| Citation: | Sandra Fernandes, Vanessa A. Mata, Luis P. da Silva. 2023: Feeding ecology of a highly aerial bird during its long breeding season. Avian Research, 14(1): 100073. DOI: 10.1016/j.avrs.2022.100073 |
Pallid Swifts (Apus pallidus), as other swifts, are birds extremely adapted to an aerial lifestyle, showing unique adaptations that allow them to fly almost continuously. The diet of these non-stopping high-altitudinal aerial birds has been mostly studied through techniques that fail to produce highly resolved prey identifications, and for that have been replaced by molecular techniques, such as DNA metabarcoding. Faecal samples of Pallid Swifts were monthly collected from a colony in the north of Portugal during the breeding season. DNA from the faecal samples was used to sex the birds and to identify the arthropods present in the diet through DNA metabarcoding. From the detected prey items, 74 families were identified belonging to 16 orders, with Hymenoptera and Hemiptera being the most frequently consumed. There were seasonal variations in diet richness, composition and prey size. Regarding the diet of males and females, although no differences were found between the diet of males and females in terms of composition and richness, there were differences in the size of arthropods preyed by the different sexes, with males feeding on larger arthropods. The large seasonal variation in Pallid Swifts' diet during the breeding season is probably a result of spatiotemporal variation in aerial prey, of which swifts likely predate opportunistically. Although no significant differences were detected in diet richness and composition between sexes, the fact that males consumed larger prey may suggest the existence of sexual dietary segregation in this group of birds. At last, several pest species were found in these swifts' diet, which, if studied through DNA metabarcoding, can be used to monitor small arthropods, including airborne pests.
Swifts (Apodiformes: Apodidae) are insectivorous birds extremely well-adapted to an aerial lifestyle, displaying unique morphological, behavioural, and physiological adaptations to this distinct way of living (Henningsson and Hedenström, 2011; Neumann and Neumann, 2016; Sachs, 2017). These traits allow these highly mobile and fast birds to fly continuously during the nonbreeding period (Liechti et al., 2013; Hedenström et al., 2016, 2019). During the breeding period, collection of material to build nests, sleeping, mating, drinking, and foraging occur during flight (Rattenborg, 2006; Henningsson et al., 2009; Orłowski and Karg, 2013; Hedenström et al., 2016). The Pallid Swift (Apus pallidus) is one of the most abundant breeding species of swifts throughout its breeding range. It is restricted to the Mediterranean region, Asia Minor, and adjoining areas (Chantler and Driessens, 2000; Keller et al., 2020), with some resident populations in Niger, Chad, and Egypt (BirdLife International, 2022). Usually, this species nests in either natural landscapes, using caves and cliffs, or in urban areas, using preferentially cavities under the eaves or ceilings of tall buildings (Cucco and Malacarne, 1987; Thibault et al., 1987; Antonov and Atanasova, 2002). The Pallid Swift often lays a second clutch in summer, leading to a long reproductive period that extends into autumn (Cramp, 1985; Antonov and Atanasova, 2001).
Previous studies have analysed the diet of the Pallid Swift through visual inspection of faeces and food boluses delivered to nestlings (Finlayson, 1979; Malacarne and Cucco, 1992; Cucco et al., 1993; Cristiano et al., 2018), and concluded that the diet of this species was mainly composed of arthropods belonging to the orders Coleoptera, Diptera, Hemiptera and Hymenoptera. Nonetheless, there is a lack of knowledge on this species' diet in its expanding northern distribution range, namely throughout the Iberian Peninsula, where it is very abundant (Keller et al., 2020). Although the diet of some swift species has already been studied, dietary differences between sexes have never been considered. Sexual dietary segregation has been described in several animal groups (Borrell et al., 2011; Mata et al., 2016; Mramba et al., 2017), namely in birds (Catry et al., 2016; da Silva et al., 2020; Massaro et al., 2020). These differences are usually the result of either sexually marked morphological traits, or differences in parental care (Lewis et al., 2005; Catry et al., 2016). Since Pallid Swift's parental care is shared by females and males (Finlayson, 1979; Malacarne et al., 1992), and this species has no sexual dimorphism, no significant differences between the diets of males and females should be expected. However, there are records of birds without major morphological sexual dimorphism, but with differences in their diet composition (da Silva et al., 2020). Moreover, the morphological dietary analyses performed have many limitations: either they are very invasive methods, or depend on samples that are already very digested, making visual identification of the prey quite difficult and sometimes even impossible (Chung et al., 2021). Therefore, these traditional approaches have been recently replaced by molecular tools, such as DNA metabarcoding (Chan et al., 2020; Chung et al., 2021). Although presenting some limitations (Taberlet et al., 2012), the ability to provide high taxonomic resolution to the consumed taxa (Jackson et al., 2014; Gibson et al., 2015), while avoiding prior knowledge of the present prey (de Sousa et al., 2019), makes this technique a good tool for diet assessment. Despite its broad application and its potential to unravel swifts' ecology, to date it has not been applied to assess the diet of any of the Palearctic swift species, whose populations, however, have been the subject of other biological and ecological studies (Hedenström et al., 2019; Cibois et al., 2022; Kearsley et al., 2022).
In this study, the feeding ecology of the Pallid Swift was analysed during its breeding season in the North of Portugal using DNA metabarcoding on faecal samples. We aimed to assess and characterize the temporal variation in diet during the breeding season, and evaluate whether differences exist between sexes regarding prey richness, niche width, prey size, and diet composition. Finally, we compared our results with those obtained in previous dietary studies on Pallid Swifts (Finlayson, 1979; Malacarne and Cucco, 1992; Cristiano et al., 2018) and other swift species (Hespenheide, 1975; Chung et al., 2021).
The studied Pallid Swift colony is located in the arcades of the Vila Nova de Famalicão City Hall (41.4100° N, 8.5203° W), Braga, Portugal. The field sampling was conducted during the 2021 breeding season, from the beginning of June to October. The majority of the birds arrive to the colony in April and start laying in May, but we did not sample during this period to avoid disturbing the colony and affecting its establishment. Birds were monthly captured and held in cotton bags for 30 min or less, from which droppings were collected. Bags were sterilized with 10% bleach for 1 h and washed after every use to minimize contamination. Droppings were transferred directly from the bags to 2 mL tubes with 96% ethanol, and stored at 4 ℃ until laboratory processing (da Silva et al., 2019). Only one sample was collected per individual in each month.
DNA was extracted from bird droppings using the Norgen Stool DNA Isolation Kit, following the manufacturer's protocol. DNA extraction was carried out in several batches of 23 samples plus one negative control in which no faecal sample was added. The extracted DNA and the negative controls were distributed in 96-well plates where the last well was left empty for PCR negative control.
Invertebrate prey items were amplified using the COI primers fwhF2 (5′-GGDACWGGWTGAACWGTWTAYCCHCC-3′) and fwhR2n (5′-GTRATWGCHCCDGCTARWACWGG-3′) (Vamos et al., 2017), modified to contain Illumina adaptors. This primer set produces a 245 bp amplicon and was originally designed to amplify the DNA of freshwater invertebrates (Vamos et al., 2017), but also performs well in the amplification of terrestrial arthropods' DNA and the amplification of degraded DNA samples, such as faecal droppings (Elbrecht et al., 2019; Mata et al., 2021). PCR reactions were carried out in volumes of 10 μL, comprising 5 μL of Multiplex PCR Master Mix (Qiagen), 0.3 μL of each 10 pM primer, 2.4 μL of H2O, and 2 μL of DNA extract. Cycling conditions consisted of a 15 min period at 95 ℃, 45 cycles of 30 s denaturation at 95 ℃, 30 s annealing at 50 ℃, and 30 s extension at 72 ℃, and a final extension period of 10 min at 60 ℃. Amplification success was checked by visually inspecting 2 μL of each PCR product on a 2% agarose gel stained with GelRed. The resulting PCR product was cleaned using a 1:0.8 ratio of AMPure XP beads (Beckman Coulter, High Wycombe, UK) according to the manufacturer's instructions, with the exception that 80% ethanol was used instead of 70%, and beads were eluted in 25 μL of 10 mM Tris, pH 8.5. Clean PCR products went through a second PCR reaction to incorporate 7 bp long indexes and P5 + P7 Illumina adaptors. This second PCR was carried out in a total volume of 14 μL, comprising 7 μL of KAPA HiFi HotStart ReadyMix, 2.8 μL of cleaned PCR product, 2.8 μL of H2O, and 0.7 μL of each Illumina adaptor. Cycling conditions were 3 min period at 95 ℃, 10 cycles of 30 s denaturation at 95 ℃, 30 s annealing at 55 ℃, and 30 s extension at 72 ℃, and a final extension period of 5 min at 72 ℃. Indexed samples were again cleaned with AMPure XP beads, as before, quantified with Epoch Microplate Spectrophotometer (Agilent Technologies, Santa Clara, USA), and then pooled at equimolar concentrations. Finally, the pool was sequenced using an Illumina MiSeq platform with a MiSeq v2 kit (500 cycles) with a target depth of 50,000 paired-end reads per sample, along with samples from other projects.
The Pallid Swifts' sex identification was performed by amplifying a small amplicon of the Z and W chromosomes using P2 (5′-TCTGCATCGCTAAATCCTTT-3′) and P8 (5′-CTCCCAAGGATGAGRAAYTG-3′) primers (Griffiths et al., 1998). This primer set was designed to amplify fragments of the (CHD) gene, providing distinct banding patterns on an agarose gel as a result of intronic regions within this gene (Quinn et al., 1990; Griffiths et al., 1998; Çakmak et al., 2017). The PCR was carried out in volumes of 11 μL, comprised of 5 μL of Multiplex PCR Master Mix (Qiagen), 0.4 μL of each primer, 2.2 μL of H2O, and 3 μL of DNA extract. P2 primers were labelled with the fluorescent dye FAM. Cycling conditions consisted of a 15 min period at 95 ℃, 20 cycles of 35 s denaturation at 95 ℃, 45 s annealing at 45 ℃, and 45 s extension at 72 ℃, followed by more 25 cycles of 35 s denaturation at 95 ℃, 45 s annealing at 47 ℃, and 45 s extension at 72 ℃ and a final extension period of 10 min at 60 ℃. PCR products were checked on an agarose gel, and, although amplification was successful, it was not possible to visually separate the different sized amplicons. Therefore, PCR products were separated by capillary electrophoresis using the automatic sequencer ABI 3130xl Genetic Analyzer. Fragments were scored against Genescan-500LIZ size Standard, using GeneMapper version 4.1 (Applied Biosystems). Male individuals showed a single fragment with about 370 bp of length, while females showed an extra fragment of about 380 bp. Each sample was sequenced three times, and sex was only assigned to samples that provided congruent results for at least two sequencing results. This should avoid false assignments resulting from allelic drop-out of the CHD-W (van der Velde et al., 2017), that can be particularly common in degraded DNA samples like bird droppings (Mitchell et al., 2012).
Bioinformatic processing of generated Illumina reads was done using the R package Metabarcoding Joining Obitools & Linkage Networks in R (MJOLNIR: https://github.com/uit-metabarcoding/MJOLNIR). Alignment of paired-end Illumina reads was done using 'illuminapairedend' from OBITools (Boyer et al., 2016) with a minimum score of 40. Afterwards, merged sequences were annotated with their corresponding sample and primer sequences were trimmed using 'ngsfilter'. In addition, sequences were filtered by size (190–220 bp) by applying the command 'obigrep'. All these steps were carried out simultaneously using the 'mjolnir2_FREYJA()' function. Chimeric sequences were removed using the 'mjolnir2_HELA()' function, which uses the uchime_denovo algorithm implemented in VSEARCH (Rognes et al., 2016), and non-chimeric sequences from different samples were merged. After this merge, reads were clustered using the 'mjolnir4_ODIN()' function, which uses SWARM (Mahé et al., 2015) to delimit Operational Taxonomic Units (OTUs), based on linkage-networks created by step-by-step aggregation. Finally, to reduce the number of erroneous OTUs (e.g., retained PCR artifacts, sequencing errors, pseudogenes, etc.) and thus achieve more realistic biodiversity metrics, the 'mjolnir2_LOKI()' function was used. This function uses the LULU (Frøslev et al., 2017) algorithm, which merges similar and highly occurring OTUs (identity higher than 84% and co-occurrence levels higher than 95%).
Taxonomic assignment of OTUs was done using BOLDigger v1.2.5 (Buchner and Leese, 2020) that queries DNA sequences against the BOLD Identification System database (https://www.boldsystems.org/) and then assigns a taxonomic identification based on the top 20 hits. OTU identifications were then manually curated. When different OTUs matched a single taxon, these were condensed into a single taxonomic unit. In case an OTU matched different species, genera, or families at a similar identity level, this was assigned to the most inclusive taxonomic rank. OTUs assigned to higher taxonomic levels than species and with more than 98% of similarity were clustered with a neighbour-joining tree (Mata et al., 2018) into distinct OTUs (e.g., Nabidae 1, Nabidae 2, etc). OTUs assigned to items that are not part of the insectivorous diet of swifts (e.g., fungi, protists, platyhelminths, birds, mammals, etc.) were categorized as "Not diet" and discarded from the analysis. The number of reads per OTU in the extraction and PCR blanks was subtracted to the associated samples to reduce possible sources of laboratory contamination. To further reduce false positives, only samples with more than 100 reads of dietary items were considered and OTUs comprising less than 1% of the total dietary reads per sample were discarded (Mata et al., 2016; Taberlet et al., 2018; Drake et al., 2022). Finally, OTUs identified at the species level were compared with a list compiled by Mata et al. (2021) to assess which species were agroforest pests.
All statistical analyses were performed on R v4.1.1 (R Core Team, 2020). Statistical significance was considered at α = 0.05. Dietary analysis was based on the OTU presence or absence per sample. Samples from the same individuals (n = 5) were considered independent because they were from different months.
The effect of sex, month, and their interaction on the number of prey taxa detected in each dropping was tested using a generalized linear model (GLM) with a Poisson distribution and a log link-function, with the base function 'glm'. To test for differences in prey size consumption between sexes and months, we conducted a literature search to characterize the size of each prey item detected. Only prey items identified to genus, species complex and species were considered, as it is not feasible to correctly assign a size to an arthropod family or order. When there was no information on the average size of a species but a range of size values was available, we used the average size of the range. For OTUs identified only to the genus level or as species complexes, we searched for information of the species known to occur in Portugal and also used their mean. Prey items used in this analysis included 85% of the OTUs and 85% of the predation events, thus covering the majority of the diet. The prey sizes obtained can be found in Appendix Table S1. The average prey size of each sample was further calculated and used as a response variable in a GLM with a Gamma distribution and an inverse link-function to test the effect of bird's sex, month of capture, and their interaction, applying the base function 'glm'. Explanatory variables significance was tested in these models using the 'Anova' function from package car (Fox and Weisberg, 2019). Pairwise comparisons were performed to identify in which pairs the observed differences occurred, using the function 'emmeans' of the emmeans package (Lenth, 2022). To evaluate the effect of prey size on the detected richness per sample, a generalized linear mixed model (GLMM) with a Poisson distribution and a log link-function was done with the function 'glmer' of the lme4 package (Bates et al., 2015), using month as random variable. This was done since the average prey richness is expected to vary across months.
The overall prey richness consumed, i.e., niche width, was estimated by sexes, and month using rarefaction curves based on Hill numbers with the function 'iNEXT' of the iNEXT package (Hsieh et al., 2016), with the triple of the lower reference sample size to minimize extrapolation bias (Chao et al., 2014). Significant differences were considered if the 95% confidence intervals between groups did not overlap (Chao et al., 2014).
The package vegan (Oksanen et al., 2020) was used to evaluate differences in prey composition among sexes and months. First, a pairwise distance matrix using the Jaccard dissimilarity index was calculated with the function 'vegdist'. This matrix was then tested using a Permutational Multivariate Analysis of Variance (PERMANOVA) with the Binomial method and 99,999 permutations using the 'adonis' function. To identify the months that differed from each other, a pairwise PERMANOVA was performed. Similarity percentage analysis was also calculated to determine the contribution of different prey groups to the observed differences in variables, using the 'simper' function.
During the Pallid Swift monitoring a total of 226 bird captures occurred, and 82 faecal samples were collected. After sequencing and bioinformatic processing, only 65 samples successfully produced dietary data. Of these 65 faecal samples, 29 were identified as females, 33 as males, and in 3 samples it was not possible to reliably assign the sex of the bird.
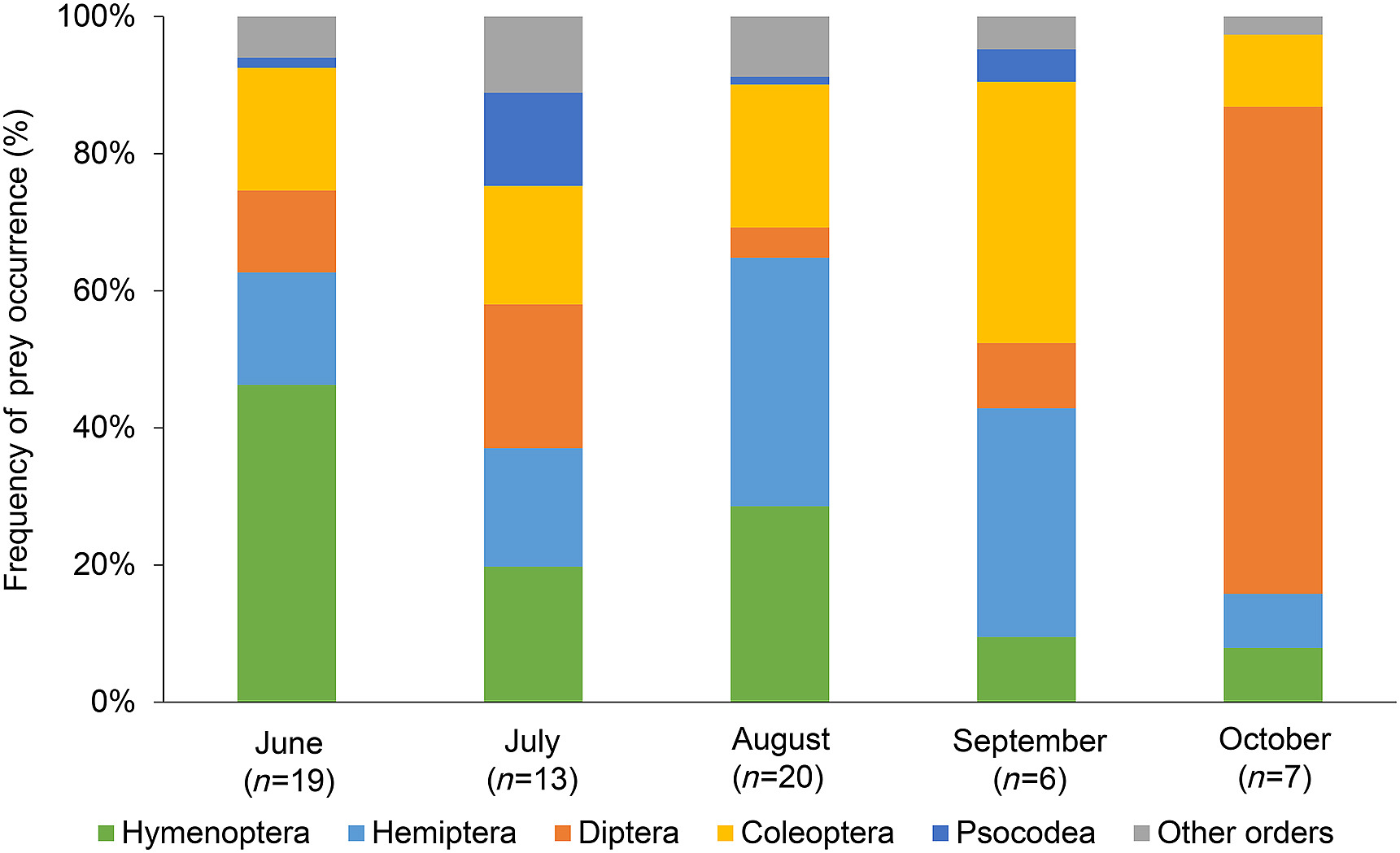
We identified 139 OTUs in the diet of the Pallid Swift, belonging to 74 different families and 16 orders. The most commonly observed OTU was Tetramorium forte (32% of the samples), followed by Lasius 1 (25%). Formicidae and Pentatomidae were the most frequent families, 62% and 20% respectively, and also represented the most detected orders Hymenoptera (66%) and Hemiptera (58%) (Appendix Table S1). The five most common orders were present throughout all the months, except for the order Psocodea which was not found in the samples collected in October (Fig. 1). In June, a substantial part of the prey interactions (46%) belonged to the order Hymenoptera, with almost all of these (96%) belonging to the family Formicidae. In July, the five most common orders were almost equitably represented in the samples collected, unlike in the other months. In August, 65% of the arthropods consumed belonged to the orders Hymenoptera and Hemiptera. Finally, the faeces collected in September and October, presented a very distinct composition. Whereas in September the orders Coleoptera and Hemiptera comprised more than 70% of the diet; in October these orders only represented 18%, with the vast majority of the diet (> 70%) being composed by Diptera.
Additionally, 17 predation events (5.7% of the total) of 11 pest species were detected, mostly agricultural pests (Appendix Table S1). The most common pest was Geomyza tripunctata, which was found in 6% of the samples.
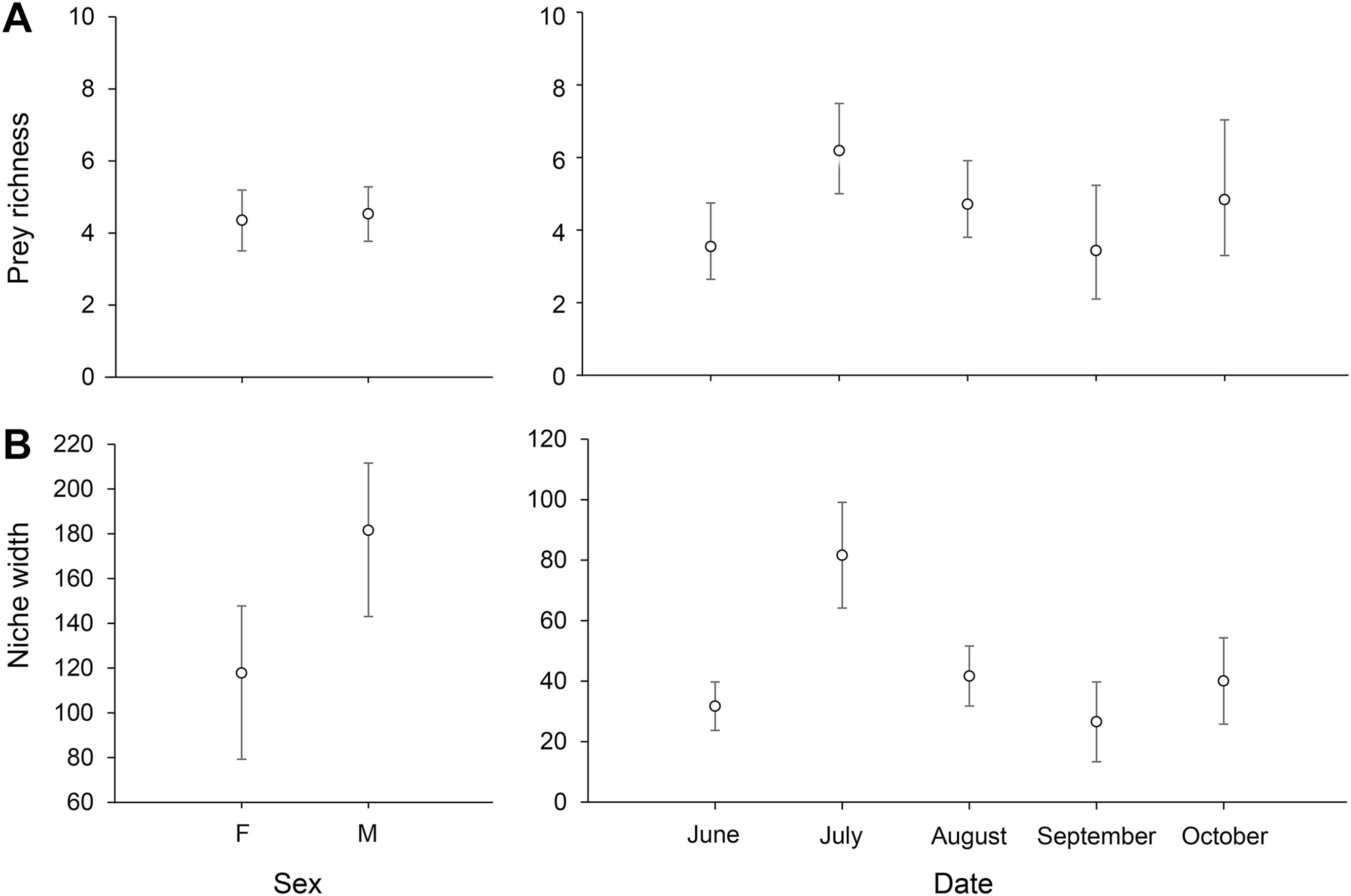
We found no differences between sexes in the number of OTUs detected per sample (χ2 = 0.199, df = 1, p = 0.656). With regard to temporal variation, differences were found in the average number of prey items detected per sample (χ2 = 14.634, df = 4, p = 0.006; Fig. 2A). The significant differences were observed between June and July (z-ratio = −3.295, p = 0.008), the months with the lowest and highest average number of prey items per sample, respectively.
Overall diet richness, i.e., the niche width, was significantly different between months, but not between sexes (Fig. 2B). Regarding the temporal variation, there were no differences between months except for July that was the month with the widest niche breadth, exhibiting more than double the average diversity values observed for the remaining breeding season. For sexes, although the differences between the niche width of males and females was not significant, males tended to ingest a higher number of prey taxa.
Arthropods between 2 and 4 mm in size were the prey on which Pallid Swifts fed the most, with size classes 4–6 and 6–8 mm being the second and third most preyed classes, respectively (Fig. 3). We found significant differences in the average size of prey consumed per sample, between sexes (χ2 = 4.871, df = 1, p = 0.027) and months (χ2 = 10.856, df = 4, p = 0.028; Fig. 4). Overall, males fed on larger prey than females (Fig. 3). Regarding the temporal variation in prey size, Pallid Swifts in July preyed on smaller sized arthropods when compared to the remaining breeding season. In turn, September was the month in which larger prey were consumed, with significant differences only detected between these two months (t-ratio = 2.882, p = 0.044). A significant inverse relationship between the ingested diversity and the prey size was also found (χ2 = 4.7, df = 1, p = 0.030), with prey diversity increasing as preys became smaller.
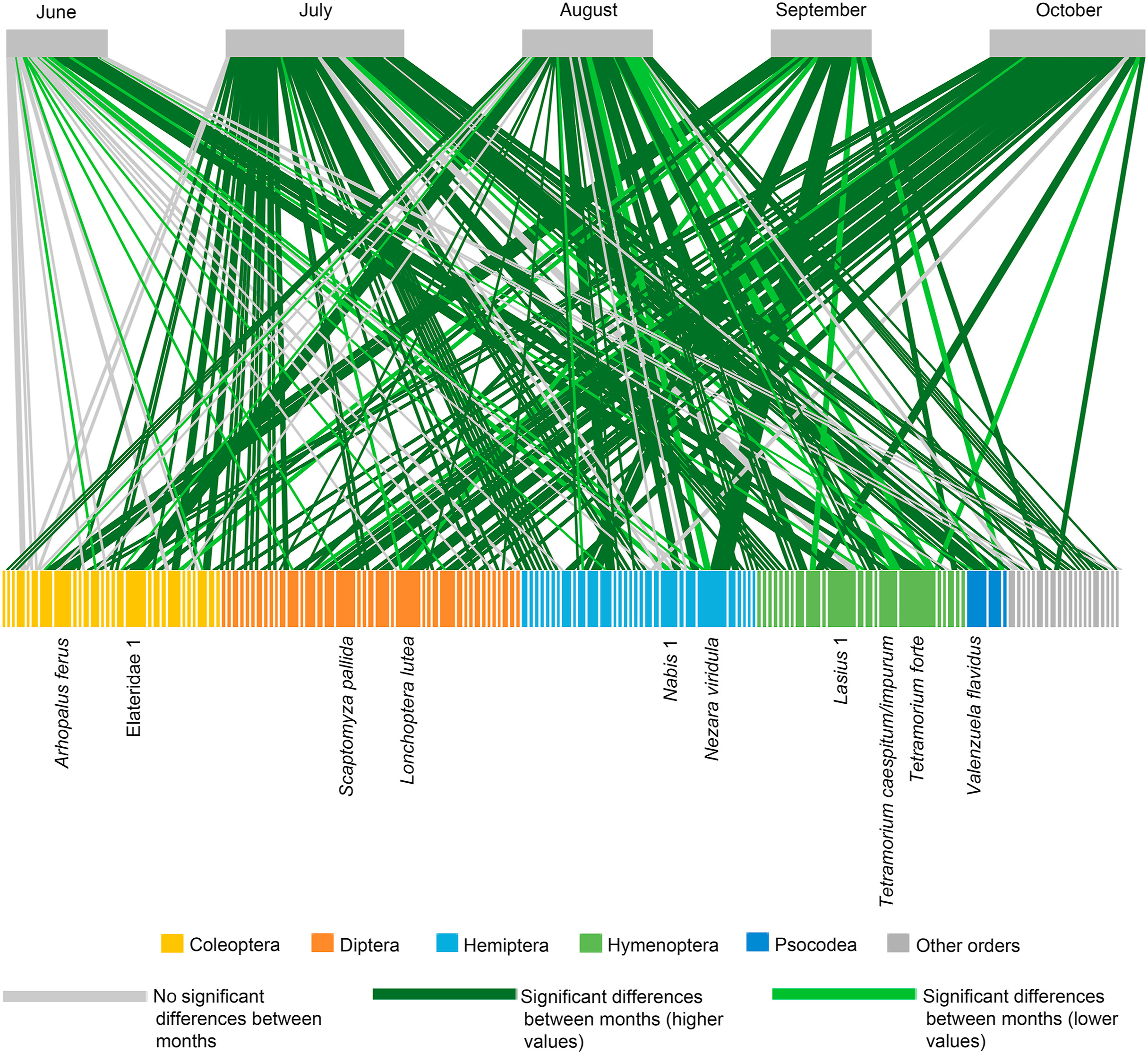
The PERMANOVA revealed no differences in niche overlap between sexes (df = 1, pseudo-F = 1.215, R2 = 0.018, p = 0.151), however, with respect to sampling month, significant differences were found in niche overlap (df = 4, pseudo-F = 2.952, R2 = 0.171, p < 0.001). These significant differences were observed between all pairs of months except September and October (Appendix Table S2).
The compositional differences at OTU level found among the diets collected over the different five months were explained by the seasonal variation of the most frequently consumed OTUs (present in more than 10% of samples; Fig. 5; Appendix Table S3). The presence of Tretamorium forte and Lasius 1 in 68% and 58% of the samples collected in June, respectively, and the negligible presence or even absence of these OTUs in the other months, explained the significant differences between the diet composition of June and the rest of the breeding season. The differences between the composition of the diet consumed in July and in the other months were due to Valenzuela flavidus, since it was present in 54% of the samples collected in July, and barely present or absent in the remaining months. The compositional differences found between August and the other four months resulted from the presence of Elateridae 1 in 50% of the samples collected in this month and the absence of this OTU in the other sampled months, with the exception of September, in which its presence was residual. In addition, Tretamorium caespitum/impurum, Nabis 1 and Macroscytus brunneus also contributed to the differences found between August and June. Finally, the marginal presence of Nezara viridula in June and August, its total absence in July and October, and its dominance in September samples (present in 67% of the faecal samples analysed), made this OTU the main responsible for the differences detected between the diets collected in September and the other months. Regarding October, the differences in diet composition between this month and the others was explained by Lonchoptera lutea and Scaptomyza pallida, both present in 57% of the samples of this month (Appendix Table S3).
Our results showed a marked seasonal variation in prey richness, size and composition throughout the breeding season, likely as a consequence of the seasonality of available arthropods, since aerial foragers rely on spatiotemporally unpredictable accumulations of aerial prey (Arbeiter et al., 2016). As swifts display an opportunistic feeding behaviour, massive arthropod assemblages lead to high intraspecific variability in these birds' diets throughout time (Cucco et al., 1993). Although no significant differences in diet richness and composition were noted between males and females, there were differences between the size of arthropods preyed by the different sexes.
Our results are similar with those found in other studies of Pallid Swifts. The study by Cristiano et al. (2018) concluded that Hemiptera and Coleoptera were the most consumed orders, while Malacarne and Cucco (1992) identified the orders Hemiptera, Hymenoptera and Diptera as the most important prey. Regarding Hymenoptera, the studies conducted by Malacarne and Cucco (1992) and Chung et al. (2021) on the diet of House Swifts (Apus nipalensis), found the same pattern as we did, with a predominance of hymenopterans, in particular ants (Formicidae), during the breeding season. The observed seasonal variation in diet was also in line to that found by Malacarne and Cucco (1992), where the vast majority of the predated arthropods in June belonged to the order Hymenoptera, while August and September were dominated by Hemiptera. The majority of Diptera in our study was recorded in October and this marked presence of dipterans at the end of the breeding season was also found by Chung et al. (2021). Unlike the other orders, Coleoptera did not show a clear seasonal variation and this pattern was also noted by Malacarne and Cucco (1992).
The prey diversity detected in our study cannot be compared with that of studies using morphological identification due to the much higher taxonomic resolution of DNA metabarcoding (da Silva et al., 2019). Until now only Chung et al. (2021) used DNA metabarcoding to study the diet of Apodidae species, and for a similar sample size, the OTU and family richness of Pallid Swifts' diet was about twice that observed in House Swifts (Chung et al., 2021). Nonetheless, the observed differences in prey richness between these two species must be interpreted carefully, because they are likely highly influenced by the use of different primer sets, which may amplify a different range of taxa and show different levels of taxonomic resolution (da Silva et al., 2019).
The optimal foraging theory suggests that natural selection promotes the most energy-efficient foraging pattern that maximises energy intake and increases fitness (Pyke, 1984). Ants are relatively weak fliers and can be found in dense aggregations during nuptial flights (Hespenheide, 1975; Levin et al., 2008), which reduces the required capture effort and energy expenditure for their predation. This might justify why swifts seem to choose ants to others arthropods present in the air column (Chung et al., 2021). In addition to prey's ease of capture and its local density, prey selection also depends on its size (Hespenheide, 1975). Thus, larger, and therefore more energetically nutritious prey, should be preferred in order to maximise energetic intake. The consumption of the largest prey was recorded in June and September, and, therefore, these were likely the months when optimal size prey items were more abundant, and, as a result, swifts appeared to concentrate on them, reducing prey richness and niche width. On the other hand, when ideal prey diminishes and environmental restrictions constrain consumers to suboptimal diets, predators often need to increase consumption rates, leading to more diversified diets (Simpson and Simpson, 1990). As in Malacarne and Cucco (1992), Pallid Swifts in this study fed mainly on prey with 2–4 mm. However, in our study the second most consumed class was 4–6 mm, while in Malacarne and Cucco (1992) it was 1–2 mm. Nevertheless, the results from this study seem to fall between those obtained by Malacarne and Cucco (1992) in Piemont and those obtained by Finlayson (1979) in Gibraltar, with the latter pointing arthropods between 4 and 6 mm in size as the most consumed by Pallid Swifts.
Sexual dietary segregation has been described in several animal groups (Borrell et al., 2011; Mata et al., 2016; Mramba et al., 2017), and particularly in birds (Catry et al., 2016; da Silva et al., 2020; Massaro et al., 2020). Marked morphological differences between sexes or differences in parental care during the breeding season are generally the drivers of this segregation. Although, to the best of our knowledge, dietary differences between sexes have never been studied in Apodidae species, the Pallid Swifts' biparental care and the lack of sexual dimorphism led us to hypothesise that no relevant differences would be found between sexes. The results partly confirmed our initial hypothesis, since no differences in diet richness or prey composition were found. However, there were significant differences in the size of consumed prey, with males preying on larger arthropods. The results obtained by compositional and prey size analysis were not congruent, since although no differences were observed in diet composition between sexes, there were differences in the size of the arthropods consumed. This non-compliance is likely related to differences in statistical power of both analyses, but a higher sample size would be required to disentangle this incongruence. For a better understanding of swifts' diet, future studies should try to extend the sampling period across several years, assessing if the same pattern is observed overtime and integrate more bird colonies, to avoid possible local and regional effects. Ideally, swifts' foraging areas would also be determined and their prey availability assessed, to study in detail prey selection by these birds.
Our work also uncovered the presence of some arthropod pests in the diet of these swifts. In addition to agricultural pests, mostly responsible for crop damage, we also found an invasive forest pest, Ctenarytaina spatulata, a psyllid responsible for economic damage to Eucalyptus spp. plantations (da Silva et al., 2022). The ability of swifts, and other aerial feeders, to provide an important regulatory service by preying on invasive and pest arthropods has already been reported in previous research (Orłowski and Karg, 2013; Cristiano et al., 2018). Besides performing an important ecosystem service, swifts can also act as bioindicators. Biosurveillance has been enhanced by the application of DNA metabarcoding, which allows an early and quick detection (Westfall et al., 2020; Montauban et al., 2021), thereby enabling the mitigation of the damaging effects that invasive and pest species may have on ecosystems. Thus, monitoring swifts' diet can be a useful tool in ecosystem monitoring (Cristiano et al., 2018).
Overall, our results suggest a sharp seasonal variation in Pallid Swifts' diet during the breeding season, with almost the whole diet being comprised of arthropods belonging to the orders Hymenoptera, Hemiptera, Coleoptera, Diptera and Psocodea. The seasonal variation found in richness, niche width, size and composition of the Pallid Swifts' prey consumption likely resulted from the spatiotemporally changes of arthropods in the air currents. Although no significant differences were found in diet richness and composition between males and females, the finding that male birds feed on larger prey items than females, raises the question of whether birds such as swifts, with no sexual dimorphism and with shared parental caretaking, may exhibit sexual dietary segregation. Our study also suggests that swifts may have an important role in pest predation.
LPS and VAM conceived the study. All authors carried the field work. SF performed the lab work and VAM the bioinformatics. Data analysis and interpretation was performed by SF and LPS with inputs from VAM. SF wrote the first draft with improvements and approval from all authors. All authors read and approved the final manuscript.
All work was carried under all the required legal requirements, namely ICNF (Portuguese Institute for the Conservation of Nature and Forests) ringing credential No. 134/2021.
The authors declare that they have no competing interests.
We gratefully acknowledge the help of all those involved in the fieldwork, specially to Vasco Flores Cruz, as well as to the Environmental Awareness Office of Câmara Municipal de Famalicão for all the support provided in the fieldwork logistics during the 2021 breeding season. We would also like to thank Susana Lopes for profiling the sex of the birds and to José Grosso-Silva for the help with prey size assessment. Lab work was supported by the project TOPDEVIL, financed by Fundação para a Ciência e a Tecnologia (FCT) and the European Regional Development Fund (FEDER) through Portugal 2020 Competitiveness and Internationalization Operational Programme (POCI), reference POCI-01-0145-FEDER-030250 and PTDC/ASP-SIL/30250/2017. VAM and LPS were also funded by FCT through the research contracts 2020.02547. CEECIND and CEECIND/02064/2017, respectively.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100073.
|
Antonov, A., Atanasova, D., 2001. Laying dates, cluch size breeding success in the Pallid Swift Apus pallidus in Sofia, Bulgaria. Avocetta 25, 299–304.
|
|
Antonov, A., Atanasova, D., 2002. Cohabitation and nest-site selection of common swift (Apus apus) and pallid swift (A. pallidus). Vogelwarte 41, 231–239.
|
|
Bates, D., Mächler, M., Bolker, B., Walker, S., 2015. Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48.
|
|
Chan, K., Tan, J., Goh, W., 2020. Taxonomic assignment of dietary arthropods in Malaysian swiftlets (Aerodramus sp.) based on DNA metabarcoding. J. Wildlife Parks 35, 73–91.
|
|
Chantler, P., Driessens, G., 2000. Swifts: A Guide to the Swifts and Treeswifts of the World, 2 ed. Pica Press, Flossmoor, IL.
|
|
Cramp, S., 1985. The Birds of the Western Palearctic, Ⅳ. Oxford Univercity Press, Oxford.
|
|
Cristiano, L., Lantieri, A., Boano, G., 2018. Comparison of Pallid Swift Apus pallidus diet across 20 years reveals the recent appearance of an invasive insect pest. Avocetta 42, 9–14.
|
|
Cucco, M., Bryant, D.M., Malacarne, G., 1993. Differences in diet of common (Apus apus) and pallid (A. pallidus) swifts. Avocetta 17, 131–138.
|
|
Cucco, M., Malacarne, G., 1987. Distribution and nest-hole selection in the breeding Pallid Swift. Avocetta 11, 57–61.
|
|
Finlayson, C., 1979. The Ecology and Behaviour of Closely Related Species in Gibraltar (With Special Reference to Swifts and Warblers). University of Oxford, Oxford.
|
|
Fox, J., Weisberg, S., 2019. An R Companion to Applied Regression. Sage Publications, Thousand Oaks.
|
|
Hespenheide, H.A., 1975. Selective predation by two swifts and a swallow in Central America. Ibis 117, 82–99.
|
|
Kearsley, L., Ranc, N., Meier, C.M., Pacheco, C.M., Henriques, P., Elias, G., et al., 2022. The aeroecology of atmospheric convergence zones: the case of pallid swifts. Oikos 2022, e08594.
|
|
Keller, V., Herrando, S., Voríšek, P., Franch, M., Kipson, M., Milanesi, P., et al., 2020. European Breeding Bird Atlas 2: Distribution, Abundance and Change. Lynx Edicions/European Bird Census Council (EBCC), Barcelona.
|
|
Levin, E., Yom-Tov, Y., Barnea, A., 2008. Frequent summer nuptial flights of ants provide a primary food source for bats. Sci. Nat. 96, 477–483.
|
|
Malacarne, G., Cucco, M., 1992. Preferenze alimentari del Rondone pallido, Apus pallidus, in Piemonte (Aves, Apodidae). Riv. Piemontese Storia Nat. 13, 89–96.
|
|
Malacarne, G., Cucco, M., Orecchia, G., 1992. Nest attendance, parental roles and breeding success in the Pallid Swift (Apus pallidus). Vogelwarte 36, 203–210.
|
|
Mata, V.A., da Silva, L.P., Veríssimo, J., Horta, P., Raposeira, H., McCracken, G.F., et al., 2021. Combining DNA metabarcoding and ecological networks to inform conservation biocontrol by small vertebrate predators. Ecol. Appl. 31, e02457.
|
|
Mata, V.A., Rebelo, H., Amorim, F., McCracken, G.F., Jarman, S., Beja, P., 2018. How much is enough? Effects of technical and biological replication on metabarcoding dietary analysis. Mol. Ecol. 28, 165–175.
|
|
Neumann, C., Neumann, C., 2016. Behavioural thermoregulation in the common swift during flight. Br. Birds 109, 10–13.
|
|
Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P., O'hara, R., et al., 2020. Community Ecology Package, 2, pp. 5–7. R package version.
|
|
Quinn, T.W., Cooke, F., White, B.N., 1990. Molecular sexing of geese using a cloned Z chromosomal sequence with homology to the W chromosome. Auk 107, 199–202.
|
|
Simpson, S., Simpson, C., 1990. The mechanisms of nutritional compensation by phytophagous insects. In: Bernays, E.A. (Ed.), Insect-plant Interactions. CRC Press, Boca Raton, pp. 111–160.
|
|
Taberlet, P., Bonin, A., Zinger, L., Coissac, E., 2018. Environmental DNA: for Biodiversity Research and Monitoring. Oxford University Press, Oxford.
|
|
van der Velde, M., Haddrath, O., Verkuil, Y.I., Baker, A.J., Piersma, T., 2017. New primers for molecular sex identification of waders. Wader Study 124, 147–151.
|