| Citation: | Yuwen Cheng, Zhixin Wen, Xingcheng He, Zhehan Dong, Mingyu Zhangshang, Dongrui Li, Yan Wang, Yong Jiang, Yongjie Wu. 2022: Ecological traits affect the seasonal migration patterns of breeding birds along a subtropical altitudinal gradient. Avian Research, 13(1): 100066. DOI: 10.1016/j.avrs.2022.100066 |
Altitudinal bird migration involves seasonal shifts up and down the altitude gradient annually. Asia as the place with the largest number of altitudinal migrants, has quite few related studies, especially for montane and temperate avifaunas. To explore the potential drivers of seasonal altitudinal migration for birds in the middle of Hengduan Mountains, we conducted a three-year investigation on breeding and non-breeding season bird communities at eight elevational bands (1200–4200 m) in the Gongga Mountains. We examined the altitudinal migration patterns and relationships between seasonal distribution shifts and species' traits of 50 species with sufficient data recorded in both seasons. We found that a large proportion of breeding birds underwent altitudinal migration and showed three migration patterns (downslope shift, upslope shift, no shift). Seasonal distribution shifts were mainly correlated with certain ecological traits. Species breeding at high and mid-elevations, nesting in scrub and being omnivorous are more likely to show downslope movements during the non-breeding season. In addition, territorially weaker species exhibited more diverse migration patterns. Notably, we found the hand-wing index (HWI) was actually more convincing than body mass in explaining altitudinal migration. These results consolidate the studies of seasonal altitudinal migration in montane birds. Our study could be used to bridge existing knowledge gaps that currently impeding effective conservation for montane avifaunas in the Hengduan Mountains.
Understanding the variation of species distribution along altitudinal gradients over space and time is crucial to assess the impact of climate change on montane species (McCain and Grytnes, 2010). The geological characteristics, physical and climatic conditions of mountains change rapidly with altitude, and these changes can affect the distribution and abundance of species along the altitudinal gradient (Körner, 2007). Birds are often treated as important indicators that reflect the state of ecosystem, because they are widely distributed, easy to detect and sensitive to environmental changes (He et al., 2022). They play an important role in the mountain ecosystem, serving as arthropod predators, seed consumers and dispersers (Proctor, 1968; Ausprey et al., 2020). Their mobility has profoundly influenced many other aspects of their ecological features and evolutional processes (e.g. strong dispersal may facilitate colonization of new regions, thereby driving range expansion [Lester et al., 2007]). Previous studies of avian movements have mostly focused on their migration (i.e. predictable, annual return movements between breeding and non-breeding ranges), and found most species of avian migrants are partial migrants rather than complete migrants (Berthold, 2002; Boyle, 2017). Such type of migratory behavior involves movement over altitudinal gradients. Most birds conduct seasonal migrations over altitudinal gradients, including short-distance upslope movement from low-altitude breeding areas to high-altitude non-breeding areas, and downslope movement from high-altitude breeding areas to low-altitude non-breeding areas (Boyle, 2017). Compared with latitudinal migrations, altitudinal migration is characterized with shorter distances such that individuals consume less energy.
Asia is home to the greatest number of altitudinal migrants around the world and it is estimated that up to 65% of the Himalayan and 58% of Taiwanese high-elevation breeding species are involved in this activity (Boyle, 2017; Tsai et al., 2021). However, this behavior remains poorly understood, especially for montane and temperate avifaunas. Under the influence of climate change and human activities, montane species are facing unique challenges such as habitat fragmentation (Liao et al., 2020). Studying the seasonal altitudinal migration patterns of birds is essential to understanding their distributional responses to seasonal changes in climate (energy), which has important conservation implications for avian biodiversity (Hsiung et al., 2018; He et al., 2019).
Birds can reduce risk of predation (Boyle, 2008a), avoid harsh climatic conditions (Nevada et al., 2004; Boyle, 2008b; Boyle et al., 2010) and track food resources via altitudinal migration (Levey, 1988; Loiselle and Blake, 1991; Liang et al., 2021). Species with different life-history strategies and ecological traits may show distinct migration patterns in response to climate change. It has been shown that ecological traits such as territoriality (Tobias et al., 2016), nest site (Boyle, 2008a; Natusch et al., 2017), diet (Boyle, 2011) and body mass (Neate-Clegg et al., 2021) could influence species' ability to shift their altitudinal ranges (Wang et al., 2021). Most studies on altitudinal migration have focused on the food-limitation hypothesis, which proposes that food abundance often varies seasonally over elevational gradients in response to fluctuations in temperature and precipitation throughout the year, and birds shift their elevational distributions seasonally as a response to the fluctuations in food availability across seasons (Hsiung et al., 2018). Consequently, species with different diets may respond differently to seasonality. For example, studies in Yunnan and Taiwan, China, have found that frugivores and nectarivores are more likely to exhibit altitudinal migration than omnivores and invertivores (Liang et al., 2021; Tsai et al., 2021). Moreover, avian territorial behaviors may also influence their distributional responses. For example, species with stronger territoriality may guard the territory year-round (Tobias et al., 2016; Sheard et al., 2020). In addition, birds may actively adopt strategies to avoid or reduce nest predation. A probably more noticeable strategy is nest-site selection, which often determines the safety of the nest (Natusch et al., 2017; Guilherme et al., 2018). Normally, predator densities are higher at low and medium altitudes, we therefore predict that variation in predation risk is related to the upslope altitudinal migration, as the risk of predation decreases with increasing elevation (Boyle, 2008a; Robinson and Robinson, 2001). Birds having nest sites lower in elevation may be at greater predation risk than those with higher nest sites, it is therefore expected that the formers would be more likely to migrate uphill during the breeding season to reduce this risk. However, while many possible drivers of seasonal altitudinal migration have been proposed, they have only been empirically examined in few cases and the generality is mostly unknown (Barçante et al., 2017; Boyle, 2017).
The causes of altitudinal migration have only been studied in a few systems and avian species. Most studies have been conducted in America, and hardly in Asia (Hsiung et al., 2018). Boyle (2017) found that altitudinal migration was prevalent, occurring in > 20% of continental North American and nearly 30% of Hawaiian species. In addition, the majority of birds that undergo altitudinal migration are frugivores or nectarivores in the Neotropics (Boyle, 2008a; Revell, 2012; Barçante et al., 2017). In China, Taiwanese researchers have modeled the behaviors based on occurrence records collected by citizen scientists and they suggested that seasonal variations in climate and food availability could be the major drivers (Tsai et al., 2021). Studies in the Gaoligong Mountains have found that species' seasonal distribution shifts were mainly associated with breeding altitudes and diets (Liang et al., 2021). Compared to long-distance latitudinal migration, altitudinal migration has been poorly studied in Asia. More studies aiming to elucidate the drivers of altitudinal migration in various regions are needed to provide a more comprehensive view of the trade-offs associated with different migratory strategies in a diversity of taxa and ecological contexts.
As one of the world's 36 biodiversity hotspots, the Hengduan Mountains are home to many altitudinal migrants (Wu et al., 2013, 2014). Local unique biogeographic (at the junction of Palaearctic and Oriental realm) and climatic features have drawn scientists' attention to the biodiversity of the area (Li et al., 2010; Liu et al., 2010). To describe seasonal altitudinal migration patterns of birds and test potential drivers influencing their movements in the middle of Hengduan Mountains, we conducted a three-year survey on bird communities in breeding and non-breeding seasons at eight elevational bands in the Gongga Mountains, and compared species' altitude ranges between the two seasons. Our aims were to explore (1) the altitudinal migration patterns of mountain birds and (2) what life-history and ecological characters of birds are the primary drivers of seasonal altitudinal changes? Specifically, we predicted that most birds will migrate uphill during the breeding season, and this behavior is strongly related to their ecological traits.
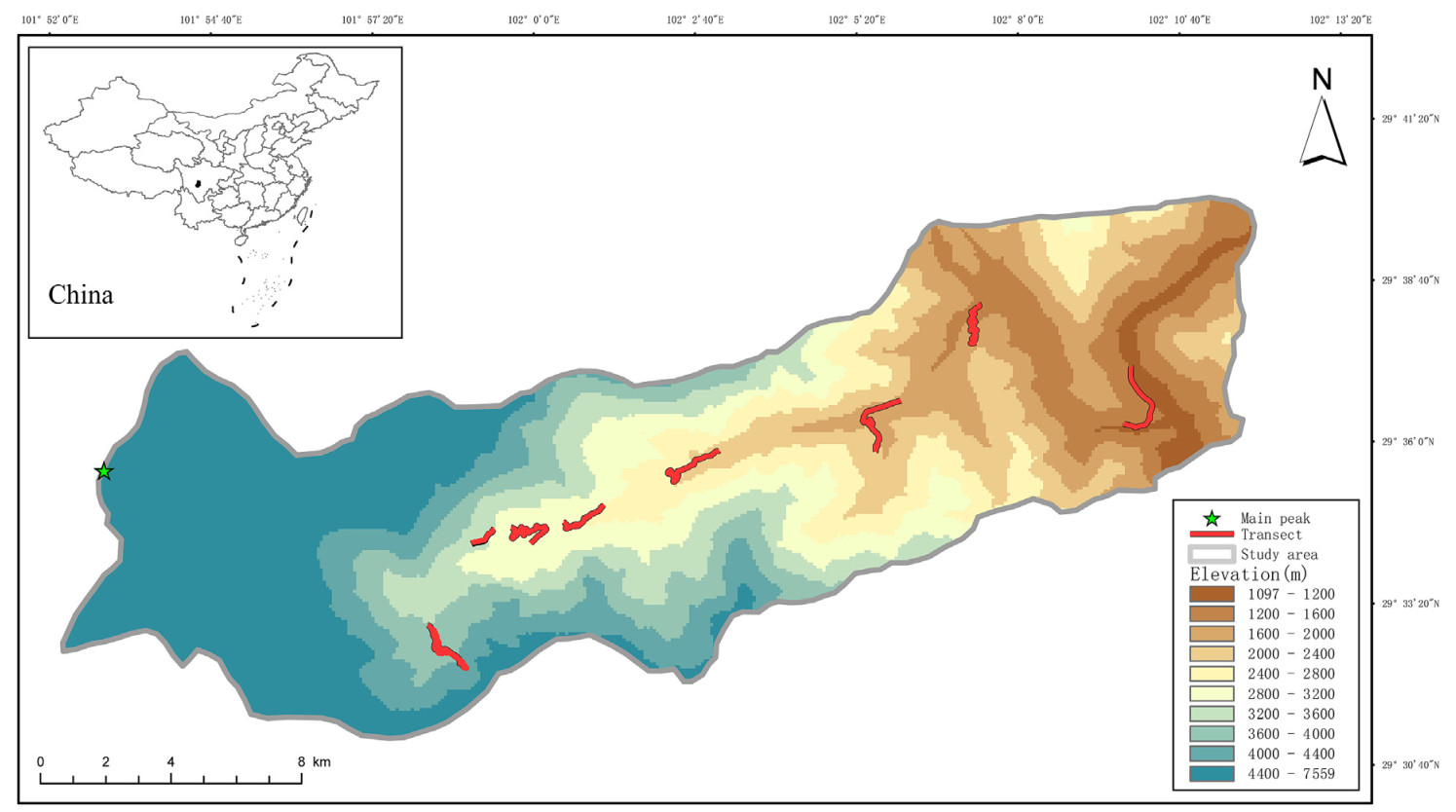
Mt. Gongga is located at the southeastern margin of the Qinghai-Tibet Plateau (29°20′–30°39′N, 101°30′–102°12′E, Fig. 1), conjoining the middle part of the Hengduan Mountains, the middle part of the Daxue Mountain and the western part of Sichuan. The main peak of Mt. Gongga is 7556 m above sea level. The region is at the transitional climatic zone of the warm and humid subtropical monsoon climate of eastern China and semi-humid climate of the eastern Qinghai-Tibet Plateau. Due to the large altitudinal coverage, the heat and moisture conditions vary with altitude and the climate at the foot of the mountain is dry and warm (Li et al., 2013). With rising altitude, the average annual precipitation of areas at 3000 m can reach 1930 mm, making the Gongga Mountain a continuous montane vertical spectrum from subtropical to alpine frigid zone. Vegetation types and soils here also show obvious vertical differentiation (Wu et al., 2017; He et al., 2022).
From May and December of 2017, 2018 and 2021, we conducted avian community surveys in the Hailuogou region along the altitudinal gradients using standard line-transects method during the breeding and non-breeding seasons. Our actual survey covered the altitudes from 1200 m (with the Dadu River as the lower altitudinal limit) to above 4200 m which is the permafrost. We divided the altitudinal gradients into eight sections at an interval of 400 m, with the bird surveys spanning the entire set of all sections (He et al., 2019). To ensure comparable efforts, we surveyed the same transects each season and the lengths were similar within the same altitudinal section, which were approximately 2–3 km. We conducted six field surveys each year consisted of two sampling seasons (i.e., breeding and non-breeding seasons). Our team was composed of two–three trained personnel who would work between dawn and morning (6:00–9:30) and between afternoon and sunset (15:30–18:30), and in each time period an uphill and a downhill survey were performed. During each survey in each season, we did four bird surveys on each transect, resulting in a cumulative total of 24 surveys per transect. By doing so, we ensured that more bird species could be observed. For each survey, we recorded vegetation types, bird occurrences and abundances, and the information of birds' locations using a GARMIN 621sc handheld GPS.
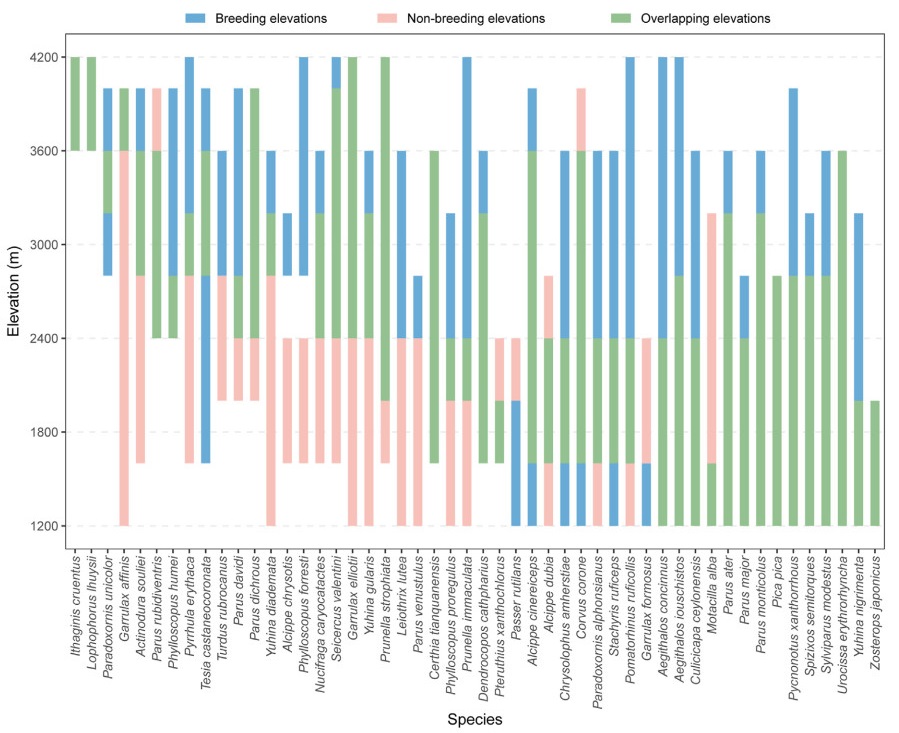
We recorded a total of 173 bird species, belonging to 9 orders and 40 families in the three-year survey (Appendix 1), and then quantified the change in the altitudinal range for each species between breeding and non-breeding seasons. First, by screening the residence type and distribution records, we excluded migratory birds that only breed in the area or spend the winter. Second, we excluded birds spotted too far away during the survey, including swifts and raptors, for which we could not estimate its actual elevation (Zhang et al., 2020; Liang et al., 2021; He et al., 2022). Finally, for comparison purpose, we focused on species that were recorded in both seasons. To improve the credibility, only the species that were recorded more than five individuals in each season were used to study species' seasonal range shifts. These treatments resulted in a sum of 50 species for further analyses.
We examined the shifts in upper range limit, lower range limit and abundance-weighted range center for the 50 species. The abundance-weighted range center of each season was calculated as:
|
Σm,nEi×PAi |
where m and n are the range limits of species A, Ei is the altitude (m) of site i and PAi is the proportion of individuals of species A at site i in its total individuals recorded along the whole gradient (Menéndez et al., 2014).
Before comparison analysis, we controlled for the potential differences in potential sampling efforts between the two seasons (the uneven samples may result in the distribution of bird observations biased towards the altitudes surveyed more often). A resampling approach was applied to standardize the sampling efforts: we randomly resampled (100 times without replacement) each altitudinal section dataset to generate the identical number of individuals between altitudinal section during each season. We randomly extracted 178 individuals from the non-breeding season using the package 'randomization' (Chen et al., 2011; Wu et al., 2017), and then compared the average to assess the range shift pattern for each of the three altitudinal locations (i.e. upper range limit, lower range limit and abundance-weighted range center). We calculated seasonal elevation shifts by subtracting the elevation of the breeding season from the non-breeding season: a positive value indicated upslope shift from breeding to non-breeding seasons, and a negative value indicated downslope shift from breeding to non-breeding seasons.
We examined the effects of species' traits on their altitudinal range shift patterns. The selected traits included flight efficiency, territoriality, breeding altitude, nest site, diet and body mass. We obtained species' territoriality from the Tobias' dataset (categorical: strong and weak) (Tobias et al., 2016). We extracted the information of hand-wing index (HWI) following Sheard et al. (2020). Hand-wing index is a proxy of avian flight efficiency and dispersal ability that measured by the ratio of Kipp's distance (the distance between the tip of the longest primary feather and the tip of the first secondary feather) to the wing chord. We classified the 50 species into low-elevation (1200–2200 m), middle-elevation (2200–3200 m) and high-elevation breeders (3200–4200 m) based on the vegetation vertical zone spectrum of Mt. Gongga and their abundance-weighted mean elevations recorded in the breeding season (He et al., 2019; Liang et al., 2021). Below 2000 m the settlement and agroforestry belts dominate; from 2000 to 3600 m there is a shift from deciduous broadleaf mixed forest belt to montane dense coniferous forest belt, and from 3600 to 4200 m there is a shrub and grass belt (Wu et al., 2013, 2014; He et al., 2019, 2022). Finally, we obtained body mass and diet (invertebrate, omnivore) data from the dataset on the life-history and ecological traits of Chinese birds (Wang et al., 2021). Body mass has received equivocal support in altitudinal migration studies. Although body mass is traditionally used as an index for dispersal ability in vertebrates, with larger size assumed to indicate greater power (Rappole, 2016), in a recent study, it was revealed that body mass did not correlate significantly with bird dispersal ability (Claramunt, 2021). Accordingly, we chose to keep the body mass as a candidate factor.
We constructed the phylogenetic generalized least squares regression models (PGLS) in packages 'caper' (Bartoń, 2013) to examine the correlations between the shifting patterns in three altitudinal locations of species and their traits. To obtain the phylogenetic information, we downloaded 5000 phylogenetic trees of 50 target species from birdtree.org (Jetz et al., 2012) and constructed the Maximum Clade Credibility tree using mean node heights with the software TreeAnnonator v1.10.4 of the 'BEAST' package (Si et al., 2017; Suchard et al., 2018). We constructed three separate PGLS models for these three measures of altitudinal shifts. In the first model, the upper-elevation limit shift was the response variable. Territoriality, flight efficiency, breeding altitude, diet and body mass were used as predictors. Then we constructed two other models with the same prediction factors, using the lower-altitude limit and the abundance-weighted range center shifts as response variables. We used the best models ranked by the Akaike's information criterion corrected for small sample sizes (AICc) and subsequently averaged the coefficient of the selected models using package 'MuMIn' (Bartoń, 2013). Finally, we used the phylogenetic paired t-tests to assess whether or not the elevations between breeding and non-breeding seasons are significantly different. All statistical analyses were conducted using the R statistical computing environment v.3.6.4 (Andy Bunn, 2017). PGLS models were built as implemented in the package 'nlme' (Pinheiro, 2012). All phylogenetic paired t-tests used the 'phyl.pairedttest' function of the 'phytools' package in R (Revell, 2012).
Our results revealed that a large proportion of resident bird species conducted seasonal altitudinal range shifts in Mt. Gongga. However, diverse patterns were performed. All 50 species showed seasonal shifts in their abundance-weighted range center and altitude limits. There were three altitudinal shift patterns among the studied species. First, most species (n = 30) bred at a higher altitude in breeding season, while migrated downward in non-breeding season. Second, some species (n = 13) tended to move higher in non-breeding seasons, as both their abundance-weighted mean elevation and upper-altitude limits showed upward movements. Finally, the altitudinal ranges of seven species did not change significantly between seasons, and the altitudes during the breeding and non-breeding seasons were mostly the same (the abundance-weighted range center altitude changes smaller than 100 m), suggesting that the partial migration might be prevalent among the migrants (Appendix 1).
The PGLS models indicated that territoriality, HWI, body mass, mean breeding elevation are important in explaining the elevational shifts from breeding to non-breeding seasons (Table 1). Using abundance-weighted mean elevation shift as an example, (1) species with strong territoriality tended to show more downward shifts from breeding to non-breeding season than species with week territoriality; (2) high-elevation breeders were more likely to show downward shifts in non-breeding seasons than low-elevation breeders, but there were no differences in shifts between high- and middle elevation breeders; (3) larger species shifted more downward than smaller species in non-breeding season; and (4) species with greater dispersal ability (as indicated by hand-wing index) showed more downward shifts in elevation in non-breeding season.
| Parameters | Estimate | SE | Z value | Pr (> z) | N containing models (< 7) | Sum of weights |
| Lower-altitude limit shift | ||||||
| Territoriality (strong vs. weak) | 682.97 | 212.53 | 3.214 | 0.002 | 1 | / |
| Mean breeding elevation (high vs. low) | 1018.83 | 272.65 | 3.737 | 0.001 | 1 | / |
| Mean breeding elevation (high vs. middle) | 617.72 | 258.52 | 2.390 | 0.021 | ||
| Diet (insectivores vs. omnivores) | −358.1 | 205.4 | 1.744 | 0.081 | 1 | 0.61 |
| Nest site (canopy vs. scrub) | −264.2 | 190.50 | 1.387 | 0.165 | 1 | 0.47 |
| Body mass | 48.58 | 159.31 | 0.305 | 0.760 | 1 | 0.262 |
| HWI | 29.64 | 13.87 | 2.137 | 0.033 | 1 | 0.77 |
| Upper-altitude limit shift | ||||||
| Territoriality (strong vs. weak) | −48.02 | 248.52 | 0.193 | 0.847 | 1 | 0.256 |
| Mean breeding elevation (high vs. low) | 216.8 | 314.5 | 0.689 | 0.491 | 1 | 0.57 |
| Mean breeding elevation (high vs. middle) | −263.7 | 298.2 | 0.884 | 0.377 | ||
| Diet (insectivores vs. omnivores) | 306.7 | 220.3 | 1.392 | 0.164 | 1 | 0.48 |
| Nest site (canopy vs. scrub) | −32.69 | 206.09 | 0.079 | 0.874 | 1 | 0.255 |
| Body mass | 332.3 | 162.3 | 2.048 | 0.040 | 1 | 0.73 |
| HWI | 22.39 | 15.06 | 1.487 | 0.137 | 1 | 0.51 |
| Abundance-weighted mean altitude shift | ||||||
| Territoriality (strong vs. weak) | 350.7 | 193.1 | 1.817 | 0.069 | 1 | 0.61 |
| Mean breeding elevation (high vs. low) | 852.35 | 203.87 | 4.181 | < 0.001 | 1 | / |
| Mean breeding elevation (high vs. middle) | 299.11 | 196.19 | 1.525 | 0.134 | ||
| Diet (insectivores vs. omnivores) | −26.37 | 170.3 | 0.155 | 0.877 | 1 | 0.255 |
| Nest site (canopy vs. scrub) | −193.4 | 169.9 | 1.139 | 0.255 | 1 | 0.4 |
| Body mass | 253.6 | 125.5 | 20.210 | 0.043 | 1 | 0.6 |
| HWI | 27.02 | 11.24 | 2.405 | 0.016 | 1 | 0.77 |
Phylogenetic paired t-test showed that territoriality, mean breeding elevation, nest site and diet significantly explained altitude variation between the breeding and non-breeding seasons (Table 2). Specifically, (1) territorially strong species varied significantly in altitude between seasons, with significant variation in abundance-weighted mean elevation shift and upper altitude limits; (2) middle-elevation breeders shifted their upper altitude limits, lower altitude limits, and abundance-weighted mean elevation, but the altitude distribution of both low and high elevation breeders did not differ significantly between the two seasons; (3) all three elevation measures for omnivores changed significantly, with insectivores also showing significant changes in abundance-weighted mean elevation shift; (4) the scrub-nesting species shifted their upper altitude limits, lower altitude limits, and abundance-weighted mean elevation shift, and the canopy-nesting species showed a downslope change in terms of abundance-weighted mean elevation shift.
| Trait groups | Altitude shifts | N | t | df | P |
| Territoriality | |||||
| Weak | Upper-altitude limit | 39 | 4.227 | 36 | < 0.001 |
| Lower-altitude limit | 39 | 1.763 | 36 | 0.086 | |
| Mean altitude | 39 | 3.239 | 36 | 0.003 | |
| Strong | Upper-altitude limit | 11 | 0.435 | 8 | 0.675 |
| Lower-altitude limit | 11 | 3.516 | 8 | 0.008 | |
| Mean altitude | 11 | 0.290 | 8 | 0.779 | |
| Mean breeding elevation | |||||
| High | Upper-altitude limit | 7 | 0.533 | 4 | 0.623 |
| Lower-altitude limit | 7 | 1.928 | 4 | 0.126 | |
| Mean altitude | 7 | 1.998 | 4 | 0.116 | |
| Middle | Upper-altitude limit | 26 | 3.959 | 23 | < 0.001 |
| Lower-altitude limit | 26 | 2.669 | 23 | 0.014 | |
| Mean altitude | 26 | 5.748 | 23 | < 0.001 | |
| Low | Upper-altitude limit | 17 | 1.146 | 14 | 0.271 |
| Lower-altitude limit | 17 | −0.702 | 14 | 0.049 | |
| Mean altitude | 17 | −1 | 14 | 0.334 | |
| Diet | |||||
| Insectivores | Upper-altitude limit | 15 | 0.561 | 12 | 0.585 |
| Lower-altitude limit | 15 | 0.402 | 12 | 0.695 | |
| Mean altitude | 15 | 2.566 | 12 | 0.025 | |
| Omnivores | Upper-altitude limit | 35 | 3.678 | 32 | < 0.001 |
| Lower-altitude limit | 35 | 3.488 | 32 | 0.001 | |
| Mean altitude | 35 | 3.750 | 32 | < 0.001 | |
| Nest height | |||||
| Canopy | Upper-altitude limit | 24 | 1.458 | 21 | 0.160 |
| Lower-altitude limit | 24 | 1.519 | 21 | 0.140 | |
| Mean altitude | 24 | 2.270 | 21 | 0.034 | |
| Scrub | Upper-altitude limit | 26 | 3.288 | 23 | 0.003 |
| Lower-altitude limit | 26 | 2.927 | 23 | 0.008 | |
| Mean altitude | 26 | 4.221 | 23 | < 0.001 | |
We described the patterns and driving factors of altitudinal migration for breeding birds in Mt. Gongga through six surveys in three years. Consistent with previous studies in Europe and North America (Boyle, 2011, 2017; Hsiung et al., 2018), our results show that altitudinal migration is common and seasonal change in food availability may be the main driving factors for the altitudinal migration. Most birds migrate downhill after breeding season at high altitudes, while some species migrate uphill and downhill in non-breeding season. This shows altitudinal migration is common in breeding birds (Fig. 2).
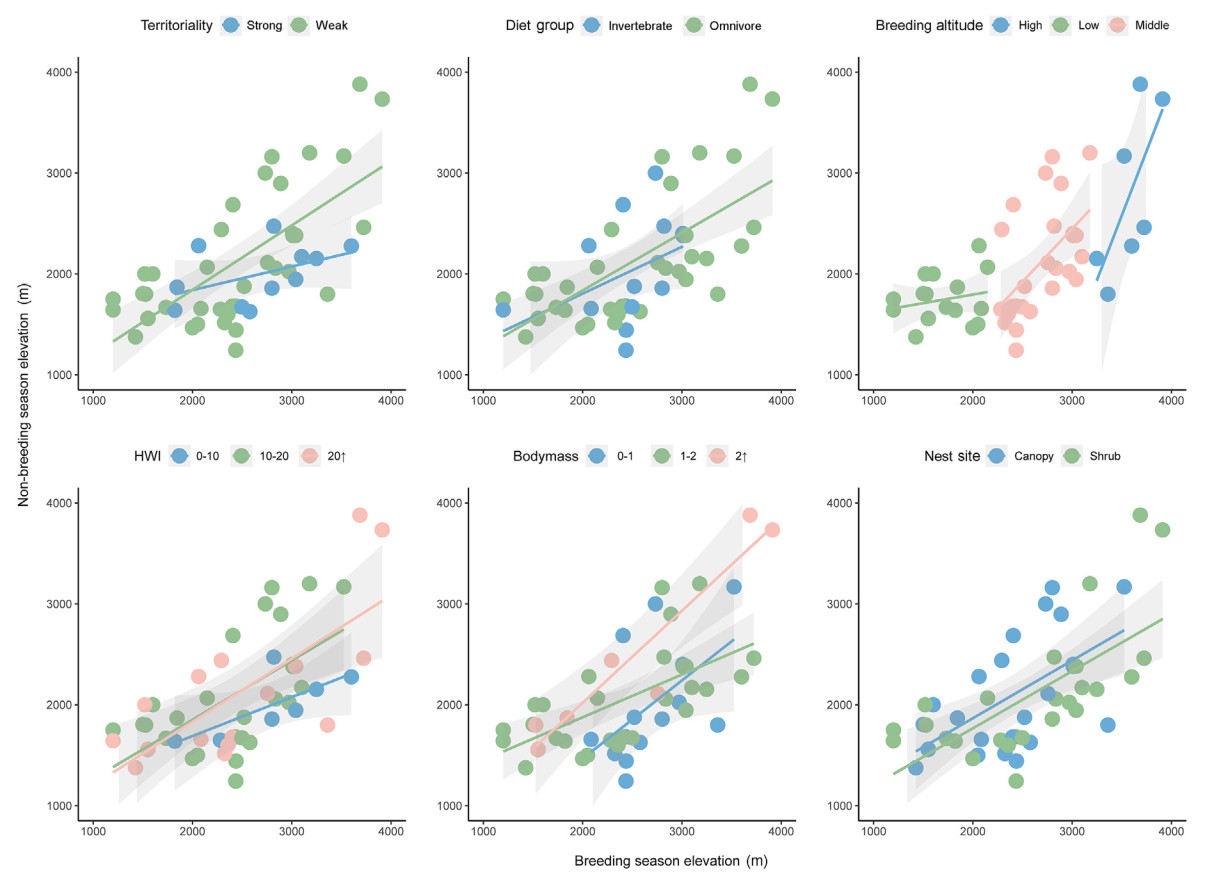
Consistent with recent studies in Taiwan and Gaoligong Mountains, high-and middle-elevation breeders showed apparent downslope movements in the non-breeding season (Fig. 3). Although not significant, approximately half of the low-elevation breeders did make an upslope movement. We found that both insectivores and omnivores tended to migrate downslope during the non-breeding season and omnivores exhibited greater range of altitudinal migration in Mt. Gongga (Table 2; Fig. 3). In Mt. Gongga, omnivorous birds fed largely on invertebrates, such that their distributions are interrelated (Liang et al., 2021; Tsai et al., 2021). In mountainous areas, invertebrate abundance normally decreases from birds' breeding to non-breeding season (Ghosh-Harihar and Price, 2014; Kimura, 2021), and this decline is particularly obvious at high altitudes. In breeding season, the invertebrate resources at the altitude are sufficient to meet birds' needs, but harsh climates and reduced food supplies over non-breeding season may drive them to move downhill (Boyle et al., 2010; Boyle, 2011; Liang et al., 2021). The demand for various food types at different periods in a year may also motivate altitudinal movements. A more diverse diet could allow omnivores to utilize habitats at different altitudes and thus perform altitudinal migrations to obtain food resources. This may explain their varying migration patterns.
Our results show that the weakly territorial species showed more diverse migration patterns, while strongly territorial species tended to migrate downhill during the non-breeding season (Table 2). Recent studies showed that strongly territorial species would expend more time defending their territories (Tobias et al., 2016; Sheard et al., 2020), thus being less likely to go through rapid distributional changes but constrained to high-altitude regions due to interactions with closely related species. For example, uphill migration during the breeding season may occur, as space is larger and predatory competition is mitigated at higher altitudes (Jankowski et al., 2010; Freeman et al., 2019). However, species showing weak territoriality would move constantly to seek for food resources (Lees and Peres, 2009; Tobias et al., 2016).
The relationship between nest structure and nest predation is controversial (Martin et al., 2017), and the nest predation risk may be higher at lower altitudes elevations (Boyle, 2008b). Our results show that migratory movements are associated with nest predation risk, with scrub-nesting birds migrating upslope and moving more distance on average than canopy-nesting birds during the breeding season to avoid nest predation (Table 1, Table 2). Nest site often determines the safety of a nest, so birds carefully select it to efficiently avoid nest predation and maladaptation (Natusch et al., 2017; Guilherme et al., 2018). The nest predation rates could generally be reduced by the increase of height-related coverage and complexity of vegetation surrounding the nest (Guilherme et al., 2018). In this context, scrub-nesting species breeding at lower altitudes would face higher nest predation pressure, leading to uphill migration during the breeding season to avoid predators such as mammals and snakes. This prediction has been confirmed by our results.
When considering the absolute rates of shifts (ignoring shift direction) and the expansions in species' upper limits, the most important predictors were HWI and wing length, as reported by Neate-Clegg et al. (2021) and Sheard et al. (2020). Our study reveals a correlation between HWI and the uphill movement (Table 2). This correlation is reasonable because uphill movement consumes more energy in the mountain system. Dispersal distances increase exponentially with flight efficiency in breeding birds of Mt. Gonga (Claramunt, 2021), which is also affected by species' migratory behavior such as seasonal altitudinal migration of birds (Bonte et al., 2012). Moreover, the possibility of estimating dispersal ability based on morphology may have applications in conservation biology, as this can be instrumental in assessing species vulnerability to habitat fragmentation and climate change (Travis et al., 2013; MacLean and Beissinger, 2017). Although our results imply a relationship between body weight and altitudinal migration, wing shape proportions and wing length were found to be more influential, which coincides with previous studies (Claramunt, 2021). It was also found that body mass was not important in explaining dispersal distances in British birds (Paradis et al., 1998; Claramunt, 2021). Most of the studies on the correlation between body mass and altitudinal migration were conducted in tropics and regions with smaller altitude span (MacLean and Beissinger, 2017; Tsai et al., 2021). The small impact of body mass on birds' altitudinal migration may be attributed to the variation in wing shape, in which larger birds have also higher aspect ratios, rather than an intrinsic effect of body mass itself (Videler, 2006; Pennycuick, 2008). Body mass has long been linked to environmental gradients via Bergmann's rule (Bergmann, 1847), which states that larger-bodied species tend to be found in colder environments. However, evidence for the existence of Bergmann's rule is variable (Blackburn and Ruggiero, 2001; Freeman, 2017), and it tends to be applied more to intraspecific variation in body mass or variation among closely related species, particularly along latitudinal gradients.
Altitudinal migration has evolved independently in different regions of the world under different environmental pressures coupled with varying life-history characteristics. These external drivers frequently interact with intrinsic factors such as seasonality of requirements due to reproduction, differences in individual condition, and competitive asymmetries among individuals to produce flexible partial migration systems. Additionally, the prevalence of partial migration implies that a combination of intrinsic and extrinsic drivers jointly shape migratory decisions at the individual level. Investigations of partial altitudinal migration patterns that focus on underlying mechanisms that lead to responses to environmental factors could help elucidate how individual characteristics affect migratory propensity. Our results showed that some breeding birds in Gongga Mountains undertook seasonal altitudinal migrations that were manifested in three distinct patterns: downslope shift, upslope shift and no shift. Main drivers influencing seasonal altitudinal migration of birds include territoriality, nest sites, HWI and diet. Our study may largely expand our understanding of the fitness trade-offs between migration strategies and how they are reflected in the population dynamics of montane species. Higher elevations cover almost the whole reserve, while lower and middle elevations include most residential and scenic area. When species shift, anthropogenic interference would undoubtedly increase threat to them. We suggest that while protecting high elevation areas, we should also strengthen the protection and restoration of forests in middle and low elevation regions, to further enhance the habitat connectivity among different altitude gradients, which may be more critical to the survival and movement of species.
Ethical approval is not required for the study because data aqusition of our research was based on an observational way and no animals were manipulated.
YC, ZW, XH and Y. Wu conceived and designed the study. YC, XH, ZD, MZ, DL and Y. Wang performed the field surveys and analyzed the data. YC, ZW, XH and Y. Wu wrote and revised the manuscript. All author read and approved the final manuscript.
The morphological and ecological data used in this study is available at https://www.nature.com/articles/s41467-020-16313-6 and https://www.biodiversity-science.net/CN/10.17520/biods.2021201.
Range information and migration data is publicly available from www.birdlife.org; climate data from www.worldclim.org; phylogenetic data from www.birdtree.org.
The authors declare that they have no competing interest.
We thank Kai Zhang, Bowei Zhu, Fei Dong, Yuke Zhang, Per Alström, Yugang Li, Gang Liu, Shaojun Mao, Qiang Xie and Xin He for their kind assistance with field data collection and suggestions for the analyses. We thank Genxu Wang and Qiao Liu in Mountain Hazards and Environmental Research Institute of Chinese Academy of Sciences; and Faming Liu, Quan Lan, and Jinhua Chen at Gongga Alpine Ecosystem Observation and Research Station for their kind help with the field work.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.avrs.2022.100066.
|
Andy Bunn, M.K., 2017. A language and environment for statistical computing. R Found. Stat. Comput. 10, 11-18.
|
|
Ausprey, I.J., Newell, F.L., Robinson, S.K., 2020. Adaptations to light predict the foraging niche and disassembly of avian communities in tropical countrysides. Ecology 102, e03213.
|
|
Barton, K., 2013. MuMIn: Multi-Model Inference. R Package, version 1.10.0.
|
|
Bergmann, C., 1847. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien 1, 595–708.
|
|
Berthold, P., 2002. Bird migration: A general survey. Auk 119, 874-875.
|
|
Claramunt, S., 2021. Flight efficiency explains differences in natal dispersal distances in birds. Ecology 102, e03442.
|
|
Pennycuick, C.J., 2008. Modelling the Flying Bird. Academic Press, New York.
|
|
Rappole, J.H., 2016. The Avian Migrant: the Biology of Bird Migration. Columbia University Press, New York.
|
|
Suchard, M.A., Lemey, P., Baele, G., Ayres, D.L., Drummond, A.J., Rambaut, A., 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 4, 1-5.
|
|
Tobias, J.A., Sheard, C., Seddon, N., Meade, A., Cotton, A.J., Nakagawa, S., 2016. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4, 00074.
|
|
Videler, J.J., 2006. Avian Flight. Oxford University Press, USA.
|
| Parameters | Estimate | SE | Z value | Pr (> z) | N containing models (< 7) | Sum of weights |
| Lower-altitude limit shift | ||||||
| Territoriality (strong vs. weak) | 682.97 | 212.53 | 3.214 | 0.002 | 1 | / |
| Mean breeding elevation (high vs. low) | 1018.83 | 272.65 | 3.737 | 0.001 | 1 | / |
| Mean breeding elevation (high vs. middle) | 617.72 | 258.52 | 2.390 | 0.021 | ||
| Diet (insectivores vs. omnivores) | −358.1 | 205.4 | 1.744 | 0.081 | 1 | 0.61 |
| Nest site (canopy vs. scrub) | −264.2 | 190.50 | 1.387 | 0.165 | 1 | 0.47 |
| Body mass | 48.58 | 159.31 | 0.305 | 0.760 | 1 | 0.262 |
| HWI | 29.64 | 13.87 | 2.137 | 0.033 | 1 | 0.77 |
| Upper-altitude limit shift | ||||||
| Territoriality (strong vs. weak) | −48.02 | 248.52 | 0.193 | 0.847 | 1 | 0.256 |
| Mean breeding elevation (high vs. low) | 216.8 | 314.5 | 0.689 | 0.491 | 1 | 0.57 |
| Mean breeding elevation (high vs. middle) | −263.7 | 298.2 | 0.884 | 0.377 | ||
| Diet (insectivores vs. omnivores) | 306.7 | 220.3 | 1.392 | 0.164 | 1 | 0.48 |
| Nest site (canopy vs. scrub) | −32.69 | 206.09 | 0.079 | 0.874 | 1 | 0.255 |
| Body mass | 332.3 | 162.3 | 2.048 | 0.040 | 1 | 0.73 |
| HWI | 22.39 | 15.06 | 1.487 | 0.137 | 1 | 0.51 |
| Abundance-weighted mean altitude shift | ||||||
| Territoriality (strong vs. weak) | 350.7 | 193.1 | 1.817 | 0.069 | 1 | 0.61 |
| Mean breeding elevation (high vs. low) | 852.35 | 203.87 | 4.181 | < 0.001 | 1 | / |
| Mean breeding elevation (high vs. middle) | 299.11 | 196.19 | 1.525 | 0.134 | ||
| Diet (insectivores vs. omnivores) | −26.37 | 170.3 | 0.155 | 0.877 | 1 | 0.255 |
| Nest site (canopy vs. scrub) | −193.4 | 169.9 | 1.139 | 0.255 | 1 | 0.4 |
| Body mass | 253.6 | 125.5 | 20.210 | 0.043 | 1 | 0.6 |
| HWI | 27.02 | 11.24 | 2.405 | 0.016 | 1 | 0.77 |
| Trait groups | Altitude shifts | N | t | df | P |
| Territoriality | |||||
| Weak | Upper-altitude limit | 39 | 4.227 | 36 | < 0.001 |
| Lower-altitude limit | 39 | 1.763 | 36 | 0.086 | |
| Mean altitude | 39 | 3.239 | 36 | 0.003 | |
| Strong | Upper-altitude limit | 11 | 0.435 | 8 | 0.675 |
| Lower-altitude limit | 11 | 3.516 | 8 | 0.008 | |
| Mean altitude | 11 | 0.290 | 8 | 0.779 | |
| Mean breeding elevation | |||||
| High | Upper-altitude limit | 7 | 0.533 | 4 | 0.623 |
| Lower-altitude limit | 7 | 1.928 | 4 | 0.126 | |
| Mean altitude | 7 | 1.998 | 4 | 0.116 | |
| Middle | Upper-altitude limit | 26 | 3.959 | 23 | < 0.001 |
| Lower-altitude limit | 26 | 2.669 | 23 | 0.014 | |
| Mean altitude | 26 | 5.748 | 23 | < 0.001 | |
| Low | Upper-altitude limit | 17 | 1.146 | 14 | 0.271 |
| Lower-altitude limit | 17 | −0.702 | 14 | 0.049 | |
| Mean altitude | 17 | −1 | 14 | 0.334 | |
| Diet | |||||
| Insectivores | Upper-altitude limit | 15 | 0.561 | 12 | 0.585 |
| Lower-altitude limit | 15 | 0.402 | 12 | 0.695 | |
| Mean altitude | 15 | 2.566 | 12 | 0.025 | |
| Omnivores | Upper-altitude limit | 35 | 3.678 | 32 | < 0.001 |
| Lower-altitude limit | 35 | 3.488 | 32 | 0.001 | |
| Mean altitude | 35 | 3.750 | 32 | < 0.001 | |
| Nest height | |||||
| Canopy | Upper-altitude limit | 24 | 1.458 | 21 | 0.160 |
| Lower-altitude limit | 24 | 1.519 | 21 | 0.140 | |
| Mean altitude | 24 | 2.270 | 21 | 0.034 | |
| Scrub | Upper-altitude limit | 26 | 3.288 | 23 | 0.003 |
| Lower-altitude limit | 26 | 2.927 | 23 | 0.008 | |
| Mean altitude | 26 | 4.221 | 23 | < 0.001 | |