| Years | August | September | ||
| Sunrise | Sunset | Sunrise | Sunset | |
| 2016 | 5:07:23 | 18:48:33 | 5:35:11 | 18:02:06 |
| 2017 | 5:07:10 | 18:48:52 | 5:34:58 | 18:02:30 |
| 2018 | 5:06:57 | 18:49:11 | 5:34:45 | 18:02:53 |
| Citation: | Hongying Xu, Zeyu Yang, Dongping Liu, Ru Jia, Lixia Chen, Boshi Liang, Zhengwang Zhang, Guogang Zhang. 2022: Autumn migration routes of fledgling Chinese Egrets (Egretta eulophotes) in Northeast China and their implications for conservation. Avian Research, 13(1): 100018. DOI: 10.1016/j.avrs.2022.100018 |
The Chinese Egret (Egretta eulophotes) is a globally threatened bird species living on the coast and islands of Liaoning, northeastern China, mainly in summer. To further protect the breeding population of Chinese Egrets, it is important to understand the current protection status of their distribution sites at pre-migration period and migration routes. Thirty-three individuals were tagged with satellite transmitters at Fantuo Island in Changhai and Xingren Island in Zhuanghe, Liaoning Province, northeastern China, in July of 2016, 2017, and 2018, to identify important distribution sites during the pre-migration period, as well as detailed migration routes. The results showed that coastal mudflats in Liaoning and the west coast of North Korea were important feeding and roosting sites for fledgling Chinese Egrets from August to September. The home range sizes in August were significantly larger than in September. The eastern coast from Shandong to Guangdong, as well as Taiwan, China, and Manila Bay and Galileo Islands in the Philippines, were important stopover sites during fall migration. Specifically, we found that the egrets’ autumn migration could be divided into four routes, i.e., sea-crossing migration (SCM), coastal migration (CM), inland migration (IM), and mixed migration (MM). The migration distance, timing, speed, and straightness of the four routes also differed. The SCM routes were the straightest, and had the fastest migration speed and shortest travel time, while the IM routes had the lowest straightness and speed, and the longest duration. Manila Bay and Bohol Island in the Philippines, the west coast of Tanintharyi in Myanmar, and the Zengwun River Estuary in Taiwan, China, were wintering sites. Our findings on the key distribution sites along pre-migration and fall migration routes, including some stopover sites, have important implications for the conservation of and global action plan development for the vulnerable Chinese Egret.
The Chinese Egret (Egretta eulophotes) is a globally threatened bird species classified as “Vulnerable” by the International Union for the Conservation of Nature (IUCN, 2016) and listed as a “First-Class National Protected Species” in China (http://www.forestry.gov.cn/main/5461/20210205/122418860831352.html). Information on its population size remains scarce. During the winter of 1992, a total of 591 Chinese Egrets were recorded in Asia, including 143 in China and 448 in Southeast Asia, according to the Asian Midwinter Waterbird Survey organized by the International Bureau of Waterfowl Research (Tian, 2002). The global population of Chinese Egrets is currently estimated at 2500 to 10,000 mature individuals; these birds winter in the Philippines, Vietnam, Malaysia, Singapore, Indonesia, and Brunei, and breed in Russia, North Korea, South Korea, and China (BirdLife International, 2021).
Zhuanghe and Changhai in Liaoning Province, northeastern China, are important breeding and habitat areas for Chinese Egrets, and are currently facing severe challenges (Yin and Lei., 2002; Zhang et al., 2018). For instance, the main breeding ground on Liaodong Peninsula is situated in the Liaoning coastal economic zone, where a large area of mudflats has been reclaimed for aquaculture, industry, and agricultural development in recent years (Zhang et al., 2017); this land-use change has resulted in severe habitat loss and heavy metal and organic pollution, threatening the breeding activities and survival of Chinese Egrets (Fisheries Administration, Ministry of Agriculture, 2015; Wang et al., 2015). Furthermore, basic knowledge of migration routes and important stopover sites of Chinese Egrets remains limited, thus this species has not been protected effectively at the flyway level because of this lack of knowledge. Therefore, understanding the current conservation status of important distribution sites during the pre-migration period along the coast of Liaoning and determining important stopover and wintering sites on its migration routes are crucial to protect the Chinese Egrets population.
To address the above issues, we identified important distribution sites in the pre-migration period and determined the migration routes and important stopover and wintering sites of the breeding population of fledgling Chinese Egrets on Liaodong Peninsula using global positioning system (GPS) tracking during the breeding period from 2016 to 2018. We also conducted spatial overlap analysis with Chinese nature reserves to determine whether the stopover sites were in protected areas of China. Based on the research results regarding important coastal stopovers and wintering areas of the egret population, we summarized the environmental problems and major challenges that these areas are currently facing to analyze the current conservation status of Chinese Egrets during the autumn migration. Finally, we propose suggestions to improve the conservation of coastal wetlands, which will effectively promote egret population growth.
Fantuo Island, Changhai (39°9′2″ N, 122°18′11″ E), located approximately 2 km west of Guanglu town in Changhai County, Liaoning Province, covers an area of 0.09 km2 and has a maximum elevation of 71.4 m. The island is mainly covered by shrubs, and this provides the most suitable nesting habitat for the egrets. Other common species on the island include the Pelagic Cormorant (Phalacrocorax pelagicus) and Black-tailed Gull (Larus crassirostris).
Xingren Island, Zhuanghe (39°31′44″ N, 123°2′29″ E), located approximately 0.5 km east of Shicheng town in Zhuanghe City, Liaoning Province, covers an area of approximately 0.25 km2 and has a maximum elevation of 46 m. The island hosts sparse vegetation with low eucalyptus trees and shrubs on the northern and eastern slopes and sparse shrubs and grasses on the southern slope; this terrain provides suitable nesting habitats for the egrets. Other common species on the island include the Black-faced Spoonbill (Platalea minor), Pelagic Cormorant (P. pelagicus) and Black-tailed Gull (L. crassirostris).
A total of 33 fledgling Chinese Egrets were captured, of which eight individuals were captured on Fantuo Island in 2016, and 25 individuals were captured on Xingren Island in 2017 and 2018. During each visit, we went to the island before dark and waited until 22:00 h to approach the nests from the top of the cliff where the egrets prefer to nest. The capture team comprised two people, one with a strong rope tied around their waist, who slowly approached the nests from the top of the cliff and then captured individual egrets by using a strong hand-held light. In total, 33 fledglings were captured from different nests to determine the different migration routes in the three consecutive breeding seasons. We tagged each egret with a GPS transmitter (YH-GTG0317, Hangzhou Yuehai Technology Ltd., China, hereafter HZYH) using the backpack method (Nagendran et al., 1994). Each transmitter weighed 10–12 g, and the harness weighed approximately 4 g, equivalent to approximately 2.3%–3.3% of an egret's body mass (range 415–690 g, n = 13).
The solar-powered transmitters were programmed to record a GPS position every 2 h, and data were received via the China Mobile Communication System. The data, decoded and downloaded through the web client, included time, longitude, latitude, and location accuracy. The location accuracy was measured using position dilution of precision (PDOP, ranging from 0.5 to 99.9). The status of transmitters that had signals that were interrupted for periods of days to months at one location, but then recovered elsewhere after interruption, was defined as signal interruption and recovery; the status of transmitters that gave persistent signals for days or months at one location, but little change of latitude and longitude and abnormal equipment temperature, was defined as individual death or equipment falling off. Finally, a total of 11,874 GPS locations were used to analyze the migration of the egrets.
The egrets in this study left their breeding islands in late July to early August and moved to coastal beaches and islands (including on the Korean Peninsula) near the breeding site, and then they left those sites in late September and migrated southward. When the egrets began to migrate south without returning to the wandering areas, the last location point of the egrets in those areas was defined as the starting point of migration (Hübner et al., 2010; Deng et al., 2019). Sites where transmitter signals indicated that the egrets stayed at one site in the winter (excluding individual deaths), were defined as wintering sites. The first location point at which the egrets arrived within the wintering sites was defined as the last point of migration (Hübner et al., 2010; Batbayar et al., 2011; Podhrázský et al., 2017). All location points from August to September before the first point of migration were used to calculate the movement distance of egrets in the pre-migration period.
During migration, sites where birds remained within 0.5 degree of latitude and where two successive fixes were less than 50 km apart were considered to be stopover sites (Kuang et al., 2020). Stopover sites were divided into resting and feeding sites (Warnock, 2010). Stops exceeding 48 h are likely foraging stopovers with the possibility of net energy gain for the birds, whereas this is quite unlikely for birds staying less than 48 h (Green et al., 2002). Therefore, the sites at which the duration of stay was ≤48 h were defined as resting sites, and sites where the duration of stay was >48 h were defined as feeding sites and considered as relatively important in the migration routes of the egrets (van Wijk et al., 2012; Kölzsch et al., 2015; Deng et al., 2019). The migration distance was obtained by calculating the actual distance between stopover sites from the first to the last point of migration (Green et al., 2002; Kölzsch et al., 2015). Additionally, the geographical location of the stopover site for each individual was the arithmetic mean of all of the geographical coordinates at each site (Giunchi et al., 2019). The straightness of the migration route was the ratio between the shortest distance (length of expected shortest route between breeding site and wintering site with constant compass bearing) and the distance of the real migration routes (Benhamou, 2004).
Important distribution sites were identified in the pre-migration period. Locations of PDOP <2.0 were used, corresponding to an accuracy of <20 m, and a velocity of <3 km/h was selected to calculate the home range and movement distance (Jia et al., 2020). A total of 2538 GPS locations were used to calculate home range size, and 2029 were used to calculate movement distance.
The daily sunrise and sunset times for Dalian were obtained for August to September from the Chinese meteorological website (https://www.51240.com). To delineate daytime and nighttime hours for each month, we chose an intermediate time for each month as a representative sunrise time, based on all sunrise times over that month, and chose the sunset time in the same way. We defined day and night cycles for each month (Table 1).
| Years | August | September | ||
| Sunrise | Sunset | Sunrise | Sunset | |
| 2016 | 5:07:23 | 18:48:33 | 5:35:11 | 18:02:06 |
| 2017 | 5:07:10 | 18:48:52 | 5:34:58 | 18:02:30 |
| 2018 | 5:06:57 | 18:49:11 | 5:34:45 | 18:02:53 |
We obtained the boundary data for the national nature reserves of China from the Resources and Environment Science and Data Center of the Chinese Academy of Sciences (http://www.resdc.cn/data.aspx?DATAID=272), and we used ArcGIS 10.2 (ESRI, Redlands, CA, USA) to conduct spatial overlap analysis with Chinese nature reserves to determine whether the stopover sites were in protected areas of China.
Data were analyzed in ArcGIS 10.2, and then the kernel density estimation (KDE; Calenge, 2011) method was used in R (version 3.3, ‘adehabitatHR’ package) to analyze the home range size (90% kernel) and core area (50% kernel) of the egrets in the pre-migration period to determine the important distribution sites. Differences in movement distance and residence days were analyzed using the Kruskal-Wallis test for the period from August to September. The differences in home range (90% and 50% KDE) and movement distance between August and September in the late breeding period were tested for significance using non-parametric Mann-Whitney U-tests. All statistical analyses were performed using SPSS Statistics 22.0 (IBM Inc., NY, USA). Data are shown as the mean ± standard error (SE).
Thirty-three Chinese Egrets were satellite-tracked from 2016 to 2018, from Fantuo and Xingren islands, of which 18 individuals successfully departed their breeding grounds. Of these 18 egrets, four individuals completed the autumn migration and arrived at their wintering grounds, and another seven individuals completed most of the autumn migration. Data for these 11 individuals were used in the analysis of migration routes. The transmitters of seven egrets stopped transmitting signals at an early migration stage and were excluded from the analysis. There was no difference in distribution locations and migration routes between the breeding populations of Chinese Egrets on Fantuo and Xingren islands.
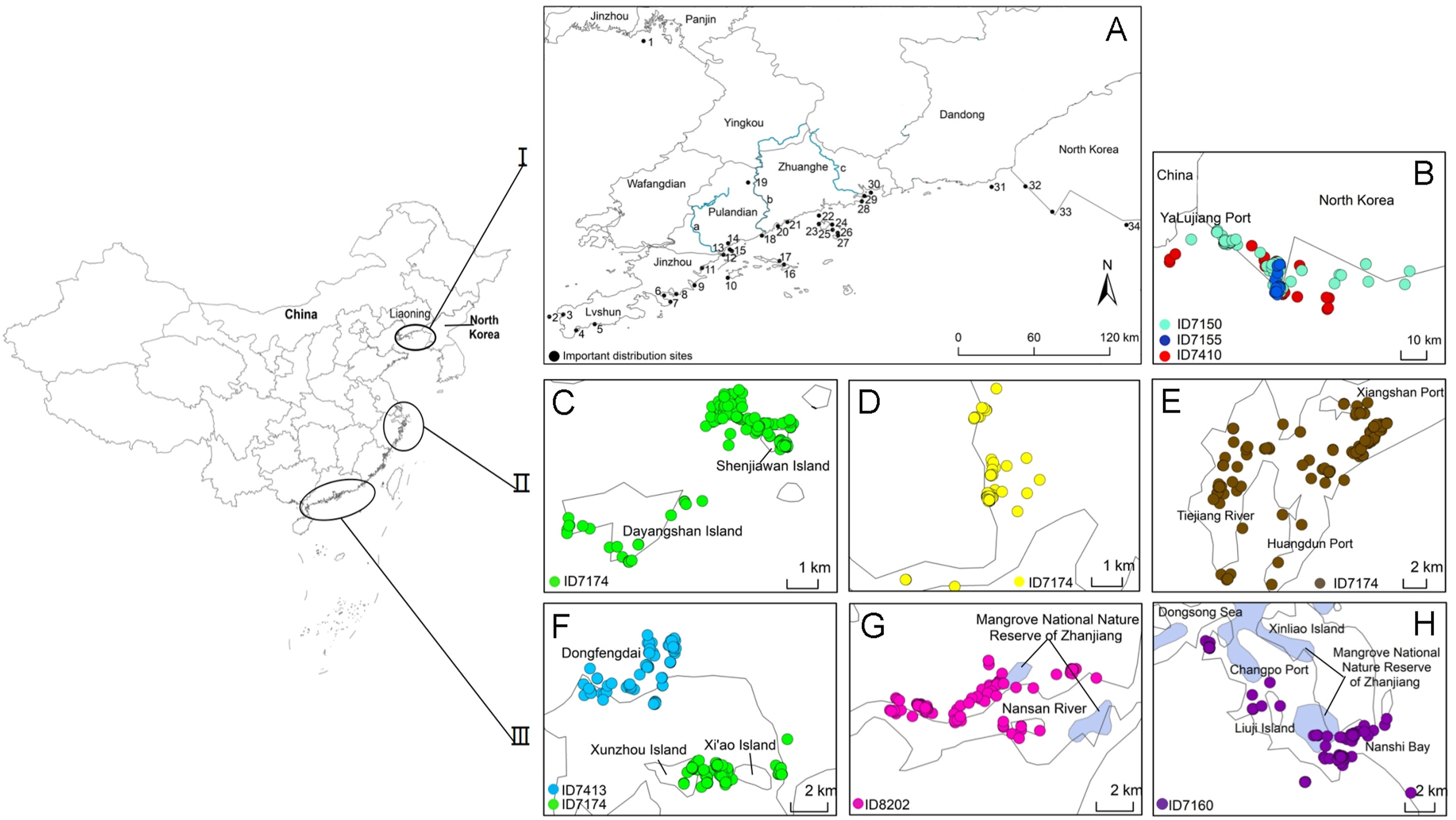
Analysis of the individual location data of 33 egrets showed that the egrets left their breeding islands in late July to early August. Then they traveled to coastal beaches and islands, including on the Korean Peninsula, near their breeding sites. Important distribution sites mainly included coastal mudflats in Liaoning, including the Yingna Estuary and Huli Estuary in Zhuanghe, the Qingshui Estuary in Pulandian, the Dengsha Estuary in Jinzhou, and Shuangdao Bay in Lvshunkou. In addition, some individuals traveled westward during their wandering period to the Liaohe Estuary in Panjin, while others went eastward to the Yalu Estuary, and then to the Cholsan Peninsula and the west coast of North Korea (Fig. 1).
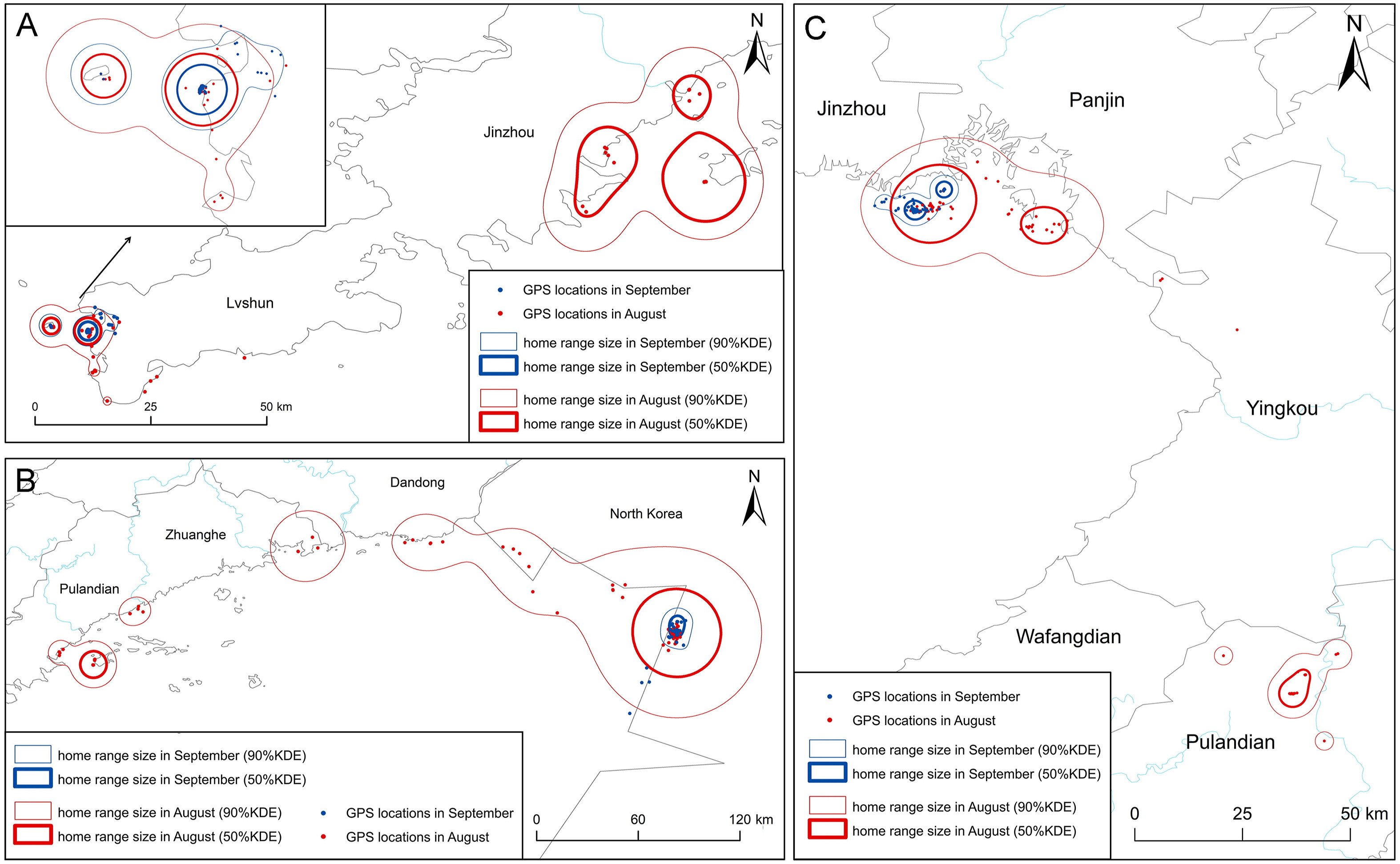
The home range sizes (50% KDE and 90% KDE) in August were 245 ± 433 km2 and 1221 ± 2706 km2, respectively, which were significantly larger than the respective home ranges in September (24 ± 21 km2 and 88 ± 80 km2; 50% KDE Z = −1.987, p = 0.047, n = 31; 90% KDE Z = −2.028, p = 0.014, n = 27). The average movement distance for August (212 ± 173 km) was greater than that recorded in September (112 ± 109 km), but there was no significant difference (p = 0.06). The egrets roosted at nighttime and foraged both during daytime and nighttime. There was no significant difference in home range and movement distance between daytime and nighttime in August and September (p > 0.05) (Fig. 2).
Chinese Egrets departed the breeding site on 5–6 October (median value, range from 22 September to 2 November, n = 14) and arrived at the wintering grounds from 11 October to 12 December (n = 4). The average migration distance (mean ± SE) from breeding ground to wintering grounds was 3175 ± 1171 km, and the mean travel time (mean ± SE) was 6 ± 1.47 days (n = 4) (Table 2).
| ID | Date departing from breeding site | Stopover sites | Coordinates of stopover sites | Duration time (h) | Migration distance (km) and days (in brackets) | Expected shortest distance (km) | Straightness | Migration speed (km/d) | Date arriving in wintering sites (duration days) | Wintering grounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inland migration | 8201 | Oct. 21, 2016 | Shihe River of Luoshan, Xingyang, Henan | 114.42° E, 32.25° N | 265 | 3055 (9) | 2428 | 0.79 | 339 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hebao Reservoir, Lingchuan, Guangxi | 110.19° E, 25.42° N | 275 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coastal migration | 7174 | Sep. 22, 2018 | Dayangshan and Xiaoyangshan Island, Hangzhou Bay, Zhejiang | 122.11° E, 30.61° N | 720 | 4684 (10) | 3627 | 0.77 | 468 | Dec. 12, 2018 (26) | The west coast of Tanintharyi, Myanmar | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zhelin Bay, Chaozhou, Guangdong | 117.05° E, 23.58° N | 841 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8215 | Oct. 5, 2016 | Zhujiang Estuary, Guangdong | 113.68° E, 22.60° N | 119 | 2758 (4) | 2124 | 0.77 | 690 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7412 | Oct. 11, 2017 | Quanzhou Bay, Fujian | 118.66° E, 24.84° N | 48 | 2169 (5) | 1880 | 0.87 | 434 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zhangjiang Estuary, Zhangzhou, Fujian | 117.47° E, 23.81° N | 72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lianyang River, Shantou, Guangdong | 116.78° E, 23.48° N | 204 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7413 | Oct. 11, 2017 | Wenzhou Bay, Zhejiang Zhelin Bay, Chaozhou, Guangdong | 120.85° E, 27.79° N 117.02° E, 23.64° N | 170 | 3357 (6) | 2821 | 0.84 | 560 | Nov. 22, 2017 (108) | Manila Bay, Philippines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 646 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sea-crossing migration | 7163 | Sep. 23, 2018 | Polilio Island, Philippines | 122.21° E, 14.85° N | 298 | 3501 (5) | 3319 | 0.95 | 700 | Oct. 16, 2018 (52) | Bohol Island, Philippines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bondoc Peninsula, Philippines | 122.64° E, 13.19° N | 119 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7151 | Oct. 1, 2018 | – | – | – | 2104 (2) | 2051 | 0.97 | 1052 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7152 | Oct. 6, 2018 | – | – | – | 2124 (3) | 1874 | 0.88 | 708 | Oct. 11, 2018 (233) | Zengwun River Estuary of Taiwan, China | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8329 | Oct. 6, 2018 | Manila Bay, Philippines | 120.84° E, 14.75° N | 690 | 3135 (3) | 2810 | 0.90 | 1045 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mixed migration | 7160 | Sep. 23, 2018 | From Dongsong Sea,Changpo Port to Nanshi Bay, Zhanjiang, Guangdong | 110.45° E, 20.57° N | 658 | 3168 (5) | 2696 | 0.85 | 634 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8202 | Oct. 6, 2016 | Nansan River, Zhanjiang, Guangdong | 110.60° E, 21.23° N | 935 | 3305 (6) | 2642 | 0.80 | 551 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others | 8200 | Nov. 2, 2016 | Jinshan Port, Yantai, Shandong | 121.70° E, 37.45° N | 62 | 693 (1) | 623 | 0.90 | 693 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| East of Chaowai Village, Yantai, Shandong | 121.13° E, 36.67° N | 61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coastal mudflat of Dafeng, Yancheng, Jiangsu | 120.75° E, 33.32° N | 164 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8203 | Sep. 28, 2016 | Sanmen Bay, Zhejiang | 121.63° E, 29.06° N | 846 | 1628 (7) | 1427 | 0.88 | 233 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7417 | Sep. 27, 2017 | South of Henggang River, Taizhou, Jiangsu | 120.04° E, 33.03° N | 93 | 1361 (4) | 1130 | 0.83 | 340 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| From Tiejiang River, Huangdun Port to Xiangshan Port, Ninghai, Zhejiang | 121.59° E, 29.50° N | 682 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7410 | – | Longjiang Estuary, Fuqing, Fujian | 119.53° E, 25.69° N | 407 | – | – | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| “Others” refer to egrets with lost transmitter signals. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The wintering grounds were located in Manila Bay (14.36° N, 120.79° E) and Bohol Island (10.24° N, 124.25° E) in the Philippines, the west coast of Tanintharyi in Myanmar (14.35° N, 97.98° E), and the Zengwun River Estuary in Taiwan, China (23.05° N, 120.05° E).
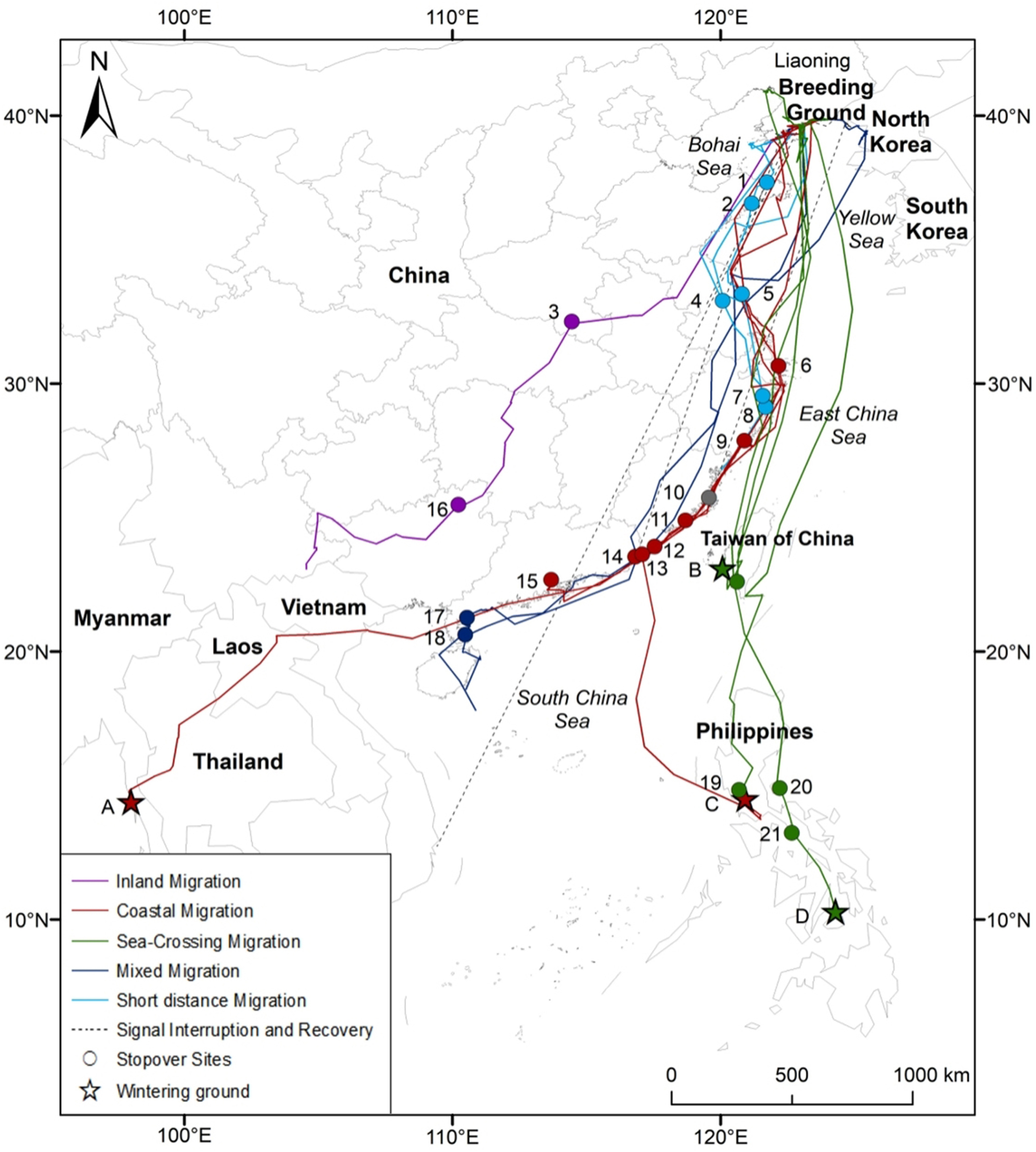
The autumn migration routes of 11 Chinese Egrets were divided into four routes according to the geographical features of the migration route, i.e., sea-crossing migration (n = 4), coastal migration (n = 4), inland migration (n = 1), and mixed migration (n = 2) (Fig. 3).
After departing the breeding ground, the egrets crossed the Yellow Sea and the East China Sea directly to Taiwan, China, or they crossed the Yellow Sea to the eastern coasts of Jiangsu and Zhejiang Provinces and then to Taiwan, China, across the East China Sea. Some individuals wintered in the Zengwun River Estuary in Taiwan, China, and others continued to migrate south across the Bashi Strait and arrived at their wintering grounds at Manila Bay and Bohol Island in the Philippines.
After departing the breeding ground, some egrets crossed the Yellow Sea directly to the coasts of Yancheng and Nantong, Jiangsu Province, and others migrated southwest across the Yellow Sea directly to the eastern coast of Shandong Province, stayed for a number of days, and continued to migrate south to the coastal area of Yancheng, Jiangsu Province, across the Yellow Sea, where there was a convergence site of two migration routes.
The egrets stayed at Yancheng for a few days or continued to migrate south, and they flew directly south to the eastern coast of Zhejiang or via the eastern coast of Shanghai. The other route was along the eastern coast of Shanghai to the eastern coast of Zhejiang, after which the egrets continued to migrate south to Quanzhou Bay, Fujian Province and finally to the Pearl River Estuary and Zhelin Bay in Guangdong after stopping for a few days. Then, some egrets migrated to the wintering site in Manila Bay in the Philippines, and others migrated westward to the west coast of Tanintharyi in Myanmar via Haiphong in Vietnam, Ramkhamhaeng National Park and Thap Salao in Thailand, or Nam Et-Phou Louey National Protected Area in Laos across the Beibu Gulf in Guangxi.
After departing the breeding grounds, the egrets migrated southwest across the Yellow Sea to Hongze Lake in Suqian, Jiangsu Province, after stopping for 1 day, and then they continued to migrate southwest to Shihe in Xinyang, Henan Province, via northern Anhui Province. After 11 days, the egrets continued to migrate to the Hebao Reservoir in Guilin, Guangxi. After 11 days, the egrets migrated to southeastern Yunnan Province via Xingyi in Guizhou Province; after this, the transmitter signals were lost.
After departing the breeding ground, the egrets crossed the Yellow Sea directly to Yancheng, Jiangsu Province, and further south, or they stopped for a few days or continued to migrate south; then turned inland following two routes: one egret (ID 8202) migrated to Lianyang River in Shantou, Guangdong Province, via central Zhejiang and Fujian Provinces, and then it continued to the Nansan River via the eastern coast of Guangdong, stayed for 39 days, and continued to migrate to Hainan Island across the Qiongzhou Strait via the eastern coast of Zhanjiang; after this, the bird's transmitter signal was lost.
One egret (ID 7160) migrated to Zhelin Bay at Chaozhou in Guangdong Province via Jinjiang in Jiangsu Province and the middle of Zhejiang and Fujian Provinces; then, it migrated to the mudflat area between Dongsong and Xinliao Islands in Zhanjiang. After 27 days, the egret migrated to Hainan Island across the Qiongzhou Strait, and then the bird's transmitter signal was lost.
During the autumn migration period, the important stopover sites for the egrets were mainly distributed on the eastern coast of China; there were 16 sites from Shandong to Guangdong and Taiwan, China, and two sites located at Xinyang in Henan and Lingchuan in Guangxi, mainly distributed in the Philippines (Table 2). Only approximately 7.04% of the important stopover sites on the autumn migration routes of the egrets were effectively protected in national protected areas, including Yancheng, Hongze Lake, Nansan River, Dongsonghai, Nanshiwan, and Zhangjiang Estuary; other important stopover sites, including Shihe River, Hangzhou Bay, Zhelin Bay, Pearl River Estuary, Quanzhou Bay, and Hebao Reservoir, face potential threats such as wetland destruction and water pollution due to a lack of effective protection.
The migration distance, timing, speed, and straightness of the four migration routes were also different. Compared with those in the other three migration routes, the SCM routes were straightest, the migration speed was the fastest, and the travel time was the shortest. There was no difference in the migration timing, speed, or route straightness between the CM and MM routes. In addition, IM had the lowest straightness, the slowest speed, and the longest duration (Table 3).
| Migration routes | Migration distance (km) | Migration days | Migration speed (km/day) | Straightness |
| Inland migration | 3055 | 9.00 | 339 | 0.79 |
| Coastal migration | 3242 ± 538 | 6.25 ± 1.31 | 538 ± 57 | 0.81 ± 0.03 |
| Sea-crossing migration | 2977 ± 303 | 3.25 ± 0.63 | 877 ± 99 | 0.93 ± 0.02 |
| Mixed migration | 3237 ± 69 | 5.50 ± 0.50 | 592 ± 41 | 0.85 ± 0.05 |
There was no significant difference in the duration at stopover sites among the four migration routes (H = 1.187, p = 0.756, df = 3) or between migration routes (p > 0.05). From highest to lowest, the duration at stopover sites was as follows: MM (162.00 ± 96.45 h), CM (118.04 ± 46.04 h), SCM (95.85 ± 54.42 h) and IM (65.60 ± 34.12 h).
There were significant differences in the home range sizes between August and September, showing a declining tendency similar to that of movement distance, which was related to the different activity routes during the pre-migration period. In August, the egrets had strong movement along the coastline of Liaoning and further to North Korea, at which time they were probably feeding to enhance their growth and development; thus, the home range size increased. In September, most individuals were concentrated at a few sites for the upcoming autumn migration, which made their home range size decline compared with that in August.
This study showed that the movement range of approximately 400 km along the coastline from Shuangdao Bay in Lvshunkou to the west coast of the Korean Peninsula, as well as the Liaohe Estuary in Panjin, contained important sites for the egrets during the pre-migration period. Xiang (2015) showed that the area of natural wetlands in these locations has declined significantly, and a large area of wetlands has been converted into farmland and residential areas. Previous studies have also found that heavy metal pollution is higher along the coast of Liaoning, and long-term colony breeding has promoted the transfer of heavy metals between wetlands and island ecosystems, which may result in heavy metal pollution of the soil on islands (Fang et al., 2010; Fisheries Administration, Ministry of Agriculture, 2015). Wang et al. (2015) analyzed the residues of organochlorine pesticides by collecting samples from 11 fish and two shellfish species in the Liaodong Peninsula between January 2011 and May 2012, and they found that hexachlorobenzene (HCB), hexachlorocyclohexane (HCH), and dichloro-diphenyl-trichloroethanes (DDTs) were the main pollutants. In addition, invasions by alien species such as the algae that cause red tide, brown tide, and green tide pose serious threats to the coastal areas of Liaoning, including Dalian Bay, Jinzhou Bay, the Yalu River Estuary, and Dayao Bay (Song et al., 2017). Because these sites provide important breeding habitats for Chinese Egrets, the egret populations are dependent on them for survival; thus, strengthening the management and protection of the coastal areas of Liaoning is very important to protect the egret population in the future.
Some islands, including Tuantuozi, Shoulong, Dachangshan, Xiaochangshan, and Ping, along the Liaoning coast, were important distribution sites during the egrets’ pre-migration period, and we suggest that field investigations be conducted on the vegetation conditions and human disturbances on these islands to determine whether the islands are breeding grounds and to assess their suitability as potential breeding grounds in the future. For these potential breeding islands, habitat restoration and artificial recruitment should be conducted, as was done for the Chinese Crested Tern (Thalasseus bernsteini) in Zhoushan, Zhejiang Province; these actions would contribute to increasing the egret population (Lu et al., 2020).
In this study, the migration routes of Chinese Egrets, including the fall migration routes and key stopover and wintering sites, were confirmed, and four migration routes, namely CM, SCM, IM, and MM, were found. Among them, three migration routes (CM, SCM and IM) were found to be the same as previous studies (Huang et al., 2021). Some important distribution sites of Chinese Egrets were found in the pre-migration period along the coastline of Liaoning; these sites represent potential breeding sites for the egrets and are very important for their effective conservation.
Long-distance migration has evolved independently in multiple taxa, enabling animals to exploit spatially and temporally discrete peaks in resources (Dingle, 1996), ultimately allowing for increased reproductive output and survival (Alerstam et al., 2003). However, migration behavior is risky and energetically costly, and selection for optimal migratory strategies is paramount to ensure that the benefits of migration outweigh the risks (Alerstam and Lindströ m, 1990). Several factors, such as weather conditions, landscape routes, flocking behaviors, and individual experience, directly affect an animal's decision regarding whether to cross the sea, while the risk of mortality increases with the absolute distance of the crossing (Kerlinger, 1989; Bildstein, 2006). In this study, four migration routes (CM, SCM, IM, and MM) were selected during the migration of Chinese Egrets, and it was speculated that the difference might have been related to their physiological status and weather conditions, which was the result of a balance between energy expenditure and acquisition.
For SCM, the travel time during migration was relatively short, with either no stops or only one to two stopover sites that were closer to the winter sites, suggesting that the juveniles are able to fly over the Pacific Ocean without a stopover (Huang et al., 2021). For CM, because of the longer travel time than that of the SCM, the egrets stopped at more than two sites, mostly in coastal bays, river estuaries, and islands such as Hangzhou Bay (ID 7174) and Zhelin Bay (ID 7174, ID 7413); additionally, egrets sometimes stopped for more than one month and then continued to migrate without stopping for energy replenishment, possibly because the abundant food resources were enough to provide a sufficient energy supply for the following migration. Two birds (ID 8202 and ID 7160) stayed longer than one month at Nansan River in Zhanjiang, although their transmitter signals were lost near the south coast of Hainan Island. Combined with the historical wintering ground (BirdLife International, 2021), we speculated that the wintering sites of the two individuals may be in the Philippines, Vietnam, or Myanmar and that they stopped at Nansan River for more days to replenish their energy to complete their longer migration.
For the egrets taking the IM routes, the number of travel days during migration was longer than those of SCM and CM and included two to three stopover sites. It was also found that the stopover sites of IM individuals were mainly lakes, rivers, and reservoirs after two weeks. Egret individuals taking MM routes had no stops during the inland migration process, and after migrating to the coast, the egrets preferred to spend more days on coastal islands, in estuaries, and in seaports.
The egrets’ selection of different migration routes is to a large extent under direct genetic control. Berthold (2002) indicated the existence of an innate migratory drive as well as genetic control of 1) the onset, duration and end of the migration period, 2) the amount of migratory activity, a genetically prescribed parameter that determines the distance over which the bird flies, 3) the migration direction, including both the maintenance of a particular direction and changes of direction (which often occur as sharp turns, flying in curves), and 4) physiological parameters, in particular fat deposition during the migratory period. In addition, factors such as the difference in the physical condition and expected destinations of the egrets, and physiography (Cordoba-Cuevas et al., 2020), may also underpin the variation in migration routes.
Our study found that most of the stopovers of the Chinese Egrets on CM, SCM, and MM routes are located in coastal areas. Coastlines may also provide leading lines, visual reference marks for navigation, making it easier to follow the traditional routes that fledglings learn during their first migration; furthermore, coasts may offer sheltered places to sit out bad weather, and allow the birds to forage and replenish their nutrient stores (Green et al., 2002).
There are many important sites that are both stopover and breeding sites along the migration routes of the egrets, such as Xizhao Island (Gu and Zhou, 2000), Huqiushan in Jiangsu (Li, 2000), and Dongzhai, as well as the Nanwan Reservoir (Zhang et al., 1994; Wen and Xia, 1996), the Wuzhishan Archipelago of Zhejiang (Wang et al., 2008), the Laiyu Archipelago of Fujian (Chen et al., 2005), and Wanheshan in Guangxi (Ye et al., 2003). These sites should be breeding grounds for the Liaoning population, and it is essential to promote genetic exchange between different geographic populations and to maintain or improve the genetic diversity of the entire population (Ma et al., 1994).
Mudflat reclamation and water pollution seriously threaten the migration routes of Chinese Egrets. For example, from 1951 to 2009, the coast of Yancheng was subject to large-scale mudflat reclamation, covering an area of 1607 km2. In 2010–2014, 222.8 km2 of new reclamation area was added (Chang et al., 2019). Over the past 30 years, the area of natural wetlands has decreased from 568.9 km2 in 1990 to 230.9 km2 in 2017 due to human activities such as land reclamation (Peng et al., 2020). Zhang et al. (2020) analyzed the spatiotemporal distribution of heavy metal pollution in the estuary of coastal wetlands in Yancheng and found that chromium, arsenic, mercury, and manganese were common pollutants. Zhelin Bay has also been seriously polluted by shipping and large-scale aquaculture breeding, with an increasing trend each year. The Pearl River Estuary also has serious heavy metal pollution, especially copper, zinc, cadmium, and arsenic, due to sea sand mining, which also results in the shrinkage of coastal wetlands (Li et al., 2011).
To effectively protect populations of Chinese Egrets, we suggest the following to address the existing threats to their breeding, stopover, and wintering sites. 1) The coastal area of Liaoning is an important breeding ground for Chinese Egrets, and the local government should protect the wetlands and strengthen the control of pollution sources. 2) The eastern coast of China hosts important stopover and breeding sites for egrets, and large-scale economic programs such as mudflat reclamation should be prohibited as much as possible to provide suitable feeding and roosting sites for egret migration. 3) For some important stopover sites, such as the Shihe River, Hangzhou Bay, Sanmen Bay, Zhelin Bay, Pearl River Estuary, Nansan River, and Lianyang River, it is important to make the local government aware of the great significance of these key sites for Chinese Egrets, and to promote the establishment of protected areas in these regions. 4) Both the Liaoning coast of China and the west coast of the Korean Peninsula are important breeding grounds for egrets, and the Philippines and Vietnam are important wintering grounds. International cooperation between these countries should be strengthened to better protect the migration routes of egret populations. 5) The local government should strengthen public education about Chinese Egrets and important wetland conservation on migration routes, especially at important stopover sites, and the conservation awareness of local fishermen should be improved.
In recent years, the decline in the population of Chinese Egrets has mainly resulted from a lack of effective protection of some important habitats at breeding and stopover sites during migration. In this study, we identified important distribution sites in the pre-migration stages of Chinese Egrets, as well as important stopover sites during migration, through the use of satellite tracking. In particular, some specific islands may be potential breeding grounds that would benefit from artificial recruitment programs in the future. These findings contribute to a better understanding of the pressures imposed on egrets from human disturbance and rapid urban and economic development, and can inform policies and global conservation action plans for the vulnerable Chinese egret.
HX, ZY and DL prepared and revised the manuscript. GZ conceived and designed the research. HX, ZY, GZ, DL, RJ and LC performed the field work. All authors read and approved the final manuscript.
The experiments complied with the current laws of China. Approval for the capture of Chinese Egret was granted by the Forestry and Grassland Bureau of Liaoning Province, China (No.10, Liaolin [2018]).
The authors declare that they have no competing interests.
We are very grateful to the Forestry and Grassland Bureau of Liaoning Province, China for granting approval to capture Chinese Egrets, and for the assistance they provided. This work was supported by the National Key Research and Development Program of China (No. 2019YFA0607103), and Program of National Forestry and Grassland Administration (No. 213023721203).
|
Alerstam, T., Lindstrom, Å., 1990. Optimal Bird Migration: the Relative Importance of Time, Energy, and Safety. Springer, Berlin, pp. 331–351.
|
|
Batbayar, N., Takekawa, J.Y., Newman, S.H., Prosser, D.J., Xiao, X., 2011. Migration strategies of Swan Geese (Anser cygnoides) from northeast Mongolia. Wildfowl 61, 90-109
|
|
Berthold, P., 2002. Bird migration: The present view of evolution, control, and further development as global. Acta Zool. Sin. 3, 291-301
|
|
Bildstein, K.L., 2006. Migrating Raptors of the World: Their Ecology and Conservation. Cornell University Press, Ithaca, NY.
|
|
Chang, M., Jin, C.N., Lin, W.B., Mao, S.F., 2019. Degradation situation, causes and ecological restoration in Jiangsu Yancheng Coastal Wetlands. J Huaihai Inst Technol Nat Sci Ed. 28, 81-86 (in Chinese)
|
|
Chen, X.L., Lin, Q.X., Zhou, X.P., 2005. Breeding Records of Egretta Eulophotes and Egretta Sacra in Fujian Province. The 8th National Congress of Ornithology Branch of Zoological Society of China and the 6th Cross-Strait Symposium on Ornithology (in Chinese).
|
|
Dingle, H., 1996. Migration: the Biology of Life on the Move. Oxford University Press, New York.
|
|
Fisheries Administration, Ministry of Agriculture, 2015. National Fishery Economic Statistics Bulletin 2014. China Fish. 6, 14-15 (in Chinese)
|
|
Gu, M.H., Zhou, Y.L., 2000. Investigation of Egretta ceulophotes in Lianshui Bird Nature Reserve. J. Nanjing Forest Univ. 24, 43–46 (in Chinese)
|
|
Huang, Z.J., Zhou, X.P., Fang, W.Z., Zhang, H.L., Chen, X.L., 2021. Autumn migration routes and wintering areas of juvenile Chinese Egrets (Egretta eulophotes) revealed by GPS tracking. Avian Res 12, 469–476
|
|
Kerlinger, P., 1989. Flight Strategies of Migrating Hawks. University of Chicago Press, Chicago.
|
|
Kuang, F., Tcoleman, J., Jhassell, C., Kleung, K.S., Maglio, G., Ke, W., et al., 2020. Seasonal and population differences in migration of Whimbrels in the East Asian-Australasian Flyway. Avain Res. 11, 246-257
|
|
Li, Z.T., 2000. Observation on nesting ecology of Nycticorax nycticorax, Ardeola bacchus and Egretta eulophotes. J Suzhou Inst Edu. 1, 100-101, 103 (in Chinese)
|
|
Li, T.J., Ma, Y., Wang, D., Wang, A.J., Zhou, Y., 2011. Status, degradation causes, and protection measures of the Pearl River Estuary seashore wetland. J Tropic. Oceanogr. 30, 77-84 (in Chinese)
|
|
Ma, Z., Melville, D.S., Liu, J., Chen, Y., Yang, H., Ren, W., et al., 1994. 2014. Rethinking China's new great wall. Science 346, 912-914
|
|
Nagendran, M., Higuchi, H., Sorokin, A.G., 1994. A Harnessing Technique to Deploy Transmitters on Cranes. Wild Bird Society of Japan, Tokyo, pp. 57–60.
|
|
Peng, X.J., Lin, X.R., Fang, J., Wang, T., Jiang, X.S., 2020. Analysis on coastline and coastal wetland changes in the Hangzhou Bay in recent 30 years. J. Ocean Technol. 39, 9-16 (in Chinese)
|
|
Tian, D.Q., 2002. A preliminary survey on the habitat of Chinese Egret in Hulu Island. Wild Anim. 2, 28-29 (in Chinese)
|
|
Wang, Z.D., Liang, B., Lu, Y.W., Chen, S.H., Fan, Z.Y., Chen, C.S., 2008. Breeding ecology and Conservation strategy for Egretta eulophotes at the Wuzhishan islands. J. Zhejiang Forest. Sci. Technol. 28, 54-57 (in Chinese)
|
|
Wang, W., Li, Q.B., Wang, C.X., Zhang, A., Zhang, X.L., Liu, X., et al., 2015. Residues and risk assessment of organochlorine pesticides in fish and shellfish samples of Liaodong Peninsula. Asian J. Ecotoxicol. 10, 135-143 (in Chinese)
|
|
Wen, Z.Z., Xia, M., 1996. The breeding ecology of the Chinese Egret (Egretta eulophotes). J. Xinyang Normal Univ. 3, 279-286 (in Chinese)
|
|
Xiang, Y.Q., 2015. Spatial and temporal change of landscape pattern and its driving force in Liaoning coastal regions. Chin. J. Ecol. 34, 2628-2635 (in Chinese)
|
|
Ye, F., Huang, C.M., Li, H.H., 2003. Breeding population of Chinese Egret in Fangcheng of Guangxi Province. Chin. J. Zool. 38, 99-99 (in Chinese)
|
|
Yin, Z.H., Lei, F.M., 2002. A preliminary study of the breeding biology of the Chinese Egret. Acta Zool. Sin. 48, 824-827 (in Chinese)
|
|
Zhang, H.B., Li, Y.F., Han, S., Zhang, Y.N., Liu, Y.Q., 2020. Spatiotemporal distribution characteristics of heavy metal pollution in rivers flowing into the sea in Yancheng coastal wetland of Jiangsu. Environ. Pollut. Control 42, 1491-1495 (in Chinese)
|
|
Zhang, C.H., Li, T.G., Li, X.M., Wu, C.B., Jiang, H.H., 2017. Multi-plan conflict measurement and its fusion method of land use in Liaoning Coastal Economic Belt: A case study of Zhuanghe, Dalian. Ocean Develop. Manag. 34, 38-42 (in Chinese)
|
|
Zhang, L.S., Liu, Z.M., Zhang, F., 1994. Breeding ecology and biology of four species of egret and heron. Acta Ecol. Sin. 14, 80-83 (in Chinese)
|
|
Zhang, H.L., Zhang, F.J., Zhang, Y.W., 2018. Study on the migration of subadult Egretta eulophotes by satellite tracking at Fantuozi Island, Dalian City. Sichuan J. Zool. 37, 519-524 (in Chinese)
|
| Years | August | September | ||
| Sunrise | Sunset | Sunrise | Sunset | |
| 2016 | 5:07:23 | 18:48:33 | 5:35:11 | 18:02:06 |
| 2017 | 5:07:10 | 18:48:52 | 5:34:58 | 18:02:30 |
| 2018 | 5:06:57 | 18:49:11 | 5:34:45 | 18:02:53 |
| ID | Date departing from breeding site | Stopover sites | Coordinates of stopover sites | Duration time (h) | Migration distance (km) and days (in brackets) | Expected shortest distance (km) | Straightness | Migration speed (km/d) | Date arriving in wintering sites (duration days) | Wintering grounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inland migration | 8201 | Oct. 21, 2016 | Shihe River of Luoshan, Xingyang, Henan | 114.42° E, 32.25° N | 265 | 3055 (9) | 2428 | 0.79 | 339 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hebao Reservoir, Lingchuan, Guangxi | 110.19° E, 25.42° N | 275 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coastal migration | 7174 | Sep. 22, 2018 | Dayangshan and Xiaoyangshan Island, Hangzhou Bay, Zhejiang | 122.11° E, 30.61° N | 720 | 4684 (10) | 3627 | 0.77 | 468 | Dec. 12, 2018 (26) | The west coast of Tanintharyi, Myanmar | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zhelin Bay, Chaozhou, Guangdong | 117.05° E, 23.58° N | 841 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8215 | Oct. 5, 2016 | Zhujiang Estuary, Guangdong | 113.68° E, 22.60° N | 119 | 2758 (4) | 2124 | 0.77 | 690 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7412 | Oct. 11, 2017 | Quanzhou Bay, Fujian | 118.66° E, 24.84° N | 48 | 2169 (5) | 1880 | 0.87 | 434 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Zhangjiang Estuary, Zhangzhou, Fujian | 117.47° E, 23.81° N | 72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lianyang River, Shantou, Guangdong | 116.78° E, 23.48° N | 204 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7413 | Oct. 11, 2017 | Wenzhou Bay, Zhejiang Zhelin Bay, Chaozhou, Guangdong | 120.85° E, 27.79° N 117.02° E, 23.64° N | 170 | 3357 (6) | 2821 | 0.84 | 560 | Nov. 22, 2017 (108) | Manila Bay, Philippines | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 646 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sea-crossing migration | 7163 | Sep. 23, 2018 | Polilio Island, Philippines | 122.21° E, 14.85° N | 298 | 3501 (5) | 3319 | 0.95 | 700 | Oct. 16, 2018 (52) | Bohol Island, Philippines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bondoc Peninsula, Philippines | 122.64° E, 13.19° N | 119 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7151 | Oct. 1, 2018 | – | – | – | 2104 (2) | 2051 | 0.97 | 1052 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7152 | Oct. 6, 2018 | – | – | – | 2124 (3) | 1874 | 0.88 | 708 | Oct. 11, 2018 (233) | Zengwun River Estuary of Taiwan, China | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8329 | Oct. 6, 2018 | Manila Bay, Philippines | 120.84° E, 14.75° N | 690 | 3135 (3) | 2810 | 0.90 | 1045 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mixed migration | 7160 | Sep. 23, 2018 | From Dongsong Sea,Changpo Port to Nanshi Bay, Zhanjiang, Guangdong | 110.45° E, 20.57° N | 658 | 3168 (5) | 2696 | 0.85 | 634 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8202 | Oct. 6, 2016 | Nansan River, Zhanjiang, Guangdong | 110.60° E, 21.23° N | 935 | 3305 (6) | 2642 | 0.80 | 551 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Others | 8200 | Nov. 2, 2016 | Jinshan Port, Yantai, Shandong | 121.70° E, 37.45° N | 62 | 693 (1) | 623 | 0.90 | 693 | – | – | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| East of Chaowai Village, Yantai, Shandong | 121.13° E, 36.67° N | 61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coastal mudflat of Dafeng, Yancheng, Jiangsu | 120.75° E, 33.32° N | 164 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8203 | Sep. 28, 2016 | Sanmen Bay, Zhejiang | 121.63° E, 29.06° N | 846 | 1628 (7) | 1427 | 0.88 | 233 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7417 | Sep. 27, 2017 | South of Henggang River, Taizhou, Jiangsu | 120.04° E, 33.03° N | 93 | 1361 (4) | 1130 | 0.83 | 340 | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| From Tiejiang River, Huangdun Port to Xiangshan Port, Ninghai, Zhejiang | 121.59° E, 29.50° N | 682 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7410 | – | Longjiang Estuary, Fuqing, Fujian | 119.53° E, 25.69° N | 407 | – | – | – | – | – | – | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| “Others” refer to egrets with lost transmitter signals. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Migration routes | Migration distance (km) | Migration days | Migration speed (km/day) | Straightness |
| Inland migration | 3055 | 9.00 | 339 | 0.79 |
| Coastal migration | 3242 ± 538 | 6.25 ± 1.31 | 538 ± 57 | 0.81 ± 0.03 |
| Sea-crossing migration | 2977 ± 303 | 3.25 ± 0.63 | 877 ± 99 | 0.93 ± 0.02 |
| Mixed migration | 3237 ± 69 | 5.50 ± 0.50 | 592 ± 41 | 0.85 ± 0.05 |