| Citation: | Yan Cai, Xiangyang Chen, Neng Wu, Canchao Yang. 2023: Chromatic and achromatic differences of melanin- and carotenoid-based plumage coloration in five minivet species (Pericrocotus spp.) under conspecific and predator visual systems. Avian Research, 14(1): 100077. DOI: 10.1016/j.avrs.2023.100077 |
Adaptive mate choice has been accepted as the leading theory to explain the colorful plumage of birds. This theory hypothesizes that conspicuous colors act as signals to advertise the qualities of the owners. However, a dilemma arises in that conspicuous colors may not only attract mates, but also alert predators. The "private channels of communication" hypothesis proposes that some intraspecific signals may not be visible to heterospecific animals because of different visual systems. To better understand the evolution of plumage colors and sexual selection in birds, here we studied the chromatic difference and achromatic differences of melanin- and carotenoid-based plumage coloration in five minivet species (Pericrocotus spp.) under conspecific and predator visual systems. We found that either the chromatic or achromatic difference among male or female minivets' plumage was consistently higher under conspecific vision than under predator vision for all five studied species of minivets. This result indicated that individual differences in plumage colors of minivets were visible to the conspecific receivers and hidden from potential predators as a result of evolution under predation risk and conspecific communication. However, males were under a higher risk of predation because they were more conspicuous than females to the vision of a nocturnal predator.
Numerous previous studies have formed a generally accepted theory of adaptive mate choice (e.g. Zahavi, 1975; Hamilton and Zuk, 1982; Clutton-Brock et al., 1997; Kappeler and van Schaik, 2002; Kruuk et al., 2002; Boesch et al., 2006; Searcy and Nowicki, 2009). However, there is a gap in the adaptive mate choice theory regarding the explanation of the colorful plumage of male birds; that is, although superior males possessing more colorful plumage than inferior males may signal their superior quality, this makes them more conspicuous to visual predators. Compared to inferior males, such a negative aspect of superior males may counteract their advantages in female mate choice. In many other animal taxa, such negative effects do not exist. For example, superior male Red Deer (Cervus elaphus) have stronger bodies and larger antlers compared to inferior males (Clutton-Brock et al., 1997; Kruuk et al., 2002), but such excessive traits do not increase predation risk. By contrast, they may enable the superior males to better defend themselves against predators. As another example, female House Mice (Mus musculus) preferred healthy males over males that were infected with viral or protozoan parasites, apparently detected through the males' odors (Kavaliers et al., 1997). Female Iberian Rock Lizards (Lacerta monticola) also used olfactory signals to evaluate the qualities of different males (Lopez et al., 2006). Because these olfactory signals were generally detected by special chemical receptors, they are specialized to conspecifics, meaning that the differences in odors among individual males can only be identified by conspecific females (Kaupp, 2010; Doty, 2015) and are unlikely to be distinguished by other species, including predators.
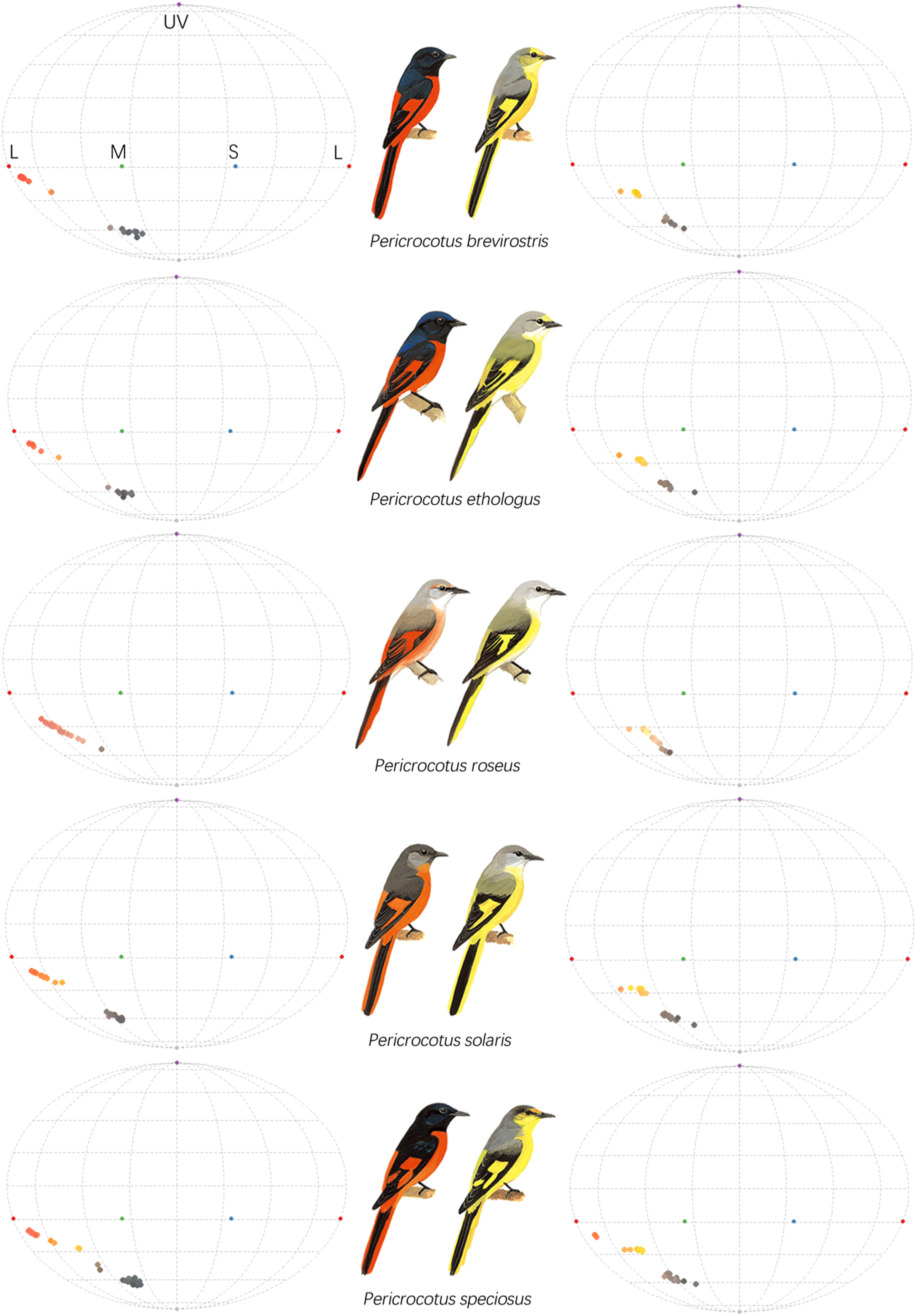
However, for colorful male birds that use ornamental plumage as a visual signal to attract females, it seems that they may inevitably incur a cost of predation, because visual detection is nearly universal among different taxa of animals. Nevertheless, since different taxa of animals may not have the same visual systems, is it possible that the differences in plumage colors of males are detectable by conspecific females but not by their predators? For example, the "private channels of communication" hypothesis proposes that some intraspecific signals may not be visible to heterospecific animals because of different visual systems (Hunt et al., 2001; Håstad et al., 2005; Stevens and Cuthill, 2007). This may help to address the dilemma between female choice and predation risk for the males of colorful birds. Here, we studied the melanin- and carotenoid-based plumage colors in five species of minivets (Pericrocotus spp.) from the perspectives of conspecific and predator visual systems. Unlike many dimorphic birds with dull plumage in females, the female minivets are also colorful and share similar patterns of plumage pigment with the males in which the red and black colors of males are replaced by yellow and gray colors (Fig. 1). Carotenoids and melanin are the two main pigments responsible for the colorful plumage of a wide variety of bird species. Carotenoids are responsible for red and yellow colors, while melanin is responsible for black and gray colors (Jawor and Breitwisch, 2003; Quesada and Senar, 2006). Therefore, the plumage colors of minivets offer a classic example of the dynamics of these two pigments. In what follows we present five predictions for this study (Table 1). First, because the plumage colors between different individuals of minivets were supposed to be identifiable from conspecific vision rather than predator vision, we hypothesized that either chromatic or achromatic difference (ΔS and ΔL, respectively) between individuals under conspecific vision should be higher than under predator vision in either males or females of the five minivets (first prediction). Similarly, the sexual dimorphism of minivets in plumage colors (i.e., ΔS and ΔL between males and females in every minivet species) should be more identifiable to conspecific vision than to predator vision (second prediction). However, because minivets are diurnal and thus should be more sensitive to chroma than luminance, we hypothesized that the predictions above (predictions 1 and 2) should be stronger in ΔS than in ΔL (third prediction). Furthermore, because in general female birds are influenced by the need for crypsis related to nesting, we hypothesized that ΔS and ΔL in females under predator vision should be maintained at a lower level than in males (fourth prediction). Finally, because we used the domestic cat as a nocturnal predator, we hypothesized that the ΔL under night-light conditions in either male or female minivets should be similar to that under daylight conditions (the fifth condition).
| Prediction | Logic | |
| 1 | ΔS and ΔL were higher under conspecific than predator vision | Plumage colors between individuals were identifiable from conspecific rather than predator vision |
| 2 | ΔS and ΔL between sexes were higher under conspecific than predator vision | Sexual dimorphism of plumage colors was identifiable from conspecific rather than predator vision. |
| 3 | Predictions 1 and 2 were stronger in ΔS than in ΔL | Minivets were more sensitive to chroma than luminance |
| 4 | ΔS and ΔL in females under predator vision were lower than in males | Females were under the need for crypsis related to nesting |
| 5 | ΔL under the night-light condition was similar to that under the daylight condition | The domestic cat was a nocturnal predator and one of the major killers of passerines |
The five studied species of minivets were the Short-billed Minivet (Pericrocotus brevirostris), the Long-tailed Minivet (P. ethologus), the Rosy Minivet (P. roseus), the Gray-chinned Minivet (P. solaris) and the Scarlet Minivet (P. speciosus) (Fig. 1). These are small passerine birds belonging to the genus Pericrocotus and the family Campephagidae. They occur in forests of southern and eastern Asia (Zheng, 2017). The males possess conspicuously colorful plumage that consists of blood-red and dark black/gray colors, while the females have corresponding yellow and gray colors (del Hoyo et al., 2013).
We quantified the plumage colors from 128 biological specimens of minivets (P. brevirostris: 10 males and 10 females; P. ethologus: 11 males and 11 females; P. roseus: 10 males and 10 females; P. solaris: 14 males and 14 females; P. speciosus: 21 males and 17 females) preserved in the Kunming Institute of Zoology, China. These specimens were collected less than 50 years ago, and thus their plumage colors are almost identical to those of living birds (Armenta et al., 2008). We measured the spectral reflectance of 30 random points for red/yellow and black/gray plumage of each individual by using a spectrophotometer (Avantes-2048; Avantes, Apeldoorn, the Netherlands) and summarized the measurements as the mean to represent each color (red/yellow or black/gray) of each individual. Then, we calculated the chromatic difference (ΔS) and achromatic difference (ΔL) between each pair of individuals under the conspecific and the predator visual systems using the receptor-noise limited (RNL) model; the chromatic and achromatic differences were noise-weighted Euclidean distance measurements of chromatic and achromatic (or luminance) distances, respectively (Vorobyev and Osorio, 1998). Therefore, ΔL was the luminance difference of the plumage, which was mainly related to night-light conditions, while ΔS was the color difference of plumage, which was only related to daylight conditions. We used the Pavo package in R (Version 4.1.0) for Windows (https://www.r-project.org/) to build the RNL model, in which the average avian UV system was used to represent the conspecific visual system. For the predator's visual system, we chose the domestic cat (noise-to-signal ratio: 0.02) as a representative nocturnal predator (Olsson et al., 2018). We used forest shade for the illuminant and green foliage for the background spectrum. For the environmental transmission spectra, we chose the default option (i.e., homogeneous transmission of 1 across all wavelengths). Furthermore, we also compared ΔL between daylight and night-light conditions based on predator vision. Robinson projection for plumage color hue that controls for brilliance was also generated to describe the plumage color and sexual dimorphism in each minivet species. For more details of the RNL model and Robinson projection, see the R documentation in the Pavo package.
To analyze our data by controlling for the phylogenetic relationships between the five studied minivets, we constructed a phylogenetic tree of these minivets based on their cytochrome b and NADH dehydrogenase subunit 2 mitochondria genes using data from GenBank (https://www.ncbi.nlm.nih.gov). We used the unweighted pair-group method using arithmetic averages (UPGMA) by MEGA (Version 4.1) for Windows (Kumar et al., 2008) to establish the phylogenetic tree, which was then used as a random effect for the following model.
We employed a phylogenetic generalized linear mixed model (PLGMM) to analyze the ΔS or ΔL of plumage colors between conspecific and predator visual systems or between daylight and night-light conditions by controlling for the phylogeny of studied minivets. The value of ΔS or ΔL was the response variable in each model. The fixed effects were the visual system (conspecific or predator), the sex (male or female), the light condition (daylight or night light), the species, and the interaction between each pair of effects. Different colors (melanin- or carotenoid-based color) were analyzed using a separate model, while the phylogenetic relationships of five studied minivets and individual identity were considered random effects. The PLGMMS was conducted using the INLA package in R (Version 4.1.0) for Windows (https://www.r-project.org/). The program performs a full Bayesian analysis on generalized additive mixed models using integrated nested Laplace Approximations (Gómez-Rubio, 2020). All tests were two-tailed, and the significance level was set to P = 0.05. However, Bayesian analyses do not provide a P value for significance; instead, they use a 95% credible interval (CI) that excludes a zero value to indicate that the effect was significant. The greater the 95% CI distance was from zero, the higher the significance.
All five studied species of minivets are sexually dimorphic according to both human eyes and avian visual modelling (Fig. 1). Both the male and female minivets possess similar patterns of melanin- and carotenoid-based plumage colors. The males have blood-red to orange-red colors that are formed from carotenoids and black to dark gray colors that are formed from melanin. In the corresponding body parts of the females, the carotenoid-based plumage is replaced by a yellow color, while the melanin-based plumage is replaced by a gray color (Fig. 1). Red and yellow colors are similar in hue but differ in chroma (i.e., color saturation), with red being more saturated than yellow. Likewise, black color is more saturated than gray color.
The inter-sex results of the PGLMM showed that the visual system, and not the species, significantly predicted the ΔS and ΔL of melanin-based plumage between sexes (Fig. 2). Such sexual dimorphism between conspecific and predator vision did not change with different species in ΔS, but was slightly significant in ΔL. In other words, the sexual dimorphism of melanin-based plumage, either chromatic or achromatic, was higher under conspecific than predator vision. However, this difference in achromatic contrast between sexes changed somewhat with different minivets. Similarly, the intra-sex results indicated that ΔS and ΔL were more distinguishable from conspecific than predator vision, but they did not differ between sex or species (Fig. 2). However, the visual difference of ΔS changed with sex in that it affected the females more than the males. Moreover, the effect of different vision, in both the inter-sex and intra-sex comparisons, was stronger in ΔS than in ΔL (Fig. 2).
According to the results of visual comparison for carotenoid-based plumage, both the ΔS and ΔL between sexes were higher for conspecific than predator vision, but ΔS was more significant (Fig. 3). Furthermore, both ΔS and ΔL did not differ between species, but the visual difference between conspecific and predator changed with species. For the results of ΔS and ΔL between individuals within sexes, both the vision and sex significantly predicted ΔS, but not ΔL (Fig. 3). A significant interaction was found between vision and sex, but not between other effects.
For the ΔL of melanin-based plumage related to daylight and night-light conditions from predator vision, the inter-sex ΔL was only predicted by the light conditions, not by the species or the interaction between light and species (Fig. 4); that is, ΔL between sexes in the daylight condition was higher than in the night-light condition. However, the ΔL between individuals within sexes did not differ between daylight and night-light conditions (Fig. 4), but it differed between sexes in that male ΔL values were higher than those of females. For the carotenoid-based plumage, the inter-sex ΔL was also significantly predicted by the light condition but not the species. However, this predictor changed with different minivet species (Fig. 5). For the intra-sex ΔL, light condition had a mild significant effect, and male ΔL was higher than female ΔL.
Our analyses revealed that both the ΔS and ΔL among male and female minivets' melanin-based plumage colors were consistently higher under conspecific vision than under predator vision for all five studied species of minivets (Fig. 2). The results for ΔS among male and female minivets' carotenoid-based plumage colors was the same, but intra-sex ΔL did not differ between conspecific and predator vision. Because minivets were diurnal, these results were mostly consistent with the first prediction, that the plumage differences between individuals would be higher from conspecific vision than from predator vision. In other words, individual differences in plumage colors were visible to the intended receivers (conspecifics) and hidden from potential predators. This was reasonable, since the plumage colors of minivets should evolve under the selection of conspecifics and predation. The ΔS and ΔL results between sexes were similar, and thus the second prediction was mostly supported in that sexual dimorphism of plumage colors in minivets was identifiable from conspecific rather than predator vision. Therefore, if the levels of intraspecific signals were related to such differences in plumage colors, the minivets would be able to display or receive such signals without increasing the risk of detection from the predator. This result therefore was also consistent with the "private channels of communication" hypothesis (Hunt et al., 2001; Håstad et al., 2005; Stevens and Cuthill, 2007).
Moreover, the effect of this visual difference on ΔS was stronger than on ΔL, which supported the third prediction and indicated that chroma was more important than illuminance for individual and sexual identification in minivets. However, the effect of visual system on ΔS changed with sex in that females were more dispersed in plumage between individuals than males; this result therefore implied that female choice may have driven the evolution of male plumage colors in one direction rather than toward diverse development (Dale et al., 2015). The prediction about the ΔS was supported but only part of the ΔL results above received support. This is understandable, because minivets are diurnal and ΔS was related to the daylight condition, while ΔL was mostly related to the nocturnal and night-light condition. The ΔL values of carotenoid-based plumage between sexes were found to be significantly higher from predator vision than conspecific vision (Fig. 3), and this effect was stronger in day than in night from the predator's vision (Fig. 5). Nevetheless, for the individual differences within sexes, the predator's detected capacity was similar between daylight and night-light conditions. This indicates that the sexual dimorphism of minivets was visible to the predator between day and night, but that the individual differences within sexes seemed similar to the predator, even under different light conditions. Therefore, the fifth prediction was partly supported. Finally, both the melanin- and carotenoid-based plumage of the males were more conspicuous to the predator than those of the females (Figs. 4, 5), indicating that males were under higher risk of predation by a nocturnal predator than femles. Therefore, the fourth prediction was partly supported, since females were relatively more difficult to see than males from a nocturnal predator's vision.
In summary, our results indicated that plumage colors between individuals, either for male or female minivets, were considerably more distinguishing under conspecific vision than under predator vision. Furthermore, the chromatic traits of plumage colors were more distinguishing than their illuminance under this circumstance. Therefore, we conclude that individual differences in plumage colors of minivets are visible to the conspecific receivers and hidden from potential predators as a result of evolution under predation risk and conspecific communication. However, females were more difficult to see, while males were under higher risk of prediction from the view of a nocturnal predator. Additionally, there were several factors that may have biased the results for the tested predictions. For example, the average avian UV system was used to represent the minivets because their spectral sensitivity was unknown. The predators would be much more diverse than domestic cats and include animals from different taxa. The environment and light conditions are diverse and changing with time. These variables would also change according to different minivet species. Therefore, the situation can be expected to be much more complicated in the real world.
To provide unambiguous evidence, we suggest the following proposals for further study. First, we should acquire specific information concerning spectral sensitivities for more avian species and predators. Second, we should investigate the predation rates of males with different levels of ornamental traits and relate them to female mate choice and the visual perception of conspecifics and predators. Additionally, if we obtain such information, it would be possible to discern whether there is a tradeoff between female mate choice and predation risk directing the evolution of ornamental plumage in male birds. Finally, based on such information, we could use meta-analyses to investigate the evolutionary scenario of colorful plumage in different taxa of male birds. Hopefully, such studies will help us to better understand the evolution of plumage colors and sexual selection in birds.
The work was conducted on the minivet specimens preserved in the Kunming Institute of Zoology, China, and no living birds were handled.
YC and CY designed the study, analyzed the data, and drafted the manuscript. YC, XC, NW and CY collected the data. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
The authors declare that they have no competing interests.
We thank the Kunming Institute of Zoology for providing specimens for spectral measurements.
|
del Hoyo, J., Elliott, A., Sargatal, J., 2013. Handbook of the Birds of the World. Lynx Edicions, Barcelona.
|
|
Doty, R.L., 2015. Handbook of Olfaction and Gustation. John Wiley & Sons, Inc., New York.
|
|
Gómez-Rubio, V., 2020. Bayesian Inference with Inla. Chapman and Hall/CRC, New York.
|
|
Kruuk, L.E.B., Slate, J., Pemberton, J.M., Brotherstone, S., Guinness, F., Clutton-Brock, T., 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683–1695.
|
|
Searcy, W.A., Nowicki, S., 2009. Sexual selection and the evolution of animal signals. In: Squire, L.R. (Ed.), Encyclopedia of Neuroscience. Academic Press, Oxford, pp. 769–776.
|
|
Zheng, G.M., 2017. A Checklist on the Classification and Distribution of the Birds of China. Science Press, Beijing. Y. Cai et al. Avian Research 14 (2023) 100077 6
|
| Prediction | Logic | |
| 1 | ΔS and ΔL were higher under conspecific than predator vision | Plumage colors between individuals were identifiable from conspecific rather than predator vision |
| 2 | ΔS and ΔL between sexes were higher under conspecific than predator vision | Sexual dimorphism of plumage colors was identifiable from conspecific rather than predator vision. |
| 3 | Predictions 1 and 2 were stronger in ΔS than in ΔL | Minivets were more sensitive to chroma than luminance |
| 4 | ΔS and ΔL in females under predator vision were lower than in males | Females were under the need for crypsis related to nesting |
| 5 | ΔL under the night-light condition was similar to that under the daylight condition | The domestic cat was a nocturnal predator and one of the major killers of passerines |