| Citation: | Ting Jin, Shuai Lu, Yunqi Wang, Junqin Hua, Zhengxiao Liu, Qian Hu, Yating Liu, Yuze Zhao, Jianqiang Li, Jiliang Xu. 2024: The clutch size, incubation behavior of Reeves's Pheasant (Syrmaticus reevesii) and their responses to ambient temperature and precipitation. Avian Research, 15(1): 100168. DOI: 10.1016/j.avrs.2024.100168 |
Weather conditions play a pivotal role in embryo development and parental incubation costs, potentially impacting the clutch size and incubation behavior of birds. Understanding these effects is crucial for bird conservation. Reeves's Pheasant (Syrmaticus reevesii) is a threatened species endemic to China, which is characterized by female-only incubation. However, there is a lack of information regarding the impact of weather conditions on clutch size and incubation behavior in this species. Using satellite tracking, we tracked 27 wild female Reeves's Pheasants from 2020 to 2023 in Hubei Province, China. We explored their clutch size and incubation behavior, as well as their responses to ambient temperature and precipitation. Clutch size averaged 7.75 ± 1.36, had an association with average ambient temperature and average daily precipitation during the egg-laying period, and was potentially linked to female breeding attempts. Throughout the incubation period, females took an average of 0.73 ± 0.46 recesses every 24 h, with an average recess duration of 100.80 ± 73.37 min and an average nest attendance of 92.98 ± 5.27%. They showed a unimodal recess pattern in which nest departures peaked primarily between 13:00 and 16:00. Furthermore, females rarely left nests when daily precipitation was high. Recess duration and nest attendance were influenced by the interaction between daily mean ambient temperature and daily precipitation, as well as day of incubation. Additionally, there was a positive correlation between clutch size and recess duration. These results contribute valuable insights into the life-history features of this endangered species.
Clutch size and incubation are important components of avian reproduction (Wiebe and Martin, 1995, 2000). As a typical life-history trait in avian species, clutch size reflects the diverse breeding investments of females. The variations in clutch size may influence offspring quality and contribute to variations in offspring survival (Martin et al., 2000; Christians, 2002; Hope et al., 2021). Incubating birds usually need to strike a balance between their own physiological needs and the thermal requirements of embryonic development (Conway and Martin, 2000a; Cresswell et al., 2003). In addition, incubation behaviors play a crucial role in determining the reproductive success and survival of incubating birds, reflecting their adaptive strategies in response to the environment (Spiegel et al., 2012). Therefore, it is essential to comprehend the life-history strategies of avian species by gaining an understanding of clutch size, incubation behavior, and potential factors that may affect them (Conway and Martin, 2000b; Evans et al., 2009; Mougeot et al., 2014). Exploring factors that influence the life-history traits and reproductive behaviors of endangered species can provide valuable insights into the underlying mechanisms of population decline and develop effective management strategies (Van Turnhout et al., 2010).
Large clutch size incurs high reproductive costs for females, including reductions in future fecundity, and survival (Williams, 2005). Clutch size may be influenced by spring weather during and before the egg-laying period (Cresswell and McCleery, 2003). Therefore, it is essential for females to maintain an optimal clutch size to effectively adapt to changes in weather conditions (Pendlebury and Bryant, 2005; Conrey et al., 2016). Besides, the incubation behavior of birds can be influenced by a variety of factors, including abiotic factors such as ambient temperature, precipitation, and solar radiation (Brown and Downs, 2003; Coe et al., 2015; Amininasab et al., 2016), as well as biotic factors such as predation risks, human disturbance, weight and age of incubating birds (Cervencl et al., 2011; Spiegel et al., 2012; Tombre et al., 2012; Brynychová et al., 2020). In species with uniparental incubation, incubating birds are usually considered to be constrained by their physiological conditions and ability to store nutrients, and must balance the time spent in foraging and incubating (Cresswell et al., 2004; Setash et al., 2021). Because foraging outside the nest may expose their eggs to the risks of cooling or overheating as the changing ambient temperatures are usually outside the optimal temperature range for embryonic development (Brown and Downs, 2003; DuRant et al., 2013; Mougeot et al., 2014). For instance, lower ambient temperatures may lead to a reduced recess frequency and duration of incubating birds (Bueno-Enciso et al., 2017). Precipitation is also assumed to affect the incubation behavior of birds, as some species, especially those with open or ground nests vulnerable to soaking, may choose to stay at the nests to protect embryos (Martin et al., 2017; Schöll et al., 2019). For example, Coe et al. (2015) have found that female Tree Swallows (Tachycineta bicolor) tend to spend less time outside their nests when precipitation occurs. Therefore, ambient temperature and precipitation play a crucial role in shaping the incubation behavior of birds.
Reeves's Pheasant (Syrmaticus reevesii), a threatened species endemic to China that lives in mountainous forests, has been listed as "Vulnerable" on the International Union for Conservation of Nature (IUCN) Red List (IUCN, 2022). A previous study on Reeves's Pheasant based on radio tracking has given us a preliminary understanding of its incubation behavior (Zhang et al., 2004). However, that study was only based on five nests from four females, and it did not analyze the relationships between environmental factors (e.g., ambient temperature and precipitation) and the incubation behavior of Reeves's Pheasant. The current limited knowledge of the reproductive ecology of Reeves's Pheasant poses a significant obstacle to the effective implementation of conservation and management strategies aimed at safeguarding its population.
To this end, our study used satellite tracking, a more effective and less intrusive tool, to monitor the incubation behavior of Reeves's Pheasant. This remote monitoring system does not need to visit targeted nests frequently during the incubation period, and can minimize the influence of human disturbance and the possible risk of nest predation. The main objective of this study was to investigate the clutch size and incubation behavior of Reeves's Pheasants and their responses to ambient temperature and precipitation. By providing essential ecological and reproductive information on Reeves's pheasant, this study will contribute valuable knowledge to the conservation of this species and related endangered pheasants.
Reeves's Pheasant is an omnivorous species weighing about 950 g (female). It is characterized by female-only incubation (Zhang et al., 2004). This species constructs open-ground nests with a clutch of 8.9 and an incubation period of 26–27 days (Zhang et al., 2004). During incubation, females must do all their own foraging, as males do not assist with food provision or nest guard (Zhang et al., 2004). Potential nest predators include mammals (e.g., Haccoon Dog Nyctereutes procyonoides, Hog Badger Arctonyx collaris, Père David's Rock Squirrel Sciurotamias davidianus), birds (e.g., Eurasian Jay Garrulus glandarius, Red-billed Blue-Magpie Urocissa erythroryncha, and Eurasian Magpie Pica pica), as well as reptiles (e.g., King Rat Snake Elaphe carinata) (Wang et al., 2016).
This study was conducted in Pingjingguan Village (31°51ʹ–31°52ʹ N, 113°54ʹ–113°55ʹ E, hereafter 'PJG') and Zhonghuashan Bird Provincial Nature Reserve (31°37ʹ–31°44ʹ N, 113°54ʹ–113°59ʹ E, hereafter 'ZHS') in Hubei Province, China (Fig. 1). PJG contains coniferous forests, deciduous broad-leaved forests and coniferous broad-leaved mixed forests at elevations of 130–850 m, with a mean annual temperature of 16 ℃ and an average annual precipitation ranging from 865 to 1070 mm. ZHS contains evergreen broad-leaved forests and coniferous broad-leaved mixed forests at elevations of 150–810 m, with a mean annual temperature of 16 ℃ and an average annual precipitation of 1022 mm. The two sites are about 22 km apart with similar climate conditions, and both are the main distribution areas of Reeves's Pheasant (Zhao et al., 2013; Zhou et al., 2015).
From 2020 to 2023, we captured 27 adult female Reeves's Pheasants (18 in PJG; 9 in ZHS) using non-injury rope techniques and attached satellite trackers (LEGO, Chengdu, China) to them before the breeding season (Lu et al., 2022). Each satellite tracker weighs about 20 g, less than 3% of the body mass of an adult female Reeves's Pheasant (Lu et al., 2022). They were set to locate targeted individuals once an hour and record the overall dynamic body acceleration (ODBA) of each individual every 10 min. Online monitoring of the tracker's power facilitated timely adjustments. When low power was detected, we modified the ODBA collection frequency online (with no impact on animals) to once every 30 min to ensure continuous data collection, thereby ensuring that the recesses of female Reeves's Pheasants would not be missed. This adjustment was made for 80 out of the 350 incubation days. ODBA can detect the kinetic energy of wild animals and help us determine whether an individual is in a state of movement, which has been tested and applied in various species such as Polar Bears (Ursus maritimus), Pelagic Cormorants (Phalacrocorax pelagicus) and Bonelli's Eagles (Aquila fasciata) (Wilson et al., 2006; Stothart et al., 2016; Pagano and Williams, 2019; Lopez-Lopez et al., 2022).
We recorded the clutch size when nests were first found. Besides, during 2020–2021, to minimize any potential disruption, we did not check the nests again after the first finding of nests unless there were anomalies in the location information or ODBA readings. In the cases where such anomalies were detected, we conducted nest examinations to determine whether predation or abandonment had occurred. From our observations during the two years, we noticed that female Reeves's Pheasants often left their nests in the afternoon (n = 212 days; Appendix Fig. S1). Therefore, in order to carry out additional studies, we specifically chose to perform periodic nest checks every two days in the afternoon from 2022 to 2023, precisely when females were most likely to leave the nest, aiming to minimize any disturbance to the nests during these inspections. Specifically, we would approach the nest from about 15 m away to detect the signal of the satellite tracker. If the signal was detected, it signified that the female was incubating eggs and the security of nest was ensured, thereby enabling us to avoid disturbing the incubating bird. Conversely, an absence of the signal indicated a possibility of foraging outside the nest, nest abandonment, or predation. In these cases, we took the opportunity to determine nest status.
The ODBA of female Reeves's Pheasants was low and stable during incubation but changed rapidly when incubation was interrupted. This was confirmed by our observation that the ODBA values elevated when the tracker site was in motion (outside the nest), and the ODBA values kept low when the tracker site was stationary (coinciding with the nest location). Thus, the beginning of incubation was determined from a sharp decline in ODBA, the interruption of incubation was determined from a sharp increase in ODBA, and constant ODBA was interpreted as continuous incubation (Fig. 2). Based on the changes in ODBA, we determined whether the females started to incubate, and then searched for their nests according to the location information and recorded clutch size.
A previous study has indicated that the egg-laying interval of Reeves's Pheasants is approximately 24 h (Zhang et al., 2004), which is similar to our observations. That study has also noted that females suddenly initiate incubation shortly after clutch completion (Zhang et al., 2004). As a result, based on the clutch size, the date of clutch completion, and the number of periodic visits by females to the nest before incubation started, the first egg-laying date was determined.
Observations and records for this study were made over a 24-h period starting at 0:00 and ending at 24:00 every day. Since the first and last incubation days were not complete monitoring days (0:00–24:00), they were not included in the analysis. For each complete monitoring day of each nest, we calculated: (1) the number of recesses, estimated as the number of times a female left the nest per day; (2) recess duration, estimated as the duration (in minutes) of each off-bout (if the female did not leave the nest during a monitoring day, we recorded the recess duration for that day as 0 min); (3) nest attendance, estimated as the proportion of time a female devoted to incubation per day.
We used the daily weather data (temporal resolution: every 1 h) from the nearest local weather station (Jigong Mountain weather station; altitude 730 m; 31.80° N, 114.07° E) provided by China Meteorological Data Service Center (http://data.cma.cn/), ~15 km away from both study areas (Fig. 1). For each nest, we calculated the average ambient temperature (averaged daily mean ambient temperature) during its egg-laying period. Additionally, we calculated the average daily precipitation during the egg-laying period for each nest, which was calculated as the total cumulative precipitation during the laying stage divided by the number of days of the laying stage. Furthermore, we collected the daily mean ambient temperature and daily precipitation of each day of incubation for all nests.
In order to analyze the effects of ambient temperature and precipitation on clutch size, we employed linear mixed-effects models (LMM) with the package lme4 (Bates et al., 2015). We took clutch size as the response variable, and took average ambient temperature and average daily precipitation during the egg-laying period of each nest as fixed effects. We did not include "the first egg-laying date" as a fixed effect due to its high correlation with "average ambient temperature" (Pearson correlation coefficients |r| > 0.70). Female identity was treated as a random effect to account for instances where individual females were involved in more than one nest (7 females had 2 breeding attempts, 1 had 3 breeding attempts. Details in Appendix Table S1). In addition, year was considered as a random effect to address the differences between years. Since clutch size was not observed to respond to the interaction between temperature and precipitation, we removed this interaction according to backward-stepwise model selection (Whittingham et al., 2006; Zhang, 2016). The resulting final model (LMM) comprised only predictors with a substantial impact on clutch size (the result of the full model is shown in Appendix Table S2 and Table S3). Average ambient temperature and average daily precipitation exhibited low collinearity (variance inflation factors VIF < 2) and low Pearson correlation coefficients |r| < 0.10.
To analyze the temporal distribution of recesses during the incubation period, we used the kernel density estimation method (package overlap, Ridout and Linkie, 2009) based on the timing of nest departures and returns.
To examine the effects of ambient temperature and precipitation on recess decisions, we employed a binomial distribution generalized linear mixed model (GLMM) with a logit link function utilizing the lme4 package (Bates et al., 2015). The response variable was whether the female left the nest (1) or not (0) during the whole day, which referred to recess decisions. In order to investigate the influence of ambient temperature and precipitation on recess duration, we employed a Poisson distribution GLMM with a logit link. Furthermore, we analyzed nest attendance through a binomial distribution GLMM with a logit link, and the dependent variable was a matrix composed of two vectors: daily incubation minutes and daily recess minutes. Across all three models, we took daily mean ambient temperature, daily precipitation, and their interaction as fixed effects. Similar to the analysis of clutch size, we removed the insignificant interaction in the final model (Whittingham et al., 2006; Zhang, 2016). Other fixed effects included day of incubation and clutch size. Day of incubation was calculated, with day 1 being the day when females started incubation for each nest. Besides, female identity and year were also treated as random factors in all three models. We did not include "date" as a fixed effect in these models because of its high correlation with "day of incubation" (Pearson correlation coefficients |r| > 0.70). There was a low collinearity and correlation among the predictor variables of the three models, with all variance inflation factor (VIF) < 2 and all Pearson correlation coefficients |r| < 0.40.
Note that, the recess behavior of female Reeves's Pheasants was not disturbed by our nest checks in 2022 and 2023, as the model analysis results were similar whether or not the datasets for 2022 and 2023 were included (also see the temporal distribution of recesses in Appendix Fig. S1, and Fig. 4B).
We reported "marginal r2 and conditional r2" to demonstrate the variation that the models could account for. Our statistical analyses were conducted in R version 4.1.3, with all values reported as mean ± SD. Furthermore, to plot illustrations with model predicted values, we generated model predictions using the "predict" function in R and reported 95% confidence intervals, while maintaining the constancy of other explanatory variables (clutch size was set to the median, and other variables were set to their respective mean values).
A total of 350 incubation days (2020: n = 132 days; 2021: n = 80 days; 2022: n = 40 days; 2023: n = 98 days) for 36 nests from 27 female Reeves's Pheasants were monitored. The average clutch size of Reeves's Pheasant was 7.75 ± 1.36 (ranging from 5 to 11). Based on four successfully hatched nests, we found that the incubation period of Reeves's Pheasant lasted for 26–27 days, with an apparent nesting success of 11.11% (the rate of nests that successfully hatched compared with the total number of nests). During the incubation period, female Reeves's Pheasants took an average of 0.73 ± 0.46 recesses (ranging from 0 to 2) every 24 h. Specifically, they took 1 recess per day during 72% of the incubation days, took no recess during 27.4% of the incubation days, and took 2 recesses per day during 0.6% of the incubation days. The average recess duration was 100.80 ± 73.37 min (ranging from 0 to 340 min), and the corresponding nest attendance was 92.98 ± 5.27% (ranging from 64.17% to 100%) (Appendix Table S1).
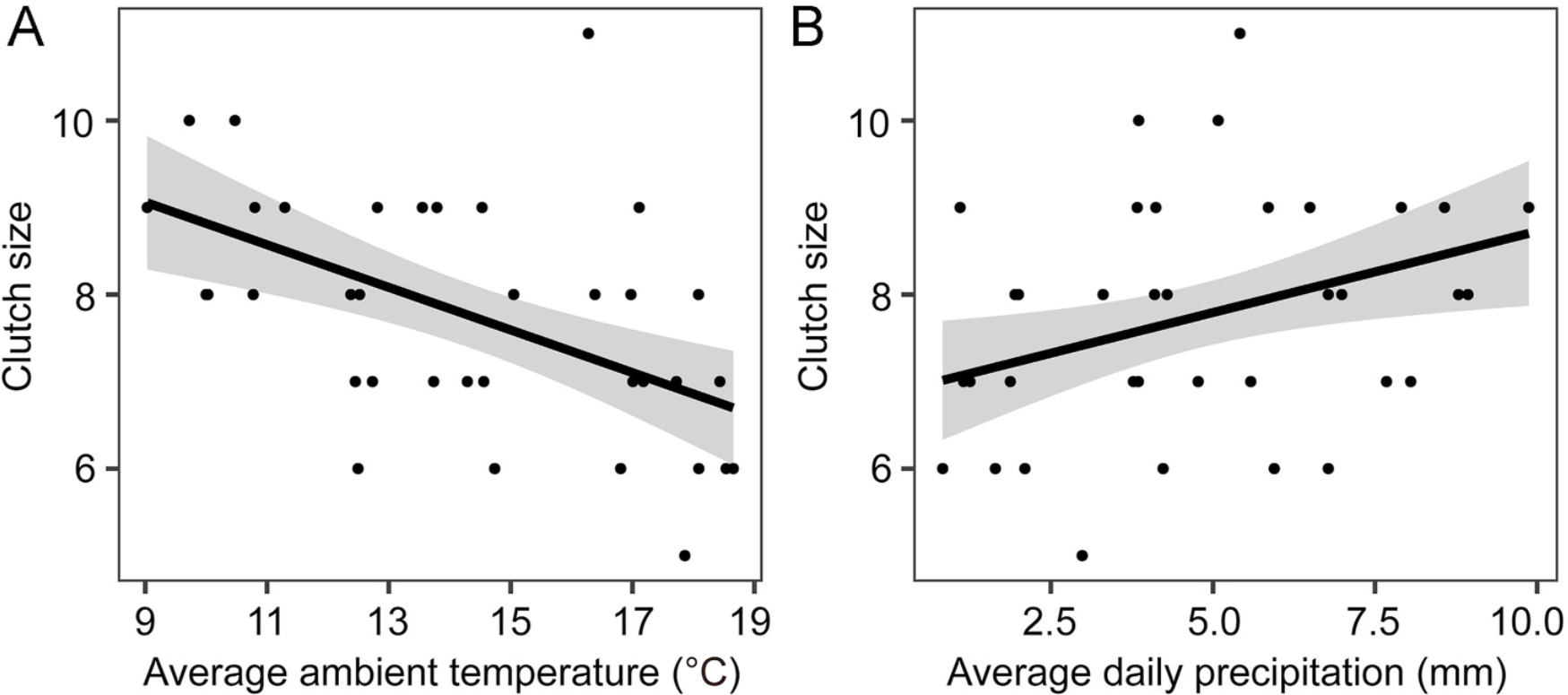
During the egg-laying period, the maximum temperature reached 33 ℃, the minimum temperature was −2 ℃, and the mean temperature was 15 ℃. A significant negative correlation was observed between clutch size and the average ambient temperature during the egg-laying period (Table 1, Fig. 3A), while a significant positive correlation was found between clutch size and the average daily precipitation during the egg-laying period (Table 1, Fig. 3B).
| Fixed effects | Estimate ± SE | t | p |
| (Intercept) | 11.235 ± 0.793 | 14.166 | < 0.001 |
| Average temperature | −0.294 ± 0.048 | −6.110 | <0.001 |
| Average daily precipitation | 0.174 ± 0.068 | 2.561 | 0.016 |
| Random effects | Variance | ||
| Female identity (Intercept) | 1.038 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.007 | ||
| Marginal r2/Conditional r2: 0.389/0.859 |
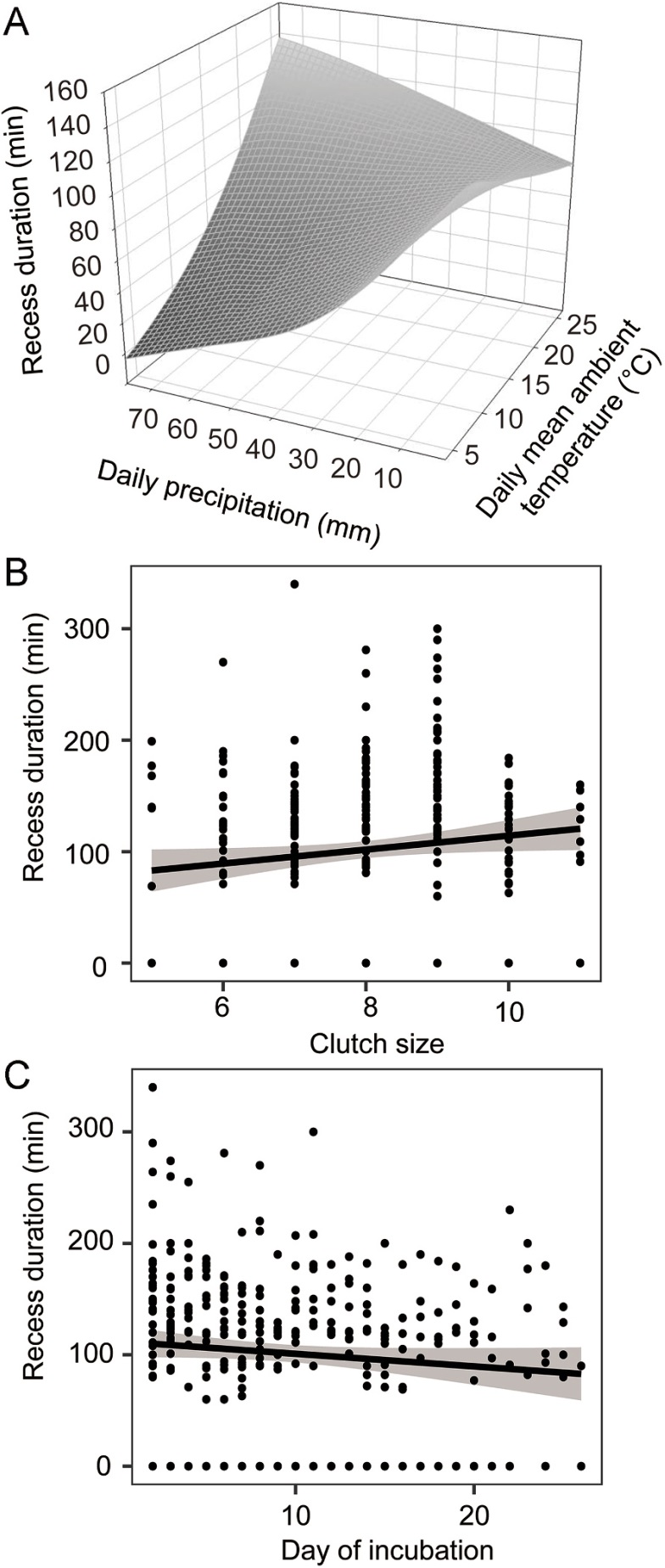
During the incubation period, the maximum temperature reached 34 ℃, the minimum temperature was 4 ℃, and the mean temperature was 19 ℃ (Fig. 4A). Approximately 60% of the cases of nest departure occurred between 13:00 and 16:00, and around 65% of the cases of nest return occurred between 15:00 and 18:00 (Fig. 4B). Notably, this recess temporal pattern corresponded with the period of elevated ambient temperatures during daylight hours.
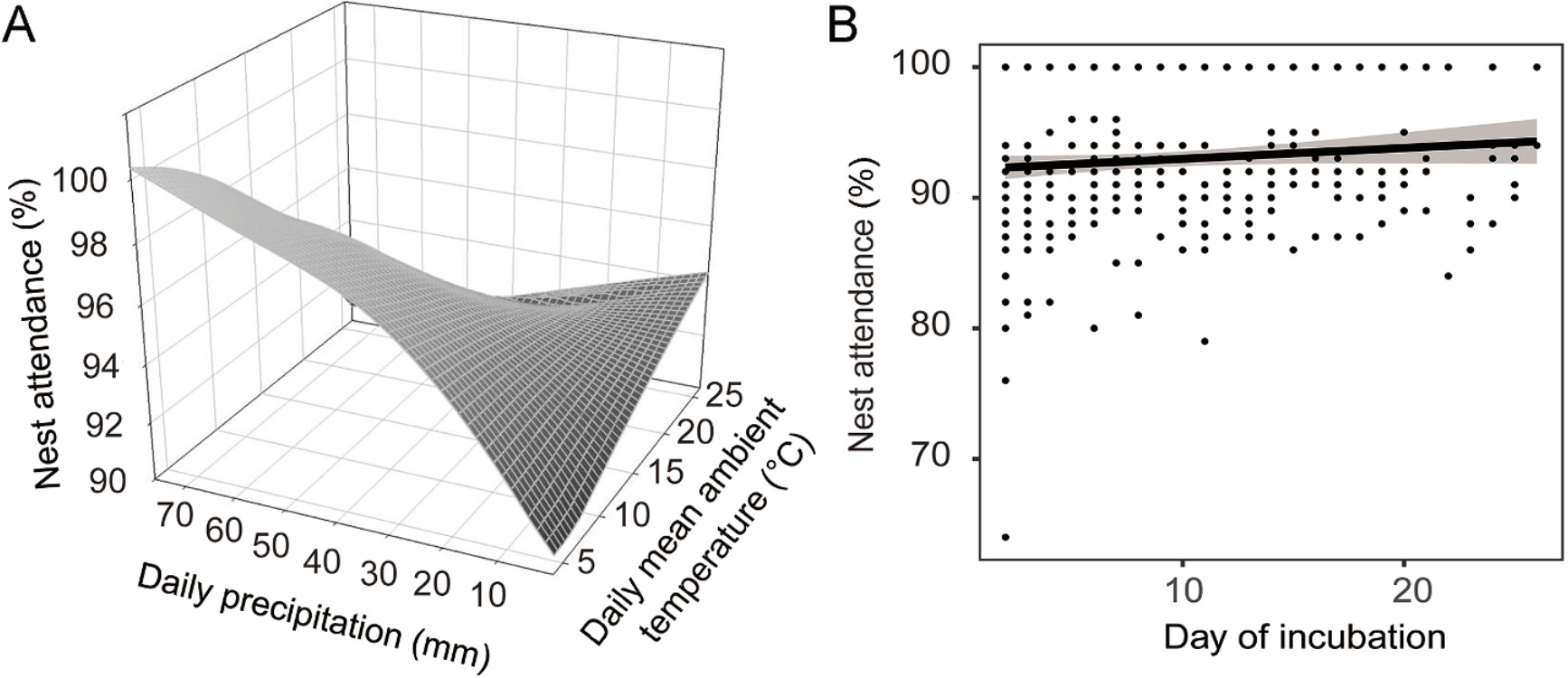
There was a significant negative correlation between daily precipitation and recess decisions, and Reeves's Pheasants rarely left their nests when daily precipitation was high (Table 2, Fig. 5). Recess duration was found to have a significant correlation with the interaction between daily mean ambient temperature and daily precipitation. Furthermore, recess duration showed a marked positive correlation with clutch size as well as a significant negative correlation with day of incubation (Table 3, Fig. 6). A significant correlation was identified between nest attendance and the interaction between daily mean ambient temperature and daily precipitation. Additionally, nest attendance displayed a marked positive correlation with day of incubation (Table 4, Fig. 7).
| Fixed effects | Estimate ± SE | z | p |
| Response: Recess decisions | |||
| (Intercept) | 0.475 ± 1.307 | 0.364 | 0.716 |
| Daily mean ambient temperature | −0.034 ± 0.038 | −0.896 | 0.370 |
| Daily precipitation | −0.029 ± 0.012 | −2.351 | 0.019 |
| Day of incubation | −0.004 ± 0.024 | −0.170 | 0.865 |
| Clutch size | 0.170 ± 0.124 | 1.375 | 0.169 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.282 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.480 | ||
| Marginal r2/Conditional r2: 0.045/0.120 | |||
| Fixed effects | Estimate ± SE | z | p |
| Response: Recess duration | |||
| (Intercept) | 4.902 ± 0.107 | 45.927 | <0.001 |
| Daily mean ambient temperature | −0.025 ± 0.002 | −12.741 | <0.001 |
| Daily precipitation | −0.045 ± 0.003 | −14.819 | <0.001 |
| Daily mean ambient temperature * Daily precipitation | 0.002 ± 0.0001 | 12.127 | <0.001 |
| Day of incubation | −0.0066 ± 0.001 | −5.092 | <0.001 |
| Clutch size | 0.026 ± 0.010 | 2.522 | 0.012 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.061 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.861 | ||
| Marginal r2/Conditional r2: 0.299/0.905 | |||
| Fixed effects | Estimate ± SE | z | p |
| Response: Nest attendance | |||
| (Intercept) | 2.081 ± 0.107 | 19.461 | <0.001 |
| Daily mean ambient temperature | 0.027 ± 0.002 | 13.759 | <0.001 |
| Daily precipitation | 0.049 ± 0.003 | 15.434 | <0.001 |
| Daily mean ambient temperature * Daily precipitation | −0.002 ± 0.0002 | −12.545 | <0.001 |
| Day of incubation | 0.008 ± 0.001 | 6.489 | <0.001 |
| Clutch size | −0.007 ± 0.010 | −0.753 | 0.452 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.076 | ||
| Year (Intercept) | 0.000 | ||
| Residual | −0.717 | ||
| Marginal r2/Conditional r2: 0.307/0.980 | |||
We found that the clutch size and incubation behavior of female Reeves's Pheasants had a significant correlation with ambient temperature and precipitation. Female Reeves's Pheasants exhibited a distinctive incubation pattern which was characterized by a low recess frequency and a high nest attendance. This study represents the first exploration of the influence of weather conditions on the reproductive behavior of Reeves's Pheasants. These findings advance our understanding of the life-history traits of Reeves's Pheasants and reveal a crucial influence of weather conditions on their reproductive behavior.
We observed that female Reeves's Pheasants laid fewer eggs in higher ambient temperatures, which may be associated with egg viability (Arnold et al., 1987; Stoleson and Beissinger, 1999; Cooper et al., 2005). In avian embryos, irregular development is expected when the ambient temperature exceeds the physiological zero (24–26 ℃) but remains below the range essential for normal embryo development (36–40 ℃). This is particularly noteworthy in the absence of contact incubation, as the incubating bird typically maintains the eggs within the optimal range of 36–40 ℃ (Webb, 1987; Veiga, 1992; Cooper et al., 2005). Reeves's Pheasants, deferring incubation until clutch completion, expose their eggs to fluctuating ambient conditions until the onset of contact incubation. The observed reduction in clutch size under warmer conditions may be attributed to that smaller clutches shorten the laying period, thereby minimizing the exposure of eggs to unfavorable thermal conditions. Moreover, considering the strong correlation between average ambient temperature and the first egg-laying date, it is reasonable to assume that clutch size potentially exhibits a seasonal decline, as observed in some other species (Decker et al., 2012; Karagicheva et al., 2016; Weiser et al., 2018). Additionally, female birds breeding multiple times may produce smaller clutches in subsequent breeding attempts (Rooneem and Robertson, 1997; Gasparini et al., 2006; Robinson et al., 2010). In this study, we could not exclude the possibility that the data of some females were not from their first nesting attempts because some females may have experienced failure in the egg-laying stage during initial breeding attempts but could not be detected by the tracking data. If this is the case, their subsequent nesting attempts, which might have smaller clutches, were more likely to occur when ambient temperature was higher. Also, some studies have shown that low-quality individuals may breed later and lay fewer eggs (Verhulst et al., 1995; Hipfner et al., 1997; Christians et al., 2001; Koenig and Walters, 2018), which could result in the negative relationship between ambient temperature and clutch size as well.
The positive relationship between clutch size and average daily precipitation during the egg-laying period could be attributed to the promoting effect of precipitation on primary and secondary productivity (e.g., the biomass of protein-rich prey), which would cause an increase in food abundance and drive females to lay more eggs (Skagen and Adams, 2012). Precipitation may inhibit the activity of certain predators such as snakes (Morrison and Bolger, 2002; Grudinskaya et al., 2022). And the reduction in predator activity during the egg-laying period may thus serve as a proximate cue for overall predation risk, influencing clutch size decisions among birds (Zanette et al., 2011; Dillon and Conway, 2017). Moreover, visually hunting corvids generally target nests with poor concealability (Weidinger, 2002), and the rapid vegetation growth following precipitation provides better hiding possibilities for ground-open nests, thereby reducing the risk of predation (Ringelman and Skaggs, 2019; Laidlaw et al., 2020). Thus, clutch size may increase due to lower predator activity during precipitation and higher nest concealment afterward (Grudinskaya et al., 2022).
Throughout the incubation period, female Reeves's Pheasants exhibited an unexpectedly low frequency of recesses, with an average of only 0.73 times per day. This differs from the typical behavior observed in most Galliforms, which generally take 2 to 7 recesses per day (Deeming, 2002). This uncommon nest activity pattern may potentially diminish the attractiveness to visually oriented predators, thereby lowering the risk of predation (Fontaine and Martin, 2006; Cervencl et al., 2011). A similar recess strategy has been also observed in Blood Pheasant (clutch size 7.8) and Sichuan Partridge (clutch size 5.0) which generally take a single recess per day (Jia et al., 2010; Fu et al., 2017). Interestingly, unlike species with lower attentiveness like Blood Pheasant (72.0%) and Sichuan Partridge (81.2%) (Jia et al., 2010; Fu et al., 2017), the female Reeves's Pheasant showed a significantly higher nest attendance (92.3%). It has been reported that high nest attendance can contribute to the rapid and stable development of embryos, ultimately leading to a shortened incubation period (Thompson and Raveling, 1987; Hepp et al., 2006; Carter et al., 2014), which may account for why Reeves's Pheasant had a shorter incubation period (26–27 days) than Blood Pheasant (37 days) and Sichuan Partridge (29 days) (Jia et al., 2010; Fu et al., 2017). Taken together, the unique incubation pattern with a low recess frequency and a high nest attendance may help alleviate predation pressure, reduce the frequency of reheating eggs, and minimize physiological costs of female Reeves's Pheasant.
The temporal distribution of recesses holds a pivotal role in shaping the survival of incubating birds and embryos, and is potentially influenced by surrounding ambient temperature (Winder et al., 2016). Previous studies have indicated that colder nighttime temperatures may cause fasting females to experience increased energy demands at dawn and subsequently forage at dusk to replenish their overnight energy reserves (Wiebe and Martin, 1997; Moiron et al., 2018; Schöll et al., 2019). If incubating females prioritize their physiological needs, recesses can potentially occur more frequently during dawn and dusk periods. However, in contrast to this expectation, our observations consistently showed that female Reeves's Pheasants exhibited a preference for leaving nests during the warmer afternoon hours. Furthermore, the timing of their recesses followed a distinct unimodal pattern in response to the changing ambient temperature.
This behavior seems to be adaptive as the females chose to forage at a higher ambient temperature, allowing their eggs to cool gradually and preventing the adverse effects of low temperatures on embryonic development. Additionally, this strategy minimized the time and effort required to reheat the eggs. The unimodal pattern of recess timing may be driven primarily by embryonic thermal needs rather than by the physiological needs of incubating females, which contrasts with the bimodal recess pattern observed in species like Chinese Grouse (Tetrastes sewerzowi) (Shi et al., 2019), Greater Prairie-chickens (Tympanuchus cupido) (Winder et al., 2016), and Greater Sage-grouse (Centrocercus urophasianus) (Coates and Delehanty, 2008).
We observed a distinct relationship between daily precipitation and recess decisions. Non-recess during higher precipitation days may serve to avoid direct wetting and cooling of the eggs. This behavior could also potentially be attributed to the reduced foraging efficiency (decreased availability of protein-rich prey) and heightened foraging costs on rainy days (Nooker et al., 2005).
The interaction between daily mean ambient temperature and daily precipitation was strongly associated with recess duration and nest attendance. In warm and dry weather, females exhibited shorter recess duration compared to cold and dry or warm and rainy weather. Furthermore, in cold and dry weather or warm and rainy weather, females had longer recess duration in comparison to warm and dry weather or cold and rainy weather. A previous study on the incubation behavior in a passerine bird has shown that foraging efficiency is reduced during low ambient temperatures and dry weather (Bambini et al., 2019). Besides, females are more susceptible to energy constraints due to increased basal metabolism and incubation costs in low temperatures (Bryan and Bryant, 1999). As a result, they need to spend more time foraging to cope with the high energy expenditure (Tulp and Schekkerman, 2006; Diez-Méndez et al., 2021). During low ambient temperatures and rainy weather, the expenses associated with foraging may surpass the energy benefits gained (Bambini et al., 2019). Furthermore, for uniparental avian species like Reeves's Pheasant which constructs open nests on the ground, the presence of females in the nest during low ambient temperatures and precipitation seems to serve as protection for their eggs, shielding them from cold weather and rain (Coe et al., 2015). On the other hand, foraging efficiency is higher in warm and dry days than in warm and rainy days (Bambini et al., 2019), thus allowing females to spend less time foraging.
As incubation progresses, embryos become more sensitive to temperature variations and less tolerant to low temperatures (Batt and Cornwell, 1972; Webb, 1987). Moreover, the heat loss rate of eggs increases gradually as embryos develop (Cooper and Voss, 2013). In our study, the variation in both recess duration and nest attendance based on day of incubation suggested that female Reeves's Pheasants adjusted their time and energy investment in incubation according to embryonic development. A similar trend has also been observed in Blood Pheasant, with nest attendance starting at around 67% at the beginning of incubation and increasing to 82% in the later stages of incubation (Jia et al., 2010). Furthermore, we found a positive correlation between clutch size and recess duration. This relationship could be attributed to the slower cooling of larger clutches compared to smaller ones, enabling females to leave nests for more extended periods (Reid et al., 2000).
Our research revealed a complex relationship between weather conditions (i.e. ambient temperature and precipitation) and both the clutch size and incubation behavior of Reeves's Pheasant, which significantly enriches our knowledge of the previously unknown life-history features and strategies of this vulnerable species. Considering its decreasing population trend and the low nest success in the wild, future studies should further investigate how ecological factors such as food availability and predation risk influence the reproductive behavior of Reeves's Pheasant. A comprehensive understanding of these factors will enable conservationists to develop targeted strategies to protect nesting sites, manage habitat, and mitigate potential threats to support successful reproduction and population growth of this species.
The study protocol and field procedures were approved by the forestry department and by the Ethics and Animal Welfare Committee from Beijing Forestry University, approval number EAWC_BJFU_2021018.
Ting Jin: Writing – original draft, Investigation, Formal analysis, Data curation. Shuai Lu: Investigation. Yunqi Wang: Investigation. Junqin Hua: Writing – review & editing, Methodology. Zhengxiao Liu: Writing – review & editing, Validation. Qian Hu: Writing – review & editing, Validation. Yating Liu: Writing – review & editing. Yuze Zhao: Writing – review & editing. Jianqiang Li: Writing – review & editing. Jiliang Xu: Writing – review & editing, Supervision, Conceptualization.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank the Forestry Department of Hubei Provinces for granting permission and support for this work, and the residents for their help in fieldwork. We thank the anonymous reviewers for their useful comments and suggestions.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2024.100168.
|
Deeming, D.C., 2002. Avian Incubation: Behaviour, Environment, and Evolution. Oxford University Press, New York.
|
|
Zhang, X.H., Xu, J.L., Zhang, Z.W., Xie, F.L., Zhang, K.Y., Zhu, J.G., 2004. A study on the incubation behavior of Reeves's pheasant (Syrmaticus reevesii) by radio tracking. J. Beijing Normal Univ. (Nat. Sci.) 2, 255-259. (in Chinese with English abstract).
|
|
Zhao, Y.Z., Wang, Z.C., Xu, J.L., Luo, X., An, L.D., 2013. Activity rhythm and behavioral time budgets of wild Reeves's pheasant (Syrmaticus reevesii) using infrared camera. Chin. Acta Ecol. Sin. 33, 6021-6027. (in Chinese with English abstract).
|
| 1. | Lu Wang, Fangqing Liu, Lan Zhao, et al. A test of genetic divergence of a bird existing in the Sichuan Basin and its surrounding mountain ranges. Avian Research, 2023. DOI:10.1016/j.avrs.2023.100144 |
| 2. | Wei Wang, Donghui Fang, Yi Shi, et al. Genome-wide SNP analysis reveals the selection signatures of two indigenous buffalo breeds in Sichuan. Conservation Genetics Resources, 2022, 14(3): 299. DOI:10.1007/s12686-022-01275-2 |
| 3. | Ningxin Gu, Guoling Chen, Jia Yang, et al. Novel microsatellite markers reveal low genetic diversity and evidence of heterospecific introgression in the critically endangered Chinese Crested Tern (Thalasseus bernsteini). Global Ecology and Conservation, 2021, 28: e01629. DOI:10.1016/j.gecco.2021.e01629 |
| 4. | Guoling Chen, Chenqing Zheng, Nelson Wan, et al. Low genetic diversity in captive populations of the critically endangered Blue-crowned Laughingthrush (Garrulax courtoisi) revealed by a panel of novel microsatellites. PeerJ, 2019, 7: e6643. DOI:10.7717/peerj.6643 |
| Fixed effects | Estimate ± SE | t | p |
| (Intercept) | 11.235 ± 0.793 | 14.166 | < 0.001 |
| Average temperature | −0.294 ± 0.048 | −6.110 | <0.001 |
| Average daily precipitation | 0.174 ± 0.068 | 2.561 | 0.016 |
| Random effects | Variance | ||
| Female identity (Intercept) | 1.038 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.007 | ||
| Marginal r2/Conditional r2: 0.389/0.859 |
| Fixed effects | Estimate ± SE | z | p |
| Response: Recess decisions | |||
| (Intercept) | 0.475 ± 1.307 | 0.364 | 0.716 |
| Daily mean ambient temperature | −0.034 ± 0.038 | −0.896 | 0.370 |
| Daily precipitation | −0.029 ± 0.012 | −2.351 | 0.019 |
| Day of incubation | −0.004 ± 0.024 | −0.170 | 0.865 |
| Clutch size | 0.170 ± 0.124 | 1.375 | 0.169 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.282 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.480 | ||
| Marginal r2/Conditional r2: 0.045/0.120 | |||
| Fixed effects | Estimate ± SE | z | p |
| Response: Recess duration | |||
| (Intercept) | 4.902 ± 0.107 | 45.927 | <0.001 |
| Daily mean ambient temperature | −0.025 ± 0.002 | −12.741 | <0.001 |
| Daily precipitation | −0.045 ± 0.003 | −14.819 | <0.001 |
| Daily mean ambient temperature * Daily precipitation | 0.002 ± 0.0001 | 12.127 | <0.001 |
| Day of incubation | −0.0066 ± 0.001 | −5.092 | <0.001 |
| Clutch size | 0.026 ± 0.010 | 2.522 | 0.012 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.061 | ||
| Year (Intercept) | 0.000 | ||
| Residual | 0.861 | ||
| Marginal r2/Conditional r2: 0.299/0.905 | |||
| Fixed effects | Estimate ± SE | z | p |
| Response: Nest attendance | |||
| (Intercept) | 2.081 ± 0.107 | 19.461 | <0.001 |
| Daily mean ambient temperature | 0.027 ± 0.002 | 13.759 | <0.001 |
| Daily precipitation | 0.049 ± 0.003 | 15.434 | <0.001 |
| Daily mean ambient temperature * Daily precipitation | −0.002 ± 0.0002 | −12.545 | <0.001 |
| Day of incubation | 0.008 ± 0.001 | 6.489 | <0.001 |
| Clutch size | −0.007 ± 0.010 | −0.753 | 0.452 |
| Random effects | Variance | ||
| Female identity (Intercept) | 0.076 | ||
| Year (Intercept) | 0.000 | ||
| Residual | −0.717 | ||
| Marginal r2/Conditional r2: 0.307/0.980 | |||