| Citation: | Michelle García-Arroyo, Miguel A. Gómez-Martínez, Ian MacGregor-Fors. 2023: Litter buffet: On the use of trash bins by birds in six boreal urban settlements. Avian Research, 14(1): 100094. DOI: 10.1016/j.avrs.2023.100094 |
Unintentional food resources in urban areas (street litter, food leftovers, overflowing trash bins) are dietary components of some urban-exploiter bird species. In this study, we report on 13 bird species in six southern Finnish cities using urban trash bins and describe differences in their activity when provided with food resources (i.e., bait) in different bin types. We used generalized linear models (GLM) and classification and regression trees (CART) to test for associations between environmental variables and bird activity at the binscapes. Bird activity at the binscapes significantly differed among all cities and among types of bins and was significantly higher after placing bait in all cases. Bins with the largest opening had more activity as opposed to those with smaller openings or lids. Corvids and gulls had the highest activity, with corvids usually being present before the bait was placed and gulls increasing their activity thereafter. These differences show that trash bin foraging is highly malleable and thus susceptible to management preventing its occurrence. Suitable waste management measures could aid in reducing the number of species close to bins and their surroundings, benefiting both bird and human health.
High food density and predictability in cities have partially explained the demographic explosions of the handful of species that have been shown to thrive in cities (Shochat, 2004; Kark et al., 2007). Yet, urban food provisioning is diverse and may vary notably across cities (Faeth et al., 2005). Active food supplementation (such as bird feeders) has received important attention and has a relevant role in urban bird ecology (Dunn, 1986; Wells et al., 1998; Kövér et al., 2019; Bonter and Greig, 2021); however, unintentionally provided food resources in urban areas, in the form of street litter, food leftovers, and/or overflowing trash bins, also provide important resources for some of these so-called “urban-exploiter species” (Auman et al., 2008; Haemig et al., 2015; Spelt et al., 2021), particularly during winter (Tryjanowski et al., 2015). While directly surveying street litter and its effect on urban birds would be a complicated task and could be obscured by a plethora of confounding factors, studying public urban trash bins is a suitable scenario to understand the role of such kind of supplementary feeding resources for birds (e.g., Skórka et al., 2016; Coccon and Fano, 2020; Bernat-Ponce et al., 2022, 2018). The presence of litter on city streets often has direct negative impacts for humans and causes unwanted interactions, not only with birds, but also with other wildlife (Przybylska et al., 2012; Schultz et al., 2013; Newsome and Van Eeden, 2017). Street litter also causes a sizable economic expense (Yle Helsinki, 2010; Niemistö, 2015; e.g., cost of littering cleaning ranges from tens of thousands for medium-sized Finnish cities up to € 300,000 for its capital, Helsinki).
Among the few studies that have focused on the role of human waste as a food resource for birds, researchers have found that some groups are particularly associated with it, including corvids (Corvidae), gulls (Laridae), mynas and starlings (Sturnidae), doves and pigeons (Columbidae), sparrows (Passeridae), and storks (Ciconiidae) (Yap et al., 2002; Mehra et al., 2017; Plaza and Lambertucci, 2017; Noreen and Sultan, 2021). Experiments have shown that Clark’s Nutcracker (Nucifraga columbiana), for instance, are capable of detecting food in containers simulating trash bins, showing a potential cognitive adaptation that makes this species more efficient when gathering food (Tornick and Gibson, 2013). This adaptation is not unique to nutcrackers, as many other corvids have been recorded, both empirically or anecdotally, to take advantage of human waste (e.g., Eurasian Magpie Pica pica, Hooded Crow Corvus cornix, Western Jackdaw Coloeus monedula) in Europe and Asia (Jerzak, 2001; Kim et al., 2012; Kövér et al., 2019), and in North America (e.g., American Crow Corvus brachyrhynchos; Townsend et al., 2019). These species are often able to discriminate between trash bins and prefer to establish their territories in areas with the most accessible ones (Jerzak, 2001; Kim et al., 2012). Another studied bird group associated with trash bins is the gulls, although not as thoroughly as corvids (e.g., Noreen and Sultan, 2021). In general, studies report that the demographic explosion of several gull species (e.g., Herring Gull Larus argentatus, Lesser Black-backed Gull Larus fuscus, Great Black-backed Gull Larus marinus, Common Gull Larus canus, Black-headed Gull Chroicocephalus ridibundus) in Europe and Asia is related to the excessive use of waste dumps for food and water resources (Harris, 1970; Mudge and Ferns, 1982; Noreen and Sultan, 2021; Spelt et al., 2021; Tryjanowski et al., 2022).
In this study we assessed the use of urban public trash bins and its immediate surroundings (referred to as “binscape” hereafter) by birds in six human settlements in southern Finland ranging from the country’s capital (Helsinki) to small urban centers (i.e., Hämeenlinna, Salo). For this, we surveyed birds in randomly found binscapes across a diverse set of urban scenarios and recorded their activities in normal conditions and after we provided food. Our goals were to: (i) identify the bird assemblage using the studied binscapes; (ii) assess if bird activity (time) varied across cities and bin types; (iii) evaluate changes in bird activity (time and behavior) with food provisioning in general and among cities, and if so, how and which species and activities were involved in the shift; (iv) evaluate how did birds use the sections of bins (i.e., top/lid, opening, 10 m radius of the immediate surroundings) for the main three bin types before and after food provisioning; and (v) evaluate how the surrounding human and environmental conditions relate to bird activity during food provision.
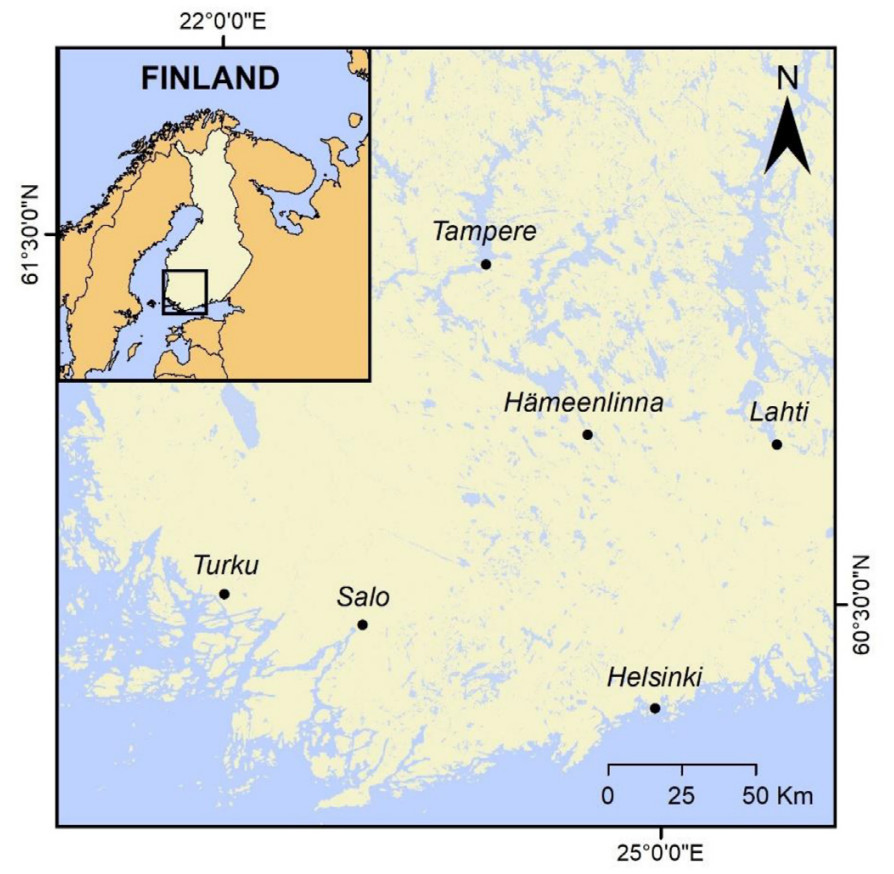
We performed this study in six urban centers located across southern Finland (Fig. 1). We surveyed bird activity in binscapes in each of the studied cities which differed in size (45 binscapes in total; Table 1). We randomly selected bins as we walked across the urban settlements, starting from downtown in all cases, only considering binscapes where enough space was available to set the recording device, which was placed on a tripod at a 25-m distance from the bins (to avoid disturbing the birds). We also selected binscapes that were placed at least 200 m away from each other, to reduce the probability of recording the same individuals in different bins (Ralph et al., 1996). At each bin location, we measured vehicle traffic during the first 10 min in a 50-m radius. At the same time, we measured noise levels using a sound meter application (Sound Meter v.2.16; Splend Apps) to retrieve the average noise value (dB). We also retrieved the number of pedestrians near the binscapes (i.e., visible on the recording) from the first 10 min of videos. Finally, we estimated the vegetation cover (%) of the surrounding area using 360° panoramic photographs of each binscape location.
| City | Location | Altitude (m a.s.l.) | Inhabitants | Number of bins studied | ||
| T1 | T2 | T3 | ||||
| Saloa | 60.384724° N, 23.128848° E | 2 | 51,600 | 4 | 1 | 0 |
| Hämeenlinna | 60.996426° N, 24.465297° E | 90 | 67,900 | 2 | 2 | 4 |
| Lahti | 60.983481° N, 25.656182° E | 106 | 120,000 | 7 | 1 | 0 |
| Turku | 60.451569° N, 22.266925° E | 9 | 194,400 | 8 | 0 | 0 |
| Tampere | 61.501403° N, 23.778682° E | 101 | 241,000 | 1 | 7 | 0 |
| Helsinki | 60.171217° N, 24.943986° E | 15 | 1,200,000b | 5 | 0 | 3 |
| a Note that eight binscapes were surveyed in all settlements except for Salo, where only five binscapes were surveyed for logistical reasons. b Considering the Helsinki Metropolitan Area |
||||||
Binscapes were located in sites with differing vegetation cover (mean ± SD = 36.1 ± 28.1%), pedestrian activity (mean ± SD = 15.6 ± 14.6), car traffic (mean ± SD = 13.7 ± 25.2), and noise (mean ± SD = 61.3 ± 5.2 dB). At each binscape we recorded two sets of videos of 10 min each. In the first video, we recorded bird activity in the binscape without any intervention from our end. Immediately after the first video was done, we approached the bin to place a bait that consisted of 100 g of fruit (i.e., ~2 cm3 apple and orange cubes) and one tablespoon of canned sardines, following Sebastián-González et al. (2020). We placed the bait on a 15 cm × 25 cm brown paper sheet (similar to that used for food wrapping) on top of all bins mimicking litter being left outside the bins. Once placed, we started the second recording.
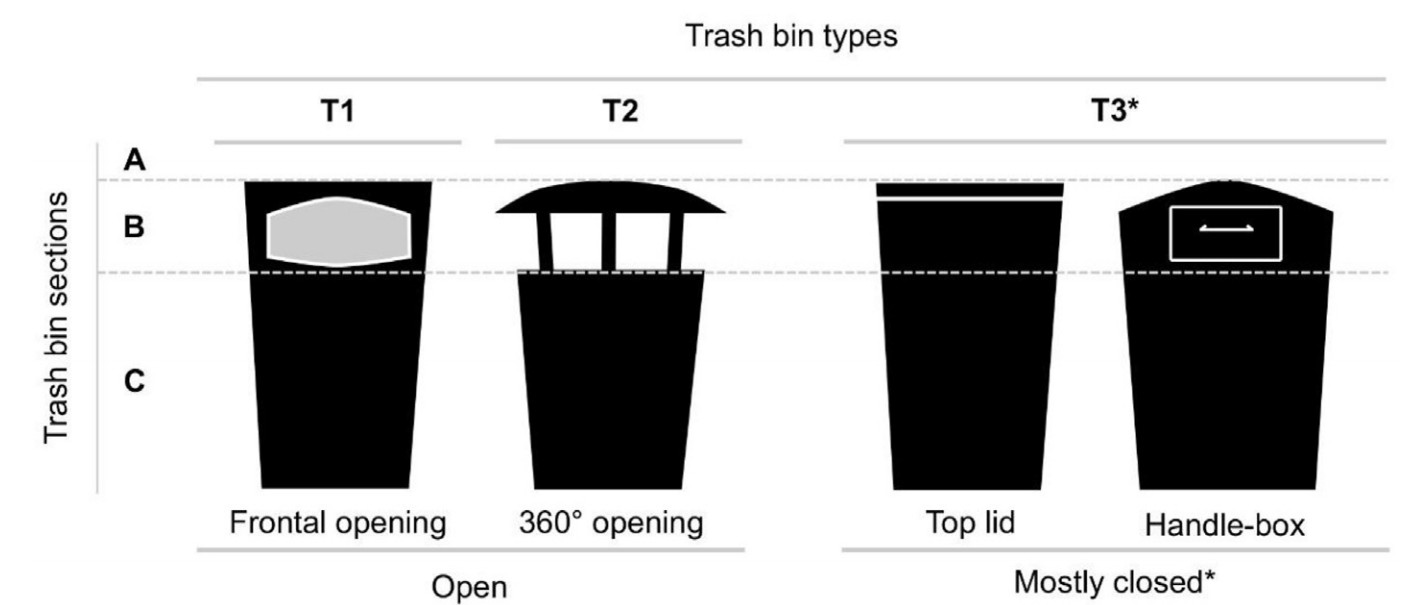
Based on our recordings, we measured the activity of each individual bird that was using the binscape. We divided each bin into sections: top/lid, opening, and 10 m radius immediate surroundings (referred to as A section, B section, and C section respectively hereafter). We recorded bird activity (time in seconds) at each section as an independent event. We considered four types of bird activity (behavior): (ⅰ) walking; (ⅱ) perching (including natural and artificial perches); and two types of foraging: (ⅲ) non-bait feeding; and (ⅳ) bait feeding. We classified bin type as: T1 (i.e., only frontal opening), T2 (i.e., 360° opening), and T3 (i.e., bins with lids, other types of bins; Fig. 2).
In order to assess if the surveyed cities and bin types (independent variables) were related to the bird activity (time) at the binscapes, we performed a generalized linear model (GLM) considering a Poisson error distribution, followed by general linear hypotheses and multiple comparisons (glht; multcomp R-package; Hothorn et al., 2008). We used a similar GLM (Poisson error distribution, followed by glht contrasts) to test: (i) changes in bird activity (time) with food provisioning in and among cities; (ii) changes in bird activity (time) among bird species and their behaviors before and after placing the baits; and (iii) changes in bird activity (time) among the sections of the three types of bins before and after placing the baits.
In order to analyze associations between city identity, vehicle traffic, pedestrian activity, and vegetation cover with bird activity (time) when the bait was in place, we ran a classification and regression tree (CART; rpart R-package; Breiman et al., 1984; Therneau and Atkinson, 2022). For this, we considered bird activity (time) as the numerical dependent variable and city identity, vehicle traffic, pedestrian activity, and vegetation cover as independent variables. CARTs use binary recursive partitioning algorithms to identify the independent variables that best explain variance of a given dependent variable (Crawley, 2013). The ‘rpart’ R-package uses ANOVAs to split the dataset into two mutually exclusive subsets at nonterminal nodes trees based on an identified threshold value of the independent variable. We used CARTs given their robustness and flexibility of not requiring specification of a data distribution, allowing for nonlinear relationships to be identified between the set of dependent and independent variables (De’ath and Fabricius, 2000). Based on the independent variables identified by the CART to relate with bird activity (time), we followed the same GLM (Poisson error distribution, followed by glht contrasts) procedure.
We recorded a total of 13 species while surveying the binscapes (Table 2). Of them, four were gulls, three were crows, two doves, two sparrows, a thrush and a wagtail, most of which are dietary generalist and widely distributed across the studied cities (pers. obs.). We recorded 11 species in the no food provisioning scenario (with the Common Wood-pigeon Columba palumbus and Herring Gull missing from the list of species recorded), and 12 species in the food provisioning one (only Eurasian Tree Sparrow Passer montanus missing from the list of birds recorded).

|
Bird activity (time) significantly differed among all cities and among the three types of bins with two exceptions (post-hoc GLM P-values <0.001; Fig. 3): Helsinki and Turku (P = 0.93), Hämeenlinna and Lahti (P = 0.98). When considering all cities and bin types, we recorded a significantly higher bird activity (time) at the binscapes after placing the bait (GLM: deviance χ21,88 = 2906.5, P < 0.001). This result remained consistent when considering city identity (GLM: deviance χ21,83 = 14186.5, P < 0.001), as well as the interaction between city and food provisioning (GLM: deviance χ21,78 = 2097.0, P < 0.001). The post-hoc test for these interactions showed that for all studied urban areas, there was a significant increase in bird activity (time) when we placed the baits on the bins (all post-hoc GLM P-values <0.001; Fig. 4A).
The assessment of the shift in bird species’ activities (behavior) before and after food provisioning showed a general increase in activity of all species (with the exception of the two Eurasian Tree Sparrow events perching for 14 s close to a studied bin; Fig. 4B). Species identity, their activities (behavior), and the interaction between them were significantly related to bird activity (time). For non-bait feeding, only the Hooded Crow showed a significant reduction in activity at the binscapes, while perching increased significantly for the Hooded Crow, Black-headed Gull, and Common Gull. In the case of walking behavior, all significant relationships were positive and involved: the Black-headed Gull, Western Jackdaw, Rock Pigeon (Columba livia), Common Gull, House Sparrow (Passer domesticus) (post-hoc GLM P-value <0.003).
Regarding the use of bin sections (i.e., A, B, and C) in the main three bin types before and after food provisioning, results show several general patterns and particularities among bin types. First, in all bin types, we recorded a significant increase of perching birds in the A section; intriguingly, we recorded a significant increase in perching birds in all sections of T2 bins (all post-hoc GLM P-values <0.001). Second, results show an increase of walking activity in the surveyed birds in the C section for all bin types (significant differences for T1 and T3, P > 0.001; non-significant trend for T2, P = 0.067). Shifts in the non-bait feeding activity were all negative and recorded at the A and C sections of T2 bins, and at the A section of T1 bins (all post-hoc GLM P-values <0.001; Fig. 5).
Lastly, city identity and traffic were the most relevant variables in explaining the activity (time) of birds in the binscapes. The CART shows that independently of traffic, small settlements (i.e., Lahti, Hämeenlinna, Salo) were associated with less bird activity (time) in the binscape, while birds in larger cities (i.e., Helsinki, Tampere, Turku) had on average higher activity when compared to the smaller settlements, but even highest in sites in larger cities with less traffic (< three vehicles in 10 min; Fig. 6). Results of the GLM confirmed that such relationships were strong and significant (city identity: deviance χ25,39 = 9688, P < 0.001; traffic: deviance χ21,38 = 1132, P < 0.001).
Reports of human litter use by birds date back to the 1960s (Noreen and Sultan, 2021). Ever since, the wide variety of litter found in city trash bins has contributed substantially to the modification of urban bird community dynamics (e.g., increasing the populations of species that exploit these resources; Marzluff et al., 1994; Nowakowski, 1996). In this study, we report that the use of binscapes by 13 bird species in six southern Finnish cities increases when provided with additional food as bait. A novel approach regarding our work was that we took into consideration different types of trash bins utilized by Finnish municipalities (Fig. 2), which to the best of our knowledge has not been considered in previous field experiments. Bin type and use differed across cities, with our results clearly showing a significantly higher use of binscapes by birds in Tampere both before and after providing the bait, where most bins were type T2. After a sizable drop-off in the bird use of the binscape in comparison with Tampere, that of Turku followed, where all bins were T1. The use of bins continued to decrease for the rest of the cities with the type of bin changing among them; for example, T3 bins were only present in Helsinki and Hämeenlinna.
Our data suggests that bin type is a relevant characteristic as birds are able to detect the presence of food resources depending on the ‘openness’ of the bin. When we contrasted the bird activity at the binscapes before and after placing the bait, we found that activity increased across all cities and bin types. Even though the bird activity at the binscapes was higher for all bin types, it was clear that for T1 (i.e., frontal opening bins) and T3 (i.e., mostly closed bins) the placement of bait was the trigger that changed bird behavior, while for T2 (i.e., 360° opening bins) birds were already engaging with the resources that those bins provide (Fig. 5). In general, after placing the bait, feeding time not only increased, but also the walking and perching time increased as more birds approached and engaged with the binscape. This type of behavior was not surprising considering that it has been documented in similar scenarios exploring the use of resources in urban contexts. It has been reported that adults of Herring Gulls are able to discern between weekdays, when the fresh waste is dumped, and the weekend, when fresh waste is scarce or unavailable (Noreen and Sultan, 2021). This type of modification of the foraging schedule due to anthropogenic food source fluctuations was also observed in Lesser Black-backed Gulls in the United Kingdom (Spelt et al., 2021). It is hypothesized that this is achieved by following visual cues like the presence of other individuals or even workers’ activity (Monaghan et al., 1986; Coulson et al., 1987; Stofberg et al., 2019). By recording an increase of the bird activity at the binscapes after the bait was placed for almost all species (with the exception of Fieldfare Turdus pilaris), we also confirmed this behavior. Which visual cues are most relevant to this shift in activity remains to be assessed, as we did not analyze these parameters. Anecdotally, we noticed that for several species, the number of individuals and the calls they emitted attracted more individuals (and other species) even after the bait was knocked off the bin or totally consumed. This type of calls and behavior around food sources has been observed in corvids, sparrows, and other species (Elgar, 1986; Bugnyar et al., 2001; Suzuki, 2012), even when recruitment is not the ultimate goal of such calls (Heinrich and Marzluff, 1991).
Previous studies have pointed out scavenger birds as the prevalent species type using bins as a food source (Eronen et al., 2022). Corvids have been identified as one of the most prolific bird groups associated with this anthropogenic resource, with up to ten species listed as users (see details in Benmazouz et al., 2021). For the three corvid species identified using binscapes in our assessment, the presence of scattered litter before our arrival was a good indicator of their engagement with the surveyed binscape and bating. In the instance in which we recorded the highest bird activity and engagement with the binscape, a T2 bin was overflowing with litter and several individuals of Hooded Crow were even diving into the bin to ‘fish’ for food. The binscape was located in the heart of a very active commercial area in Tampere, thus explaining the amount of food-related litter and maybe the crows’ habituation to this resource. During this particular event, the individuals were not flushed away by the area’s intense traffic (i.e., 44 vehicles in 10 min) nor the presence of bikes, pedestrians and their dogs (i.e., 55 pedestrians in 10 min), even when traffic was one of the most relevant variables explaining the amount of bird activity (as seen in other studies assessing small water resources; Tryjanowski et al., 2022).
In another display of bold behavior, when we were removing the leftovers of the bait after we finished the survey of a T1 bin located in the main square of the city of Lahti, a Western Jackdaw perched on the recording device/tripod approximately 1 m away from us to inspect our actions. This type of behavior that involves both boldness and habituation to human disturbance displayed by corvids could be explained by a decrease in persecution over time (Vuorisalo et al., 2003). Hooded Crows were previously considered a pest species in Finnish cities and nest removal and destruction was encouraged during the 1960s and early 1970s, but this practice eventually stopped and population growth began in late 1970s (Vuorisalo et al., 2001, 2003). It is notable that birds living in highly urbanized environments tend to be bolder than those living in non-urban areas, thus the presence of humans around the food resources in the binscapes does not constitute a deterrent for these bird species in the urban context (Morelli et al., 2022).
Similarly with what has been previously reported in the literature on the use of human food waste (Noreen and Sultan, 2021), in our study, corvids and gulls had the highest activity time in the assessed binscapes. However, the most important difference between their activities was that corvids were usually present before the bait was placed and gulls increased their activity after the bait was placed. We found that the four species of gulls we recorded in our study were an important component of the binscape in sites near waterfronts, particularly on piers that also accommodated touristic restaurants or food stands nearby. Populations of some gull species have been experiencing an increase in urban areas due to the high variety of nesting and feeding resources available on cities (e.g., Common Gull; Kubetzki and Garthe, 2007; Noreen and Sultan, 2021). In a comparative study, males of Silver Gull (Chroicocephalus novaehollandiae) from urban populations were heavier and had a larger body condition than their counterparts in the non-urban area, representing a 10% difference in body mass between both groups (Auman et al., 2008). Changes in the foraging behavior of gulls and other birds inhabiting urban areas has been highly documented in the literature (Noreen and Sultan, 2021). Urban colonies of Herring gulls developed more site fidelity, carrying out their foraging trips in urban parks, shopping centers, and parking lots, in contrast with colonies in a low degree of urbanization that foraged at the sea and took longer trips (Fuirst et al., 2018). This urban versus not-urban difference was also observed when assessing birds using small water resources during summer in a northern temperate zone (Tryjanowski et al., 2022).
In comparison with the recorded corvids and gulls, the activity from the other six species recorded was much lower. In the case of the House Sparrow, activities increased in time after the bait was placed but individuals were flushed by pedestrians (mean = 15.25 pedestrians in 10 min) and thus sparrows ceased to feed on the binscape in most cases. The presence of other species did not seem to deter their interest in the binscape but sparrows almost always stayed in the ground or surroundings and not directly on the bin. The amount of food resources might explain the willingness of House Sparrows to approach or not a site when other species, such as corvids, are also present (Skórka et al., 2016). Surprisingly, species that are considered highly urban, like the Rock Pigeon, hardly engaged with the bait during our study, but remained foraging on the ground and the bin’s surroundings and were mainly flushed by larger species. However, positive correlations between the average number of houses and organic waste produced in them and the number of Rock Pigeons have also been reported (Buijs and Van Wijnen, 2001), thus highlighting the association between this species and the use of human litter as a resource. Other species not as frequently mentioned in this type of assessments were those that we considered “indirectly associated species”: those individuals that were present either before or after, or before and after, that did not interact with the bin or the bait, yet were walking, perching, or foraging in the surroundings (thus, inside the binscape). In our study, this list includes the White Wagtail (Motacilla alba), Fieldfare, Common Wood-pigeon, and Eurasian Tree Sparrow.
Beyond the well-known ecological issues related with urban trash bins, the consumption of litter can have harmful effects on birds creating important nutrient imbalance, spreading infectious diseases (e.g., West Nile Virus), and increasing the prevalence of bacteria and fungi (Julian et al., 2002; Vlahović et al., 2010; LaDeau et al., 2011; Burt et al., 2021). Some studies have focused on the Eurasian Magpie as a good bioindicator of heavy metal accumulation in different tissues and feathers in urban areas (Dmowski and Golimowski, 1993; Zarrintab et al., 2016). A parsimonious explanation of heavy metal presence on magpie’s tissues involves the contamination by ingestion of anthropogenic food, having harmful effects in many cases (Zarrintab and Mirzaei, 2018; Iemmi et al., 2021). Moreover, the importance of litter in urban areas lies in the fact that this kind of food represents a fundamental part (~23–40%) of the diet of many omnivorous birds such as the Magpie (Jerzak, 2001). This was not the species with the highest feeding time in our study but the same contamination by ingestion could be occurring to any other of the species we recorded consuming litter.
Moreover, the use of bins as feeding sites in cities can lead to an increase in the abundance of gull and corvid species which in turn would make human-wildlife conflicts more frequent. In many cases these interactions are perceived as negative, making the human population feel aversion towards these birds as they become a nuisance for several reasons (e.g., noise, increase of feces in urban structures, litter scattering, aggressive behaviors; Benmazouz et al., 2021). Given the already high economic cost of litter management for Finnish cities, the emergence of negative human-wildlife interactions due to the bird use of trash bins, including potential disease transmission to poultry (Pitkänen, 2022; Siltanen, 2022), and the involvement of other wildlife groups (such as urban mammals; Rantanen, 2017; Korpela, 2022) could pose an additional economic burden.
Our findings show that the use of binscapes by birds in southern Finland differs among cities, bin types, and when bait is provided, with way more species using the micro-system than previously reported. Having found differences in bin type, for instance, shows this phenomenon is thus highly malleable and therefore susceptible to being properly managed to prevent its occurrence. To avoid human-wildlife conflicts and to keep gulls, corvids, and pigeons away from their roofs, docks, and tables urbanites have come up with the use of many deterrents (Yle Turku, 2011; Jämsen, 2015). Regarding food resources, however, the most effective way has always been to not leave litter for birds to consume (Koskinen, 2014). Our results show that bins with large openings are more susceptible to bird litter consumption, thus we suggest avoiding the use of bins with 360° openings. Interestingly, bins with lids showed no difference in activity to the ones with just small frontal openings, which are already the type of bins most commonly found around these cities. We also highlight how activity increases when food resources are left outside of the bins (in this study, using bait as a proxy), thus making it essential that urbanites dispose of their litter in a proper manner to avoid attracting unwanted bird attention. One example of making this process easier is cities in Europe implementing underground receptacles of this type of waste, with a decrease in the abundance of birds around them (i.e., House Sparrows; Bernat-Ponce et al., 2022). Suitable waste management measures could aid in reducing the number of these species close to bins and other sites, benefiting both bird and human health (Chong et al., 2012). To this end, these measures are important for reducing the occurrence of negative interactions with the aim of avoiding the transmission and proliferation of potentially zoonotic diseases and reducing the noxious elements on these birds’ diets to humans and in the wildlife food chain in general.
Michelle García-Arroyo: Conceptualization, Methodology, Investigation, Data curation, Writing–Original draft, Visualization, Funding acquisition. Miguel A. Gómez-Martínez: Methodology, Investigation, Writing–Original draft. Ian MacGregor-Fors: Conceptualization, Methodology, Formal analysis, Resources, Writing–Original draft, Visualization, Supervision. All authors read and approved the final manuscript.
The present research was mostly observational. The bait provided in trash bins was comprised of fresh fruit and sardines (no artificial colors, flavors, or other substances added). No animal was subjected to any procedure likely to cause pain, suffering, distress, or lasting harm in compliance with ethical standards.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are thankful to Eleanor S. Diamant and the two anonymous reviewers for their helpful comments that enhanced the quality and clarity of our manuscript. This work was supported by funds from the Otto A. Malm Foundation (to MG-A) and the University of Helsinki Lahti fund (to MG-A).
|
Breiman, L., Friedman, J., Stone, C.J., Olshen, R.A., 1984. Classification and Regression Trees. CRC Press, Boca Raton, Florida.
|
|
Chong, K.Y., Teo, S., Kurukulasuriya, B., Chung, Y., Rajathurai, S., Lim, H., et al., 2012. Decadal changes in urban bird abundance in Singapore. Raffles Bull. Zool. 25, 181-188.
|
|
Crawley, M.J., 2013. The R Book, second ed. Wiley, Chichester, West Sussex.
|
|
Dmowski, K., Golimowski, J., 1993. Feathers of the magpie (Pica pica) as a bioindicator material for heavy metal pollution assessment. Sci. Total Environ. 139-140, 251-258.
|
|
Dunn, E.H., 1986. Feeder counts and winter bird population trends. Am. Birds 40, 61-66.
|
|
Eronen, E., Johnson, D., Wan, K.M., 2022. Understanding Trash Foraging Practices In Bird Populations on The UBC Vancouver Campus (Student Research Report). University of British Columbia, Vancouver.
|
|
Heinrich, B., Marzluff, J.M., 1991. Do common ravens yell because they want to attract others? Behav. Ecol. Sociobiol. 28, 13-21.
|
|
Jerzak, L., 2001. Synurbanization of the magpie in the Palearctic. In: Marzluff, J.M., Bowman, R., Donnelly, R. (Eds.), Avian Ecology and Conservation in an Urbanizing World. Springer US, Boston, MA, pp. 403-425.
|
|
Kim, S., Srygley, R.B., Lee, J.Y., Lee, S., Choe, J.C., 2012. Urban and natural components of Korean Magpie (Pica pica sericea) territories and their effects on prey density. Pol. J. Ecol. 60, 407-417.
|
|
Marzluff, J.M., Boone, R.B., Cox, G.W., 1994. Historical changes in populations and perceptions of native pest bird species in the West. Stud. Avian Biol. 15, 202-220.
|
|
Mehra, S.P., Mehra, S., Uddin, M., Verma, V., Sharma, H., Singh, T., et al., 2017. Waste as a resource for avifauna: review and survey of the avifaunal composition in and around waste dumping sites and sewage water collection sites (India). Int. J. Waste Resour. 7, 289.
|
|
Noreen, Z., Sultan, K., 2021. A global modification in avifaunal behavior by use of waste disposal sites (waste dumps/rubbish dumps): a review paper. Pure Appl. Biol. 10, 603-616.
|
|
Nowakowski, J.J., 1996. Changes in the breeding avifauna of Olsztyn [NE Poland] in the years 1968-1993. Acta Ornithol. 1, 39-44.
|
|
Ralph, C.J., Geupel, G.R., Pyle, P., Martin, T.E., DeSante, D.F., 1996. Handbook of Field Methods for Monitoring Landbirds. U.S.D.A., Forest Service, Pacific Southwest Research Station. Gen. Tech. Rep. PSW-GTR-144, Albany.
|
|
Vlahović, K., Prukner-Radovčić, E., Horvatek, D., Pavlak, M., Gomerčić, T., Rumiha, Z., et al., 2010. Bacterial and fungal flora in faecal samples from rooks (Corvus frugilegus) in the City of Zagreb, Croatia. Vet. Arh. 80, 81-92.
|
|
Vuorisalo, T., Andersson, H., Hugg, T., Lahtinen, R., Laaksonen, H., Lehikoinen, E., 2003. Urban development from an avian perspective: causes of hooded crow (Corvus corone cornix) urbanisation in two Finnish cities. Landsc. Urban Plann. 62, 69-87.
|
|
Wells, J.V., Rosenberg, K.V., Dunn, E.H., Tessaglia-Hymes, D.L., Dhondt, A.A., 1998. Feeder counts as indicators of spatial and temporal variation in winter abundance of resident birds. J. Field Ornithol. 69, 577-586.
|
| City | Location | Altitude (m a.s.l.) | Inhabitants | Number of bins studied | ||
| T1 | T2 | T3 | ||||
| Saloa | 60.384724° N, 23.128848° E | 2 | 51,600 | 4 | 1 | 0 |
| Hämeenlinna | 60.996426° N, 24.465297° E | 90 | 67,900 | 2 | 2 | 4 |
| Lahti | 60.983481° N, 25.656182° E | 106 | 120,000 | 7 | 1 | 0 |
| Turku | 60.451569° N, 22.266925° E | 9 | 194,400 | 8 | 0 | 0 |
| Tampere | 61.501403° N, 23.778682° E | 101 | 241,000 | 1 | 7 | 0 |
| Helsinki | 60.171217° N, 24.943986° E | 15 | 1,200,000b | 5 | 0 | 3 |
| a Note that eight binscapes were surveyed in all settlements except for Salo, where only five binscapes were surveyed for logistical reasons. b Considering the Helsinki Metropolitan Area |
||||||

|