| Citation: | George Sangster, Edward L. Braun, Ulf S. Johansson, Rebecca T. Kimball, Gerald Mayr, Alexander Suh. 2022: Phylogenetic definitions for 25 higher-level clade names of birds. Avian Research, 13(1): 100027. DOI: 10.1016/j.avrs.2022.100027 |
Knowledge of the higher-level phylogenetic relationships of birds has grown substantially during the past two decades due to the application of genomic data. However, the nomenclature of higher-level taxa has not become more stable, due to the lack of regulation of taxon names above the level of superfamily by the ICZN, and the usage of rank-based nomenclature, which is not tied to clades in a phylogeny. Lack of regulation and the instability of rank-based nomenclature impede effective communication among systematists. We review support for higher-level avian clades using a set of 10 phylogenomic data sets, and identify clades that are supported by congruency of at least four of these. We provide formal definitions of the names of these clades based on the rules of the recently published PhyloCode. The names of 25 clades are here defined using minimum-crown-clade (n = 23), minimum-clade (n = 1) and maximum-crown-clade (n = 1) definitions. Five new names are introduced here: Dinocrypturi, Pteroclimesites, Musophagotides, Phaethoquornithes and Pelecanes. We also review diagnostic apomorphies of the relevant clades, and identify known synonyms and homonyms. By establishing a formal link between higher-level taxon names and well-supported phylogenetic hypotheses, our phylogenetic definitions will provide a solid basis for the stabilization of avian higher-level nomenclature.
Since the publication of the first comprehensive multi-locus phylogeny of birds in 2006 (Ericson et al., 2006), avian phylogenomic studies have clarified many aspects of the avian tree of life. Many higher-level relationships are now congruently supported by multiple phylogenies (Fig. 1). In contrast, the nomenclature of higher-level taxa has not become more stable. There are two main reasons for this. First, the names of higher-level taxa (i.e. above the level of superfamily) are not regulated by the International Code of Zoological Nomenclature (ICZN, 1999) and this is reflected by how such names are sometimes being introduced and used. Some names have been introduced informally without any indication of how the names should be applied in a slightly different phylogeny (e.g. 'Conglomerati', Slack et al., 2007; 'Coronaves', 'Metaves', Fain and Houde, 2004).
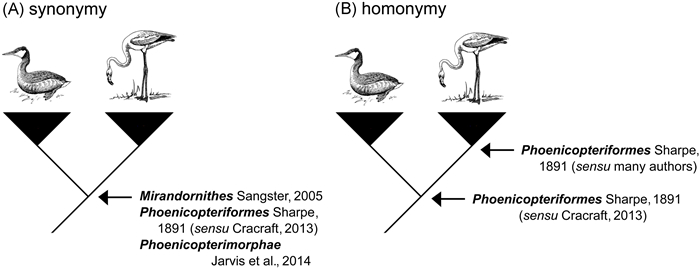
A second reason why instability may occur even if there is agreement about phylogenetic relationships is the use of taxonomic ranks. If a taxonomist intends to name a higher taxon using rank-based nomenclature, he/she must designate a single type taxon and provide a statement about the rank of the taxon. Rank-based names are not connected to a specified clade. Future workers are allowed to emend the content of taxa depending on their views about the appropriate rank of the relevant taxa. Thus, the inclusiveness of a taxon, and hence the meaning of the taxon name, is potentially unstable, even in situations where phylogenetic relationships are undisputed. This is illustrated in Fig. 2. The sister relationship of flamingos and grebes was first documented in 2001 (van Tuinen et al., 2001) and has been corroborated overwhelmingly by subsequent phylogenomic studies (e.g. Hackett et al., 2008; McCormack et al., 2013; Yuri et al., 2013; Jarvis et al., 2014; Prum et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021). The flamingo-grebe clade was formally named Mirandornithes in 2005 (Sangster, 2005), but within a couple of years its nomenclature had become unstable due to the introduction and re-use of other names (Fig. 2). As a consequence of this, and the lack of restrictions to the inclusiveness of a taxon, taxon names are more closely associated with ranks than with actual taxa.
The lack of restrictions to the inclusiveness of a taxon often leads to situations where different authors use the same name for different taxa (homonymy) and use different names for the same taxon (synonymy, Fig. 2). Homonymy is a problem because information associated with a name refers to different taxa. One has to know to which taxon the name is, or has been, applied. Failure to recognize homonymy may lead to misinterpretation of biological information. Synonymy is also a problem because a search for information about a taxon means that one has to know all names of a taxon and must repeat the search effort for all of these synonyms. Failure to recognize all synonyms of a taxon may result in relevant literature being overlooked. Synonymy and homonymy pose serious problems for information retrieval and communication in biology. These problems underscore that the rules of rank-based nomenclature do not promote explicitness, universality nor stability with regard to the phylogenetic meanings of taxon names (De Queiroz and Gauthier, 1994).
An alternative system that aims to avoid the problems of rank-based nomenclature has been developed by De Queiroz and Gauthier, 1990, De Queiroz and Gauthier, 1992. This system is now known as 'phylogenetic nomenclature'. Phylogenetic nomenclature has the same basic goals as rank-based nomenclature: to provide unambiguous methods for (i) applying names to taxa, (ii) selecting a single accepted name for a taxon (from multiple homonyms or synonyms), and (iii) promoting nomenclatural stability and continuity, as long as this does not contradict new systematic conclusions (De Queiroz, 2005). This system differs from rank-based nomenclature in several important ways. First and foremost, phylogenetic nomenclature is rankless. As a consequence, taxonomic names do not depend on, nor vary with, their phylogenetic position. As there are no mandatory ranks, each clade only has one valid name.
Second, taxonomic names are explicitly defined in terms of ancestry and descent. Each name is defined using at least two reference points on a cladogram (two taxa, or a taxon and an apomorphy). This means that the limits of the taxon to which the name refers are fixed. The contents of the clade to which the name refers are determined empirically, and therefore one needs a reference phylogeny to determine the meaning of the name. Each name explicitly refers to a clade; therefore, all taxa are monophyletic, and only the contents of the clade are subject to change.
The rules of phylogenetic nomenclature are codified in the PhyloCode, of which the printed version was officially published in spring 2020 (Cantino and De Queiroz, 2020). The starting date of the PhyloCode coincides with the publication of 'PhyloNyms' (De Queiroz et al., 2020), a major volume that provides phylogenetic definitions for many widely used clade names. Names defined in PhyloNyms or in subsequent publications are available under the PhyloCode. PhyloNyms includes definitions of eleven clade names of birds, including four higher-level clades (Aves, Galloanserae, Mirandornithes and Daedalornithes). Several others have been published subsequently (Chen and Field, 2020; Sangster and Mayr, 2021). Names proposed before the starting date of the PhyloCode (e.g. Gauthier and De Queiroz, 2001) are unavailable and remain subject to instability until these are formally defined.
In this paper, we define 25 names of higher-level clades of birds that are well-corroborated by comprehensive phylogenomic studies using the rules and recommendations of the PhyloCode. The scope of this paper is restricted to 'supra-ordinal' names because these are almost all supported by the same set of studies.
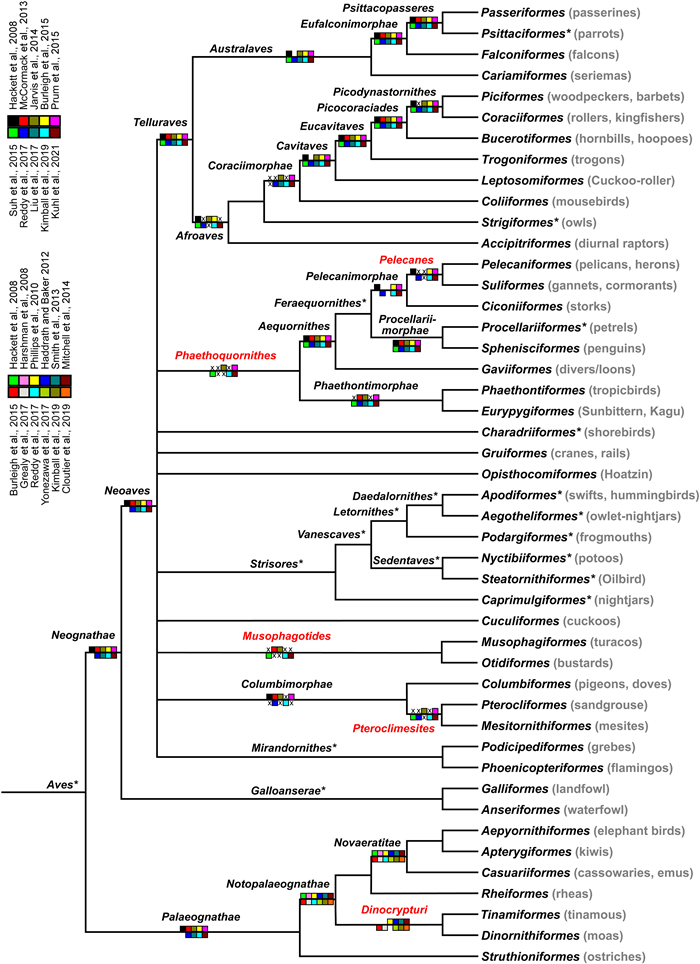
Genomic support for phylogenetic relationships among major clades of birds was evaluated using a set of 10 phylogenomic studies published between 2008 and 2021 (Table 1). For palaeognath clades, we also consulted a set of eight other works (i.e. Harshman et al., 2008; Phillips et al., 2010; Haddrath and Baker, 2012; Smith et al., 2013; Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017; Cloutier et al., 2019; see Table 2). Relationships congruently supported by a minimum of four phylogenomic studies were used to construct a consensus phylogeny. In addition to a full consideration of these studies we also assessed relationships that were not as strongly corroborated with other studies that largely analysed published data and/or did not present a preferred cladogram (Kimball et al., 2013; Gilbert et al., 2018; Houde et al., 2019; Braun and Kimball, 2021). For these studies, we focused on what we felt were the figures that provided the most robust and distinct information (i.e., Figures 1 and 4 from Kimball et al., 2013, Figure 3a from Gilbert et al., 2018, Figure 11d from Houde et al., 2019, and Figures 4c and 5 from Braun and Kimball, 2021).
| Source | Data type(s) | Palaeognathae | Neognathae | Neoaves | Columbimorphae | Pteroclimesites | Musophagotides | Phaethoquornithes | Phaethontimorphae |
| Hackett etal. (2008) | 19 nuclear loci | 100% ML bootstrap | 96% ML bootstrap | 96% ML bootstrap | < 70% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered |
| McCormack etal. (2013) | 1541 ultra-conserved elements | > 0.95 PP, > 70% bootstrap | > 0.95 PP, > 70% bootstrap | Not recovered | > 0.95 PP, > 70% bootstrap | Not recovered | 96% ML bootstrap | ||
| Jarvis etal. (2014) | 8251 exon loci, 2516 intron loci, 3769 ultra-conserved elements | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 55% exaML bootstrap | 70% exaML bootstrap | 100% exaML bootstrap |
| Burleigh etal. (2015) | 25 nuclear loci, mitochondrial DNA | 95% ML bootstrap | 95% ML bootstrap | 93% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered | Not recovered |
| Prum etal. (2015) | 259 anchored nuclear loci | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | Not recovered | 1.0 PP | 1.0 PP |
| Suh etal. (2015) | 2118 retroposon presence/absence loci | Not recovered | 8 retroposons | 7 retroposons | 5 retroposons | 20 retroposons | |||
| Reddy etal. (2017) | 54 nuclear loci | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | < 70% ML bootstrap | Not recovered | Not recovered | < 70% ML bootstrap |
| Liu etal. (2018) | 63 nuclear protein-coding loci | 100% ML bootstrap | 100% ML bootstrap | 100% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered | 100% ML bootstrap |
| Kimball etal. (2019) | supertree | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 85% MRP bootstrap | 100% MRP bootstrap | 25% MRP bootstrap | 43% MRP bootstrap | 100% MRP bootstrap |
| Kuhl etal. (2021) | 3′UTR sequences (2.5 million analyzable patterns) | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) | Not recovered | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) |
| Source | Aequornithes | Procellariimorphae | Pelecanimorphae | Pelecanes | Telluraves | Afroaves | Coraciimorphae | ||
| Hackett et al.(2008) | 89% ML bootstrap | 98% ML bootstrap | 81% ML bootstrap | 88% ML bootstrap | 98%ML bootstrap | < 70%ML bootstrap | Not recovered | ||
| McCormack et al.(2013) | >0.95 PP, >70% bootstrap | 99% ML bootstrap | >0.95 PP, 66% bootstrap | Not recovered | Not recovered | ||||
| Jarvis et al.(2014) | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | ||||
| Burleigh et al.(2015) | Not recovered | 99% ML bootstrap | 77% ML bootstrap | 73% ML bootstrap | 49% ML bootstrap | 50% ML bootstrap | Not recovered | ||
| Prum et al.(2015) | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | Not recovered | 1.0 PP | ||
| Suh et al.(2015) | 16 retroposons | 212 retroposons | 64 retroposons | 16 retroposons | Not recovered | ||||
| Reddy et al.(2017) | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | < 70% ML bootstrap | ||
| Liu et al.(2018) | 99% ML bootstrap | 80% ML bootstrap | 97% ML bootstrap | Not recovered | < 70% ML bootstrap | ||||
| Kimball et al.(2019) | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 72% MRP bootstrap | ||
| Kuhl et al.(2021) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | ||
| Source | Cavitaves | Eucavitaves | Picocoraciae | Picodynastornithes | Australaves | Eufalconimorphae | Psittacopasserae | ||
| Hackett et al.(2008) | 85% ML bootstrap | 71% ML bootstrap | 98% ML bootstrap | 98% ML bootstrap | 64% ML bootstrap | 73% ML bootstrap | 77% ML bootstrap | ||
| McCormack et al.(2013) | >0.95 PP, >70% bootstrap | >0.95 PP, >70% bootstrap | Not recovered | >0.95 PP, >70% bootstrap | >0.95 PP, >70% bootstrap | ||||
| Jarvis et al.(2014) | 100% exaML bootstrap | 72% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | ||
| Burleigh et al.(2015) | 76% ML bootstrap | 69% ML bootstrap | 89% ML bootstrap | 85% ML bootstrap | 72% ML bootstrap | 59% ML bootstrap | 47% ML bootstrap | ||
| Prum et al.(2015) | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | ||
| Suh et al.(2015) | 124 retroposons | 16 retroposons | 6 retroposons | 5 retroposons | 176 retroposons | 88 retroposons | 16 retroposons | ||
| Reddy et al.(2017) | ≥70% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | ≥95% ML bootstrap | ||
| Liu et al.(2018) | 71% ML bootstrap | 93% ML bootstrap | 95% ML bootstrap | 100% ML bootstrap | < 70% ML bootstrap | 85% ML bootstrap | 92% ML bootstrap | ||
| Kimball et al.(2019) | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | ||
| Kuhl et al.(2021) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | ||
| Source | Data type(s) | Notopalaeognathae | Novaeratitae | Dinocrypturi |
| Hackett etal. (2008) | 19 nuclear loci | 100% ML bootstrap | 96% ML bootstrap | |
| Harshman etal. (2008) | 20 nuclear loci | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP | |
| Phillips etal. (2010) | mitochondrial DNA | 44% ML bootstrap, 0.99 PP | 92% ML bootstrap, 1.0 PP | 99% ML bootstrap, 1.0 PP |
| Haddrath and Baker (2012) | 27 nuclear loci | 1.0 PP | 1.0 PP | 1.0 PP |
| Smith etal. (2013) | 60 nuclear loci, mitochondrial DNA | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Mitchell etal. (2014) | mitochondrial DNA | 63% ML bootstrap, 1.0 PP | 87% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Burleigh etal. (2015) | 25 nuclear loci, mitochondrial DNA | 86% ML bootstrap | 100% ML bootstrap | 44% ML bootstrap |
| Grealy etal. (2017) | 154 nuclear loci, mitochondrial DNA | 80% ML bootstrap, 1.0 PP | 57% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Reddy etal. (2017) | 54 nuclear loci | ≥95% ML bootstrap | ≥95% ML bootstrap | |
| Yonezawa etal. (2017) | Morphology | 92% ML bootstrap, 0.99 PP | 100% ML bootstrap, 0.94 PP | 96% ML bootstrap, 0.99 PP |
| Cloutier etal. (2019) | 20, 850 nuclear loci | 100% ML bootstrap | 100% ML bootstrap | 100% ML bootstrap |
| Kimball etal. (2019) | supertree | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap |
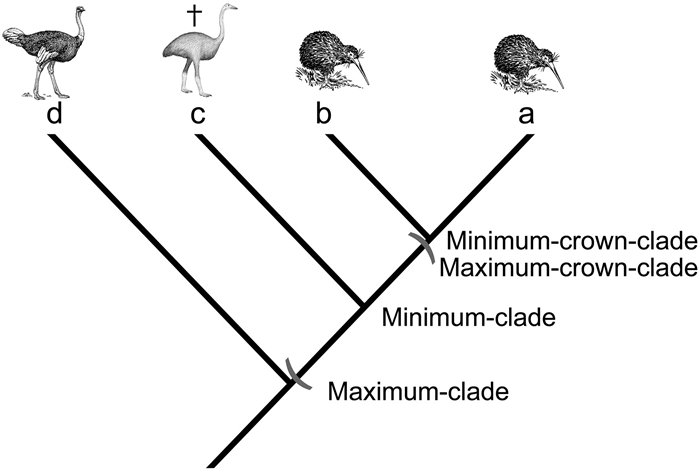
We selected the most appropriate phylogenetic definition based on the state of knowledge about the clade, and our wish to maximize the explicitness of the definition. For instance, if the relationships within a clade are well-known and its sister-taxon is also well-known, we adopted a minimum-crown-clade or minimum-clade definition because this explicitly defines the origin of the clade as that of a known clade. On the other hand, if the relationships within the clade are poorly known but its sister-taxon is well-known (e.g. Neoaves), we selected a maximum-crown-clade definition (Fig. 3).
Reference phylogenies were selected based on their topology and taxonomic completeness. In most cases, we used Prum et al. (2015) and Kuhl et al. (2021).
Diagnostic apomorphies were located in the extensive morphological data set published by Livezey and Zusi (2006, 2007). We list these data 'as is'. Thus, we did not verify these data, and in some cases, we cannot vouch for their accuracy.
The accounts follow the same format as that of Phylonyms, except that we also include a paragraph on homonyms. All names, including five that are newly proposed in this work, are included in RegNum (https://www.phyloregnum.org), the official registry of clade names (Cellinese and Dell, 2020).
A total of 25 clades met our criteria for naming. In one case (the basal relationships among Notopalaeognathae), two topologies were each supported by at least four studies. Although detailed evaluations of the evidence clearly supported one of these over the other (Cloutier et al., 2019, but see Simmons et al., 2022), we have refrained from naming the relevant clade. The position of elephantbirds as the sister of the kiwis was supported by only three studies (Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017), one of which is mitogenomic rather than phylogenomic data, and thus we have left the kiwi–elephantbird clade unnamed. We have also refrained from naming the Strigiformes–Coraciimorphae clade due to uncertainty about the position of Strigiformes (Braun and Kimball, 2021). The topology of the avian consensus tree used in this paper is very similar to that of Suh (2016) and differs only in the position of Coliiformes and the lack of sufficient support for a sister-relationship of Cuculiformes and (Otidiformes + Musophagiformes) (Fig. 1). The consensus tree includes a basal polytomy of ten clades. As pointed out by Suh (2016), there is strong disagreement among phylogenomic studies about the initial divergence of Neoaves and this may well represent a hard polytomy.
Registration number: 695.
Definition: The least inclusive crown clade containing Tetrao major (now Tinamus major) Gmelin, 1789 (Tinamiformes) and Struthio camelus Linnaeus, 1758 (Struthioniformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Tinamus major (Gmelin, 1789) & Struthio camelus Linnaeus, 1758).
Etymology: Derived from the Greek words παλαιός (palaios), meaning old, ancient, and γνάθος (gnathos), meaning jaw, which refer to the skeletal anatomy of the palate, which is considered to be more primitive than that in Neognathae.
Reference phylogeny: For the purpose of applying the definition of Palaeognathae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Struthioniformes (ostriches, 2 extant species), Rheiformes (rheas, 2 extant species), Apterygiformes (kiwis, 5 extant species), Casuariiformes (cassowaries, 3 extant species, and Emu), and Tinamiformes (tinamous, 47 extant species).
This clade also includes the extinct groups Dinornithiformes (moas) and Aepyornithiformes (elephant birds). Accounts of these groups are given in Worthy and Holdaway (2002), Hume and Walters (2012), Mayr (2017), Hansford and Turvey (2018) and Torres and Clarke (2018). It is not clear if Lithornithiformes are part of Palaeognathae (Houde, 1988; Livezey and Zusi, 2007; Yonezawa et al., 2017; Nesbitt and Clarke, 2016; Worthy et al., 2017).
Diagnostic apomorphies: Diagnostic apomorphies are (characters and states are indicated by their number-letter combination in Livezey and Zusi, 2006, 2007): (1) Os quadratum, processus orbitalis, articulatio pterygoideus marginalis ventralis, present (540b); (2) Os prearticulare, processus prearticularis—tuberculum (dorsale) insertii m. pseudotemporalis superficialis—present and prominent (tuberculum verae), on margo dorsalis of processus rostralis prearticulare and at approximate midpoint of margines ventralis et dorsalis mandibulae, typically medial to fenestra caudale mandibulae within fossa aditus canalis mandibulae (631b); (3) Rostrum (symphysis) mandibulae, pars symphysialis and anteriormost segment of pars intermedia, dorsoventral compression of rami producing essentially flat apex (especially obvious in rostral perspective), associated with virtual absence of crista tomialis rostrally, present (656b); (4) Ramus mandibulae, partes symphysialis et intermedia, shallow sulci indicative of rhamphothecal patterns, present (659b); (5) Articulatio metacarpophalangealis alulae, approximate position with respect to facies cranialis ossis metacarpale Ⅱ (in repose), angulus diagonal (approximating 45°), alula approaching diagonality with respect to ossa metacarpalia, associated with facies articularis phalangealis spheroidal and subdiagonal to axis majoris phalangis alularis (1750b); (6) Extremitas distalis femoris, condylus lateralis, crista supracondylaris lateralis, tuberculum m. gastrocnemialis lateralis, eminentia present, vaguely indicated (2029b); (7) Junctura (articulatio) tibiofibularis, os fibulare (calcaneum), status definitivum absent by failure of os fibulare to ossify (2436c); (8) Testa, limitates stratorum primus et secundus, aprismatic (2945b).
Mayr and Clarke (2003) noted that in palaeognathous birds the mesethmoid reaches beyond the nasofrontal hinge.
Synonyms: The following names are approximate synonyms: Struthioniformes (sensu Garrod, 1874; Bock and Bühler, 1990), Struthiones (sensu Garrod, 1874), Palaeognathiformes (sensu Cracraft, 1981), Eoaves (sensu Sibley and Ahlquist, 1990), Ratitae (sensu Sibley et al. 1988; Sibley and Ahlquist, 1990; Kurochkin, 1995).
Homonyms: There are no homonyms.
Comments: Monophyly of the clade formed by the ostriches, rheas, kiwis, cassowaries, emus and tinamous was already supported by early molecular data sets (Ho et al., 1976; Prager and Wilson, 1976; Sibley and Ahlquist, 1990; Harshman, 1994) and analyses of morphological characters (Meise, 1963; Cracraft, 1986, 1988; Elzanowski, 1995; Kurochkin, 1995; Livezey and Zusi, 2007). Reciprocal monophyly of Palaeognathae and Neognathae is now overwhelmingly supported by phylogenomic data (Table 1).
Arguments for non-monophyly proposed by Feduccia (1985), Houde and Olson (1981) and Olson (1985) were based on differences among Palaeognathae (i.e. Ratitae and Tinamiformes) and the belief that the defining characters of Palaeognathae are primitive. The first argument is not acceptable under phylogenetic methodology and the second holds true for only some of the characteristics of palaeognathous birds.
The definition proposed here agrees with current usage of the name Palaeognathae (Cracraft, 1988, 2013; Cracraft and Mindell, 1989; Kurochkin, 1995; Elzanowski, 1995; Livezey and Zusi, 2007). A definition of the name Palaeognathae was previously given by Gauthier and De Queiroz (2001), who also used Tinamus major and Struthio camelus as internal specifiers.
In Linnean classifications, this clade has been ranked at the level of family (Garrod, 1874), cohort (Garrod, 1874; Livezey and Zusi, 2007), order (Cracraft, 1981), superorder (del Hoyo et al., 1992), infraclass (Sibley and Ahlquist, 1990; Cracraft, 2013), and parvclass (Sibley et al., 1988; Sibley and Ahlquist, 1990; Kurochkin, 1995).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Palaeognathae in recent molecular analyses (Harshman et al., 2008; Phillips et al., 2010; Haddrath and Baker, 2012; Smith et al., 2013; Baker et al., 2014; Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017; Cloutier et al., 2019).
Palaeognathae is sometimes erroneously spelt Paleognathae (e.g. Gussekloo and Zweers, 1999; Baker and Pereira, 2009).
Registration number: 696.
Definition: The least inclusive crown clade containing Struthio americanus (now Rhea americana) Linnaeus, 1758 (Rheiformes), Tetrao major (now Tinamus major) Gmelin, 1789 (Tinamiformes) and Apteryx australis Shaw, 1813 (Apterygiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Rhea americana (Linnaeus, 1758), Tinamus major Gmelin, 1789 & Apteryx australis Shaw, 1813).
Etymology: The derivation of the name was given by Yuri et al. (2013) as follows: "Notopalaeognathae is from the Greek notos (southern) and Palaeognathae from the Greek word palaios (ancient, old) and gnathidion (jaw), the latter two words referring to the birds classified by Pycraft (1900) as having a "primitive" (palaeognathous) palate. Noto refers to the distribution of these taxa on fragments of the ancient southern supercontinent Gondwana. It also refers to the exclusion of Struthioniformes (ostriches) from this clade since they historically had a widespread Eurasian distribution."
Reference phylogeny: For the purpose of applying the definition of Notopalaeognathae, Figure 1A in Cloutier et al. (2019) should be regarded as the primary reference phylogeny. Figure 1 in Yonezawa et al. (2017) may be regarded as a secondary reference phylogeny.
Composition: Notopalaeognathae includes all Palaeognathae except the ostriches.
Diagnostic apomorphies: Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed only one potential apomorphy: Acetabulum, foramen acetabuli, bilateral compression and dorsal position relative to synsacrum in combination creating dorsally deep bilateral recessi—termed recessus acetabulo-synsacralis by Livezey and Zusi (2006)—visible through acetabulae (lateral perspective) and/or between ossa coxae et synsacrum (ventral perspective), present (1773b). This character state is not found in Tinamidae.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Evidence for a clade comprising all Palaeognathae except ostriches was first found by Hackett et al. (2008) and Harshman et al. (2008), and subsequently corroborated by Phillips et al. (2010), Haddrath and Baker (2012), Smith et al. (2013), Baker et al. (2014), Mitchell et al. (2014), Grealy et al. (2017), Yonezawa et al. (2017) and Cloutier et al. (2019).
The position of Rheidae among Palaeognathae is not congruently resolved, and three alternative positions have been inferred during the past decade: (i) as the sister-group to all other Palaeognathae, except ostriches (Figure 1 in Harshman et al., 2008; Phillips et al., 2010; Mitchell et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Grealy et al., 2017; Yonezawa et al., 2017), (ii) as the sister-group to cassowaries, Emu and kiwis (Novaeratitae) (Haddrath and Baker, 2012; Baker et al., 2014; Reddy et al., 2017; Cloutier et al., 2019; Kimball et al., 2019), and (iii) as the sister-group to tinamous (Figure 2 in Harshman et al., 2008) or tinamous and moas (Smith et al., 2013). As a consequence, a definition with three internal specifiers was selected.
Registration number: 697.
Definition: The least inclusive crown clade containing Apteryx australis Shaw, 1813 (Apterygiformes) and Struthio Casuarius (now Casuarius casuarius) Linnaeus, 1758 (Casuariiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Apteryx australis Shaw, 1813 & Casuarius casuarius (Linnaeus, 1758)).
Etymology: Novaeratitae is from the Latin words novus (new) and ratis (a raft). Novae refers to the three regions where this clade is found, namely New Guinea (novaeguineae), Australia (novaehollandiae), and New Zealand (novaeseelandiae) and ratis refers to the unkeeled sternum of these birds (Yuri et al., 2013).
Reference phylogeny: For the purpose of applying the definition of Novaeratitae, Figure 1a in Cloutier et al. (2019) should be regarded as the primary reference phylogeny. Figure 1 in Yonezawa et al. (2017) may be regarded as a secondary reference phylogeny.
Composition: Apterygiformes (kiwis, 5 extant species) and Casuariiformes (cassowaries, 3 extant species, and Emu).
The clade also includes the extinct Aepyornithiformes (elephant birds). The latter group comprises the taxa Mullerornis, Vorombe and Aepyornis. Accounts of the elephant birds are given in Hume and Walters (2012), Mayr (2017), Hansford and Turvey (2018) and Torres and Clarke (2018).
Diagnostic apomorphies: Diagnostic apomorphies are (characters and states are indicated by their number-letter combination in Livezey and Zusi, 2006, 2007): (1) Os carpi radiale, os proprius et facies articularis metacarpalis, vestigial, meniscoid, typically substantially smaller and of reduced functionality than os carpi ulnare (1562b); (2) Os metacarpale primus (I, alulare), absent (1580e); (3) Os metacarpale secundus (Ⅱ, majus), closely synchondrotic with ossa metacarpalia I et Ⅲ, spatium intermetacarpale obsolete or absent (1581c); (4) Phalanges digiti I (alularis, primus) manus, zero, phalanges alulae lacking entirely (1677f); (5) Phalanges digiti minus (Ⅲ, tertius), zero, despite retention of os metacarpale Ⅲ, typically vestigial (1679e); (6) Phalanx proximalis digiti I (alulae, primus), absent (1693b); (7) Junctura interphalangealis (proximalis) digiti majoris manus, synchondrosis (1756d); (8) M. rhomboideus superficialis, situs origii costae vertebrales, facies laterales (2512b); (9) M. biceps brachii, insertio(nes), one, insertio radialis only (2574d); (10) M. flexor (meta)carpi ulnaris, pars (caudalis) remigialis, vestigial or absent (2611b); (11) M. interosseus dorsalis (volaris), corpus, vestigial or absent, typically associated with reduction of spatium intermetacarpale (2622c); (12) M. interosseus ventralis (palmaris), absent (2624b); (13) M. extensor brevis alulae (pollicus), absent (2628b); (14) M. abductor alulae (pollicus), vestigial or absent (2629b); (15) M. adductor alulae (pollicus), absent (2632b); (16) M. flexor digiti minoris, absent (2635b). Many of these apomorphies are related to forelimb reduction and flightlessness. Given that a close relationship between the New Zealand kiwi and the Malagasy elephant birds implies multiple origins of flightlessness in this clade, it is possible that these character states developed convergently.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: The monophyly of a clade formed by kiwis, emus and cassowaries is supported by at least 11 modern studies (Hackett et al., 2008; Harshman et al., 2008; Phillips et al., 2010; Haddrath and Baker, 2012; Smith et al., 2013; Baker et al., 2014; Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017; Cloutier et al., 2019; Kimball et al., 2019). The only recent study that did not recover a kiwi-emu-cassowary clade was Prum et al. (2015); reanalysis of the Prum et al. (2015) data in a multispecies coalescent framework does yield this clade (Braun and Kimball, 2021).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Novaeratitae (Phillips et al., 2010; Haddrath and Baker, 2012; Smith et al., 2013; Baker et al., 2014; Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017; Cloutier et al., 2019).
Registration number: 698.
Definition: The smallest clade containing Tetrao major (now Tinamus major) Gmelin, 1789 (Tinamiformes) and Dinornis Novae-Zealandiae (now Dinornis novaezealandiae) Owen, 1843 (Dinornithiformes). This is a minimum-clade definition. Abbreviated definition: min ∇ (Tinamus major (Gmelin, 1789) & Dinornis novaezealandiae Owen, 1843).
Etymology: Derived from the Greek δεινος (deinos), meaning terrible, and the Greek κρυπτικός (krypticos), from which Pycraft's (1900) name for the tinamous (Crypturi) was derived. The name combines elements of the names of the two main clades.
Reference phylogeny: For the purpose of applying the definition of Dinocrypturi, Figure 1 in Yonezawa et al. (2017) should be regarded as the primary reference phylogeny. Figure 2C in Grealy et al. (2017) may be regarded as a secondary reference phylogeny.
Composition: Dinocrypturi includes the tinamous (Tinamiformes, 46 extant species; Gill et al., 2020) and moas (Dinornithiformes, 10 extinct species; Gill, 2010).
Diagnostic apomorphies: No unambiguous morphological apomorphies are known, but it is noted that the Dinornithidae and Tinamidae are the only palaeognathous birds in which an ossified supratendinal bridge is consistently present on the tarsometatarsus (see, however, Mayr, 2019 for the occurrence of this feature in the Eocene Palaeotididae).
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Evidence for a sister-group relationship of tinamous and moas was first documented by Phillips et al. (2010) and subsequently corroborated by Haddrath and Baker (2012), Smith et al. (2013), Baker et al. (2014), Mitchell et al. (2014), Grealy et al. (2017), Yonezawa et al. (2017), Cloutier et al. (2019), Urantówka et al. (2020), and Gordon et al. (2021).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Dinocrypturi (Phillips et al., 2010; Haddrath and Baker, 2012; Smith et al., 2013; Baker et al., 2014; Mitchell et al., 2014; Grealy et al., 2017; Yonezawa et al., 2017; Cloutier et al., 2019; Urantówka et al., 2020).
The name Dinornis giganteus, used in several molecular papers (Grealy et al., 2017; Yonezawa et al., 2017), is a junior synonym of Dinornis novaezealandiae Owen, 1843 (Gill, 2010).
Registration number: 699.
Definition: The least inclusive crown clade containing Phasianus (now Gallus) gallus (Linnaeus, 1758) (Galliformes) and Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Gallus gallus (Linnaeus, 1758 & Passer domesticus (Linnaeus, 1758)).
Etymology: Derived from the Greek words νέος (néos) meaning "new", and γνάθος (gnathos), meaning "jaw", which together refer to the skeletal anatomy of the palate, which is considered to be more advanced than that in Palaeognathae.
Reference phylogeny: For the purpose of applying the definition of Neognathae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: All Aves except Palaeognathae, i.e. a total of > 10, 000 extant bird species (Dickinson and Remsen, 2013; Dickinson and Christidis, 2014; Gill et al., 2020).
Diagnostic apomorphies: Diagnostic apomorphies are (characters and states are indicated by their number-letter combination in Livezey and Zusi, 2006, 2007): (1) Sutura frontoparietalis, absent, rendered indiscernable by synostosis (213b); (2) Os quadratum, processus mandibularis quadrati, facies articularis pterygoidea (facies ventralis in those taxa having two), condylar, tubercular, or jugo-sublinear (523c); (3) Junctura interpalatina et articulatio palatino-rostroparasphenoidalis ("palatorostralis"), preclusion by medial interposition of ossa vomera throughout length of palatum osseum, absent (579b); (4) Junctura pterygopalatina, articulatio pterygo-palatina simplex (601b); (5) Sutura costouncinata, typus ligamentosus, absent, suturae typically indiscernable and synostosis complete in adults (1096b); (6) Corpus sterni, facies muscularis sterni, lineae intermusculares (dorsomedialis), present, bilateral pair in approximate parallel with axis medianus sterni (1106b); (7) Extremitas distalis humeri, facies caudalis, sulcus tendinis m. humerotricipitalis (sensu stricto), present (1487c); (8) Tuberculum preacetabulare (processus pectinealis), os principalis, os ilium (1809b); (9) Synchondrosis (caudalis) ilioischiadica (et fenestra ilioischiadica definitivum), present (1953b); (10) Extremitas proximalis tibiotarsi, caput tibiae, facies articulares medialis et lateralis, distinctness of mutual delimitation by area interarticularis et fossae retrocristales, marked (2068b); (11) Extremitas proximalis tibiotarsi, caput tibiotarsi, facies articularis fibularis, present, short jugum extending distal from margo capitis et/aut distinct lateral extension of rima capitis (2108c); (12) Os tibiale (astragalus), medial extent and contribution to tibiotarsus relative to extremitas distalis tibiotarsi, condylae lateralis et medialis restricted, forming only part of tibiotarsus, extremitas distalis tibiotarsi, typically only condylus lateralis (2209b); (13) Os pretibiale, absent (2216b); (14) Os tarsi distale proprius, contribution to extremitas proximalis tarsometatarsus proprius (i.e., laminar, dorsal, subangular corona for termini proximales ossa tarsalia, present, contributes to both lamina et hypotarsus (2217b).
Synonyms: Carinatae (sensu Sclater, 1880; Sharpe, 1891; Gadow, 1893; Prager and Wilson, 1980) and Neoaves (sensu Sibley and Ahlquist, 1990) are approximate synonyms.
Homonyms: Wetmore (1960) used the name Neognathae for a group comprising all Aves except Spheniscidae (penguins).
Comments: Evidence for a close relationship of all crown group birds except Palaeognathae was presented by Pycraft (1900), and supported by studies of morphological characters (Cracraft, 1986, 1988; Kurochkin, 1995; Mayr and Clarke, 2003; Livezey and Zusi, 2007), immunological distances among transferrins (Prager and Wilson, 1976; Prager et al. 1976), α-crystallin sequences (Stapel et al., 1984; Hedges et al., 1995; Caspers et al., 1997), 12S and 16S rRNA sequences (Hedges et al. 1995) and more recently by a series of phylogenomic studies (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Neognathae in recent molecular analyses, i.e. between Galloanserae and Neoaves (Hackett et al., 2008; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021). A definition of the name Neognathae was previously given by Gauthier and De Queiroz (2001), who used a stem-modified node-based (maximum-crown-clade) definition, with Pluvialis apricaria as an internal specifier and Struthio camelus and Tinamus major as external specifiers.
The definition proposed here agrees with current usage of the name Neognathae (Cracraft, 1988, 2013; Cracraft and Mindell, 1989; Yuri et al., 2013; Jarvis et al., 2014).
In Linnean classifications the clade defined here as Neognathae has been ranked at the level of infraclass (Sibley and Ahlquist, 1990; Cracraft, 2013), parvclass (Kurochkin, 1995), and cohort (Livezey and Zusi, 2007).
Registration number: 700.
Definition: The most inclusive crown clade including Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes) but not Phasianus gallus (now Gallus gallus) Linnaeus, 1758 (Galliformes). This is a maximum-crown-clade definition. Abbreviated definition: max crown ∇ (Passer domesticus (Linnaeus, 1758) ~ Gallus gallus (Linnaeus, 1758)).
Etymology: The prefix neo-, from the Greek νέος (néos) meaning "new", and the Latin aves, meaning birds.
Reference phylogeny: For the purpose of applying the definition of Neoaves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: All Aves except Palaeognathae and Galloanserae, i.e. a total of > 9600 extant bird species (Dickinson and Remsen, 2013; Dickinson and Christidis, 2014; Gill et al., 2020).
Diagnostic apomorphies: Diagnostic apomorphies are (characters and states are indicated by their number-letter combination in Livezey and Zusi, 2006, 2007): (1) Extremitas omalis coracoidei, processus glenoidalis coracoidei, facies (sulcus) articularis humeralis (labrum glenoidale), primary position (dorsoventral and lateromedial dimensions) relative to processus acrocoracoideus, dorsolateral (1280c); (2) Apparatus copulationis — Mm. retractores phalli caudalis et cranialis (organa masculina) aut mm. levator cloacae et dilator cloacae (organa feminina)— absent (2502b); (3) M. entepicondylo-ulnaris, absent (2586b); (4) Epididymis, ductulus conjugens testis, absent (2893b); (5) Apparatus copulationis, phallus protrudens, basis phalli, lymphobulbus phalli, corpus vasculare phalli, glomera corporis vascularis phalli, absent (2895b); (6) Apparatus copulationis, phallus protrudens, basis phalli, vasa lymphatica cloacalia, absent (2896b); (7) Proctodeum, phallus femininus, absent (2900d).
Synonyms: Plethornithae Groth and Barrowclough 1999 is an approximate synonym.
Homonyms: Neoaves (sensu Sibley and Ahlquist, 1990) also included Galloanserae.
Comments: Monophyly of a clade comprising all birds except Palaegnathae and Galloanserae is well supported by several early molecular data sets (Ho et al., 1976; Prager and Wilson, 1976; Stapel et al., 1984; Mindell and Honeycutt, 1989; Sibley and Ahlquist, 1990; Cooper and Penny, 1997; Mindell et al., 1997) and is now very strongly supported by phylogenomic data sets (Table 1).
The name Neoaves was proposed by Sibley et al. (1988) for a clade comprising all extant birds except Palaeognathae and Galloanserae. At the time, they considered Galloanserae to be the sister-taxon of Palaeognathae and combined the two groups under the name Eoaves. The grouping of Galloanserae with Palaeognathae was later replaced (Sibley and Ahlquist, 1990) by the now well corroborated topology that places Palaeognathae sister to Neognathae, which consists of two major clades, Galloanserae and Neoaves. Unfortunately, in their revised classification, Sibley and Ahlquist (1990) did not use the familiar and widely accepted names Palaeognathae and Neognathae but used instead their own names Eoaves and Neoaves in a new sense.
Groth and Barrowclough (1999) considered that Sibley and Ahlquist's change of the meaning of the name Neoaves was confusing and proposed the name Plethornithae for the clade consisting of all extant birds except Palaeognathae and Galloanserae. However, although Sibley and Ahlquist's (1990) subsequent use of the name Neoaves was unfortunate and may cause confusion, it does not invalidate this name. Thus, Neoaves does not have to be replaced by Plethornithae. The latter name is a junior synonym of Neoaves.
A definition of Neoaves was previously given by Gauthier and De Queiroz (2001), who used a stem-modified node-based (maximum-crown-clade) definition, with Passer domesticus as an internal specifier and Gallus gallus and Anser anser as external specifiers.
Comprehensive phylogenomic studies show no congruent support for the basal dichotomy at the base of Neoaves (reviewed by Suh, 2016). This, in combination with evidence for incomplete lineage sorting (Suh et al. 2015; Houde et al. 2020) suggests that the base of Neoaves represents a hard polytomy (Suh, 2016). For this reason, a maximum-crown-clade definition was selected.
Registration number: 701.
Definition: The least inclusive crown clade containing Columba oenas Linnaeus, 1758 (Columbiformes), Mesites variegata (now Mesitornis variegatus) I. Geoffroy Saint-Hilaire, 1838 (Mesitornithiformes) and Tetrao (now Pterocles) alchata Linnaeus, 1766 (Pterocliformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Columba oenas Linnaeus, 1758 & Mesitornis variegatus (Geoffroy Saint-Hilaire, 1838) & Pterocles alchata (Linnaeus, 1766)).
Etymology: Derived from the Latin Columba, meaning pigeon or dove, and the Greek μορφή (morphe), meaning shape or form.
Reference phylogeny: For the purpose of applying the definition of Columbimorphae, Figure 1 in Prum et al. (2015) should be regarded as the primary reference phylogeny. Figure 1 in Jarvis et al. (2014) may be regarded as a secondary reference phylogeny.
Composition: This clade includes Columbiformes (331 extant species), Mesitornithiformes (3 extant species) and Pterocliformes (16 extant species) (Gill et al. 2020).
Diagnostic apomorphies: Columbiformes and Pterocliformes share numerous derived morphological characteristics and were considered closely related by many earlier authors (e.g., Stegmann, 1968; Mayr and Clarke, 2003). It is, however, difficult to characterize a clade that also includes the Mesitornithiformes with derived characters. Here we note that all three taxa exhibit a notarium and a humerus with a strongly developed and proximodistally elongated tuberculum dorsale. This tubercle serves for the attachment of musculus supracoracoideus and is usually strongly developed in birds capable of powerful flight. Its occurrence in the Mesitornithiformes is particularly remarkable, because mesites have very poor flight capabilities.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Monophyly of Columbiformes, Mesitornithiformes and Pterocliformes was first documented by Hackett et al. (2008) and subsequently recovered in five other phylogenomic studies (Table 1). Cuculiformes is sometimes recovered as part of this clade; specifically, Cuculiformes is nested within Columbimorphae in several Jarvis et al. (2014) trees (Figure 4C, Figure S14B, and Figure S14D) and in the primary tree in Kuhl et al. (2021).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Columbimorphae in recent molecular analyses (Jarvis et al., 2014; Prum et al., 2015; Reddy et al., 2017; Kimball et al., 2019).
Registration number: 702.
Definition: The least inclusive crown clade containing Mesites variegata (now Mesitornis variegatus) I. Geoffroy Saint-Hilaire, 1838 (Mesitornithiformes) and Tetrao (now Pterocles) alchata Linnaeus, 1766 (Pterocliformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Mesitornis variegatus (Geoffroy Saint-Hilaire, 1838) & Pterocles alchata (Linnaeus, 1766)).
Etymology: Derived from the scientific names of sandgrouse and mesites, which in turn are derived from the Greek πτερός (pteros, feather, wing) and κλεις (kleis, key), and μεσί (mesi, middle), respectively.
Reference phylogeny: For the purpose of applying the definition of Pteroclimesites, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: This clade includes Mesitornithiformes (mesites, 3 extant species) and Pterocliformes (sandgrouse, 16 extant species) (Dickinson and Remsen, 2013).
Diagnostic apomorphies: Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed two potential apomorphies: (1) Corpus sterni, margo caudalis sterni, trabeculae caudolateralis, intermediana, et mediana, relative caudal extents caudal to margo caudalis proprius trabecula mediana ≥ trabecula caudolateralis ≥ trabecula intermedia (1192e); this character state is otherwise only found in Afrotis and Passeriformes; (2) Scapus (corpus) pubis (dorsal perspective), pars intermedia, flexible, filamentous, extremely reduced in diameter (1932b). The latter character state is otherwise only found in some members of Galliformes, Suliformes, Geococcyx and Falconidae (Livezey and Zusi, 2006, Livezey and Zusi, 2007.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: The phylogenetic affinities of the mesites (Mesitornithiformes) and sandgrouse (Pterocliformes) have long been unclear due to incongruence among studies. Mesites have been found close to Gruiformes (Sibley and Ahlquist, 1990; Livezey, 1998), Cuculidae (Mayr and Ericson, 2004), and Turnicidae (Tunicidae) (Livezey and Zusi, 2007), whereas sandgrouse have been associated with Charadriiformes (Fjeldså, 1976; Sibley and Ahlquist, 1990) or Columbiformes (Livezey and Zusi, 2007; Mayr and Clarke, 2003). A sister-relationship of mesites and sandgrouse was first documented by Jarvis et al. (2014) and subsequently recovered in six other phylogenomic studies (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Pteroclimesites in recent molecular analyses (Jarvis et al., 2014; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 703.
Definition: The least inclusive crown clade containing Otis tarda Linnaeus, 1758 (Otidiformes) and Musophaga violacea Isert, 1789 (Musophagiformes) but not Ardea Grus (now Grus grus) Linnaeus, 1758 (Gruiformes) or Mesites variegata (now Mesitornis variegatus) I. Geoffroy Saint-Hilaire, 1838 (Mesitornithiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Otis tarda Linnaeus, 1758 & Musophaga violacea Isert, 1789 ~ Grus grus (Linnaeus, 1758) & Mesitornis variegatus (I. Geoffroy Saint-Hilaire, 1838).
Etymology: derived from the New Latin muso- (from Musa, a genus of bananas and plantains), and the Latin phaga (eater) and ōtis (bustard).
Reference phylogeny: For the purpose of applying the definition of Musophagotides, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Jarvis et al. (2014) may be regarded as a secondary reference phylogeny.
Composition: This clade includes Otidiformes (bustards, 26 species) and Musophagiformes (turacos, 23–33 species) (Dickinson and Remsen, 2013; Gill et al., 2020; Perktaş et al., 2020).
Diagnostic apomorphies: Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed one potential apomorphy: (1) vomer (synostotic ossa vomeris), processus pterygoideus, vestigial (467b). This state is only shared with Galliformes.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Otidiformes has long been associated with Gruiformes in pre-cladistic classifications (reviewed by Sibley and Ahlquist, 1990). However, current phylogenomic studies support a close relationship with Musophagiformes (McCormack et al., 2013; Jarvis et al., 2014; Suh et al., 2015; Kimball et al., 2019; Kuhl et al., 2021). The phylogeny of Prum et al. (2015) implies that Cuculiformes is also part of Musophagotides.
A minimum-crown-clade definition was selected because there is congruent support for a dichotomy within Musophagotides in recent molecular analyses (McCormack et al., 2013; Jarvis et al., 2014; Suh et al., 2015; Kimball et al., 2019; Kuhl et al., 2021). The external specifiers Grus grus (Gruiformes) and Mesitornis variegatus (Mesitornithiformes) were selected to prevent the name from being applied to conflicting phylogenomic topologies in which Gruiformes (Burleigh et al., 2015, Reddy et al., 2017, Figure 5 in Braun and Kimball, 2021) or Mesitornithiformes (Liu et al., 2018) would be part of Musophagotides.
Registration number: 704.
Definition: The least inclusive crown clade containing Phaëthon (now Phaethon) aethereus Linnaeus, 1758 (Phaethontiformes) and Pelecanus onocrotalus Linnaeus, 1758 (Pelecaniformes) but not Hirundo (now Apus) apus Linnaeus, 1758 (Strisores), Charadrius hiaticula Linnaeus, 1758 (Charadriiformes) or Musophaga violacea Isert, 1789 (Musophagiformes) or Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Phaethon aethereus Linnaeus, 1758 & Pelecanus onocrotalus Linnaeus, 1758 ~ Apus apus (Linnaeus, 1758) & Charadrius hiaticula Linnaeus, 1758 & Musophaga violacea Isert, 1789) & Passer domesticus (Linnaeus, 1758).
Etymology: Derived from the Greek Phaethon, which in Greek mythology was an epithet or surname of Helios (the sun) but was also used as the name of a son of Helios by Clymene, the Latin noun aequor, meaning expanse of water, and the Greek noun ορνις (ornis), meaning bird.
Reference phylogeny: For the purpose of applying the definition of Phaethoquornithes, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: This clade includes Phaethontimorphae (5 extant species) and Aequornithes (315–355 extant species) (Dickinson and Remsen, 2013; Gill et al., 2020).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: Natatores (sensu Livezey and Zusi, 2007) included Mirandornithes but did not include Eurypygiformes.
Homonyms: There are no homonyms.
Comments: This name is coined in accordance with a specific phylogenetic hypothesis, which is reflected by our proposed definition. This name is not applicable to topologies in which this grouping is not monophyletic (Hackett et al., 2008; McCormack et al., 2013; Burleigh et al., 2015; Reddy et al., 2017; Liu et al., 2018).
A minimum-crown-clade definition was selected because there is congruent support for the basal dichotomy within Phaethoquornithes in recent molecular analyses (Jarvis et al., 2014; Prum et al., 2015; Suh et al., 2015; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 705.
Definition: The least inclusive crown clade containing Phaëthon (now Phaethon) aethereus Linnaeus, 1758 (Phaethontiformes), Eurypyga helias Pallas, 1781 and Rhynochetos jubatus J. Verreaux and Des Murs, 1860. This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Phaethon aethereus (Linnaeus, 1758) & Eurypyga helias Pallas, 1781 & Rhynochetos jubatus J. Verreaux and Des Murs, 1860).
Etymology: Derived from the Greek Φαέϑων (Phaethon), which in Greek mythology was an epithet or surname of Helios (the sun) but was also used as the name of a son of Helios by Clymene, and the Greek μορφή (morphe), meaning shape or form.
Reference phylogeny: For the purpose of applying the definition of Phaethontimorphae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: This clade includes five extant species: Phaethon aethereus (Red-billed Tropicbird), P. lepturus (White-tailed Tropicbird), P. rubricauda (Red-tailed Tropicbird), Eurypyga helias (Sunbittern) and Rhynochetos jubatus (Kagu).
An account of the extinct group Prophaethontidae was given by Mayr (2017, 2022).
Diagnostic apomorphies: No morphological apomorphies are known (Livezey and Zusi, 2006, 2007). We also note that one of the authors of the current paper (GM) questions whether the results of current molecular studies correctly reflect the true evolutionary history of the involved taxa (see Mayr, 2017).
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: The phylogenetic affinities of the tropicbirds (Phaethon) were long unclear due to incongruence among studies (Cracraft et al., 2004; Fain and Houde, 2004; Ericson et al., 2006; Hackett et al., 2008). Modern phylogenomic studies support a close relationship to Sunbittern (Eurypyga helias) and Kagu (Rhynochetos jubatus) (McCormack et al., 2013; Yuri et al., 2013; Jarvis et al., 2014; Prum et al., 2015; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
The name Phaethontimorphae has not been widely adopted yet, but it has been used for this clade by Jarvis et al. (2014), Suh (2016), Liu et al. (2018), Braun et al. (2019), and Braun and Kimball (2021).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Phaethontimorphae in recent molecular analyses (Jarvis et al., 2014; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 706.
Definition: The least inclusive crown clade containing Pelecanus onocrotalus Linnaeus, 1758 (Pelecaniformes) and Colymbus Immer (now Gavia immer) Brünnich, 1764 (Gaviiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Pelecanus onocrotalus Linnaeus, 1758 & Gavia immer (Brünnich, 1764)).
Etymology: Derived from from the Latin noun aequor, meaning expanse of water, and the Greek noun ορνις (ornis), meaning bird.
Reference phylogeny: For the purpose of applying the definition of Aequornithes, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Aequornithes includes the divers (Gaviidae), storm-petrels (Hydrobatidae, Oceanitidae), albatrosses (Diomedeidae), petrels and diving-petrels (Procellariidae), penguins (Spheniscidae), storks (Ciconiidae), frigatebirds (Fregata), darters (Anhinga), cormorants (Phalacrocoracidae), gannets and boobies (Sulidae), herons (Ardeidae), ibises (Threskiornithidae), pelicans (Pelecanidae), Shoebill (Balaeniceps rex) and Hamerkop (Scopus umbretta). This clade comprises 315–355 extant species, listings of which are given in Dickinson and Remsen (2013) and Gill et al. (2020).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: Aequornithia Cracraft, 2013 is an approximate synonym.
Homonyms: There are no homonyms.
Comments: A clade of aquatic and semi-aquatic birds, including the divers, storm-petrels, albatrosses, petrels (including diving-petrels), penguins, storks, frigatebirds, darters, cormorants, gannets and boobies, herons, ibises, pelicans, Shoebill and Hamerkop was first documented by Ericson et al. (2006) and further supported by several phylogenomic studies (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Aequornithes (i.e. between divers and all other extant members of the clade) in recent molecular analyses (Hackett et al., 2008, McCormack et al., 2013; Jarvis et al., 2014; Burleigh et al., 2015; Kuramoto et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 707.
Definition: The least inclusive crown clade containing Diomedea demersa (now Spheniscus demersus) Linnaeus, 1758 (Sphenisciformes) and Procellaria aequinoctialis Linnaeus, 1758 (Procellariiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Spheniscus demersus (Linnaeus, 1758) & Procellaria aequinoctialis Linnaeus, 1758).
Etymology: Derived from the taxonomic name Procellaria which is "a modern adjectival form of the Latin word for a storm (procella) and gives the meaning of 'creatures of the storm' as is appropriate" (Marchant and Higgins, 1990: 557), and the Greek μορφή (morphe, shape, form).
Reference phylogeny: For the purpose of applying the definition of Procellariimorphae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Includes Spheniscidae (penguins), Diomedeidae (albatrosses), Hydrobatidae (northern storm-petrels), Oceanitidae (southern storm-petrels), Procellariidae (petrels, shearwaters, and diving petrels).
Accounts of the extinct group Diomedeoididae are provided by Mayr and Smith (2012) and Mayr (2017, 2022).
Diagnostic apomorphies: Diagnostic apomorphies are (characters and states are indicated by their number-letter combination in Livezey and Zusi, 2006, 2007): (1) Os lacrimale, processus orbitalis, lamina medialis, perforatio laminae obliquus, present (196b); (2) Os lacrimopalatinum, present, sublinear (722b); (3) Os humerus, virtual linearity independent of relative elongation, present, linear, elongate (1347b); (4) Digitus IV pedis, phalanx proximalis, basis phalangis, tuberculum flexorium, prominently symmetrical bilobation (proximal perspective), present, tuberculum typically bilobate (2404b); (5) Aponeurosis carpo-alularis dorsalis, present, broad and robust (2630b); (6) Naris externum, ducti tubulares (bilaterally paired ostia externa), comprising ostium efferens glandulae et ostium efferens respiratorium, present in early ontogeny, absent in juvenile and definitive semaphorants (2744b); (7) Pennae auriculares, plumae auriculares rostrales et caudales, present, acoustically redirecting buttress in taxa possessing or lacking facial auditory discs (2933b).
Synonyms: Austrodyptornithes Yuri et al., 2013 is an approximate synonym.
Homonyms: There are no homonyms.
Comments: The relationships of Sphenisciformes and Procellariiformes were long uncertain due to conflicting relationships inferred from limited morphological or molecular data (Sibley and Ahlquist, 1990; Cooper and Penny, 1997; García-Moreno et al., 2003; Mayr and Clarke, 2003; Chubb, 2004; Cracraft et al., 2004; Poe and Chubb, 2004; Bourdon, 2005; Smith, 2010). Among early studies, only McKitrick (1991a, 1991b) and Livezey (2001) had found a sister-group relationship of Sphenisciformes and Procellariiformes. Modern phylogenomic data now overwhelmingly support the monophyly of Sphenisciformes + Procellariiformes (Table 1).
The name Austrodyptornithes was proposed by Yuri et al. (2013) but this name refers to the same clade that Livezey and Zusi (2007) had named Procellariimorphae. Both names are currently in use; for instance, Procellariimorphae was used by Cracraft (2013), Jarvis et al. (2014) and Suh (2016), and Austrodyptornithes was used by Ksepka and Phillips (2015), Kuramoto et al. (2015), Kimball et al. (2019) and Kooijman (2020). We have selected Procellariimorphae over Austrodyptornithes based on the (informal) priority of the former.
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Procellariimorphae in recent molecular analyses (Hackett et al., 2008; McCormack et al., 2013; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 708.
Definition: The least inclusive crown clade containing Pelecanus onocrotalus Linnaeus, 1758 (Pelecaniformes), Pelecanus leucogaster (now Sula leucogaster) Boddaert, 1783 (Suliformes) and Ardea ciconia (now Ciconia ciconia) Linnaeus, 1758 (Ciconiiformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Pelecanus onocrotalus Linnaeus, 1758 & Sula leucogaster (Boddaert, 1783) & Ciconia ciconia (Linnaeus, 1758)).
Etymology: Derived from the Greek πελεκάν (pelecan, pelican) and the Greek μορφή (morphe, shape, form).
Reference phylogeny: For the purpose of applying the definition of Pelecanimorphae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Pelecanimorphae includes the storks (Ciconiidae), frigatebirds (Fregatidae), darters (Anhingidae), cormorants (Phalacrocoracidae), gannets and boobies (Sulidae), herons (Ardeidae), ibises (Threskiornithidae), pelicans (Pelecanidae), Shoebill (Balaeniceps rex) and Hamerkop (Scopus umbretta).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: Pelecaniformes Sharpe, 1891 (sensu Cracraft, 2013) is an approximate synonym.
Homonyms: Livezey and Zusi (2007) used the name for a group that comprised Pelagornithidae ("Odontopterygidae"; now considered to be galloanserine birds; Mayr, 2017, 2022), Balaenicipitidae, Phaethontidae, Fregatidae, Pelecanidae, Sulidae, Phalacrocoracidae and Anhingidae, but which excluded Ciconiidae, Ardeidae and Threskiornithidae. Cracraft (2013) used Pelecanimorphae as a redundant name for his Pelecaniformes (see above).
Comments: Monophyly of a clade formed by storks, frigatebirds, cormorants, gannets, herons, ibises and pelicans is supported by congruence of multiple phylogenomic data sets (Table 1).
The name Pelecaniformes has long been associated with a polyphyletic group comprising the tropicbirds (Phaethontidae), pelicans (Pelecanidae), gannets and boobies (Sulidae), cormorants (Phalacrocoracidae), darters (Anhingidae) and frigatebirds (Fregatidae) (e.g. Howard & Moore 1991). After the clarification of relationships among these groups, several authors have restricted the name Pelecaniformes to a group comprising pelicans, Shoebill, Hamerkop, herons and ibises, have applied the name Suliformes to the frigatebirds, darters, cormorants, gannets and boobies, and have restricted the name Ciconiiformes to the storks (Yuri et al., 2013; Mayr, 2017; Kimball et al., 2019; Kuhl et al., 2021). We follow these authors here. Following Cracraft (2013), we use the name Pelecanimorphae for the clade comprising these three major groups.
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal subdivision within Pelecanimorphae in recent molecular analyses (Hackett et al., 2008; Yuri et al., 2013; Burleigh et al., 2015; Kuramoto et al., 2015; Prum et al., 2015; Reddy et al., 2017; Kimball et al., 2019; Kuhl et al., 2021).
The name Pelecanimorphae is sometimes ascribed to Huxley (1867) (e.g. Livezey and Zusi, 2007) but Huxley (1867) did not actually mention this name in his work. Instead, he included the pelicans (Pelecanidae) with other totipalmate birds in a group called Dysporomorphae.
Registration number: 754.
Definition: The least inclusive crown clade containing Pelecanus onocrotalus Linnaeus, 1758 (Pelecaniformes) and Pelecanus leucogaster (now Sula leucogaster) Boddaert, 1783 (Suliformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Pelecanus onocrotalus Linnaeus, 1758 & Sula leucogaster (Boddaert, 1783)).
Etymology: Derived from the Greek πελεκάν (pelecan, pelican).
Reference phylogeny: For the purpose of applying the definition of Pelecanes, Figure 1 in Prum et al. (2015) should be regarded as the primary reference phylogeny. Figure 3 in Kuhl et al. (2021) may be regarded as a secondary reference phylogeny.
Composition: Pelecanes includes the frigatebirds (Fregatidae), darters (Anhingidae), cormorants (Phalacrocoracidae), gannets and boobies (Sulidae), herons (Ardeidae), ibises (Threskiornithidae), pelicans (Pelecanidae), Shoebill (Balaeniceps rex) and Hamerkop (Scopus umbretta).
Diagnostic apomorphies: Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed one potential apomorphy: Extremitas proximalis tibiotarsi, caput tibiotarsi, crista cnemialis lateralis, crista patellaris, truncate and crescentiform, imbedded within vertex lateroproximalis of unified cristae cnemiales, at obtuse angulus with corpus tibiotarsi (2105f). This character state is only shared with Phaethon and Uria. An elongated hallux may also represent a synapomorphy of Pelecanes (Mayr, 2017).
Synonyms: there are no synonyms.
Homonyms: there are no homonyms.
Comments: Monophyly of a clade formed by frigatebirds, cormorants, gannets, herons, ibises and pelicans is supported by congruence of multiple phylogenomic data sets (Table 1; see also Kuramoto et al. 2015).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal subdivision within Pelecanes in recent molecular analyses (Hackett et al., 2008; Yuri et al., 2013; Burleigh et al., 2015; Kuramoto et al., 2015; Prum et al., 2015; Reddy et al., 2017; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 709.
Definition: The least inclusive crown clade containing Falco Nisus (now Accipiter nisus) Linnaeus, 1758 (Accipitriformes) and Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Accipiter nisus (Linnaeus, 1758) & Passer domesticus (Linnaeus, 1758)).
Etymology: Derived from the Latin words telluris (the earth, earth, land) and aves (birds) (Yuri et al., 2013).
Reference phylogeny: For the purpose of applying the definition of Telluraves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Telluraves comprises all members of Afroaves and Australaves.
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: This clade was first recovered by Ericson et al. (2006) and is now strongly supported by congruence of phylogenomic data sets (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Telluraves in recent molecular analyses (Hackett et al., 2008; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 710.
Definition: The least inclusive crown clade containing Falco Nisus (now Accipiter nisus) Linnaeus, 1758 (Accipitriformes), Loxia colius (now Colius colius) Linnaeus, 1766 (Coliiformes) and Picus viridis Linnaeus, 1758 (Piciformes) but not Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Accipiter nisus (Linnaeus, 1758) & Colius colius (Linnaeus, 1766) & Picus viridis Linnaeus, 1758) ~ Passer domesticus (Linnaeus, 1758).
Etymology: Derived from the word Africa, and the Latin aves, meaning birds. The name ('African birds') refers to the inferred African origin of the clade (Ericson, 2012).
Reference phylogeny: For the purpose of applying the definition of Afroaves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 3 in Reddy et al. (2017) may be regarded as a secondary reference phylogeny.
Composition: Includes Accipitriformes (hawks and allies), Strigiformes (owls), Coliiformes (mousebirds), Leptosomiformes (cuckoo-rollers), Trogoniformes (trogons), Bucerotiformes (hornbills and allies), Upupiformes (hoopoes and allies), Coraciiformes (rollers and allies), and Piciformes (woodpeckers and allies).
The clade also includes the extinct Teratornithidae, a group related to hawks and allies (Mayr, 2017), the extinct Sandcoleidae, which are stem group representatives of mousebirds (Houde and Olson, 1992; Ksepka and Clarke, 2010; Mayr, 2017, 2022), the extinct Ogygoptyngidae and Protostrigidae, and other groups related to owls (Mayr, 2009, 2017, 2022), Messelirrisoridae, a group related to hoopoes and wood hoopoes (Mayr, 2017), Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, possible stem group representatives of the Piciformes (Mayr, 2017, 2022).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: Coracornithes Fürbringer, 1888 is a partial synonym. Coracornithia Cracraft, 2013 was derived from that name and is an approximate synonym.
Homonyms: There are no homonyms.
Comments: A clade comprising hawks and allies, owls, mousebirds, cuckoo-roller, trogons, hornbills and allies, rollers and allies, and woodpeckers and allies was first recovered by Ericson et al. (2006). This clade is now overwhelmingly supported by phylogenomic data (Table 1).
The name Afroaves was introduced by Ericson (2012) and is now in wide use (Yuri et al., 2013; Jarvis et al., 2014; Brusatte et al., 2015; Suh, 2016). As a consequence, we have selected this name rather than Coracornithia Cracraft, 2013.
The basal dichotomy within Afroaves differs among recent molecular analyses. Most studies support a dichotomy between Accipitriformes and all other Afroaves (Hackett et al., 2008; Jarvis et al., 2014; Burleigh et al., 2015; Reddy et al., 2017; Kimball et al., 2019; Kuhl et al., 2021) but one study inferred a dichotomy between Coliiformes and all other Afroaves (Suh et al. 2015, this issue is further reviewed by Suh, 2016). A node-based (minimum-crown-clade) definition was selected that reflects both hypotheses. In two recent phylogenomic studies, Accipitriformes was sister to a large clade that included not only mousebirds, owls, cuckoo-roller, trogons, hornbills, rollers, woodpeckers but also Australaves (Prum et al., 2015; Liu et al., 2018).
Registration number: 711.
Definition: The least inclusive crown clade containing Loxia (now Colius) colius Linnaeus, 1766 (Coliiformes) and Picus viridis Linnaeus, 1758 (Piciformes) but not Falco nisus (now Accipiter nisus) Linnaeus, 1758 (Accipitriformes) or Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Colius colius (Linnaeus, 1766) & Picus viridis Linnaeus, 1758) ~ Accipiter nisus (Linnaeus, 1758) & Passer domesticus (Linnaeus, 1758).
Etymology: From the Greek κορακίας (korakías), derived from κόραξ (korax), meaning raven or crow, and the Greek μορφή (morphe), meaning shape or form.
Reference phylogeny: For the purpose of applying the definition of Coraciimorphae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Includes Coliiformes (mousebirds), Leptosomiformes (cuckoo-rollers), Trogoniformes (trogons), Bucerotiformes (hornbills and allies), Upupiformes (hoopoes and allies), Coraciiformes (rollers and allies), and Piciformes (woodpeckers and allies).
The clade also includes the extinct taxa Sandcoleidae, a group related to mousebirds (Houde and Olson, 1992; Ksepka and Clarke, 2010; Mayr, 2017), Ogygoptyngidae, Protostrigidae, and other groups related to owls (Mayr, 2009, 2017), Messelirrisoridae, a group related to hoopoes and wood hoopoes (Mayr, 2017), Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, a stem group related to Piciformes (Mayr, 2017).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Monophyly of a clade formed by mousebirds, cuckoo-rollers, trogons, hornbills and allies, rollers and allies, and woodpeckers and allies is supported by congruence of multiple phylogenomic data sets (Table 1). Whereas most phylogenomic studies support a closer position of Coliiformes to Coraciiformes than to Accipitriformes and Strigiformes, two analyses placed Coliiformes sister to a clade that includes Accipitriformes, Strigiformes and all other Afroaves (the ultraconserved element tree in Jarvis et al., 2014 and the Suh et al., 2015 retroposon study). Thus, we have added the external specifier Accipiter nisus (Accipitriformes) to prevent the name from being applied to that conflicting phylogenomic topologies.
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Coraciimorphae in recent molecular analyses (Jarvis et al., 2014; Prum et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021). This is also present in Kimball et al. (2013), Gilbert et al. (2018), and Houde et al. (2019).
Registration number: 712.
Definition: The least inclusive crown clade containing Cuculus (now Leptosomus) discolor Hermann, 1783 and Picus viridis Linnaeus, 1758 (Piciformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Leptosomus discolor (Hermann, 1783) & Picus viridis Linnaeus, 1758).
Etymology: Derived from the Latin cavus (hollow, hole), referring to the cavity-nesting habits of the clade, and aves (birds).
Reference phylogeny: For the purpose of applying the definition of Cavitaves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Leptosomiformes (cuckoo-rollers), Trogoniformes (trogons), Bucerotiformes (hornbills and allies), Upupiformes (hoopoes and allies), Coraciiformes (rollers and allies), and Piciformes (woodpeckers and allies).
The clade also includes the extinct taxa Messelirrisoridae, a group related to hoopoes and wood hoopoes (Mayr, 2017), Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, a stem group related to Piciformes (Mayr, 2017).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Monophyly of a clade formed by cuckoo-rollers, trogons, hornbills and allies, rollers and allies, and woodpeckers and allies is supported by congruence of multiple phylogenomic data sets (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Cavitaves in recent molecular analyses (Hackett et al., 2008; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 713.
Definition: The least inclusive crown clade containing Trogon viridis Linnaeus, 1766 (Trogoniformes) and Picus viridis Linnaeus, 1758 (Piciformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Trogon viridis Linnaeus, 1766 & Picus viridis Linnaeus, 1758).
Etymology: Derived from the Latin cavus (hollow, hole), refering to the cavity-nesting habits of the clade, and aves (birds) (Yuri et al., 2013) combined with the Greek eu (well, good) to indicate that this group corresponds to the core Cavitaves.
Reference phylogeny: For the purpose of applying the definition of Eucavitaves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Trogoniformes (trogons), Bucerotiformes (hornbills and allies), Upupiformes (hoopoes and allies), Coraciiformes (rollers and allies), and Piciformes (woodpeckers and allies).
The clade also includes the extinct taxa Messelirrisoridae, a group related to hoopoes and wood hoopoes (Mayr, 2017), Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, a stem group related to Piciformes (Mayr, 2017).
Diagnostic apomorphies: No morphological apomorphies are known.
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Monophyly of a clade formed by trogons, hornbills and allies, rollers and allies, and woodpeckers and allies is supported by congruence of multiple phylogenomic data sets (Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Eucavitaves in recent molecular analyses (Hackett et al., 2008; McCormack et al., 2013; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 714.
Definition: The least inclusive crown clade containing Buceros rhinoceros Linnaeus, 1758 (Bucerotiformes), Coracias garrulus Linnaeus, 1758 (Coraciiformes) and Picus viridis Linnaeus, 1758 (Piciformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Buceros rhinoceros Linnaeus, 1758 & Coracias garrulus Linnaeus, 1758 & Picus viridis Linnaeus, 1758).
Etymology: Derived from the Latin picus (woodpecker), and the Greek κορακίας (korakías), derived from κόραξ (korax), meaning raven or crow.
Reference phylogeny: For the purpose of applying the definition of Picocoraciades, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Bucerotiformes (hornbills and allies), Upupiformes (hoopoes and allies), Coraciiformes (rollers, kingfishers, motmots, bee-eaters, todies, ground rollers) and Piciformes (woodpeckers, honeyguides, toucans, barbets, puffbirds and jacamars).
The clade also includes the extinct taxa Messelirrisoridae, a group related to hoopoes and wood hoopoes (Mayr, 2017), Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, a stem group related to Piciformes (Mayr, 2017).
Diagnostic apomorphies: Mayr (2014) identified a well-defined sulcus for the tendon of m. extensor longus alulae on the radial carpal as a synapomorphy. Mayr (2008a) also listed a mandible that projects beyond the upper beak in hatchlings, reduced ventral secondary coverts, and a marked medial parahypotarsal fossa in which the medial margin forms a sharp ridge as potential synapomorphies, citing Manegold (2005).
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: Molecular phylogenetic studies have shown that Coraciiformes (sensu Voous, 1977; del Hoyo et al. 2001) is not monophyletic and that some taxa in Coraciiformes are more closely related to Piciformes than to other taxa traditionally included in Coraciiformes (Ericson et al., 2006; Brown et al., 2008; Hackett et al., 2008; McCormack et al., 2013; Yuri et al., 2013; Burleigh et al., 2015; Prum et al., 2015; Reddy et al., 2017). The name Coraciiformes has become restricted to the bee-eaters (Meropidae), todies (Todidae), motmots (Momotidae), rollers (Coraciidae), ground rollers (Brachypteraciidae) and kingfishers (Alcedinidae), whereas the clade comprising hoopoes (Upupidae), woodhoopoes (Phoeniculidae) and hornbills (Bucerotidae) has become known under the names Bucerotiformes (Sangster et al., 2013; Yuri et al., 2013; Cracraft, 2013) and Bucerotes (Mayr, 2011).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Picocoraciae in recent molecular analyses (Hackett et al., 2008; McCormack et al., 2013; Yuri et al., 2013; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017).
The name was spelled 'Picocoraciae' by Mayr (2011) and subsequent authors. It is here amended to Picocoraciades to make it grammatically correct (Coraciades is the correct plural of Coracias).
Registration number: 715.
Definition: The least inclusive crown clade containing Coracias garrulus Linnaeus, 1758 (Coraciidae), Gracula (now Alcedo) atthis Linnaeus, 1758 (Alcedinidae) and Picus viridis Linnaeus, 1758 (Piciformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Coracias garrulus Linnaeus, 1758 & Alcedo atthis (Linnaeus, 1758) & Picus viridis Linnaeus, 1758).
Etymology: Derived from the Latin picus (woodpecker), the Greek δυνάστης (dynastes, lord, master, ruler) in reference to the "king" in kingfishers (Yuri et al. 2013), and the Greek noun ορνις (ornis), meaning bird.
Reference phylogeny: For the purpose of applying the definition of Picodynastornithes, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Coraciiformes (rollers, kingfishers, motmots, bee-eaters, todies, ground rollers) and Piciformes (woodpeckers, honeyguides, toucans, barbets, puffbirds and jacamars).
The clade also includes the extinct taxa Primobucconidae, Eocoraciidae and Geranopteridae, three stem group taxa related to rollers and ground-rollers (Mayr, 2017) and Sylphornithidae, a stem group related to Piciformes (Mayr, 2017).
Diagnostic apomorphies: A bifurcated scapular acromion is a potential synapomorphy of Picodynastornithes, although this character state is absent in Galbulae (Mayr, 2009, 2022).
Synonyms: There are no synonyms.
Homonyms: There are no homonyms.
Comments: There is strong, congruent support for a sister relationship of Coraciiformes and Piciformes (e.g. Sibley and Ahlquist, 1990; Ericson et al., 2006, Table 1).
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Picodynastornithes in recent molecular analyses (Hackett et al., 2008; Yuri et al., 2013; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 716.
Definition: The least inclusive crown clade containing Palamedea (now Cariama) cristata Linnaeus, 1766 (Cariamiformes) and Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Cariama cristata (Linnaeus, 1766) and Passer domesticus (Linnaeus, 1758)).
Etymology: Derived from the Latin australis, meaning southern, and the Latin aves, meaning birds.
Reference phylogeny: For the purpose of applying the definition of Australaves, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: The clade comprises the extant groups Cariamiformes (2 extant species, seriemas), Falconiformes (64 species, Dickinson and Remsen, 2013), Psittaciformes (376 species, Dickinson and Remsen, 2013) and Passeriformes (6063 species, Dickinson and Christidis, 2014).
The clade also includes the extinct Idiornithidae, Bathornithidae, and Phorusrhacidae, which are stem group representatives of the Cariamiformes (Angst et al. 2013; Degrange et al., 2015; Mayr, 2017, 2022), Quercypsittidae, a stem-group of Psittaciformes (Ksepka et al., 2011; Mayr, 2017), Halcyornithidae, Messelasturidae and Vastanavidae, which are stem groups of Psittaciformes or Psittacopasseres or perhaps even outside the total-group of Psittacopasseres (Ksepka et al., 2011, 2019; Mayr, 2015, 2017, 2021), Psittacopedidae, which is a possible stem-group of Passeriformes (Ksepka et al., 2019; Mayr, 2020), and the taxon Zygodactylidae, which is believed to represent the extinct sister-group of passerines (Mayr, 2008b, see also Mayr, 2015, 2017).
Diagnostic apomorphies: No morphological apomorphies are known. Suh et al. (2011) identified two retroposons that are apomorphic for this clade. A subsequent study identified a larger number of retroposons (Suh et al., 2015).
Synonyms: Passerimorphae (sensu Cracraft, 2013) is an approximate synonym.
Homonyms: There are no homonyms.
Comments: The existence of a clade formed by seriemas, falcons, parrots and passerines was first documented by Ericson et al. (2006), and further supported by subsequent phylogenomic studies (Table 1, see also Wang et al. 2012). The name was originally spelled 'Australavis' by Ericson (2012); it was emended to Australaves by Yuri et al. (2013). The name Australaves is now in wide use (e.g. Jarvis et al., 2014; Brusatte et al., 2015; Prum et al., 2015; Suh, 2016).
A node-based (minimum-crown-clade) definition was selected because there is multiple genomic support for the basal dichotomy within Australaves (i.e. between Cariamidae and Eufalconimorphae) in recent molecular analyses (Hackett et al., 2008; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Suh et al., 2015; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 717.
Definition: The least inclusive crown clade containing Falco subbuteo Linnaeus, 1758 (Falconiformes) and Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Falco subbuteo Linnaeus, 1758 and Passer domesticus (Linnaeus, 1758)).
Etymology: Derived from the Greek eu (well, good, true), the Latin falco (falcon) and the Greek μορφή (morphe), meaning shape or form.
Reference phylogeny: For the purpose of applying the definition of Eufalconimorphae, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: The clade comprises the extant groups Falconiformes (64 species, Dickinson and Remsen, 2013), Psittaciformes (376 species, Dickinson and Remsen, 2013) and Passeriformes (6063 species, Dickinson and Christidis, 2014).
The clade also includes the extinct Quercypsittidae, a stem-group of Psittaciformes (Ksepka et al., 2011; Mayr, 2017), Halcyornithidae, Messelasturidae and Vastanavidae, which are stem groups of Psittaciformes or Psittacopasseres or may be outside the total-group of Psittacopasseres (Ksepka et al., 2011, 2019; Mayr, 2015, 2017, 2021), Psittacopedidae, which is a possible stem-group of Passeriformes (Ksepka et al., 2019; Mayr, 2020), and the taxon Zygodactylidae, which is believed to represent the extinct sister-group of passerines (Mayr, 2008b, see also Mayr, 2017).
Diagnostic apomorphies: Suh et al. (2011) identified three retroposons that are apomorphic for this clade. A subsequent study identified a larger number of retroposons (Suh et al., 2015). Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed no morphological apomorphies. However, Pyle (2013) observed that falcons and parrots share a distinctive wing molt sequence that may therefore be a synapomorphy of Eufalconimorphae which is lost in Passeriformes.
Synonyms: The name Australaves was used by Ericson (2012) for a clade that also included Cariamidae, the latter being depicted as the sister taxon of Falconidae. Under the definitions in this paper, the names Australaves and Eufalconimorphae would refer to the same clade if Cariamidae were indeed the sister taxon of Falconidae (as in Wang et al., 2012). However, all subsequent phylogenomic studies have placed Cariamidae as the sister to the falcon-parrot-passerine clade. As a consequence, Eufalconimorphae is here applied to its original clade and Australaves is here applied to a more inclusive clade that also includes Cariamidae.
Homonyms: There are no homonyms.
Comments: Evidence for this clade was first presented by Hackett et al. (2008) with moderate support. The clade received further support from a retroposon study (Suh et al., 2011) and all subsequent phylogenomic studies (Table 1).
Yuri et al. (2013) noted there is a potential for confusion due to the prior use of Falconimorphae for the non-monophyletic group comprising Accipitriformes and Falconidae. Yuri et al. (2013) also noted that the taxon Eufalconimorphae includes many non-raptorial taxa (Psittaciformes and Passeriformes) and excludes a raptorial taxon (Cariamiformes). They concluded that it seems inappropriate to use Eufalconimorphae and they instead recommended using Australaves. However, modern evidence shows that seriemas are not sister to falcons (contra Ericson, 2012; Wang et al., 2012), and that Eufalconimorphae and Australaves are not synonyms.
A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Eufalconimorphae in recent molecular analyses (Hackett et al., 2008; Suh et al., 2011, 2015, 2015; McCormack et al., 2013; Jarvis et al., 2014; Prum et al., 2015; Kimball et al., 2019; Kuhl et al., 2021).
Registration number: 718.
Definition: The least inclusive crown clade containing Psittacus erithacus Linnaeus, 1758 (Psittaciformes) and Fringilla domestica (now Passer domesticus) Linnaeus, 1758 (Passeriformes). This is a minimum-crown-clade definition. Abbreviated definition: min crown ∇ (Psittacus erithacus Linnaeus, 1758 and Passer domesticus (Linnaeus, 1758)).
Etymology: Derived from the Latin psittacus, meaning a parrot, and the Latin passer, meaning a sparrow.
Reference phylogeny: For the purpose of applying the definition of Psittacopasseres, Figure 3 in Kuhl et al. (2021) should be regarded as the primary reference phylogeny. Figure 1 in Prum et al. (2015) may be regarded as a secondary reference phylogeny.
Composition: Psittaciformes (376 recent species, Dickinson and Remsen, 2013) and Passeriformes (6063 recent species, Dickinson and Christidis, 2014).
The clade also includes the extinct Quercypsittidae, a stem-group of Psittaciformes (Ksepka et al., 2011; Mayr, 2017), Psittacopedidae, which is a possible stem-group of Passeriformes (Mayr, 2015, 2020; Ksepka et al., 2019), and the taxon Zygodactylidae, which is believed to represent the extinct sister-group of passerines (Mayr, 2008b, see also Mayr, 2017). It is not clear whether Halcyornithidae, Messelasturidae and Vastanavidae are members of this clade (Ksepka et al., 2019; Mayr, 2015, 2017, 2021).
Diagnostic apomorphies: Suh et al. (2011) identified six retroposons that are apomorphic for this clade. A larger number of retroposons was identified in a subsequent study (Suh et al. 2015). Our examination of the morphological data set of Livezey and Zusi (2006, 2007) revealed no morphological apomorphies. However, a possible synapomorphy is the separation of the accessory trochlea of the fourth toe from the main body of this trochlea by a furrow, a character state that is shared between parrots and stem-Passeriformes but not found (i.e. likely lost) in crown Passeriformes (Mayr, 2015).
Synonyms: Passerimorphae (sensu Jarvis et al., 2014) is an approximate synonym.
Homonyms: There are no homonyms.
Comments: A clade comprising Psittaciformes and Passeriformes is supported by congruence of multiple phylogenomic data sets (Table 1). A node-based (minimum-crown-clade) definition was selected because there is congruent support for the basal dichotomy within Psittacopasserae in recent molecular analyses (Hackett et al., 2008; Suh et al., 2011, 2015; Wang et al., 2012; McCormack et al., 2013; Jarvis et al., 2014; Burleigh et al., 2015; Prum et al., 2015; Reddy et al., 2017; Liu et al., 2018; Kimball et al., 2019; Kuhl et al., 2021).
The name was spelled 'Psittacopasserae' by Suh et al. (2011) and subsequent authors. It is here amended to Psittacopasseres to make it grammatically correct (Passeres is the correct plural of Passer).
Not applicable.
The authors declare that they have no competing interests.
We are very grateful for the extensive and constructive comments of the two reviewers.
GS – Conceptualization, Investigation, Writing - Original Draft; ELB, USJ, RTK, GM, AS – Validation, Writing - Review & Editing. All authors read and approved the final manuscript.
|
Baker AJ, Pereira SL. Ratites and tinamous (Paleognathae). In: The TimeTree of Life (Ed. Hedges SB, Kumar, S. Oxford University Press). Pp. 412-414; 2009
|
|
Bock, W.J., Bühler, P., 1990. The evolution and biogeographical history of the paleognathous birds. In: 100th International DO-G Meeting. Current Topics in Avian Biology, Bonn, pp. 31–36
|
|
Braun EL, Cracraft J, Houde P. Resolving the avian tree of life from top to bottom: The promise and potential boundaries of the phylogenomic era. In: Kraus R (editor). Avian genomics in ecology and evolution. Springer, Cham, pp. 151-210; 2019
|
|
Cantino PD, De Queiroz K. International Code of Phylogenetic Nomenclature (PhyloCode). Boca Raton: CRC Press; 2020
|
|
Clarke JA, Mindell DP, de Queiroz K, Hanson M, Norell MA, et al. Aves. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1247-1253; 2020
|
|
Cracraft J. Toward a phylogenetic classification of the recent birds of the world (Class Aves). Auk. 1981;98:681-714
|
|
Cracraft J. The major clades of birds. pp. 339-361 in Benton MJ (ed.). The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds. Systematics Association Special volume 35A, Clarendon Press, Oxford; 1988
|
|
Cracraft J. Avian higher-level relationships and classification: Nonpasseriforms. In: Dickinson EC, Remsen JV Jr (editors). The Howard and Moore complete checklist of the birds of the world. Fourth edition, vol. 1: Non-passerines. Aves Press, London, pp. xxi-xliii; 2013
|
|
Cracraft J, Mindell DP. The early history of modern birds: a comparison of molecular and morphological evidence. Pp. 389-403 in Fernholm B, Bremer K, Jornvall H (eds.). The Hierarchy of Life. Elsevier, Amsterdam; 1989
|
|
Cracraft J, Barker FK, Braun M, Harshman J, Dyke GJ, et al. Phylogenetic relationships among modern birds (Neornithes): towards an avian tree of life, in Cracraft J, Donoghue M (eds), Assembling the Tree of Life, pp. 468-489; 2004
|
|
De Queiroz K. Linnaean, rank-based, and phylogenetic nomenclature: restoring primacy to the link between names and taxa. Symb Bot Ups. 33:127-140; 2005
|
|
De Queiroz K, Cantino P, Gauthier J (eds). Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press; 2020
|
|
del Hoyo J, Elliott A, Sargatal J (eds.). Handbook of the Birds of the World. Vol. 1. Ostrich to Ducks. Lynx Edicions, Barcelona; 1992
|
|
del Hoyo J, Elliott A, Sargatal J (eds.). Handbook of the Birds of the World. Vol. 6. Mousebirds to hornbills. Lynx Edicions, Barcelona; 2001
|
|
Dickinson EC, Remsen JV Jr. The Howard and Moore Complete Checklist of the Birds of the World (4th edition). Vol 1: Non-passerines. Aves Press, London; 2013
|
|
Dickinson EC, Christidis, L. The Howard and Moore Complete Checklist of the Birds of the World (4th edition). Vol 2: Passerines. Aves Press, London; 2014
|
|
Elzanowski A. Cretaceous birds and avian phylogeny. Courier Forschungsinst Senckenb. 1995;181:37-53
|
|
Feduccia A. The morphological evidence for ratite monophyly: fact or fiction. Proc Int Ornithol Congr 1985;18:184-190
|
|
Fjeldsa J. The systematic affinities of the sandgrouse, Pteroclididae. Vidensk Medd Dansk Naturh Foren 1976;139:179-243
|
|
Gadow H. Vogel. II. Systematischer Theil. In Bronn, H.G. Klassen und Ordnungen des Thier-Reichs. Leipzig: C.F. Winter Pt 4; 1893
|
|
Gauthier J, De Queiroz K. Feathered dinosaurs, flying dinosaurs, crown dinosaurs, and the name “Aves”. In: Gauthier JA, Gall LF (eds), New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom, Yale Peabody Museum, New Haven, pp. 7-41; 2001
|
|
Gill BJ (Convener). Checklist of the Ornithological Society of New Zealand . Checklist of the Birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington: Te Papa Press, OSNZ; 2010
|
|
Houde P. Palaeognatous birds from the early Tertiary of the northern Hemisphere. Publ Nuttall Ornithol Club. 1988;22:1-148
|
|
Houde P, Olson SL. A radiation of coly-like birds from the Eocene of North America (Aves: Sandcoleiformes new order). Natural History Museum of Los Angeles County, Science Series. 1992;36:137-160
|
|
Hume JP, Walters M. Extinct birds. London: Bloomsbury; 2012
|
|
Huxley TH. On the classification of birds; and on the taxonomic value of the modifications of certain of the cranial bones observable in that class. Proc Zool Soc London. 1867:415-472
|
|
ICZN. International code of zoological nomenclature. Fourth edition. London: International Trust for Zoological Nomenclature; 1999
|
|
Kurochkin EN. Synopsis of mesozoic birds and early evolution of class Aves. Archaeopteryx. 1995;13:47-66
|
|
Manegold A. 2005. Zur Phylogenie und Evolution der „Racken”-, Specht- und Sperlingsvogel („Coraciiformes”, Piciformes und Passeriformes: Aves). PhD Dissertation. Berlin
|
|
Marchant S, Higgins P. Handbook of Australian, New Zealand & Antarctic Birds (Vol. 1). Melbourne: Oxford University Press; 1990
|
|
Mayr G. Avian higher-level phylogeny: well-supported clades and what we can learn from a phylogenetic analysis of 2954 morphological characters. J Zool Syst Evol Res. 2008a;46:63-72
|
|
Mayr G. Paleogene Fossil Birds. Heidelberg, Springer; 2009
|
|
Mayr G. Avian Evolution: The Fossil Record of Birds and its Paleobiological Significance. Chichester, Wiley-Blackwell; 2017
|
|
Mayr G. Hindlimb morphology of Palaeotis suggests palaeognathous affinities of the Geranoididae and other “crane-like” birds from the Eocene of the Northern Hemisphere. Acta Palaeontol Polon. 2019;64:669-678
|
|
Mayr G. Paleogene fossil birds, 2nd edition. Heidelberg, Springer; 2022
|
|
Mayr G, Ericson PGP. Evidence for a sister group relationship between the Madagascan mesites (Mesitornithidae) and cuckoos (Cuculidae). Senckenb Biol. 2004;84:119-135
|
|
McKitrick MC. Phylogenetic analysis of avian hindlimb musculature. Misc Publ Mus Zool Univ Michigan. 1991a;179:1-85
|
|
Meise W. Verhalten der Straussartigen Vogel und Monophylie der Ratitae. Proc Int Ornithol Congr. 1963;8:115-125
|
|
Mindell DP. Galloanserae. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1255-1257; 2020
|
|
Mindell DP, Honeycutt RL. Variability in transcribed regions of ribosomal DNA and early divergences in birds. Auk. 1989;106:539-548
|
|
Mindell DP, Sorenson MD, Huddleston CJ, Miranda HC, Knight A, et al. Phylogenetic relationships among and within select avian orders based on mitochondrial DNA. In Mindell DP (ed.). Avian molecular evolution and systematics. San Diego: Academic Press, pp. 213-247; 1997
|
|
Olson SL. The fossil record of birds. Avian Biol. 1985;8:79-238
|
|
Prager EM, Wilson AC. Phylogenetic relationships and rates of evolution in birds. Acta IOC. 1980:1209-1214
|
|
Sangster G. Mirandornithes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1265-1267; 2020a
|
|
Sangster G. Charadriiformes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1269-1272; 2020b
|
|
Sangster G. Procellariiformes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1273-1276; 2020c
|
|
Sangster G. Strigiformes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1277-1280; 2020d
|
|
Sangster G. Psittaciformes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1285-1288; 2020e
|
|
Sangster G. Daedalornithes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1289-1291; 2020f
|
|
Sangster G. Apodiformes. In: De Queiroz K, Cantino PD, Gauthier, J (eds) Phylonyms: a Companion to the PhyloCode. Boca Raton: CRC Press, Taylor & Francis Group, pp. 1293-1296; 2020g
|
|
Sclater PL. Remarks on the present state of the systema avium. Ibis. 1880;22(4):340-350, 399-411.
|
|
Sharpe RB. A review of recent attempts to classify birds. Proc. 2nd Int. Ornithol. Congr., Budapest; 1891
|
|
Sibley CG, Ahlquist JE. Phylogeny and Classification of Birds. New Haven: Yale Univ. Press; 1990
|
|
Wetmore A. A classification for the birds of the world. Smithson Misc Coll. 1960;139(11):1-37
|
|
Worthy TH, Holdaway RN. The lost world of the moa: prehistoric life of New Zealand. Bloomington, IN: Indiana University Press; 2002
|
| Source | Data type(s) | Palaeognathae | Neognathae | Neoaves | Columbimorphae | Pteroclimesites | Musophagotides | Phaethoquornithes | Phaethontimorphae |
| Hackett etal. (2008) | 19 nuclear loci | 100% ML bootstrap | 96% ML bootstrap | 96% ML bootstrap | < 70% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered |
| McCormack etal. (2013) | 1541 ultra-conserved elements | > 0.95 PP, > 70% bootstrap | > 0.95 PP, > 70% bootstrap | Not recovered | > 0.95 PP, > 70% bootstrap | Not recovered | 96% ML bootstrap | ||
| Jarvis etal. (2014) | 8251 exon loci, 2516 intron loci, 3769 ultra-conserved elements | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 55% exaML bootstrap | 70% exaML bootstrap | 100% exaML bootstrap |
| Burleigh etal. (2015) | 25 nuclear loci, mitochondrial DNA | 95% ML bootstrap | 95% ML bootstrap | 93% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered | Not recovered |
| Prum etal. (2015) | 259 anchored nuclear loci | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | Not recovered | 1.0 PP | 1.0 PP |
| Suh etal. (2015) | 2118 retroposon presence/absence loci | Not recovered | 8 retroposons | 7 retroposons | 5 retroposons | 20 retroposons | |||
| Reddy etal. (2017) | 54 nuclear loci | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | < 70% ML bootstrap | Not recovered | Not recovered | < 70% ML bootstrap |
| Liu etal. (2018) | 63 nuclear protein-coding loci | 100% ML bootstrap | 100% ML bootstrap | 100% ML bootstrap | Not recovered | Not recovered | Not recovered | Not recovered | 100% ML bootstrap |
| Kimball etal. (2019) | supertree | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 85% MRP bootstrap | 100% MRP bootstrap | 25% MRP bootstrap | 43% MRP bootstrap | 100% MRP bootstrap |
| Kuhl etal. (2021) | 3′UTR sequences (2.5 million analyzable patterns) | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) | Not recovered | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) | 100% (SH-aLRT) |
| Source | Aequornithes | Procellariimorphae | Pelecanimorphae | Pelecanes | Telluraves | Afroaves | Coraciimorphae | ||
| Hackett et al.(2008) | 89% ML bootstrap | 98% ML bootstrap | 81% ML bootstrap | 88% ML bootstrap | 98%ML bootstrap | < 70%ML bootstrap | Not recovered | ||
| McCormack et al.(2013) | >0.95 PP, >70% bootstrap | 99% ML bootstrap | >0.95 PP, 66% bootstrap | Not recovered | Not recovered | ||||
| Jarvis et al.(2014) | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | ||||
| Burleigh et al.(2015) | Not recovered | 99% ML bootstrap | 77% ML bootstrap | 73% ML bootstrap | 49% ML bootstrap | 50% ML bootstrap | Not recovered | ||
| Prum et al.(2015) | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | Not recovered | 1.0 PP | ||
| Suh et al.(2015) | 16 retroposons | 212 retroposons | 64 retroposons | 16 retroposons | Not recovered | ||||
| Reddy et al.(2017) | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | < 70% ML bootstrap | ||
| Liu et al.(2018) | 99% ML bootstrap | 80% ML bootstrap | 97% ML bootstrap | Not recovered | < 70% ML bootstrap | ||||
| Kimball et al.(2019) | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 72% MRP bootstrap | ||
| Kuhl et al.(2021) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | ||
| Source | Cavitaves | Eucavitaves | Picocoraciae | Picodynastornithes | Australaves | Eufalconimorphae | Psittacopasserae | ||
| Hackett et al.(2008) | 85% ML bootstrap | 71% ML bootstrap | 98% ML bootstrap | 98% ML bootstrap | 64% ML bootstrap | 73% ML bootstrap | 77% ML bootstrap | ||
| McCormack et al.(2013) | >0.95 PP, >70% bootstrap | >0.95 PP, >70% bootstrap | Not recovered | >0.95 PP, >70% bootstrap | >0.95 PP, >70% bootstrap | ||||
| Jarvis et al.(2014) | 100% exaML bootstrap | 72% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | 100% exaML bootstrap | ||
| Burleigh et al.(2015) | 76% ML bootstrap | 69% ML bootstrap | 89% ML bootstrap | 85% ML bootstrap | 72% ML bootstrap | 59% ML bootstrap | 47% ML bootstrap | ||
| Prum et al.(2015) | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | 1.0 PP | ||
| Suh et al.(2015) | 124 retroposons | 16 retroposons | 6 retroposons | 5 retroposons | 176 retroposons | 88 retroposons | 16 retroposons | ||
| Reddy et al.(2017) | ≥70% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | ≥95% ML bootstrap | < 70% ML bootstrap | ≥95% ML bootstrap | ||
| Liu et al.(2018) | 71% ML bootstrap | 93% ML bootstrap | 95% ML bootstrap | 100% ML bootstrap | < 70% ML bootstrap | 85% ML bootstrap | 92% ML bootstrap | ||
| Kimball et al.(2019) | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap | ||
| Kuhl et al.(2021) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | 100%(SH-aLRT) | ||
| Source | Data type(s) | Notopalaeognathae | Novaeratitae | Dinocrypturi |
| Hackett etal. (2008) | 19 nuclear loci | 100% ML bootstrap | 96% ML bootstrap | |
| Harshman etal. (2008) | 20 nuclear loci | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP | |
| Phillips etal. (2010) | mitochondrial DNA | 44% ML bootstrap, 0.99 PP | 92% ML bootstrap, 1.0 PP | 99% ML bootstrap, 1.0 PP |
| Haddrath and Baker (2012) | 27 nuclear loci | 1.0 PP | 1.0 PP | 1.0 PP |
| Smith etal. (2013) | 60 nuclear loci, mitochondrial DNA | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Mitchell etal. (2014) | mitochondrial DNA | 63% ML bootstrap, 1.0 PP | 87% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Burleigh etal. (2015) | 25 nuclear loci, mitochondrial DNA | 86% ML bootstrap | 100% ML bootstrap | 44% ML bootstrap |
| Grealy etal. (2017) | 154 nuclear loci, mitochondrial DNA | 80% ML bootstrap, 1.0 PP | 57% ML bootstrap, 1.0 PP | 100% ML bootstrap, 1.0 PP |
| Reddy etal. (2017) | 54 nuclear loci | ≥95% ML bootstrap | ≥95% ML bootstrap | |
| Yonezawa etal. (2017) | Morphology | 92% ML bootstrap, 0.99 PP | 100% ML bootstrap, 0.94 PP | 96% ML bootstrap, 0.99 PP |
| Cloutier etal. (2019) | 20, 850 nuclear loci | 100% ML bootstrap | 100% ML bootstrap | 100% ML bootstrap |
| Kimball etal. (2019) | supertree | 100% MRP bootstrap | 100% MRP bootstrap | 100% MRP bootstrap |