe that rainy periods are beneficial for pipit nestlings' growth due to an overall positive effect on arthropod abundance, although it should be kept in mind that extreme rain events can cause nest flooding. For example, during the study period, seven out of 93 pipit nests were flooded after precipitation events between 25 and 106 mm (Colombo and Segura, 2023), so extremely rainy breeding seasons may not be beneficial for populations.
In contrast, finches were not affected by pre-hatching precipitation, suggesting that seed abundance depends more on rainfall from the previous seasons or year (Crowley and Garnett, 1999; Blendinger and Ojeda, 2001). Further research on the seeds selected by finches and their availability would greatly improve our understanding of the influence of weather on seed-eating passerines.
Although weather effects are complex to analyze due to the large number of factors involved (Mainwaring and Hartley, 2016), we found that nestling growth of insectivorous grassland birds can be influenced by intra-seasonal variation in temperature and precipitation. These results provide insights into the potential impacts of weather fluctuations on nesting neotropical grassland birds, which have not been deeply studied in this aspect of their breeding biology. Knowing how grassland birds react to weather is helpful to predict their future responses and their likelihood of survival in the current context of global climate change (Sauve et al., 2021). Further studies on nestling provisioning and food availability would be a good complement to fully address the mechanisms underlying these patterns. Additionally, given the very low nest success of grassland passerines in the region (Colombo et al., 2021; Colombo and Segura, 2023), studying post-fledging survival would be valuable to understand the implications of reduced nestling asymptotic size in cold and dry weather for their populations. Current projections of climate change in the Pampas region predict an increase in mean temperature (Müller et al., 2021). It is also expected that the frequency and intensity of extreme weather events caused by El Niño – La Niña oscillations will increase in all South America (Cai et al., 2020). The recent extreme drought in Argentina from 2019 to 2023 (Naumann et al., 2021) is an alarming example of this trend, which could severely impact nestling growth of insectivorous grassland birds. In this scenario, it is very important to continue studying the effects of weather changes on all aspects of grassland birds' biology and to closely monitor their populations.
Fieldwork methodology and bird handling were approved by the state environmental agency Organismo Para el Desarrollo Sostenible (OPDS Research permit #17717, Dirección de Áreas Naturales Protegidas, Buenos Aires province, Argentina).
Martín Alejandro Colombo: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Adrián Jauregui: Writing – review & editing, Validation, Software, Methodology, Investigation, Formal analysis. Luciano N. Segura: Writing – review & editing, Visualization, Validation, Project administration, Investigation, Funding acquisition, Conceptualization.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank M.L. Shaw for allowing us to conduct this study in Estancia 'Luis Chico'. We also thank E. Gonzalez, C. Tiernan, A. Wolf, B. Vidrio, A. Valencia, T. Lansley, C. Dudley, A. Banges, M. Gilles, A. Hodges, L. Haag, S. Musgrave, A. Miller, B. Ewing, K. Depot, and K. McPartlin for help with data collection. We appreciate the improvements in English usage made by K. Depot.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100173.
| Time scale | Measure | Parameter | Variable | Estimate ±SE | df | t | p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immediate | Tarsus length | A | Intercept | 24.865±0.234 | 152 | 106.05 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T | 0.104±0.046 | 152 | 2.27 | 0.02 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.103±0.003 | 152 | 33.06 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 4.010±0.266 | 152 | 24.45 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 17.738±0.414 | 152 | 42.84 | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T | 0.215±0.086 | 152 | 2.49 | 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.129±0.005 | 152 | 21.75 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.452±0.196 | 152 | 12.51 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pre-hatching | Tarsus length | A | Intercept | 24.684±0.232 | 152 | 106.34 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pp | 0.006±0.002 | 152 | 2.89 | 0.004 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.099±0.003 | 152 | 37.77 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 3.45±0.12 | 152 | 14.08 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 17.493±0.390 | 153 | 44.79 | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| k | Intercept | 0.131±0.006 | 153 | 22.27 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.539±0.200 | 153 | 12.67 | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| We fitted mixed-effects models to a Richard's equation with the parameters A (upper asymptote), k (maximum relative growth rate), d (shape parameter), and fixed W0 (size at age=0). Immediate time scale=4 days within the growing period. Pre-hatching scale=30 days prior to hatching. In the initial model for each measure, we included precipitation (pp) and mean minimum temperature (T) as predictor variables for A and k at each different time scale, while d was considered constant within species. We only kept significant effects in the final models. SE=standard error, df=degrees of freedom, t=test statistic, p=p-value of each estimate. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Measure | Parameter | Variable | Estimate ±SE | df | t | p | |||||||||||||||||||||||||||||||||||
| Tarsus length | A | Intercept | 18.862±0.165 | 345 | 114.09 | – | |||||||||||||||||||||||||||||||||||
| k | Intercept | 0.116±0.004 | 345 | 31.90 | – | ||||||||||||||||||||||||||||||||||||
| d | Intercept | 6.29±0.599 | 345 | 10.52 | – | ||||||||||||||||||||||||||||||||||||
| Body mass | A | Intercept | 14.335±0.434 | 345 | 33.03 | – | |||||||||||||||||||||||||||||||||||
| k | Intercept | 0.123±0.005 | 345 | 25.86 | – | ||||||||||||||||||||||||||||||||||||
| d | Intercept | 2.56±0.185 | 345 | 13.85 | – | ||||||||||||||||||||||||||||||||||||
| We fitted mixed-effects models to a Richard's equation with the parameters A (upper asymptote), k (maximum relative growth rate), d (shape parameter), and fixed W0 (size at age=0) and evaluated the effects of minimum temperature and precipitation at the immediate time scale (4 days within the growing period) and the pre-hatching scale (30 days prior to hatching) and sequentially discarded non-significant parameters. SE=standard error, df=degrees of freedom, t=test statistic, p=p-value of each estimate. | |||||||||||||||||||||||||||||||||||||||||