| Citation: | Sook-Young Cho, Hyun-Young Nam, Se-Young Park, Chang-Yong Choi. 2022: Sexual dimorphism and sex-differential migration of Little Buntings (Emberiza pusilla) at an East Asian stopover site. Avian Research, 13(1): 100014. DOI: 10.1016/j.avrs.2022.100014 |
Sex differences in morphology provide key information for understanding a species' morphological adaptations in relation to the evolution of sexual selection. In migratory birds, morphological traits have adapted to long-distance travel, and sexual dimorphism is typically related to sex-differential migration phenology. Little Buntings (Emberiza pusilla) have one of the longest migrations and are the least dichromatic species among the Emberiza buntings. In this study, we measured sexual size dimorphism and sexual dichromatism of Little Buntings in relation to the spring arrival dates at a stopover site in Korea. Wing length was the most important predictor for identifying sex; the wings of males were longer than those of females. Males also had a significantly stronger chestnut color of the head feathers than females, but this color difference was more prominent in the spring than in the fall. Males arrived earlier than females by four days. Arrival dates correlated with both size and color, but unlike other bunting species previously studied in the same area, there was no clear sex-differential trend in the relationship between arrival date and morphological characteristics. Seasonal differences in the degree of sexual dichromatism suggest that chestnut plumage coloration can be used as a social or sexual signal of males in the breeding season. The correlation of size and color to early arrival regardless of sex may indicate that a preference for assortative mating exists or that a sex-differential migration strategy is not clearly defined in the early stages of northward migration. Our findings on the sexual dimorphism of Little Buntings provide insight into the evolution of the sex-differential migration of buntings in the East Asian Flyway.
Sexual dimorphism, generally measured as sexual size dimorphism (SSD; the difference in size between sexes) and sexual dichromatism (SD; the difference in the plumage color between sexes), are key insights into a species' morphological adaptation in relation to the evolution of sexual selection (Björklund, 1990; Owens and Hartley, 1998). Particularly in migratory birds, of which morphological traits have been adapted to long-distance travel (Winkler and Leisler, 1992), sexual dimorphism may reflect sex-differential strategies in migration phenology (Francis and Cooke, 1986; Kissner et al., 2003; Saino et al., 2010).
Although the details remain unclear, buntings in the genus Emberiza have long-distance migrations that occur throughout Europe, Asia, and Africa (Byers et al., 1995). Comparisons of morphological adaptations according to migration ecology in this group are useful because there are many species in a closely related group (Alström et al., 2008) that vary in the degree of sexual dimorphism. Most of these species show strong sexual dimorphism in their plumage during the breeding season (Byers et al., 1995), but the Little Bunting (Emberiza pusilla) is regarded as the least sexually dichromatic species out of the 14 species that regularly occur in Korea (Nam et al., 2011). They are reported to be sexually size dimorphic, but morphological sex determination in the field is not reliable in general, because the difference is slight and the ranges of the size in both sexes overlap considerably (Svensson, 1992; Byers et al., 1995). In addition, this species is one of the northernmost breeding birds with a wide range from northern Scandinavia to northeastern Russia (Byers et al., 1995), and breeding populations are also found in northeastern China (Mackinnon and Philipps, 2000). Their wintering grounds range from eastern Nepal and northern India to southern China, Korea, and northern Southeast Asian countries (Byers et al., 1995; Lee et al., 2000; Mackinnon and Philipps, 2000; Tomek, 2002; Brazil, 2009). Therefore, this species is a long-distance migrant with less prominent sexual dimorphism, which will be an important piece of bridging the knowledge gap in our understanding of morphological adaptation to sexual selection and migration.
In this study, we measured sex-dependent variation in size and plumage coloration of the Little Bunting in East Asia to evaluate whether these traits are related to the phenology during northward spring migration. The results of this study will provide insight into morphological adaptations in relation to sex-differential migration phenology in the East Asian Emberiza buntings that have various degrees of sexual dimorphism and migration distances.
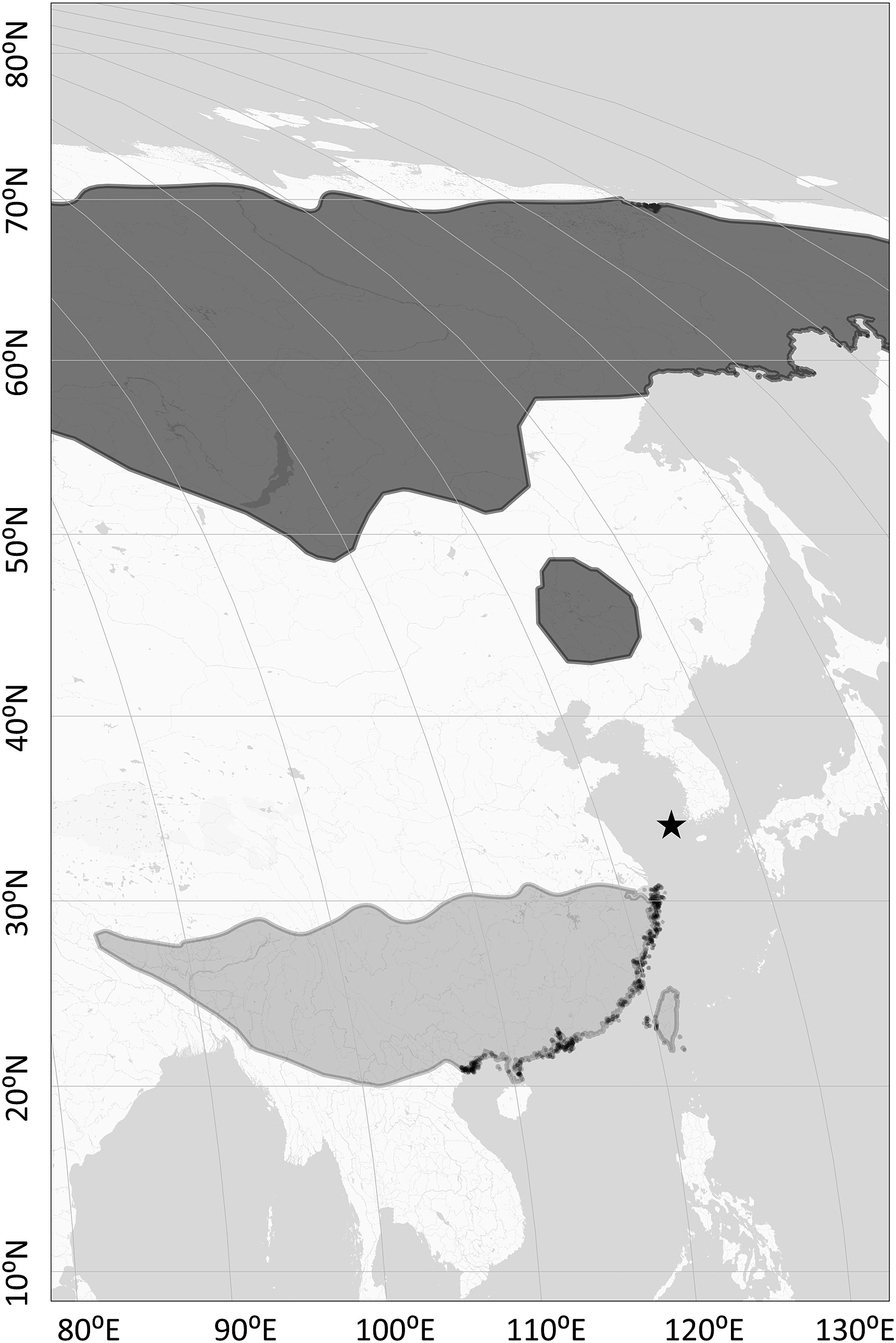
The study site, Heuksan-do (34°41ʹ N, 125°25ʹ E), is an island located about 90 km away from the southwestern tip of mainland Korea (Fig. 1) and it is one of the major stopover sites for migratory passerines in the East Asian flyway (Nam et al., 2011), on which a constant bird banding program runs throughout the year at a small wetland. We used 16 mist nets (mesh size: 30–36 mm, length: 12 m) daily from March 1 to May 31 in spring and August 16 to November 30 in autumn. The mist nets were monitored and managed every 30–60 min from 6:00 a.m. to 12:00 p.m. We captured a total of 155 Little Buntings during the three migration seasons (spring and autumn in 2012, and spring in 2013) and randomly collected measurement data from 127 birds among them. Out of the selected 127 birds, 124 were analyzed after excluding date outliers (arrived too early or too late based on the multivariate Mahalanobis distance, n = 2), failure in molecular sexing (n = 2), or both (n = 1). Only 11 (5 females, 6 males) of the 124 individuals were adult (older than 1st summer plumage), and the effect of age on the wing length was marginal in females (Wilcoxon two-sample test; z = 2.00; p = 0.046) and almost absent in males (z = 0.42; p = 0.674). Therefore, age was not involved as a variable.
We banded birds with individual aluminum bands and measured nine morphological characteristics during the routine banding procedure. To detect sexual size dimorphism, wing (maximum wing length), tail, and total lengths were measured to the nearest 0.1 mm using rulers, and tarsus (minimum tarsus length), head (head and bill length), bill to skull length (bills), bill to nostril length (billn), and bill depth and width at the nostrils were measured to the nearest 0.01 mm using calipers. Body masses were weighed with electric scales (precision: 0.01 g). All bird banding procedures and measurements of the morphological characteristics followed the standard banding methods of the Korea National Park Service (Nam et al., 2011, 2014) with appropriate bird banding center permits issued by the local government (Shinan County Office) and the Korean Ministry of Environment.
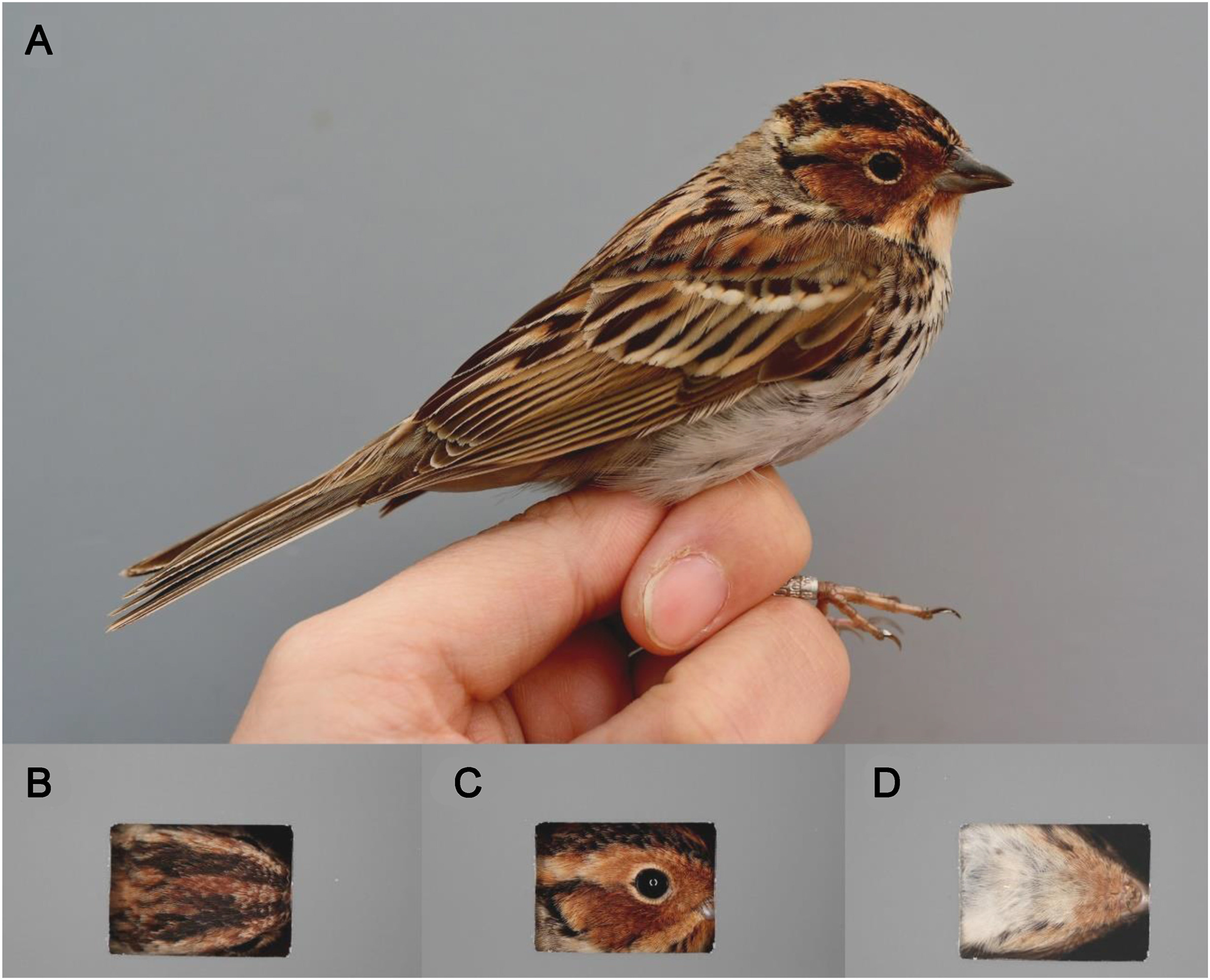
Plumage color was measured using a digital imagery technique (Badyaev et al., 2001; McGraw et al., 2002; Oh and Badyaev, 2006; Giraudeau et al., 2012, 2016; Lendvai et al., 2013; McKay, 2013; Luttrell et al., 2015). We photographed the birds using a digital single lens reflex (DSLR) camera (EOS 5D Mark II, Canon Inc., Japan) and a flashlight (MR-14EX, Canon Inc., Japan) in a small, shaded chamber (approximately 80 cm × 60 cm × 50 cm) covered by grey cardboard. Then, we placed grey cardboard with a rectangular hole (2 cm × 3 cm) on the head, where the chestnut color was revealed, to confine the plumage of interest. Three photos were taken of each of the dorsal, lateral, and ventral head parts revealed through the hole (Fig. 2). To control any external light effects, we took the photos with a constant distance between the bird and the camera, and we used constant camera settings including the light exposure (F7.1), shutter speed (1/6400 s), and sensitivity (ISO 400). Nine images per bird were processed in Photoshop CS3 software (Adobe Inc., CA, USA). We selected four random 101 × 101-pixel areas on a grey background using the color sampler tool, and then calculated their averaged brightness and adjusted them to the value of the background brightness being 50% (128 in the RGB value). After controlling the color of the background, we used the color sampler tool to pick four random 3 × 3-pixel areas from six plumage parts in the crown, supercilium, ear covert, lore, chin, and throat from three sides of the head. Color scores were calculated as the averaged values of hue, saturation, and brightness (Lendvai et al., 2013; Giraudeau et al., 2016).
We collected one outermost tail feather (usually the rightmost one) for molecular sexing and preserved it at −20 ℃ until lab analysis. DNA was extracted from the quill roots of the collected tail feathers using a commercial extraction kit (DNeasy Blood & Tissue Kit, Qiagen, Germany), and two chromobox-helicase-DNA-binding genes (CHD-W and CHD-Z) were amplified by polymerase chain reaction using P2 and P8 primers (Griffiths et al., 1998).
All statistical procedures were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA; SAS Institute Inc, 1999).
To describe sexual size dimorphism, we performed a t-test with the morphological measurements by sexes, as all measurements met the normality and homogeneity of variance assumptions. To compare the degree of sexual size dimorphism in each measurement on a different scale, we also calculated the effect size (Cohen's d; Cohen, 1988):
|
|
where M1 and M2 are the means of males and females respectively, and Spooled represents the standard deviations for the two groups.
Stepwise discriminant function analysis (DFA) was conducted for the best size parameters for separating sexes using the PROC STEPDIC procedure (SAS Institute Inc, 1999) with a significance level of 0.05 for entering and staying. A pooled covariance matrix was used in the discriminant function (Bartlett's test; χ2 = 0.237, df = 1, p = 0.626; Morrison, 1976) because the selected parameter met the variances of homogeneity. We performed the canonical discriminant analysis with selected variables from the previous stepwise DFA using PROC DISCRIM (SAS Institute Inc, 1999) to calculate a discriminant score of each individual (Bavoux et al., 2006; Dmitrenok et al., 2007) and drew a canonical function. In order to estimate the proportion of correctly classified individuals, we used the re-substitution and jackknife cross-validation (leave-one-out) methods (Dechaume-Moncharmont et al., 2011).
We used a mixed model to test the differences in color and arrival dates by sex, size, and seasons with the PROC MIXED procedure (SAS Institute Inc, 1999). Year was treated as a random effect in the models because data from two springs were used in the statistics. We conducted principal component analysis (PCA) with 18 color variables from hue, saturation, and brightness of the 6 plumage parts to extract the reduced number of color components (Mougeot et al., 2007) using PROC PRINCOMP procedure (SAS Institute Inc, 1999).
To select morphological variables that affected the arrival dates, we built models on the arrival dates including four variables (wing length, color PC1, color PC2, and sex) and their interactions. We started with a full model that includes all variables and their interactions and eliminated the variables and interactions that did not significantly contribute to the goodness of model fit based on the AICc (corrected Akaike information criterion; Burnham and Anderson, 2002; Kristensen et al., 2012). For this model selection, we used the PROC GLMSELECT procedure (SAS Institute Inc, 1999) with backward selection and confirmed their significance level using the PROC MIXED procedure (SAS Institute Inc, 1999).
A total of 124 Little Buntings were molecularly sexed. Among them, 60 were females and 64 were males, and the sex ratio was not different from 1:1 both in the spring (n = 51; Z = 0.140; p = 0.889) and in the autumn (n = 73; Z = −0.585; p = 0.558).
In general, males had longer wings, tails, and total lengths, and were heavier than females (Table 1). Nevertheless, wing length was the only variable that remained significant after stepwise DFA (canonical correlation = 0.746; F1,122 = 152.81; p < 0.001), and was selected as the morphological trait best discriminating females and males. According to linear DFA with wing length, we acquired the correct classification rates of 91.7% for females and 86.0% for males using both re-substitution and jackknife cross-validation methods. Canonical discriminant function (D) was calculated as follows to help with sex determination in the field:
|
|
where the probability of being female is higher than that of being male when D < 0 and vice versa.
| Variable | Female | Male | t | p | Effect size (d) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n | Mean | SD | Range | n | Mean | SD | Range | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wing (mm) | 60 | 69.3 | 1.5 | 65.6–74.2 | 64 | 72.7 | 1.6 | 67.8–76.5 | −12.36 | <0.001 | 1.49 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tail (mm) | 60 | 56.2 | 2.1 | 51.2–61.8 | 64 | 58.7 | 2.2 | 53.0–62.5 | −6.41 | <0.001 | 1.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tarsus (mm) | 60 | 17.45 | 0.46 | 16.59–18.62 | 64 | 17.6 | 0.55 | 16.45–19.31 | −1.92 | 0.057 | 0.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Head (mm) | 60 | 26.6 | 0.65 | 25.11–29.39 | 64 | 26.71 | 0.54 | 25.47–28.19 | −1.05 | 0.297 | 0.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill from skull (mm) | 60 | 12.23 | 0.44 | 11.11–13.76 | 64 | 12.34 | 0.39 | 11.59–13.36 | −1.46 | 0.148 | 0.26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill from nostril (mm) | 60 | 6.74 | 0.30 | 6.12–7.48 | 64 | 6.79 | 0.30 | 6.25–7.78 | −0.96 | 0.340 | 0.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total length (mm) | 60 | 138.1 | 2.9 | 130.0–145.0 | 64 | 141.9 | 3.2 | 134.0–149.5 | −6.97 | <0.001 | 1.07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill depth (mm) | 56 | 4.67 | 0.20 | 4.16–5.20 | 55 | 4.69 | 0.20 | 4.32–5.15 | −0.48 | 0.633 | 0.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill width (mm) | 56 | 3.92 | 0.31 | 3.28–4.91 | 55 | 3.92 | 0.30 | 3.31–5.16 | −0.03 | 0.976 | −0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass (g) | 60 | 12.80 | 0.85 | 11.13–15.68 | 64 | 13.74 | 1.02 | 12.1–16.1 | −5.55 | <0.001 | 0.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Effect sizes are presented to compare relative differences in the size dimorphism among measurements with different size scales. Effect size was calculated as Cohen's d effect size: (mean of males – mean of females)/pooled standard deviation (Cohen, 1988). SD indicates a standard deviation of the measurements. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We extracted two principal components that explained approximately 56% of the total variation. The first principal component (PC1; 32% of the total variation) was primarily explained as a higher value of hue (more directed to orange than red) and less saturated color. The second principal component (PC2; 24% of the total variation) was primarily explained as higher brightness. Males had significantly lower values of PC1 (F1,119 = 41.35; p < 0.001) and PC2 (F1,119 = 6.55; p = 0.012), which indicated more red-directed, more saturated, and darker color. Plumage color showed seasonal differences (PC1: F1,119 = 14.79; p < 0.001; PC2: F1,119 = 66.62; p < 0.001), and the difference in color by sexes was dependent on the season in PC2 (interaction of sex and color: F1,119 = 4.95; p = 0.028) (Fig. 3).
Individuals with longer wings had lower PC1 values (more directed to red and more saturated color) in both sexes (Table 2). Individuals with longer wings tended to have lower PC2 values (darker color), but the trend was season-dependent because it was significant only during the spring season (Table 2).
| Effect | Color PC1 | Color PC2 | ||||||
| Num df | Den df | F | p | Num df | Den df | F | p | |
| Size | 1 | 116 | 5.90 | 0.017 | 1 | 116 | 3.18 | 0.077 |
| Sex | 1 | 116 | 5.80 | 0.018 | 1 | 116 | 0.17 | 0.677 |
| Season | 1 | 116 | 3.28 | 0.073 | 1 | 116 | 67.91 | <0.001 |
| Size × Sex | 1 | 116 | 0.20 | 0.657 | 1 | 116 | 4.21 | 0.042 |
| Size × Sex × Season | 2 | 116 | 1.17 | 0.314 | 2 | 116 | 4.59 | 0.012 |
Males arrived at the study site ahead of females with a difference of 3.6 days (F1,48 = 4.14; p = 0.048). The average arrival date of females was 125.54 ± 6.56 (May 5), while that of males was 121.96 ± 5.74 (May 1). The best parsimonious model that explained the arrival dates included one fixed effect (size), and the two interactions of size and color and sex and color (AICc = 226.98; Table 3). Individuals with longer wings arrived earlier (F1,45 = 13.19; p < 0.001). In addition, larger wings that interacted with darker plumage color were related to earlier arrival (F1,45 = 5.69; p = 0.021). Darker individuals arrived earlier but the degree of relationship was different between the sexes (F1,45 = 8.69; p < 0.001; Table 3).
| Parameter | df | Estimate | SE | t |
| Intercept | 1 | 119.726 | 1.244 | 96.27 |
| Size | 1 | −2.805 | 0.772 | −3.63 |
| Size × Color PC2 | 1 | −0.876 | 0.367 | −2.39 |
| Color PC2 × Sex (female) | 1 | −3.697 | 0.887 | −4.17 |
| Color PC2 × Sex (male) | 1 | −0.663 | 0.657 | −1.01 |
The sex of Little Buntings is not easily identifiable in the field, but the difference in size between the sexes confirmed by molecular sexing proved that wing length is the best predictor of sex based on a wing length cutoff of 71.1 mm. Due to the difficulty in identifying sex by plumage color during the autumn migration season (sex determination rate based on morphology in the field: 95.6% in spring, 34.8% in autumn, n = 361; H.Y. Nam, unpublished data), sex determination by wing length, which correctly determines sex 88.8% regardless of season, is more helpful in autumn migration seasons.
The results of our study also showed sexual dichromatism. Although the general morphological description on sex differences indicates that males have more chestnut color on their heads with more black in their lateral crown stripes than females, the results of this study provided the first quantitative evidence that males have more red-directed, more saturated, and darker color on their heads, especially in the spring. Our digital imagery analysis also suggested that the seasonal difference in the coloration was greater in males than in females. These results may support the theory that the chestnut plumage coloration of males may act as a social signal of dominance in males (Rohwer, 1975; Maynard Smith and Harper, 1988) or as a quality signal for preference by females (Zuk et al., 1990; Safran and McGraw, 2004) during the breeding season. Their chestnut plumage coloration, the key component of sexual dichromatism, is assumed to be melanin-derived (McGraw, 2006). Melanin-based plumage coloration is generally known to reflect individual body condition (Siefferman et al., 2007), survival rate against environmental stress (Galván et al., 2013), food availability, parasitic resistance (Piault et al., 2009), juvenile growth (Fargallo et al., 2007), and female preference (reviewed in Hill, 2006). Our results that bigger individuals had more red-directed, more saturated, and darker color, also supported the theory that plumage coloration may indicate body condition measured by their size.
Regarding the season of migration, the difference in the plumage color was greater in spring than in autumn. In the Emberiza species, the breeding plumage coloration is generally attained in two different ways: abrasion of feather tips and pre-breeding molt (Jenni and Winkler, 1994; Tökölyi et al., 2008). The head color obscured by pale fringes in freshly molted feathers would appear in the spring as pale fringes that had worn off during the wintering period. On the other hand, we also observed that most individuals underwent partial pre-breeding molting of their head feathers during spring migration seasons, as previously noted (Byers et al., 1995). In particular, the feathers of the lateral and ventral portions of the head grew during the spring migration. Therefore, both feather abrasion and partial pre-breeding molt likely formed the breeding plumage colors of the Little Buntings. These two progressive changes in head feathers resulted in prominent changes in color when they finally arrived at their breeding grounds, and this especially helped males to attain more red-directed, more saturated, and darker color for the breeding season, which is competitive to other males or attractive to females.
In this study, we confirmed the protandrous migration of Little Buntings, with males migrating and arriving earlier than females. In addition, like other prominent, sexually-dichromatic bunting species previously studied (Nam et al., 2011), the wing, tail, and total lengths were the important traits that explained size variation in Little Buntings. To explain the relationship between sexual dimorphism and protanrdy, several non-mutually exclusive hypotheses have been proposed (reviewed by Morbey and Ydenberg, 2001; Rubolini et al., 2004). For example, the early arrival of larger males, that are the generally ‘chosen’ sex in the passerines, may occur because of the advantages of less cost from the harsh weather in wintering, the early arrival seasons (Møller, 1994; Weatherhead and Clark, 1994; Kissner et al., 2003), the intrasexual competition over territory (Ketterson and Nolan, 1983), or the enhancement of mating opportunities (Møller, 1994). A previous study on the five East-Asian buntings showed the relationship between size and arrival dates was only shown in males (Nam et al., 2011). However, in this study, bigger Little Bunting individuals arrived earlier regardless of sex. We also found that the relationship between plumage color and arrival dates was significant in both sexes and that individuals with good quality color (red-directed, saturated, and dark) arrived earlier. These findings might be explained by the advantage of early breeding after an early arrival and are also applied to females as well as males (Cristol, 1995; Smith and Moore, 2005), therefore assortative mating is favored (MacDougall and Montgomerie, 2003; Silva et al., 2008; Rowe and Weatherhead, 2011). However, the results of our study conducted at the stopover site might not have reflected the migration strategies by the sexes at the breeding ground exactly as the sex-difference of arrival dates is higher in the northern stopover site (Wobker et al., 2021), because the breeding latitude of the Little Buntings was the highest among the studied bunting species and, therefore, they had the longest distance to go (KNPRI, 2013).
In a previous study (Nam et al., 2011), the Yellow-browed Bunting (Emberiza chrysophrys), which bred at the highest latitude among the five species that were studied, showed the least difference in arrival dates between the sexes. Like the Yellow-browed Bunting, Little Buntings are one of the highest breeders in the East Asian Emberiza buntings and showed a lesser prominent degree of protandry. Males could lessen their stopover period at the next stopover site and advance their arrival dates (Nilsson et al., 2013; Monti et al., 2018), or the relatively low level of protandry in this species may have related to least sexual dichromatism due to low pressure of mating competition (Rubolini et al., 2004). Possibilities of intraspecific variation among populations in migration phenology which is related to different migration schedules (Choi et al., 2019) or in size which is related to different migration distances (Copete et al., 1999) also can be considered. Further study of the migration strategies, morphological traits, and plumage colors of the Little Buntings along their migration routes and in their entire distribution ranges throughout their entire annual life cycle would help bridge the knowledge gap on the evolutionary adaptation to sexual selection and migration of East Asian buntings, which are currently experiencing a rapid population decline and conservation crisis (Tamada et al., 2014; Kamp et al., 2015; Yong et al., 2015; Edenius et al., 2017; Choi et al., 2019).
Morphological traits of the migrating Little Buntings revealed that the species is sexually size dimorphic and dichromatic. More distinctive dichromatism in the spring than in the fall may indicate its role as a social signal in male-male competition or sexual signaling in mate choice for the upcoming breeding season. However, unlike the results of a previous study on the other Emberiza bunting species, the proven tendency of the early arrival of bigger and darker individuals was not dependent on sex in Little Buntings, which showed the least SD. We suggested the following two possibilities: selection pressures for early arrival acted similarly not only on males but on females as well, or sex-differential migration strategies were not fully detected on a stopover site in a relatively lower latitude far from the breeding grounds of Little Buntings. Due to their location at an extreme breeding latitude and the sexual dichromatism of East Asian buntings, our results will provide important insight into how various environmental factors related to breeding latitude and migration distance affect the evolution of sexual dimorphism.
CYC and HYN conceptualized the topic and acquired the fund. HYN and SYC designed methodology and conducted analyses. SYC, HYN, and SYP performed fieldwork. HYN and SYC were the major contributors in writing the first draft, and HYN and CYC reviewed and edited the manuscript. All authors read and approved the final manuscript.
The authors confirm that this study was conducted under the ethical guidelines and protocols of the Korea National Park Service (KNPS) during the entire fieldwork procedure (Nam et al., 2014) and all necessary permission for bird banding was obtained.
The authors declare that they have no competing interests.
The authors thank Seung-Yeon Lee, Gi-Chang Bing, Seul-Gi Seo, Chang-uk Park for their contributions to fieldwork and valuable comments. The earlier draft was improved by comments and advice from Pengjun Cheng and two anonymous reviewers. The main fieldwork was conducted by the 2012–2013 Migratory Bird Research Project of the Korea National Park Research Institute, and the data analysis was supported by the New Faculty Startup Fund from Seoul National University (Grant No. 500-20200268). This research was also supported by the National Research Foundation of Korea (NRF) grants funded by the Republic of Korea Government (Ministry of Education; NRF-2018R1D1A1B07050135 & NRF-2019R1I1A1A01063760).
|
Badyaev, A.V., Hill, G.E., Dunn, P.O., Glen, J.C., 2001. Plumage color as a composite trait: developmental and functional integration of sexual ornamentation. Am. Nat. 159, 221-235
|
|
Brazil, M., 2009. Birds of East Asia: China, Taiwan, Korea, Japan, and Russia. Princeton University Press, Princeton.
|
|
Burnham, K.P., Anderson, D.R., 2002. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach. Springer-Verlag, New York.
|
|
Byers, C., Olsson, U., Curson, J., 1995. Buntings and Sparrows. Pica Press, Sussex.
|
|
Choi, C-Y., Nam, H-Y., Park, J-G., Bing, G-C., 2019. Migration pattern of Yellow-throated buntings revealed by isotope-based geographic assignment. Int. J. Geogr. Inf. Sci. 34, 504-519
|
|
Cohen, J., 1988. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic, New York.
|
|
Francis, C.M., Cooke, F., 1986. Differential timing of spring migration in wood warblers (Parulinae). Auk 103, 548-556
|
|
Hill, G.E., 2006. Female mate choice for ornamental coloration. In: Hill, G.E., McGraw, K. (Eds.), Bird Coloration II – Function and Evolution. Havard University Press, Massachusetts, pp. 137–200.
|
|
Jenni, L., Winkler, R., 1994. Moult and Ageing of European Passerines. Academic Press, London.
|
|
KNPRI, 2013. 2012–2013 Annual Report of Migratory Bird Research. Korea National Park Research Institute, Wonju.
|
|
Kristensen, D.L., Erikstad, K.E., Reiertsen, T.K., Moum, T., Barrett, R.T., Jenni-Eiermann, S., 2012. Are female offspring from a single-egg seabird more costly to raise? Behav. Ecol. 24, 136-143
|
|
Lee, W.-S., Koo, T.-H., Park, J.-Y., 2000. Field Guide to the Birds of Korea. LG Evergreen Foundation, Seoul.
|
|
Mackinnon, J., Phillipps, K., 2000. A Field Guide to the Birds of China. Oxford University Press, New York.
|
|
Maynard Smith, J., Harper, D.G.C., 1988. The evolution of aggression: can selection generate variability? Philos. T. Roy. Soc. B. 319, 557-570
|
|
McGraw, K.J., 2006. Mechanics of melanin-based coloration. In: Hill, G.E., McGraw, K. (Eds.), Bird Coloration I – Mechanisms and Measurements. Harvard University Press, Massachusetts, pp. 243–294.
|
|
Morrison, D.F., 1976. Multivariate Statistical Methods. McGraw-Hill, New York.
|
|
Nam, H.-Y., Cho, S.-Y., Kim, H.-J., Park, J.-G., Choi, C.-Y., Kwon, Y.-S., 2014. Bird Banding Manual for Constant Effort Surveys in National Parks of Korea. Korea National Park Research Institute, Wonju.
|
|
SAS Institute Inc, 1999. SAS/STAT® User's Guide, Version 8. SAS Institute Inc., Cary.
|
|
Svensson, L., 1992. Identification Guide to European Passerines. British Trust for Ornithology, Norfolk.
|
|
Tomek, T., 2002. The birds of North Korea: Passeriformes. Acta Zool. Cracov. 45, 1-235
|
|
Winkler, H., Leisler, B., 1992. On the ecomorphology of migrants. Ibis 134, 21-28
|
| Variable | Female | Male | t | p | Effect size (d) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n | Mean | SD | Range | n | Mean | SD | Range | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wing (mm) | 60 | 69.3 | 1.5 | 65.6–74.2 | 64 | 72.7 | 1.6 | 67.8–76.5 | −12.36 | <0.001 | 1.49 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tail (mm) | 60 | 56.2 | 2.1 | 51.2–61.8 | 64 | 58.7 | 2.2 | 53.0–62.5 | −6.41 | <0.001 | 1.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tarsus (mm) | 60 | 17.45 | 0.46 | 16.59–18.62 | 64 | 17.6 | 0.55 | 16.45–19.31 | −1.92 | 0.057 | 0.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Head (mm) | 60 | 26.6 | 0.65 | 25.11–29.39 | 64 | 26.71 | 0.54 | 25.47–28.19 | −1.05 | 0.297 | 0.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill from skull (mm) | 60 | 12.23 | 0.44 | 11.11–13.76 | 64 | 12.34 | 0.39 | 11.59–13.36 | −1.46 | 0.148 | 0.26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill from nostril (mm) | 60 | 6.74 | 0.30 | 6.12–7.48 | 64 | 6.79 | 0.30 | 6.25–7.78 | −0.96 | 0.340 | 0.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total length (mm) | 60 | 138.1 | 2.9 | 130.0–145.0 | 64 | 141.9 | 3.2 | 134.0–149.5 | −6.97 | <0.001 | 1.07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill depth (mm) | 56 | 4.67 | 0.20 | 4.16–5.20 | 55 | 4.69 | 0.20 | 4.32–5.15 | −0.48 | 0.633 | 0.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bill width (mm) | 56 | 3.92 | 0.31 | 3.28–4.91 | 55 | 3.92 | 0.30 | 3.31–5.16 | −0.03 | 0.976 | −0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass (g) | 60 | 12.80 | 0.85 | 11.13–15.68 | 64 | 13.74 | 1.02 | 12.1–16.1 | −5.55 | <0.001 | 0.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Effect sizes are presented to compare relative differences in the size dimorphism among measurements with different size scales. Effect size was calculated as Cohen's d effect size: (mean of males – mean of females)/pooled standard deviation (Cohen, 1988). SD indicates a standard deviation of the measurements. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Effect | Color PC1 | Color PC2 | ||||||
| Num df | Den df | F | p | Num df | Den df | F | p | |
| Size | 1 | 116 | 5.90 | 0.017 | 1 | 116 | 3.18 | 0.077 |
| Sex | 1 | 116 | 5.80 | 0.018 | 1 | 116 | 0.17 | 0.677 |
| Season | 1 | 116 | 3.28 | 0.073 | 1 | 116 | 67.91 | <0.001 |
| Size × Sex | 1 | 116 | 0.20 | 0.657 | 1 | 116 | 4.21 | 0.042 |
| Size × Sex × Season | 2 | 116 | 1.17 | 0.314 | 2 | 116 | 4.59 | 0.012 |
| Parameter | df | Estimate | SE | t |
| Intercept | 1 | 119.726 | 1.244 | 96.27 |
| Size | 1 | −2.805 | 0.772 | −3.63 |
| Size × Color PC2 | 1 | −0.876 | 0.367 | −2.39 |
| Color PC2 × Sex (female) | 1 | −3.697 | 0.887 | −4.17 |
| Color PC2 × Sex (male) | 1 | −0.663 | 0.657 | −1.01 |