| Citation: | Xingyi Jiang, Yanyun Zhang. 2024: Sounding the alarm: Functionally referential signaling in Azure-winged Magpie. Avian Research, 15(1): 100164. DOI: 10.1016/j.avrs.2024.100164 |
Functionally referential signals are a complex form of communication that conveys information about the external environment. Such signals have been found in a range of mammal and bird species and have helped us understand the complexities of animal communication. Corvids are well known for their extraordinary cognitive abilities, but relatively little attention has been paid to their vocal function. Here, we investigated the functionally referential signals of a cooperatively breeding corvid species, Azure-winged Magpie (Cyanopica cyanus). Through field observations, we suggest that Azure-winged Magpie uses referential alarm calls to distinguish two types of threats: ‘rasp’ calls for terrestrial threats and ‘chatter’ calls for aerial threats. A playback experiment revealed that Azure-winged Magpies responded to the two call types with qualitatively different behaviors. They sought cover by flying into the bushes in response to the ‘chatter’ calls, and flew to or stayed at higher positions in response to ‘rasp’ calls, displaying a shorter response time to ‘chatter’ calls. Significant differences in acoustic structure were found between the two types of calls. Given the extensive cognitive abilities of corvids and the fact that referential signals were once thought to be unique to primates, these findings are important for expanding our understanding of social communication and language evolution.
The meaning of animal signals is an ongoing focus within the field of animal communication (Smith, 2017). Referential signals are of particular interest, as they are reliably associated with specific objects or events in the environment and convey information to receivers about these referents (Macedonia and Evans, 1993). The “waggle dance” of honeybee and the alarm calls of Vervet Monkey (Cercopithecus aethiopsre) are two classic examples of referential signaling through visual and auditory modalities, respectively. Honeybees transmit information about the location of food and water sources to conspecifics through physical movements (von Frisch, 1974). Vervet Monkeys have distinct alarm calls for the threats posed by leopards, eagles, and snakes that assist receivers in obtaining critical information about potential dangers (Struhsaker, 1967), and represent functionally referential alarms. Functionally referential alarm calls not only provide an excellent context for understanding animal communication but are also considered a vital connection for studying the evolution of human language (Fedurek and Slocombe, 2011); therefore, they have attracted extensive attention, especially in nonhuman primates (e.g., Pereira and Macedonia, 1991; Zuberbühler, 2003; Slocombe and Zuberbühler, 2005). More recently, some studies have found that birds that rely heavily on vocalization also produce such signals. For example, Great Tits (Parus major) give acoustically distinctive alarm calls for the Large-billed Crow (Corvus macrorhynchos) and the Japanese Rat Snake (Elaphe climacophora), which are its two main nest predators during the breeding season (Suzuki, 2011). In contrast to calls highly specific to certain predators, a more widespread phenomenon in birds is calls that indicate the spatial location of predators, which includes distinguishing between aerial and terrestrial threats (e.g., Grieves et al., 2014; LaPergola et al., 2023). These sophisticated alarm systems can help birds detect and evade predators and, to some extent, enhance their fitness (Bradbury and Vehrencamp, 2011).
According to the social complexity hypothesis, groups with complex social systems need more complex communication systems to regulate relationships and interactions among members (Freeberg, 2006). Complex social systems are those in which individuals frequently interact in many different contexts (e.g., reproduction, aggression and foraging) (Freeberg et al., 2012), which can be assessed in terms of group size, group density, diversity in roles or status of group members (Hailman et al., 1985). Vocal complexity can be measured in terms of vocal repertoire size, information (bits) in a vocal signaling system or in the diversity of ways that vocal signals are used by group members (Freeberg, 2006). These extensive social interactions have benefits for individuals, including access to more food resources and reduced risk of predation (Krause and Ruxton, 2002). They are also commonly attributed to effective vocal communication, which enhances social cohesion among group members (Bolt, 2020; Bouchard and Zuberbühler, 2022).
Corvids (family Corvidae) have varying degrees of sociality and are especially well known for their exceptional cognitive abilities (Bond et al., 2003; Emery and Clayton, 2004). Importantly, as songbirds (oscines), corvids possess a large and flexible vocal repertoire that conveys various information (Rosa et al., 2016; Tanimoto et al., 2017). However, the features and structures of crow song are quite different from the songs of other songbirds (Brown, 1985). As corvids have complex social relationships and are capable of imitating human speech, in-depth research into their vocalizations may provide insights into the evolution of human language (Emery and Clayton, 2004; Clayton and Emery, 2015).
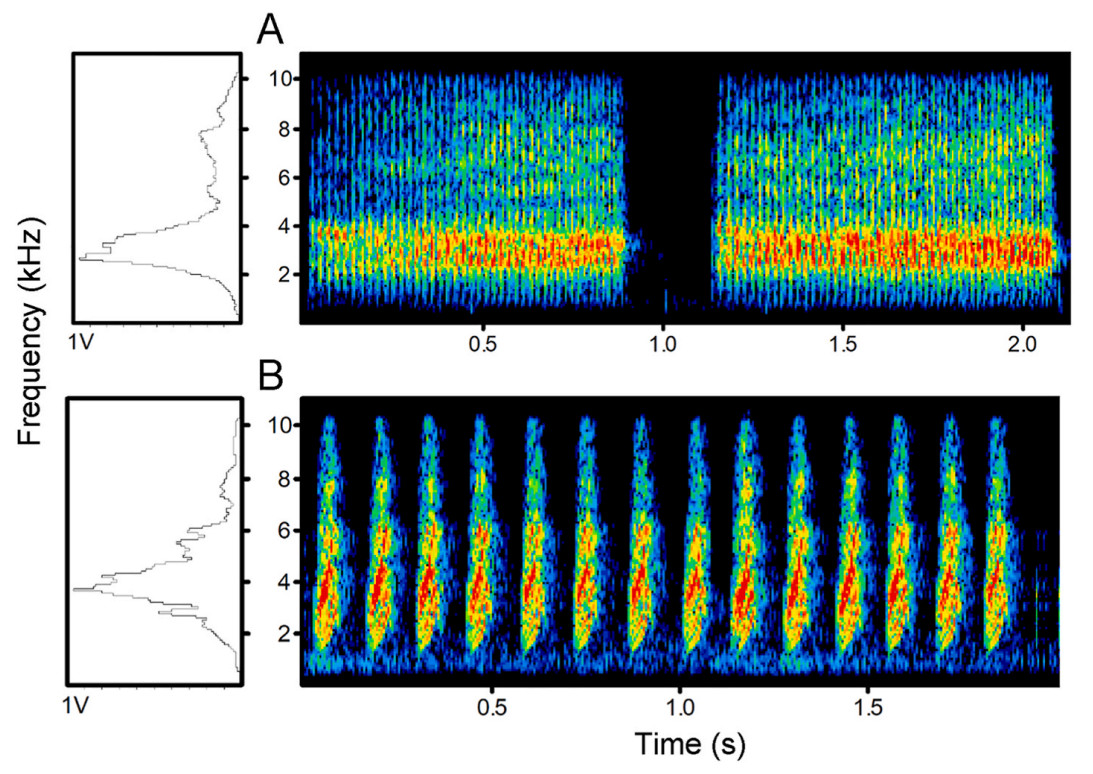
Here, we examined alarm calls in the Azure-winged Magpie (Cyanopica cyanus), a corvid species. Although there has not been a quantitative description of their social complexity, they are known to frequently exhibit cooperative breeding (Ren et al., 2016) and continue to live in groups during the nonbreeding season, which is a fundamental aspect of social complexity (Freeberg, 2006). We infer that they have a certain level of social complexity and may have intricate communication systems. A recent study provided a detailed description of the repertoire of Azure-winged Magpies and found that they enrich their call types by combining different notes (Wang et al., 2023). Based on our field observations, we find that Azure-winged Magpies use two distinct alarm calls to differentiate between terrestrial and aerial predators; specifically, ‘rasp’ calls are thought to indicate terrestrial predators, while ‘chatter’ calls are thought to indicate aerial predators (Fig. 1). The vocalizations we define as “chatter calls” have also been documented in Wang’s study (J type). However, unlike our research, they define these vocalizations through observation as calls associated with localization (Wang et al., 2023). According to the 3-criteria standard described in Evans et al. (1993), for signals to be truly referential, they must exhibit distinct acoustic structural differences as the first criterion. Secondly, calls need to be stimulus-specific and consistently linked to a given stimulus. Thirdly, referential signals should elicit different escape responses.
Therefore, we initially compared the acoustic features of rasp and chatter calls. Next, we describe instances of these calls given in natural circumstances during two years of field observations and whether specific calls are associated with specific classes of predators. Finally, we use playback experiment to test whether rasp and chatter calls independently elicit appropriate escape behaviors to evade ground-based and aerial predators, respectively.
The Azure-winged Magpie is widely distributed in the Palearctic and Oriental realms. Fieldwork was conducted from 2022 to 2023 in China, including urban parks in five cities (Haikou, Guangzhou, Changsha, Nanjing, and Beijing), and breeding sites in Gannan Tibetan Autonomous Prefecture (Gannan). The breeding population is located at the northeast of the Tibetan Plateau (102.5° E, 34.6° N, elevation: 3400 m), and this population has been monitored and banded since 2011 (Gao et al., 2021). These magpies build nests in high densities (20–180 nests/ha) on Sea Buckthorn (Hippophae rhamnoides) and Goat Willow (Salix caprea) (Ren et al., 2016).
Individual vocalizations were collected from the breeding population in Gannan from May 2023 to July 2023 to eliminate geographic variation effects. The rasp calls were collected by an approaching observer: the observer prioritized selecting a target individual banded with color rings to avoid repeatedly collecting recordings of the same individual, approached while holding a ZOOM Handy Recorder (ZOOM, Japan) connected to a Sennheiser MKH416P48 directional microphone (Sennheiser, Germany), with a resolution of 16 bits and a sample rate of 44.1 kHz. The observer approached at a constant speed and maintained visual contact with the target individual. When the target individual started to alarm, the observer stopped approaching while maintaining visual contact, and its continuous alarm calls were recorded for 2 min. If vocalizations were recorded from an individual without color rings, then no further recordings of vocalizations from unbanded individuals were made for playback within a 2 km radius of the recording site. Chatter calls were incidentally recorded when raptors were circling. In such circumstances where the appearance of raptors is unpredictable, the observer conducts recordings randomly and determines the identity of vocalizing individuals through color rings (if banded) during the recording process. These two types of calls are structurally distinct when viewed on spectrograms and can be distinguished by human listeners in the field.
Recordings were transformed into spectrograms using AvisoftSASLab Pro 5.3.2–16 (Avisoft Bioacoustics, Germany), with the following parameters: sampling frequency = 22.050 kHz, FFT length = 256 points, window = Hamming, frame size = 100%, overlap = 50%, frequency resolution = 172 Hz, and temporal resolution = 2.9 ms. Based on the requirements of this study and the characteristics of the call, automatic measurement was applied to measure a total of 29 variables (Table 1).
| Abbreviations | Meanings |
| Duration | Length of note |
| Peak freq (end) | Peak frequency at the end of a note |
| Peak ampl (end) | Peak amplitude at the end of a note |
| Bandw (end) | Bandwidth at the end of a note |
| Quart 25 (end) | First quartile frequency at the end of a note |
| Quart 50 (end) | Second quartile frequency at the end of a note |
| Quart 75 (end) | Third quartile frequency at the end of a note |
| Entropy (end) | Entropy at the end of a note |
| Peak freq (centre) | Peak frequency at the centre of a note |
| Peak ampl (centre) | Peak amplitude at the centre of a note |
| Bandw (centre) | Bandwidth at the centre of a note |
| Quart 25 (centre) | First quartile frequency at the centre of a note |
| Quart 50 (centre) | Second quartile frequency at the centre of a note |
| Quart 75 (centre) | Third quartile frequency at the centre of a note |
| Entropy (centre) | Entropy at the centre of a note |
| Peak freq (max) | Maximum peak frequency |
| Peak ampl (max) | Maximum peak amplitude |
| Bandw (max) | Maximum bandwidth |
| Quart 25 (max) | Maximum first quartile frequency |
| Quart 50 (max) | Maximum second quartile frequency |
| Quart 75 (max) | Maximum third quartile frequency |
| Entropy (max) | Maximum entropy |
| Peak freq (mean entire) | Peak frequency of the entire note |
| Peak ampl (mean entire) | Peak frequency of the entire amplitude |
| Bandw (mean entire) | Bandwidth of the entire note |
| Quart 25 (mean entire) | First quartile frequency of the entire note |
| Quart 50 (mean entire) | Second quartile frequency of the entire note |
| Quart 75 (mean entire) | Third quartile frequency of the entire note |
| Entropy (mean entire) | Entropy of the entire note |
In the nonbreeding seasons of 2022 (March) and 2023 (February to April) and the breeding season of 2023 (May to July), regular periods of observation (07:00–19:00) were conducted in which field observers opportunistically documented alarm calls while conducting observations and routine nest monitoring. Since humans represent a terrestrial threat, when documenting alarm calls not caused by humans, observers maintained a distance of at least 15 m from the vocalizing individuals to avoid disturbing, ensuring that they did not exhibit alarm behaviors in response to the observer. For each observation, the date, territory identity, identity of the Azure-winged Magpie (if banded), call type, and visible stimulus were recorded.
All playback experiment were conducted in Gannan from May 2023 to July 2023. The design of the playback experiment included editing alarm calls, playing calls back to the focal magpies, video-recording their behavior, and categorizing behavioral responses. The vocalizations used for playback are sourced from field recordings with high signal-to-noise ratio. We selected recordings from four individuals (two males and two females) for rasp call playbacks and another four individuals (two males and two females) for chatter call playbacks. Control stimuli were generated by synthesizing white noise using Adobe Audition 2023 (Adobe Inc.), and AvisoftSASLab Pro 5.3.2–16 (Avisoft Bioacoustics, Germany) was utilized to create the alarm call stimuli. As the vocalizations of Azure-winged Magpies are continuously distributed from low to high-frequency ranges, and most recordings were collected in situations where the signaler was close to the microphone and background noise was low, we did not filter the sound. For each rasp call playback, we trimmed 30 s of continuous vocalization from the raw recordings, retaining their natural rates and intervals to better simulate real situations. Due to the varying duration and intervals of the notes in each individual’s vocalizations, the resulting playback audio ranged from 25 to 32 s, containing 16 to 28 notes. Regarding chatter calls, from pre-experiment observations, we noticed that chatter calls do not sustain for long periods like rasp calls, and receivers respond rapidly to chatter calls (within less than 5 s). Consequently, the playback duration for chatter call stimuli was shortened to 10–13 s, containing 5–6 bouts. Each bout comprises 7–30 notes, maintaining their natural intervals and call rates. The duration of white noise was set at 30 s. All playback volumes were adjusted to 80–85 dB (at 1 m).
We conducted two sets of playback experiments. The playback procedure for the two sets were essentially identical, with the only difference being that the individual’s position: the first set of experiment focused on birds that had perched on the ground, and the second targeted birds perched in high, exposed positions (on transmission line or on top of vegetation higher than 2 m). For each set, we tested the response to three types of stimuli: rasp calls, chatter calls, and white noise. The playback process was as follows: during daytime, the researcher entered the known territories, and then followed the activities of a group of birds to identify their approximate range. A speaker (BEE ME HERE, BV210) was then suspended on a 1–1.5 m high branch within the activity area, covered with leaves or nearby vegetation. The researcher retreated 20 m and waited for the Azure-winged Magpies to resume their normal activities (e.g., foraging or emitting contact calls on the ground). The individuals appearing within a 15-m range of the speaker were defined as the focal bird. Simultaneously, there might have been other individuals active beyond this 15-m range, which were counted as group members. Then a 30 s pretrial period began to ensure that the focal individuals were in a stable state. If other predators appeared or the focal birds’ position changed, we stopped the pretrial period and waited for another suitable opportunity. When the pretrial period was successfully completed, we played the predetermined stimulus from the speaker connected to a playback device (iPhone XS Max). Observations continued until the bird resumed its normal activities (usually within 1 min). We video-recorded the focal birds during the pretrial, trial, and posttrial periods using a JVC GC-P100BAC (JVCKENWOOD, Corp, China) video camera. A Songmeter micro (Wildlife Acoustics Inc., Maynard, MA, USA) was used for vocalization recording. For each trail, the observer recorded the type of playback (chatter, rasp, or control), the distance between the focal individual and the speaker at the start of playback, the number of focal individuals, and the number of group members. To avoid dear-enemy effects, playback stimuli were randomly selected after excluding vocalizations from nearby individuals.
Video analysis was conducted using Adobe Audition 2023 for both video and audio data. Using the “preview video frames during audio clip editing” feature, recordings were transformed into spectrograms, and the start of the playback and reaction times were recorded. The frame rate was adjusted to 12 frames per second. After muting the audio in the video, the immediate response of the focal bird to the playback was categorized blindly.
We used a discriminate function analysis (DFA) to test whether rasp and chatter calls are acoustically distinct. To identify the features that best separate them in acoustic space, we simplified the model by using stepwise model selection (P = 0.1 to enter, P = 0.2 to remove).
To assess whether rasp and chatter calls were associated with different types of threats, we constructed a generalized linear mixed model (GLMM) with a binomial distribution, using call type (rasp or chatter) as the response variable, stimulus (aerial or terrestrial threat) as a fixed effect, and territory identity and year as random effects.
To identify variables predicting the response of the focal individuals, we used a multinomial logistic regression model. The predictive variables included position, stimulus type, number of group members, number of focal individuals, and individual distance from the speaker. The interaction between position and stimulus type was examined. The ANOVA function was used to test model assumptions. Additionally, ANOVA was conducted to determine if there were differences in response times to different alarms by focal individuals, followed by post hoc comparisons. Statistical analyses were performed in IBM SPSS Statistics version 27.0 (IBMCorp., Armonk, N.Y., USA) R software version 4.3.1 (R Core Team, 2023).
We analyzed 210 notes of rasp calls (from 7 individuals) and 224 notes of chatter calls (from 7 individuals). Eight variables were selected in the stepwise procedure for DFA (Table 1): duration, quartile 25 (end), peak frequency (centre), quartile 75 (centre), peak frequency (max), quartile 50 (max), entropy (max) and bandwidth (mean entire). The discriminant functions were statistically significant (Wilks’ λ = 0.08, χ2 = 820.866, P < 0.001). The DFA correctly classified 100% of calls.
From 2022 to 2023, a total of 195 rasp calls and 35 chatter calls were documented from 51 territories. Overall, 92.8% of rasp calls occurred in the presence of terrestrial threats, whereas 97.2% of chatter calls occurred in the presence of aerial threats (GLMM: estimate ± S.E. = 18.3 ± 5.15, z = 3.55, P < 0.001). Terrestrial threats were mostly humans, cats, dogs, rabbits, cows, or a plastic sparrowhawk model on the ground, while aerial threats were primarily Accipitridae species, Carrion Crows (Corvus corone), and Eurasian Magpies (Pica serica).
The first set of experiments tested individuals that had landed on the ground. The average group size was 10 (ranging from 2 to 18), with an average of 1 focal individual (ranging from 1 to 3). We classified their responses as “fly up”, “look around”, “fly to cover”, and “no response”. (Fig. 2). Responses significantly differed according to the stimuli. Specifically, in response to the rasp alarm, Azure-winged Magpies exhibited a higher proportion of “fly up” responses (N = 13/15), while in response to the chatter stimulus, there was a higher proportion of “fly to cover” responses (N = 14/15). No significant response was observed to white noise (N = 12/14).
In the second set of experiments involving individuals in highly exposed positions, the average group size was eight individuals (ranging from 1 to 17), and the average focal size was 2 individuals (ranging from 1 to 9). Responses were classified as “fly up”, “fly toward the speaker”, “dive down”, and “no response”. (Fig. 3). In the presence of the chatter stimulus, all target individuals quickly exhibited “dive down” responses (N = 21/21). Similarly, there was no response to white noise (N = 17/19) or rasp calls (N = 14/19). In addition, some individuals showed a tendency to approach the speaker during rasp playbacks (N = 5/19).
Among the factors considered, including position, stimuli type, group size, focal individual numbers, and distance to the speaker, both position (χ2 = 67.614, df = 5, P < 0.001) and stimuli type (χ2 = 142.395, df = 10, P < 0.001) were found to significantly influence the response type of focal individuals. The interaction effect was nonsignificant (χ2 = 7.136, df = 10, P = 0.7125). Azure-winged Magpies exhibited differences in response times to different stimuli (F = 4.976, P = 0.01). While there was no difference in response times to chatter stimuli between the two experiments (P = 0.78), response times to chatter stimuli were significantly shorter than those to rasp stimuli in both experiments (P = 0.01 and P = 0.04, respectively) (Fig. 4).
We provide evidence of functionally referential signals in Azure-winged Magpies in terms of acoustic structure, context specificity and behavioral responses to playback stimuli.
In Azure-winged Magpies, the rasp and chatter calls can be effectively distinguished, which aligns with the outcomes of the linear discriminant functions in other study (Wang et al., 2023). A step-wise model revealed that several acoustic features including duration, frequency, bandwidth, and entropy contributed significantly to the primary differences. Rasp calls featuring a wide bandwidth and a relatively longer duration, which is consistent with previous spectrographic and quantitative descriptions of a typical terrestrial predator-specific alarm call (e.g., Grieves et al., 2014; Cunningham and Magrath, 2017; Wang et al., 2023); this facilitates sound localization, attracting conspecifics to gather and provide assistance (Brown, 1982). In the case of aerial alarms, the duration of a single note in the chatter call is very brief. This aids receivers in reacting swiftly and makes it challenging for predators to localize the signaler, thereby reducing the risk of the signaler becoming prey (Jurisevic and Sanderson, 1998). Previous studies have also reported a trend of short- and high-frequency characteristics of aerial alarms (Greene and Meagher, 1998; Wood et al., 2000).
In addition to being acoustically distinct, rasp and chatter calls were given in specific natural and experimental contexts. Overall, the context of alarm call production is depended to predator’s position but not predator type. The chatter call appears to be given only to raptors in flight, whereas the rasp call is given to a variety of general terrestrial threats. However, in this study, when documenting airborne threats, the presence of raptors was unpredictable. Despite the observers’ efforts to confirm the absence of other types of predators in the vicinity, the presence of surrounding bushes prevented us from completely ruling out the presence of other predators while only documenting visible cases of raptors. Researchers have utilized the gliding and landing processes of the raptor-like models to simulate varying spatial positions, thereby contributing to a more controlled context (Cunningham and Magrath, 2017). Therefore, further research would be necessary to clarify this point.
In our playback experiments, despite the absence of any other cues about danger, magpies responded appropriately to the types of playbacks. Focal individuals, whether active on the ground or perched at high position, reacted to chatter calls by quickly hiding in the bushes, almost without looking around to confirm the actual presence of a predator, and have significantly more short response time. These reactions were never triggered by rasp and control stimuli, appear to be appropriate anti-predatory strategy of avoiding aerial predators (Grieves et al., 2014). For individuals perched at heights, their lack of notable response to rasp calls is understandable. Rasp calls typically signify ground-based predators that do not pose an immediate threat to individuals positioned far above the ground. In addition, we also observed that alarm calls indicating rasp calls prompted some focal individuals to fly toward the speaker, and during field observations, some rasp calls were used when individuals detected food. Terrestrial alarms are typically produced in response to a stationary or slow-moving predator (Marler, 1955), are commonly used as a mobbing call (e.g., Igic and Magrath, 2014; Cunningham and Magrath, 2017; Dutour et al., 2021), and have the distinctive, general property of attracting conspecific and heterospecific individuals and inciting mobbing behavior (Magrath et al., 2015). Consequently, rasp calls may serve as a means of instigating mobbing behavior, gathering individuals around the predator’s location to deter the predator before it begins hunting and also convey information about the location of food resources in a more neutral context.
Animals are generally believed to have two types of alarm systems, namely, functionally referential and urgency-based alarm systems. Urgency-based alarm systems are typically observed in species that have lower social complexity and live in relatively open habitats (Blumstein and Armitage, 1997; but see Fernández et al., 2023). However, the habitat in which Azure-winged Magpie live is relatively complex. In our study site, they inhabit and nest in dense sea buckthorn and willow bushes (Ren et al., 2016). Distinguishing predators using different vocalizations can be crucial. This helps in obtaining accurate predator-related information, even in situations where visual information transmission is hindered, and enables them to make the most appropriate anti-predatory response. In addition, Butler et al. (2022) revealed that life history characteristics influence the use of alarm calls in birds, and the breeding system can predict alarm call behavior. Specifically, cooperatively breeding species are more likely to emit different types of alarm calls than noncooperatively breeding species (Leighton, 2017). In previous studies, some researchers have discovered functionally referential alarm calls in several cooperatively breeding species. For example, the joint-nesting Smooth-billed Ani (Crotophaga ani) produces ‘chlurp’ calls in response to flying raptorial birds, and they give ‘ahnee’ alarms in response to terrestrial threats (Grieves et al., 2014); the communally breeding Greater Ani (Crotophaga major) gives “high cackle” calls in response to aerial threats and “scold” calls in response to general nonaerial predators (LaPergola et al., 2023). Cooperative breeding implies more frequent interactions among individuals and more complex social relationships, leading to additional and potentially even more challenging problems compared to species with lower social complexity (Freeberg et al., 2012). For instance, these species are more likely to be noticed by predators. In such situations, informing other individuals about common goals or recruiting unified behaviors through vocal signals contributes to efficient cooperation, avoiding confusion within the group.
Despite the increasing interest in study the communicative systems of social birds, much of the research on alarm systems primarily focuses on mammals rather than birds (e.g., Bolt et al., 2015; Berthet et al., 2022; Deshpande et al., 2023). As corvids have extensive cognitive abilities and complex social interactions, their vocalizations are excellent models for understanding the evolution of language and cooperative behavior strategies. The results presented here suggest that social corvid species Azure-winged Magpies use functionally referential signals to transmit predator information to group members. In addition to referential signals, it may be interesting to examine whether other signaling systems are used in their communication and whether they take turns in sentinel duty or if it is based on the social hierarchy.
This study was approved by the Ethic and Animal Welfare Committee, College of Life Science, Beijing Normal University on animal ethics (approval number: CLS-EAW-2021–020).
Xingyi Jiang: Writing – original draft, Investigation, Formal analysis. Yanyun Zhang: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to express our gratitude to Dr. Bo Du from Lanzhou University for assistance with fieldwork. We are grateful to Dr. Canwei Xia for the helpful assistance with data analysis.
|
Bradbury, J.W., Vehrencamp, S.L., 2011. Principles of Animal Communication, second ed. Sinauer, Sunderland, MA.
|
|
Brown, C.H., 1982. Ventroloquial and locatable vocalizations in birds. Z. Tierpsychol. 59, 338–350.
|
|
Hailman, J.P., Ficken, M.S., Ficken, R.W., 1985. The ‘chick-a-dee’ calls of Parus atricapillus: a recombinant system of animal communication compared with written English. Semiotica 56, 191224.
|
|
Krause, J., Ruxton, G.D., 2002. Living in Groups. Oxford University Press, Oxford.
|
|
Pereira, M.E., Macedonia, J.M., 1991. Ringtailed lemur anti-predator calls denote predator class, not response urgency. Anim. Behav. 26, 760–777.
|
|
Struhsaker, T.T., 1967. Auditory communication among vervet monkeys. In: Altmann, S. A. (Ed.), Social Communication Among Primates. University of Chicago Press, Chicago, pp. 281–324. Cercopithecus aethiops.
|
|
Zuberbühler, K., 2003. Referential signaling in non-human primates: cognitive precursors and limitations for the evolution of language. Adv. Study Behav. 33, 265–307.
|
| Abbreviations | Meanings |
| Duration | Length of note |
| Peak freq (end) | Peak frequency at the end of a note |
| Peak ampl (end) | Peak amplitude at the end of a note |
| Bandw (end) | Bandwidth at the end of a note |
| Quart 25 (end) | First quartile frequency at the end of a note |
| Quart 50 (end) | Second quartile frequency at the end of a note |
| Quart 75 (end) | Third quartile frequency at the end of a note |
| Entropy (end) | Entropy at the end of a note |
| Peak freq (centre) | Peak frequency at the centre of a note |
| Peak ampl (centre) | Peak amplitude at the centre of a note |
| Bandw (centre) | Bandwidth at the centre of a note |
| Quart 25 (centre) | First quartile frequency at the centre of a note |
| Quart 50 (centre) | Second quartile frequency at the centre of a note |
| Quart 75 (centre) | Third quartile frequency at the centre of a note |
| Entropy (centre) | Entropy at the centre of a note |
| Peak freq (max) | Maximum peak frequency |
| Peak ampl (max) | Maximum peak amplitude |
| Bandw (max) | Maximum bandwidth |
| Quart 25 (max) | Maximum first quartile frequency |
| Quart 50 (max) | Maximum second quartile frequency |
| Quart 75 (max) | Maximum third quartile frequency |
| Entropy (max) | Maximum entropy |
| Peak freq (mean entire) | Peak frequency of the entire note |
| Peak ampl (mean entire) | Peak frequency of the entire amplitude |
| Bandw (mean entire) | Bandwidth of the entire note |
| Quart 25 (mean entire) | First quartile frequency of the entire note |
| Quart 50 (mean entire) | Second quartile frequency of the entire note |
| Quart 75 (mean entire) | Third quartile frequency of the entire note |
| Entropy (mean entire) | Entropy of the entire note |