| Citation: | Tihana Hamzaj, Brendan Graham, Katherine Bonilla, Ignacio Gutiérrez, Luis Sandoval. 2022: Intruder familiarity and not duet similarity influences the territory defense in a year-round territorial bird species. Avian Research, 13(1): 100032. DOI: 10.1016/j.avrs.2022.100032 |
To avoid unnecessary energy expenditures in territorial defense, many species (e.g., insects, amphibians, birds, and mammals) have developed the capability to distinguish between different intruder types using visual, acoustic, and/or chemical signals. Determining the mechanism used for intruder recognition is key to understanding the dynamics of territorial behaviors. In birds that use vocalizations for territorial defense, the frequency and duration of vocalizations or the familiarity with the intruder may be the main mechanism used for intruder recognition. Here, we conducted a playback experiment with territorial White-eared Ground-sparrows (Melozone leucotis), to analyze if territorial pairs recognize intruders using frequency and duration characteristics (a bird's response is based on how structurally similar the intruders' duets are to their own) or by familiarity with the intruders (neighbors vs. non-neighbors). We focused on duets because this species uses duets exclusively for territorial defense. We broadcasted a duet from a territorial neighbor, two duets from non-neighbors (with different frequency and duration characteristics), and a duet from a control species in 39 territorial pair from three populations. During playback we measured five behavioral responses: latency of the first vocalization, latency of the approach to the speaker, time spent close the speaker (within 5 m), number of individuals that approached the speaker, and the number of vocalizations. We found that territorial White-eared Ground-sparrow pairs responded stronger to neighbors than non-neighbors, and this response is not influenced by duet duration or frequency characteristics. This result suggests that neighbors represent a greater territorial threat for White-eared Ground-sparrows than non-neighbors. Further work is necessary to understand how common this observation is for tropical species that defend small territories year-round.
Communication between individuals is important for attracting mates, advertising danger or food, defending territories, or maintaining contact (Hall, 2004; Sandoval et al., 2013; Tobergte and Curtis, 2013). In particular, communication appears to be a key component of territory and resource defense (Bradbury and Vehrencamp, 1998; Catchpole and Slater, 2008), because it allows for individuals to signal that a particular area or resource has an owner and may reduce future physical interactions between individuals. Physical interactions are energetically expensive and therefore need to be prevented (Hall, 2004; Fedy and Stutchbury, 2005). Territorial behavior varies temporally (Tinbergen, 1936; Nice, 1941; Stamps, 1994; Giuggioli et al., 2011); whereas some species defend territories or resources for short periods (Nice, 1941; Osborne and Bourne, 1977; Woltmann and Sherry, 2011), other species defend territories and resources year-round (Wikelski et al., 2000; Fedy and Stutchbury, 2005). Territorial behavior intensity may also change over time (Bodmer, 1990; Wikelski et al., 2000; Fedy and Stutchbury, 2005; Sosa-López et al., 2017) due to changes in the abundance of resources, breeding stage (i.e., non-reproductive vs. reproductive periods), hormone levels, and pair bond strength (Skutch, 1969; Møller, 1990; Wikelski et al., 2000; Fedy and Stutchbury, 2005). For example, territorial defense is negatively correlated with food abundance since a display of strong territorial behavior when resources are abundant will produce an unnecessary energy expenditure (Carpenter et al., 1983; Burker and Nol, 1998; Duca et al., 2006). Territorial behavior may intensify for small periods of time when individuals benefit from strong territorial display, as during the pre-breeding season when individuals fight over territorial limits (Gill et al., 2007; Sosa-López et al., 2017) or during the breeding season where individuals guard their mates to avoid extra-pair copulations or pair usurpation (Møller, 1990; MacDougall-Shackleton and Robertson, 1995; Juárez et al., 2020).
To avoid unnecessary energy expenditures in territorial defense, many species (e.g., insects, amphibians, birds, and mammals) have developed the capability to distinguish between different territory intruder types using characteristics of territorial signals (e.g., vocal or visual signals) or through familiarity with the neighbors (Fisher, 1954; Temeles, 1994; Lovell and Lein, 2004). In birds, where vocal communication is primarily used for territory defense (Stamps, 1994; Giuggioli et al., 2011), it is expected that those species might use distinctive characteristics in frequency, duration, intensity, or complexity of acoustic signals to differentiate between intruders and respond to the threat of each intruder accordingly (Fisher, 1954; Temeles, 1994; Lovell and Lein, 2004). This has been found for Meadow Pipits (Anthus pratensis), where intruders are recognized using the characteristics of the introductory motif of the song, which varies in duration between individuals (Elfström, 1990). Additionally, Field Sparrow (Spizella pusilla) territorial males can distinguish between intruders based on the frequency characteristics of the songs (Nelson, 1989). Finally, Sylvia warblers also recognize threats for territory usurpation (from conspecific males) using the frequency and duration of the songs (Matyjasiak, 2005).
Territorial individuals may also use the familiarity (daily interactions and habituation) with the intruder to respond according to their identity; because territorial individuals will have daily interactions with neighboring individuals but not with non-neighbors. For example, territorial birds share boundaries with other individuals and reduce the intensity and aggressiveness of the response to neighbors to reduce energetic expenditures in a phenomenon known as the "dear enemy effect" (Fisher, 1954; Lovell and Lein, 2004). In this case intruder recognition may be based on the familiarity with the location of neighboring individual and not with the acoustic characteristics of the signal (Falls and Brooks, 1975; Stoddard et al., 1991). This has been demonstrated in playback experiments with White-throated Sparrows (Zonotrichia albicollis) and Song Sparrows (Melospiza melodia), where the neighbor songs were reproduced from the opposite side of the natural territory boundaries, and responses toward songs reproduced on the opposite side of territory boundaries were of higher intensity and aggressiveness compared to responses towards songs reproduced from the existing side of the territory boundaries (Falls and Brooks, 1975; Stoddard et al., 1991).
Examining how mechanisms of intruder recognition interact is key to our understanding of territorial establishment and defense. In this study our main objective was to analyze whether a year-round territorial bird species from the Neotropics discriminates between intruders using duet characteristics (i.e., duets' frequency and duration) or familiarity with potential intruders (i.e., neighbors and non-neighbors). We studied White-eared Ground-sparrows (Melozone leucotis), because this sparrow uses duets exclusively for territory defenses year-round (Sandoval et al., 2016, 2018; Juárez et al., 2020). Recognizing intruders using duet characteristics or familiarity will be advantageous for territory defense (primary functions of duets), especially in year-round territorial species, and allow pairs to avoid unnecessary energy expenditure against less threatening intruders (Fisher, 1954; Lovell and Lein, 2004). We tested two hypotheses on the use of duets in this species to determine the mechanisms used for intruder recognition. First, White-eared Ground-sparrow territorial pairs assess and distinguish the threat of intruders based on familiarity (i.e., neighbors vs. non-neighbors). We predict a less aggressive response by pairs towards the duets of neighboring pairs compared to non-neighboring pairs. In this scenario neighbors have already established their territories and through previous interactions with neighbors, both pairs recognize each other even when their duets are structurally different. The pairs will exhibit a reduced response towards their neighbors' duets and will save energy by avoiding unnecessary territorial disputes, as predicted for the dear enemy effect (Fisher, 1954; Lovell and Lein, 2004). If familiarity is not used to recognize neighbors in territory defense, we consequently predict the same level of aggressiveness towards neighbor and non-neighbor duets independently of duet characteristics. Second, White-eared Ground-sparrow territorial pairs assess and distinguish the threat of intruders based on duet characteristics (e.g., duration and frequency). We predict a more aggressive response towards duets that have more similar duration and frequency characteristics with duets of the territorial pair; these responses will occur independently from neighbors or non-neighbor status, as has been shown for Medium Ground-finches (Geospiza fortis), where territorial males respond stronger to songs from their own population (Podos, 2007). If duet characteristics are not used to recognize intruders, however, we predict the same level of aggression towards pairs with different degrees of duet distinctiveness.
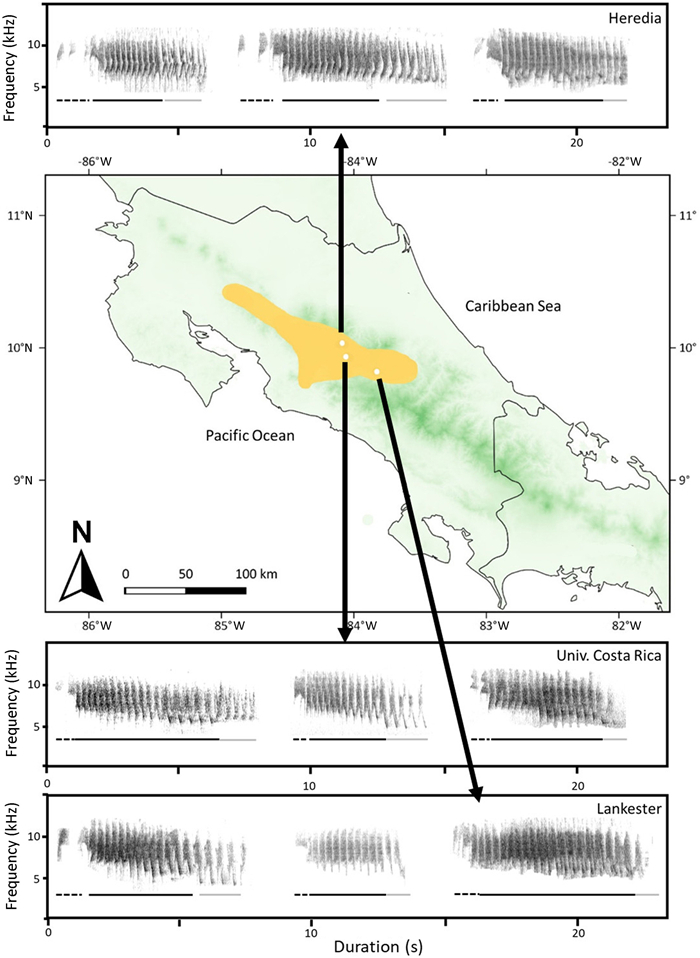
Contrary to the majority of species that produce duets using male and female solo songs (Wiley and Wiley, 1977; Molles and Vehrencamp, 2001), White-eared Ground-sparrows produce duets with a vocalization that is spectrotemporally different from male solo songs and used only for duetting (Sandoval et al., 2016). This probably originates from the fact that females do not sing solo songs in this species (Sandoval et al., 2016). Consequently, females contribute to territory and pair defense by duetting (Sandoval et al., 2018; Méndez and Sandoval, 2021). Both sexes initiate duets with equal frequency and produce polyphonal duets (duets that overlap in time and frequency; Fig. 1; Sandoval et al., 2013, 2016). Duets in this species have three sections: the introduction, started by one individual (male or female) which is composed of one to six elements; the middle part, that is composed of noisy broadband elements produced by the two individuals (the second individual starts to vocalize in this section); and the final part, produced by the second individual with distinctive elements that have a lower maximum frequency compared to the middle part (Fig. 1; Sandoval et al., 2016; Méndez and Sandoval, 2021). Previous research with White-eared Ground-sparrow has demonstrated that duets vary in frequency and duration characteristics among pairs by 11% based on discriminant function analysis (N = 36; Sandoval et al., 2016).
We conducted playback experiments in three populations of White-eared Ground-sparrows in the Central Valley, Costa Rica during the breeding season, from April 30th to June 12th, 2018. Populations were located at: (1) Universidad de Costa Rica campus, San José province (09°56ʹ N, 84°05ʹ W, 1200 m), (2) Getsemaní, Heredia province (10°01ʹ N, 84°05ʹ W, 1350 m), and (3) Jardín Botánico Lankester, Cartago province (9°50ʹ N, 83°53ʹ W, 1370 m). The White-eared Ground-sparrow populations studied in this playback experiment are part of a long-term study with color-banded individuals (Sandoval et al., 2013, 2016), allowing us to ensure that the same pairs were not resampled on different days. From March to June 2018, each pair was recorded for one to two days using a solid-state recorder (Marantz PMD661, sampling rate of 44.1 kHz, accuracy of 24 bit, and file format WAVE) and a shotgun microphone (Sennheiser ME66/K6) using the focal recording method, when this species is most vocally active between 04:55 and 06:00 h (Sandoval et al., 2016). We collected two to 15 duets from each pair. We quantified the spectrotemporal characteristics (minimum and maximum frequency, frequency of maximum amplitude, frequency range in Hz, duration in seconds) of duets using Raven Pro 1.6 (Cornell Laboratory of Ornithology, NY, USA), with a frequency resolution of 188 Hz and a temporal resolution of 5.8 ms in a Hann window of 256 kHz sampling and 50% overlap. We obtained the measurements values using a visual assessment of the spectrogram, wave and power spectrum windows following the technique suggested for other authors previously (Podos, 2001). We used the spectrogram window to identify duets, the wave spectrum to measure time and power spectrum to measure frequencies.
We used complete White-eared Ground-sparrow duets (Sandoval et al., 2016) recorded in March and April 2018 with the same equipment and focal recording method described above to create our playbacks. All duets used for playback stimuli came from different pairs, and all duets used for playback had a high signal-to-noise ratio and no overlap with other sounds. In several instances we had two or more duets from a given pair. For these cases, we assigned each duet a number from 1 to n, and then randomly selected a duet for a playback using the random number generator in Excel. We filtered all sounds below 5 kHz and above 11.5 kHz using the FFT filter in Adobe Audition 1.0 (Adobe System Incorporated, CA, USA). We standardized amplitude by normalizing duets to −1 dB with the same software. The filtered and normalized duets were repeated four times per minute following the rate used in previous territory studies for this species (Sandoval et al., 2013; Méndez and Sandoval, 2021). We created four stimuli: (1) neighbor duet: a duet from a pair that shares a border with the pair receiving the stimulus, (2) non-neighbor duet from the same population: a duet from a pair at least two territories apart with the pair receiving the stimulus, (3) non-neighbor duet from another population (minimum straight line distance between population was 13 km), and (4) control duet from Cabanis's Wren (Cantorchilus modestus). We used Cabani's wren for the control duet because they shars territorial areas with White-eared Ground-sparrows but have different diets and ecological requirements from White-eared Ground-sparrows. Each stimulus consisted of 2 min of playback followed by 5 min of silence. The four stimuli were presented in a consecutive sequence for a total of 28 min of experiment per pair, following previous playback experiment designs (e.g., Bolton, 2007; Geberzahn et al., 2009; Ripmeester et al., 2010; Sandoval et al., 2013; Sosa-López et al., 2016). The order of playback stimulus presentation to each territorial pair varied systematically. We presented each stimulus in each position (i.e., first, second, third, and fourth position) the same number of times within a population. The majority of pairs (71%) that approached the speaker returned to their original activities before the 2 min of stimulus ended, and no birds were observed within 3 m of the speaker at the end of the 5 min of silence. As in previous playback experiments for this ground-sparrow species (Sandoval et al., 2013, 2018), we broadcasted playbacks using an active loudspeaker (Anchor Audio Minivox, frequency response: 100–15, 000 Hz) and a portable audio player (iPod nano, Apple, CA, USA). To avoid any effect of differences in stimulus volume, we played back all stimulus at a constant volume of 80 dB SPL measured at 1 m distance to the loudspeakers using a digital sound level meter (Sper Scientific Digital Mini Sound Meter NIB – 850014), with weighting A and a fast response setup, as has been conducted in other playback experiments.
The experiments were conducted on 39 pairs in total: 16 from the University of Costa Rica campus, 14 from Getsemaní, and 9 from Lankester Botanical Garden. We mounted the loudspeaker 1.5 m high on a pole 5 m inside of the boundary shared by pairs for each experimental playback session. Of the 39 pairs examined, 35 shared borders with only one neighbor, while the other four pairs shared borders with two neighbors on opposites side of the territory. The approach used in this study followed the approach and distances reported from other experiments that tested intruder responses for other bird species (Wiley and Wiley, 1977; Temeles, 1994; Lovell and Lein, 2004; Dingle and Salbbekoorn, 2018). Given that territories are not temporally static (Hixon et al., 1983; Møller, 1990; Butchart et al., 1999), we located the speaker inside the territorial pair to ensure that the stimulus was perceived as a territorial intrusion (Wiley and Wiley, 1977; Lovell and Lein, 2004). Additionally, with this approach we were confident that the pair could hear the stimulus from this position because the territory average of the White-eared Ground-sparrows is 0.17 ha (SE ± 0.03), which is approximately 42 × 42 m in length (Juárez et al., 2020). Additionally, our single speaker experiment followed the design of previous playback experiments for this species (Sandoval et al., 2013, 2018) that showed aggressive responses of territorial pairs to duet stimuli, with both members of each pair producing duets near each other. Orange flagging tape (length: 10 cm) was positioned 3 m on both sides of the loudspeaker. These markings served as a spatial reference for behavioral responses during the trials and allowed for more accurate measurements of the time that took it to approach the speaker and total time spent near the speaker. This is especially important given how dense the vegetation (thicket habitats and coffee plantations; Sandoval and Mennill, 2012) is that this species inhabits. The observer was located 8 m away from the speaker, motionless, to reduce the observer effect on the ground-sparrow responses. Experiments were conducted between 06:00 and 09:30 h. Of the 7 min conducted for each playback stimulus, the first 2 min were of playback; the following 3 min of silence plus the first 2 min were our observation period, where we measured the following five behavioral responses: (1) latency from the start of the playback to the subjects' first vocalization (in seconds); a value of 301 s was assigned for no vocalizations; (2) latency of approaching to within 3 m of the speaker, referred to as the marked area (in seconds), a value of 301 s was assigned for no approach; (3) duration within the marked area (in seconds), a value of 0 s was assigned for no presence at all; (4) total number of individuals approaching inside the marked area (vary from 0 to 2 individuals); and (5) total number of vocalizations. It was possible to make measurements 1 to 4 directly in the field using a timer and counting the number of individuals that approached. The number of vocalizations was the total number of vocalizations made during the first 5 min of the stimuli. As in previous playback studies (Koloff and Mennill, 2011; Sandoval et al., 2013, 2018; Wu et al., 2021), we used the protocol of replacement 'no responses' with the maximum time possible value plus 1 unit (301 s), this is a solution to include 'no response' as part of our analysis and have a more robust sample size. As the last 2 min were considered recovery time, we stopped recording the behavior of the pair, but in all cases, both pair members returned during this time or before to conduct their prior behavior and abandoned the 3 m area around the speaker.
To analyze if intruder recognition occurs using duets characteristics (i.e., frequency and duration) or familiarity with potential intruders (i.e., neighbors and non-neighbors), we combined four of the five behavioral responses into multivariate responses through a principal component analysis of the correlation matrix without rotation (KMO test: latency of approach = 0.70 s; latency of first vocalization = 0.81 s; duration within the marked area = 0.72 s; number of individuals = 0.83; Bartlett's Test of Sphericity: χ2 = 265.63, df = 6, p < 0.001). This first principal component had an eigenvalue of 2.78 and explained 69.42% of the total variance in the original variables (Appendix Table S1, S2). We called this multivariate response "aggressive response" (here after) because the first principal coordinate had a negative relationship with the latency of approach (r = −0.55; meaning faster approximation to the speakers) and latency of first vocalization (r = −0.37; meaning that the vocalizations were initiated faster), and a positive relationship with the duration within the marked area (r = 0.54; meaning longer durations closer to the speaker) and the number of individuals that approached the speaker (r = 0.52; meaning more individuals approaching the speaker). Conversely, the number of vocalizations was analyzed independently, because the Kaiser-Meyer-Olkin-Criterium (KMO) had a value of 0.38, indicating that the data was not useful for inclusion in a factor analysis like principal component analysis.
Before conducting any statistical analyses, we tested whether the presentation order of stimuli produced differences in pair responses. If the successive stimuli presentation has an effect on the intensity of response, we expected to find more aggressive responses in each stimulus compared to the previous one, independently of the stimulus type used in each position. This will occur when the time to recover between stimuli is too short, resulting in pairs responding faster and quicker to each successive stimulus. Therefore, we analyzed pair responses (PC1 and number of vocalizations) with two general linear mixed-effect models (GLMM) with a negative binomial distribution and a logit link function. We used a negative binomial distribution because it is appropriate for skewed and zero-bounded variables (Mun, 2008). We included stimuli order as our independent variable and pair identity as a random effect. Because these analyses showed no effect of stimulus order (PC1: t95 = −0.14, p = 0.89 and number of vocalizations: t95 = 0.77, p = 0.44), we did not included order as a variable in our statistical analysis. To analyze if intruder recognition is based on neighbor familiarity we conducted two models (GLMM with negative binomial distribution and a logit link function) to analyze the effect of duet stimulus (independent variable with four levels: neighbor, non-neighbor, non-neighbor from other population, and control) in the aggressive response of territorial pairs (PC1 and number of vocalizations as dependent variables), and pair identity as a fixed-effect factor to control for the effect that each pair received four stimuli.
The first step to determine whether duet similarity is used to recognize intruders per territorial pair is to analyze how duet similarity varies between populations. For this, we analyzed the effect of distance on duet similarity using a Mantel test with 10, 000 permutations. For this test we used Euclidian distance in both matrices, the first matrix included the geographic distances between the center of the pairs' territories and the second matrix included duet similarity using duration and frequency values. We also conducted a MANOVA to compare duet similarity between populations. The purpose of this analysis was to provide another measurement of duet similarity, in case duet similarity is not influenced by geographic distance. Following these two analyses we conducted another two GLMMs with negative binomial distribution and a logit link function using duet similarity estimated under the Euclidian distance of the Mantel test as our independent continuous variable. We used PC1 and number of vocalizations as dependent variables in each GLMM, and pair identity as a fixed-effect factor. Principal component analysis was conducted with the program JMP (version 7.0; SAS Institute, Cary, NC, USA). Bartlett's test of sphericity and KMO tests were conducted in R (R Core Team, 2021) with the package "psych" (Revelle, 2021). GLMMs were conducted in R with the package "MASS" (Venables and Ripley, 2002) and "nlme" (Pinheiro et al., 2021). Mantel test and MANOVA were conducted in PAST 4.05.
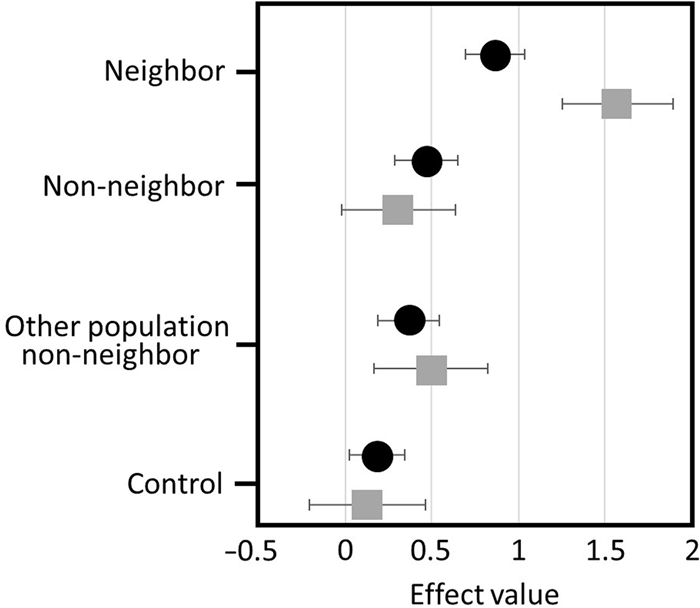
Our results were based on 32 pairs. Seven pairs (2 out of 13 at Getsemaní and 5 out of 16 at the University of Costa Rica campus) did not respond to any of our stimuli. When we analyzed familiarity in intruder recognition, we found differences in the intensity of the aggressive response (PC1) and number of vocalizations that White-eared Ground-sparrows produced according to the type of stimulus received. White-eared Ground-sparrows showed the strongest aggression towards neighbor simulated duets (t93 = 5.17, p < 0.001) with an effect of 0.87 (SD ± 0.17) higher than control stimulus (Fig. 2). The level of aggression response for non-neighbors (t93 = 2.61, p = 0.01) and non-neighbors from other population duets (t93 = 2.02, p = 0.04) was lower than responses to neighbors, but higher than response towards control stimuli, for an effect of 0.47 (SD ± 0.18) and 0.37 (SD ± 0.18) respectively (Fig. 2). Overall, the aggression of responses for control duets (t93 = 1.27, p = 0.21; effect of 0.19, SD ± 0.16; Fig. 2) was very low. When compared across treatments, territorial pairs only produced more vocalizations in response to the neighbor stimulus (t93 = 4.88, p < 0.001), with an effect of 1.57 (SD ± 0.32), which was higher than the response to the control stimuli (Fig. 2). The number of vocalizations in response to non-neighbor (t93 = 0.96, p = 0.34), non-neighbors from other population (t93 = 1.51, p = 0.14), and control duets (t93 = 0.39, p = 0.70) were very low and comparable in effect values (0.31 ± 0.33, 0.50 ± 0.33, and 0.13 ± 0.33; Fig. 2).
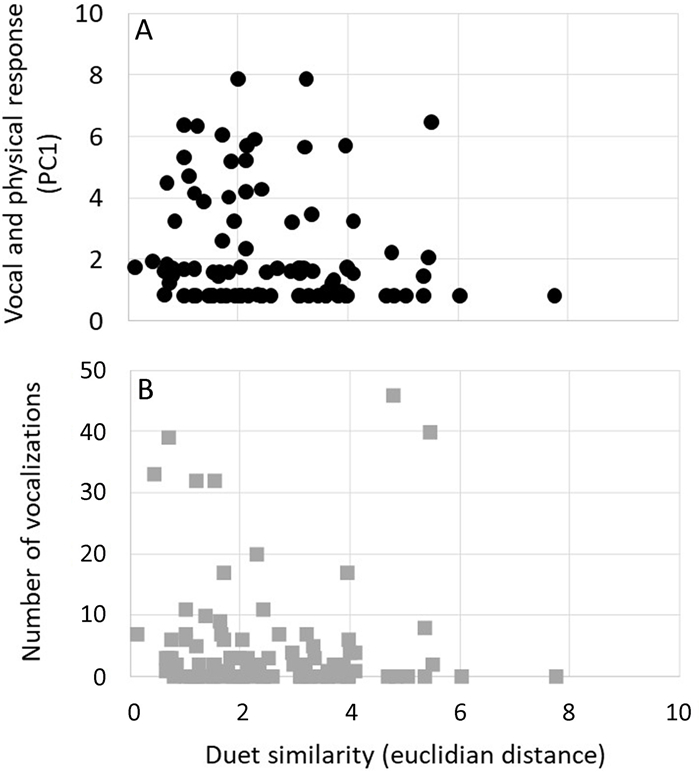
Duet similarity is not related to distance (Mantel: r = 0.10, p = 0.10), but duets were more similar within than between populations (MANOVA: F10, 96 = 2.11, p = 0.04). We found no relationship between the intensity of the aggressive response (PC1) and duet similarity (t93 = −1.76, p = 0.08) with a lower effect of −0.10 (SD ± 0.06; Fig. 3). The number of vocalizations, however, increased in response to more similar duets (t93 = −2.76, p = 0.008; with an effect value of −0.31 (SD ± 0.11; Fig. 3).
Our results showed that White-eared Ground-sparrows used familiarity for intruder recognition rather than acoustic characteristics of duets (i.e., frequency and duration). The level of aggression towards neighbors, however, was the highest compared to aggression towards non-neighbors (from the same or another population), contrary to the predictions of the dear enemy effect hypothesis (Fisher, 1954; Temeles, 1994). According to this hypothesis, a more aggressive response to non-neighbors is expected, followed by neighbors, and finally the control, to reduce energy expenditure towards familiar neighbors, which have defined territory borders.
The dear enemy effect has been demonstrated in Stripe-backed Wrens (Campylorhynchus nuchalis), another year-round territorial species that uses duets for territory defense. Stripe-backed Wrens exhibit more aggressive behaviors against non-neighbor duets than neighbor duets (Wiley and Wiley, 1977). In this case, the authors justified the aggressive response against non-neighbors as a behavior to defend the territory from possible intruders (Wiley and Wiley, 1977). We found the opposite pattern in White-eared Ground-sparrows. The reason for this observation may occur because neighbors are a higher threat than non-neighbors in terms of territorial theft or mate usurpation (Ferkin, 1988; Temeles, 1989, 1990; Sandoval et al., 2018). This type of response also occurs for Meadow Voles (Microtus pennsylvanicus), where the probability of encountering a non-neighbor male in their own territory is lower in comparison to encountering a neighbor. Therefore, males demonstrate more aggressive behavior towards neighbors than non-neighbors (Ferkin, 1988). A similar phenomenon may occur with White-eared Ground-sparrows that share territory borders with several pairs over-years (Juárez et al., 2020), however, the majority of White-eared Ground-sparrows observed in this study share territorial borders with only a single pair during each breeding season (pers. obs.). Therefore, the probability of encountering a non-neighbor pair inside the territory is relatively low, and fighting may be costly, especially if they are only passing through (Ferkin, 1988). Additionally, if a new pair tries to create a territory in an established neighborhood, this new pair will not only be a threat to a single pair, but to all pairs that share territory borders within the target area.
White-eared Ground-sparrow pairs were able to discriminate between neighbor and non-neighbor duets from the same population with low pair distinctiveness (Sandoval et al., 2016), but unable to distinguish between different non-neighbors. This result suggests that White-eared Ground-sparrow use familiarity instead of the duet acoustic characteristics we measured in this study to discriminate potential competitors from non-competitors (Falls and Brooks, 1975; Stoddard et al., 1991). We cannot rule out, however, that White-eared Ground-sparrow territorial pairs are using other characteristics not analyzed in this study (e.g., duet energy entropy or duet coordination) to recognize non-neighbors from their own and other populations. This phenomenon is not rare among bird species (e.g., medium ground-finches, Moltoni's warbler: Sylvia moltonii, or Hypocnemis antbird) where individuals are capable of recognizing their own population or specific individuals during playbacks, despite the inability to separate songs based on song measurements (Tobias and Seddon, 2003; Podos, 2007; Brambilla et al., 2008).
How common the observations reported in this study are among long-lived tropical bird species with year-round territories (Skutch, 1969; Stutchbury and Morton, 2001; Fedy and Stutchbury, 2005; Battiston et al., 2015) is poorly known, especially for duetting species. Given, that acoustic characteristics of duets are also used to explain other behavioral hypotheses aside from the dear-enemy effect (Hall, 2004, 2009; Dahlin and Benedict, 2014), including this variable in future experiments will help to understand the relationship between function and structure for duets. This approach will provide more support to the growing body of evidence on the multiple functions of duets for tropical bird species (Hall, 2004, 2009; Dahlin and Benedict, 2014). Territories in the tropical habitats are not only defended by males and pairs, but they are also defended by family or non-family groups, females alone, during the breeding, or outside of the breeding season (Skutch, 1969; Stutchbury and Morton, 2001; Fedy and Stutchbury, 2005). Therefore, to develop a better understanding of the territorial behavior related to the mechanism of intruder recognition, a broad understanding of the species interactions during territorial defense is necessary.
White-eared Ground-sparrows used familiarity with intruders as the main mechanism for the recognition of threats. Contrary to the dear enemy effect hypothesis, territorial ground-sparrow pairs produced more aggressive responses against neighbors than non-neighbors. This behavior may be the result of the original habitat inhabited by this species, young successional vegetation in forest gaps, landslides, and rivers edges (Sandoval and Mennill, 2012), or because neighbor pairs may pose a risk for pair usurpation or extra-pair copulation (Sandoval et al., 2018). The isolation of habitats and reduced area (Schemske and Brokaw, 1981) for territory establishment make non-neighbors rare over-years. The characteristics of these habitats may increase fights for territory boundaries between neighbor's over-time to secure more area and access to resources, making neighboring pairs more threatening than non-neighboring pairs. The availability of White-eared Ground-sparrow habitat has increased over the last 500 years (approximately) following increased human settlements inside this species' distribution. These developments have likely increased the pairs' abundances and the probability of encountering non-neighbor pairs, yet territorial pairs still recognize neighbors as more dangerous for territory usurpation. On the other hand, the risk of losing a mate or reproduction opportunities with neighbor pair members, suggests that duets are also used by White-eared Ground-sparrows for mate guarding and pair-bonding, and not just for territorial defense.
TH and LS designed the experiment. TH, KB, IG, and LS conducted the data collection. BG and LS analyzed data. TH, BG, and LS wrote the manuscript. All authors read and approved the final manuscript.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Our study was approval by the Investigation Commission of Escuela de Biología, Universidad de Costa Rica, according to the Act on Experiments on Animals (Disposition No. 289 from 2005). Our playback experiments were kept brief to minimize disturbance and impact of the tested subjects.
The authors declare that they have no competing interests.
We are thankful for the Universidad de Costa Rica and Jardín Botánico Lankester for allowing us to conduct the field work on their properties. LS is also thankful for the partial time support of the Escuela de Biología. We are thankful to the two anonymous reviewers and the editor for all the constructive comments in a previous draft of this manuscript, which help to improve it. Financial support was provided by the Vicerrectoría de Investigación Universidad de Costa Rica through the project numbers B9123, B9469, and C1085.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100032.
|
Bradbury, J.W., Vehrencamp, S.L., 1998. Principles of Animal Communication. Sinauer Associates, Sunderland
|
|
Bolton, M., 2007. Playback experiments indicate absence of vocal recognition among temporally and geographically separated populations of Madeiran storm-petrels Oceanodroma castro. Ibis 149, 255-263
|
|
Catchpole, C.K., Slater, P.J.B., 2008. Bird Song Biological Themes and Variations. Cambridge University Press, Cambridge
|
|
Fisher, J., 1954. Evolution and bird sociality. In: Huxley, J., Hardy, A.C., Ford, E.B. (Eds.), Evolution as a Process. Allen & Unwin, London, pp. 71-83
|
|
MacDougall-Shackleton, E.A., Robertson, R.J., 1995. Mate guarding tactics used by great crested flycatchers. Wilson Bull. 107, 757-761
|
|
Matyjasiak, P., 1995. Birds associate species-specific acoustic and visual cues: recognition of heterospecific rivals by blackcaps. Behav. Ecol. 16, 467-471
|
|
Mun, J., 2008. Understanding and choosing the right probability distributions. In: Mun, J. (Ed.), Advanced Analytical Models: Over 800 Models and 300 Applications from the Basel II Accord to Wall Street and Beyond. John Wiley, New Jersey, pp. 899-917
|
|
Sandoval, L., Mennill, D.J., 2012. Breeding biology of White-eared Ground-Sparrows (Melozone leucotis), with a description of a new nest type. Ornit. Neotrop. 23, 225-234
|
|
Skutch, A.F., 1969. Life Histories of Central American Birds III. Pacific Coast Avifauna-Cooper Ornithological Society, Berkeley
|
|
Stutchbury, B.J.M., Morton, E.S., 2001. Behavioral Ecology of Tropical Birds. Academic Press; San Diego
|
|
Temeles, E.J., 1990. Northern harriers on feeding territories respond more aggressively to neighbors than to floaters. Behav. Ecol. Sociobiol. 26, 57-63
|
|
Tobias, J.A., Seddon, N., 2003. Signal design and perception in Hypocnemis antbirds: evidence for convergent evolution via social selection. Evolution 63, 3168-3189
|
|
Venables, W.N., Ripley, B.D., 2002. Modern Applied Statistics with S. Springer, New York
|