| Citation: | Xiyuan Guan, Xiaodong Rao, Gang Song, Daiping Wang. 2022: The evolution of courtship displays in Galliformes. Avian Research, 13(1): 100008. DOI: 10.1016/j.avrs.2022.100008 |
Species in Galliformes have elaborate ritual courtship displays, often including strutting, fluffing of tail or head feathers, and vocal sounds that serve as excellent examples of sexual selection. According to the male orientation to the female while either posturing or moving, these courtship displays of gallinaceous species can be classified into three categories: 1) ‘frontal displays’, 2) ‘lateral displays’, and 3) ‘both frontal and lateral displays’. Questions regarding which category of displays is the ancestral state and the evolutionary history of courtship displays in Galliformes remain unanswered. We collected and classified 131 species in terms of their courtship displays into the three categories listed above and carried out a large-scale comparative analysis to reveal the evolutionary trajectory of this trait. We found that the ancestral state of courtship displays of Galliformes involves both relatively short and straightforward frontal and lateral elements (i.e., the category of ‘both frontal and lateral displays’). Furthermore, ancestral trait reconstructions suggest that transitions from ‘lateral displays’ to ‘frontal displays’ occurred more frequently than the other way around (i.e., from ‘frontal displays’ to ‘lateral displays’). In addition, some transitions occurred from ‘both frontal and lateral displays’ to ‘lateral displays’ but not from ‘both frontal and lateral displays’ to ‘frontal displays’. Ancestral state reconstruction of courtship displays at the root of the Galliformes phylogeny supports the ‘both frontal and lateral displays’ first scenario. This original state then evolved towards two extremes, either ‘frontal displays’ or ‘lateral displays’, with more complicated and elaborate display components. Moreover, subsequent transitions occurred from ‘lateral displays’ to ‘frontal displays’ much more frequently than the other way around during the evolutionary history, indicating positive selection of ‘frontal displays’.
Courtship display – a suite of behaviours displayed by an individual to attract and eventually reproduce with an individual of the opposite sex – is an essential feature of animals, fulfilling a variety of crucial functions such as sex recognition, sexual stimulation, synchronization of mating behaviour, affecting female choice process and moderation of female aggression (Bastock, 1967; Mitoyen et al., 2019). Research efforts to investigate courtship displays in the animal kingdom have mainly focused on the visually conspicuous dances and acoustic calls of birds, especially species in Galliformes (Dinsmore, 1970; Prum, 1990; Fiske et al., 1998). According to the male orientation to the female while either posturing or moving and the male body's symmetricity during displaying, courtship displays of gallinaceous species can be classified into three categories: 1) ‘frontal displays’, 2) ‘lateral displays’, and 3) ‘both frontal and lateral displays’ (del Hoyo et al., 1994). Specifically, the male faces the female with spreading the wings or tail in the category of ‘frontal displays’, which tends to be symmetrical (Fig. 1A). On the other hand, ‘lateral displays’ often involve spreading wings and the tail in such an unsymmetrical way that the bird appears more significant when viewed from the side (McGowan et al., 2020, Fig. 1B). In the third category, courtship displays of these species include elements of ‘both frontal and lateral displays’ (del Hoyo et al., 1994).
Galliformes is an order of birds that includes turkey, chicken, quail, and other landfowl. This avian group features heavy-bodies with ground feeding and is adapted to almost any environment except for innermost deserts and perpetual ice (Tian et al., 2018; del Hoyo et al., 2020). Galliformes contains 295 species divided into five families: Megapodiidae (incubator birds like malleefowl and brush-turkeys); Cracidae (including chachalacas and curassows); Numididae (guineafowls); Odontophoridae (New World quails), and Phasianidae (including chicken, quail, partridges, pheasants, turkeys, peafowl (peacocks) and grouses) (del Hoyo et al., 2020). Some species in this order are economically and culturally valuable as they are domesticated for a long history for food and appeared in ancient literature and artworks (Fuller and Garson, 2000; Persons et al., 2016).
Gallinaceous species have been model organisms for studying sexual selection, with the classic system being the plumage patterns and large tail-coverts of peacock (Pavo cristatus). It is well known that the male peacock's sexual display is among the most spectacular in nature and consists of both a glittering train and crest plumage along with various behavioural traits (Jaiswal et al., 2021). It was, in fact, Charles Darwin's fascination with the peacock that shaped his theory of sexual selection (Darwin, 1871). Furthermore, as Darwin wrote, “What a contrast is presented between the sexes by the polygamous peacock or pheasant, and the monogamous guinea-fowl or partridge!” (Darwin, 1871), which indicates that there is an extraordinary diversity of sexual dimorphism and dimorphic behaviour across all species in Galliformes. Indeed, species in this order have elaborate ritual courtship displays – often including strutting, fluffing of tail or head feathers, and vocal sounds – that serve as excellent examples of sexual selection. However, even though some species within Galliformes have elaborate and sophisticated courtship displays, on the other hand, some species have courtship displays that are rather short and straightforward in this avian group. Therefore, this contrast (i.e., rather short and straightforward displays to extremely elaborate and sophisticated displays) offers a unique opportunity to study the evolution of courtship displays using comparative methods and further makes Galliformes an interesting group for studying mating behaviours under the theory of sexual selection. One of the key and fundamental questions regarding courtship displays remaining unknown is: which category of courtship displays is the ancestral trait, and what is the evolutionary history of courtship displays in Galliformes?
Here, we investigate the origin of courtship displays and their evolutionary history over the galliform phylogeny. With 131 gallinaceous species' courtship displays, we first classified them into three categories (i.e., ‘both frontal and lateral displays’, ‘frontal displays’, and ‘lateral displays’). Then we undertook a larger-scale comparative analysis with ancestral state and transition rate analyses by phylogenetic reconstruction of this trait. Our primary aim is to summarize and complement more courtship display data in Galliformes. At the same time, we aim to reveal the ancestral state and evolution of this trait; propose potential selection drives on courtship displays in this avian group.
We assessed all 295 gallinaceous species for their courtship displays. Data on courtship displays (description notes, live videos or pictures) are from five sources that are: 1) description notes of sexual behaviour in the ‘Behaviour’ or ‘Breeding’ section; or 2) courtship display videos from the ‘Multimedia’ section of the species account from Birds of the World (del Hoyo et al., 2020); 3) courtship videos from YouTube obtained by searching with the keywords ‘species name’ + ‘mating’ or ‘display’ or ‘breeding’ or ‘sexing’ or ‘courtship’; 4) courtship videos or pictures from other website media (e.g., Baidu, Youku) or personal communication; 5) for those species for which we cannot obtain any valuable data from the above four sources, we searched the literature on their courtship displays from Google Scholar. Birds were classified into the following three categories according to their courtship displays: 1) ‘frontal displays’, in this category, the male typically faces the female with spreading the wings or tail and tends to do so symmetrically (e.g., Tragopan caboti); 2) ‘lateral displays’, in contrast to ‘frontal displays’, displays by species in this category often involve spreading wings and the tail in such an unsymmetrical way that the bird appears more significant when viewed from the side (usually the wing close to the female is dropping and the farther wing is stretching above the body, e.g., Polyplectron malacense); 3) ‘both frontal and lateral displays’, where courtship displays in this category include both elements of frontal and lateral displays (e.g., Acryllium vulturinum). For those species recorded with description notes either from Birds of the World or the published literature, we implemented the criterion that their courtship displays were clearly identified as ‘frontal displays’, ‘lateral displays’ or ‘both frontal and lateral displays’ (e.g., Sichuan Partridge Arborophila rufipectus, from Liao and Hu (2010) stating that this species has ‘lateral displays’). On the other hand, for those species with pictures or videos of courtship displays (either from Birds of the World, YouTube, or other websites), we implemented the criterion that the video or picture clearly showed the species' courtship displays.
After classifying courtship displays, we matched the scientific names from Birds of the World (del Hoyo et al., 2020) with the species names from a phylogenetic information source (www.birdtree.org, Jetz et al., 2012), which we used for further statistical analyses. By doing so, we ended up with 131 species for which we had data on their courtship displays. The 131 species included 12 species with courtship display description notes, 10 species with courtship display videos from the website of Birds of the World, 76 species with courtship videos from YouTube, six species with courtship videos or pictures from other website media or personal communication and 27 species from the literature.
All analyses were carried out within the R statistical environment (R Development Team, 2021). First, we used the ‘ace’ function in the R package ‘ape’ (Paradis and Schliep, 2019) to reconstruct ancestral states at internal nodes of the tree. Specifically, we tested three different models: 1) the ‘all rates different’ model (‘ARD’ model) to allow independent estimates for transitions to and from the three states; 2) the ‘all rates equal’ model (‘ER’ model), and 3) a model assuming symmetrical transition rates between states (“SYM” model’) to estimate the ancestral state of this discrete trait (i.e., ‘frontal displays’, ‘lateral displays’ or ‘both frontal and lateral displays’). We ultimately chose the best fit model using the likelihood ratio test.
Second, we used stochastic character mapping with the function ‘make.simmap’ in the R package ‘phytools’ (Revell, 2012) to estimate evolutionary transitions between three states: ‘frontal displays’, ‘lateral displays’ or ‘both frontal and lateral displays’. This allowed us to estimate the number of times gallinaceous species' courtship displays evolved from a reconstructed ancestral state and transitions between the other two categories of this trait. To account for phylogenetic uncertainty, stochastic maps were generated for 100 phylogenetic trees (Jetz et al., 2012), and the results represent an average across these trees. Again, we used three models with the function (i.e., ‘ARD’ model, ‘ER’ model, and ‘SYM’ model) to estimate the evolutionary transitions between three states, and the likelihood ratio test was used to assess the performance of these three models.
For all 131 (44% of Galliformes) species for which courtship displays were recorded from data sources, there were three species of Megapodiidae, 13 species of Cracidae, one species of Numididae, 20 species of Odontophoridae, and 94 species of Phasianidae (Table 1). Of these, 24 species (18%) had ‘both frontal and lateral displays’, 89 species (68%) had ‘frontal displays’, and 18 species (14%) had ‘lateral displays’ (Table 1).
| Family | Species | Displays | Source |
| Megapodiidae | Alectura lathami | Both | BOW video |
| Megapodiidae | Leipoa ocellata | Frontal | Youtube |
| Megapodiidae | Macrocephalon maleo | Frontal | Youtube |
| Cracidae | Ortalis vetula | Frontal | Youtube |
| Cracidae | Ortalis garrula | Frontal | Youtube |
| Cracidae | Ortalis canicollis | Frontal | Youtube |
| Cracidae | Penelope albipennis | Frontal | BOW description |
| Cracidae | Penelope obscura | Frontal | Literature |
| Cracidae | Pipile pipile | Frontal | Youtube |
| Cracidae | Pipile jacutinga | Frontal | Literature |
| Cracidae | Penelopina nigra | Frontal | Youtube |
| Cracidae | Mitu salvini | Frontal | Youtube |
| Cracidae | Mitu mitu | Frontal | Youtube |
| Cracidae | Crax rubra | Frontal | Youtube |
| Cracidae | Crax alector | Frontal | BOW description |
| Cracidae | Crax fasciolata | Frontal | BOW video |
| Numididae | Acryllium vulturinum | Both | Personal video |
| Odontophoridae | Oreortyx pictus | Lateral | BOW description |
| Odontophoridae | Colinus virginianus | Lateral | Literature |
| Odontophoridae | Callipepla squamata | Both | Literature |
| Odontophoridae | Callipepla californica | Both | BOW description |
| Odontophoridae | Callipepla gambelii | Both | BOW description |
| Odontophoridae | Odontophorus gujanensis | Both | Literature |
| Odontophoridae | Odontophorus capueira | Both | Literature |
| Odontophoridae | Odontophorus melanotis | Both | Literature |
| Odontophoridae | Odontophorus atrifrons | Both | Literature |
| Odontophoridae | Odontophorus erythrops | Both | Literature |
| Odontophoridae | Odontophorus hyperythrus | Both | Literature |
| Odontophoridae | Odontophorus melanonotus | Both | Literature |
| Odontophoridae | Odontophorus speciosus | Both | Literature |
| Odontophoridae | Odontophorus dialeucos | Both | Literature |
| Odontophoridae | Odontophorus strophium | Both | Literature |
| Odontophoridae | Odontophorus columbianus | Both | Literature |
| Odontophoridae | Odontophorus leucolaemus | Both | Literature |
| Odontophoridae | Odontophorus balliviani | Both | Literature |
| Odontophoridae | Odontophorus stellatus | Both | Literature |
| Odontophoridae | Odontophorus guttatus | Both | Literature |
| Phasianidae | Arborophila rufipectus | Lateral | Literature |
| Phasianidae | Rheinardia ocellata | Both | Youtube |
| Phasianidae | Argusianus argus | Both | Youtube/Literature |
| Phasianidae | Pavo cristatus | Frontal | BOW video |
| Phasianidae | Pavo muticus | Frontal | Youtube |
| Phasianidae | Afropavo congensis | Frontal | Youtube |
| Phasianidae | Haematortyx sanguiniceps | Frontal | Youtube |
| Phasianidae | Galloperdix spadicea | Frontal | Youtube |
| Phasianidae | Galloperdix lunulata | Frontal | Youtube |
| Phasianidae | Polyplectron napoleonis | Lateral | BOW video |
| Phasianidae | Polyplectron malacense | Lateral | BOW video |
| Phasianidae | Polyplectron schleiermacheri | Lateral | Youtube |
| Phasianidae | Polyplectron germaini | Lateral | Youtube |
| Phasianidae | Polyplectron inopinatum | Lateral | BOW video |
| Phasianidae | Polyplectron chalcurum | Lateral | Youtube |
| Phasianidae | Polyplectron bicalcaratum | Lateral | Youtube |
| Phasianidae | Ammoperdix griseogularis | Frontal | Youtube |
| Phasianidae | Ammoperdix heyi | Frontal | Youtube |
| Phasianidae | Synoicus chinensis | Both | Youtube |
| Phasianidae | Coturnix japonica | Frontal | Literature |
| Phasianidae | Coturnix coturnix | Frontal | Literature |
| Phasianidae | Coturnix delegorguei | Frontal | Literature |
| Phasianidae | Coturnix coromandelica | Frontal | Literature |
| Phasianidae | Coturnix pectoralis | Frontal | Literature |
| Phasianidae | Alectoris graeca | Frontal | Youtube |
| Phasianidae | Alectoris chukar | Frontal | Youtube |
| Phasianidae | Alectoris philbyi | Frontal | Youtube |
| Phasianidae | Alectoris rufa | Frontal | Youtube |
| Phasianidae | Alectoris melanocephala | Frontal | Youtube |
| Phasianidae | Alectoris barbara | Frontal | Youtube |
| Phasianidae | Tetraogallus caucasicus | Frontal | Youtube |
| Phasianidae | Tetraogallus caspius | Frontal | Youtube |
| Phasianidae | Tetraogallus tibetanus | Frontal | BOW video |
| Phasianidae | Tetraogallus himalayensis | Frontal | BOW description |
| Phasianidae | Pternistis natalensis | Frontal | Youtube |
| Phasianidae | Pternistis harwoodi | Frontal | Literature |
| Phasianidae | Pternistis swainsonii | Both | BOW description |
| Phasianidae | Pternistis afer | Frontal | BOW video |
| Phasianidae | Francolinus francolinus | Frontal | Youtube |
| Phasianidae | Dendroperdix sephaena | Lateral | Youtube |
| Phasianidae | Bambusicola fytchii | Frontal | Youtube |
| Phasianidae | Bambusicola thoracicus | Frontal | Youtube |
| Phasianidae | Gallus gallus | Lateral | Youtube |
| Phasianidae | Peliperdix coqui | Lateral | Literature |
| Phasianidae | Ithaginis cruentus | Frontal | Baidu |
| Phasianidae | Lophophorus impejanus | Frontal | Youtube |
| Phasianidae | Lophophorus sclateri | Frontal | Youtube |
| Phasianidae | Lophophorus lhuysii | Frontal | Youtube |
| Phasianidae | Lerwa lerwa | Frontal | BOW description |
| Phasianidae | Tetraophasis szechenyii | Frontal | Baidu |
| Phasianidae | Tragopan melanocephalus | Frontal | Youtube |
| Phasianidae | Tragopan satyra | Frontal | Youtube |
| Phasianidae | Tragopan temminckii | Frontal | Youtube |
| Phasianidae | Tragopan caboti | Frontal | Youtube |
| Phasianidae | Syrmaticus reevesii | Lateral | Youtube/xinhuanet |
| Phasianidae | Syrmaticus soemmerringii | Frontal | Youtube |
| Phasianidae | Syrmaticus mikado | Frontal | Youtube |
| Phasianidae | Syrmaticus ellioti | Frontal | BOW video |
| Phasianidae | Syrmaticus humiae | Frontal | Youtube |
| Phasianidae | Chrysolophus pictus | Lateral | Youtube |
| Phasianidae | Chrysolophus amherstiae | Lateral | Youtube |
| Phasianidae | Phasianus colchicus | Frontal | Youtube |
| Phasianidae | Crossoptilon crossoptilon | Frontal | Baidu |
| Phasianidae | Crossoptilon mantchuricum | Frontal | Youtube |
| Phasianidae | Crossoptilon auritum | Frontal | Baidu |
| Phasianidae | Lophura nycthemera | Frontal | Youtube |
| Phasianidae | Lophura leucomelanos | Frontal | Youtube |
| Phasianidae | Lophura diardi | Frontal | Youtube |
| Phasianidae | Lophura bulweri | Lateral | Youtube |
| Phasianidae | Lophura swinhoii | Frontal | Youtube |
| Phasianidae | Lophura erythrophthalma | Lateral | Youtube |
| Phasianidae | Lophura ignita | Frontal | Youku |
| Phasianidae | Perdix perdix | Frontal | Youtube |
| Phasianidae | Pucrasia macrolopha | Frontal | BOW video |
| Phasianidae | Tetrao urogalloides | Frontal | Youtube |
| Phasianidae | Tetrao urogallus | Frontal | BOW description |
| Phasianidae | Tetrao tetrix | Frontal | Youtube |
| Phasianidae | Tetrao mlokosiewiczi | Frontal | Youtube |
| Phasianidae | Tetrastes bonasia | Frontal | Youtube |
| Phasianidae | Bonasa umbellus | Frontal | Youtube |
| Phasianidae | Centrocercus urophasianus | Frontal | Youtube |
| Phasianidae | Centrocercus minimus | Frontal | Youtube |
| Phasianidae | Falcipennis falcipennis | Frontal | Youtube |
| Phasianidae | Falcipennis canadensis | Frontal | Youtube |
| Phasianidae | Lagopus lagopus | Frontal | BOW description |
| Phasianidae | Lagopus muta | Frontal | BOW description |
| Phasianidae | Lagopus leucura | Frontal | BOW description |
| Phasianidae | Dendragapus obscurus | Frontal | Youtube |
| Phasianidae | Dendragapus fuliginosus | Frontal | Youtube |
| Phasianidae | Tympanuchus phasianellus | Frontal | Youtube |
| Phasianidae | Tympanuchus cupido | Frontal | Youtube |
| Phasianidae | Tympanuchus pallidicinctus | Frontal | Youtube |
| Phasianidae | Meleagris gallopavo | Frontal | Youtube |
| Phasianidae | Meleagris ocellata | Frontal | Youtube |
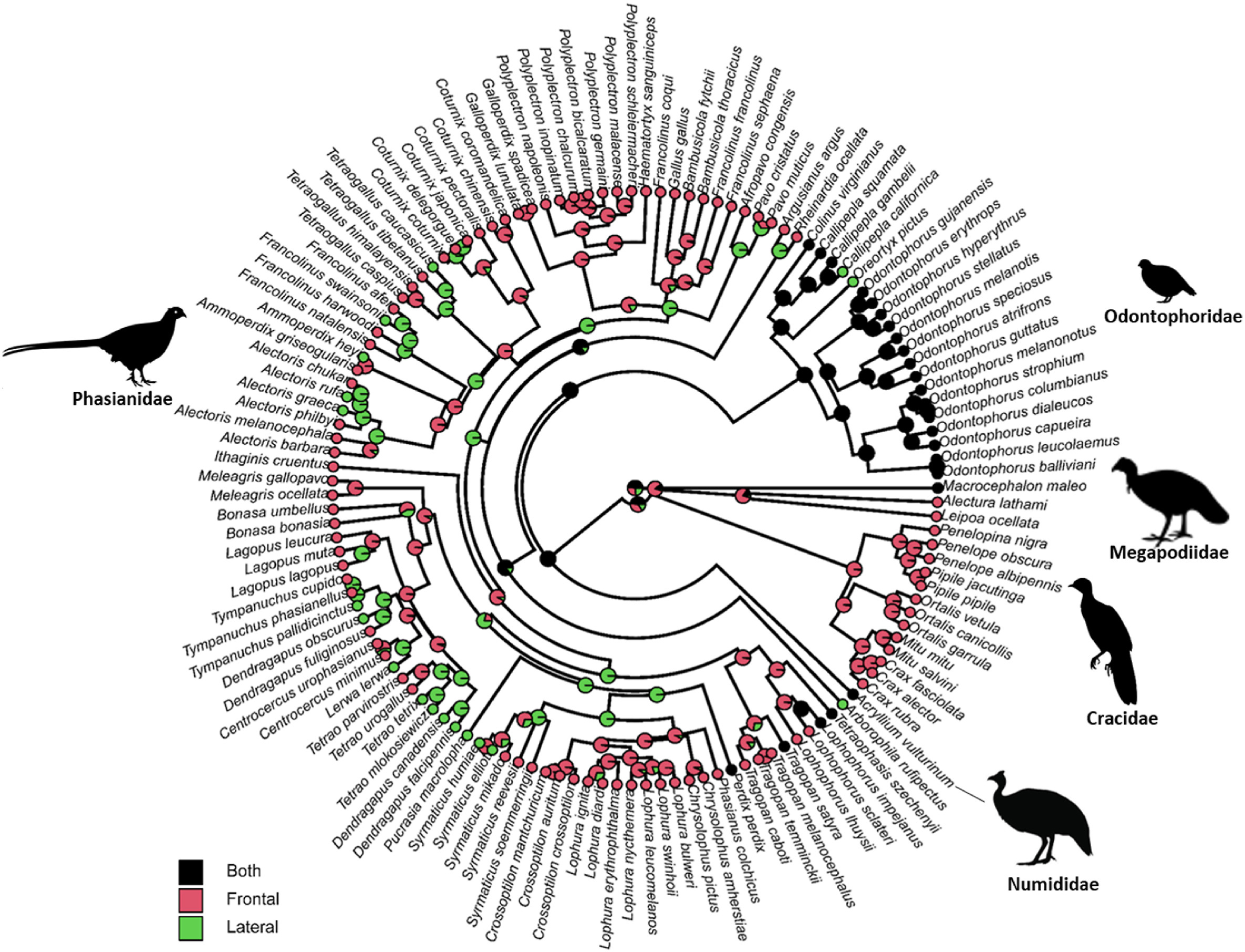
There is a strong phylogenetic signal of courtship displays in this order (Fig. 2, λ close to one). In detail, all species in Cracidae had ‘frontal displays’. For species in Megapodiidae and Numididae, courtship displays had either ‘both frontal and lateral displays’ or ‘frontal displays’. For those species in Odontophoridae, most of them had ‘both frontal and lateral displays’, except for two species with ‘lateral displays’. However, for those species in ‘Phaslanidae’, courtship displays had a diverse pattern: species of grouse, turkeys, peafowls, monals, and tragopans mainly had ‘frontal displays’; on the other hand, species of peacock-pheasants, and some pheasants (e.g., Chrysolophus pictus, Chrysolophus amherstiae and Gallus gallus) had ‘lateral displays’ (Fig. 2).
The ancestral state analysis using three models (i.e., ‘ARD’, ‘ER’, and ‘SYM’ models) to reconstruct the ancestral state of courtship displays showed that the ‘ARD’ was the best fit model (likelihood value: −55.3) compared with the ‘ER’ (−62.2, likelihood ratio test, p = 0.02) and the ‘SYM’ model (−61.1, likelihood ratio test, p = 0.01). This ancestral state reconstruction (‘ARD’ model’) revealed that the most likely state at the root of the phylogeny was the category of ‘both frontal and lateral displays’ (probability of root being ‘both frontal and lateral displays’: 0.69, Fig. 2).
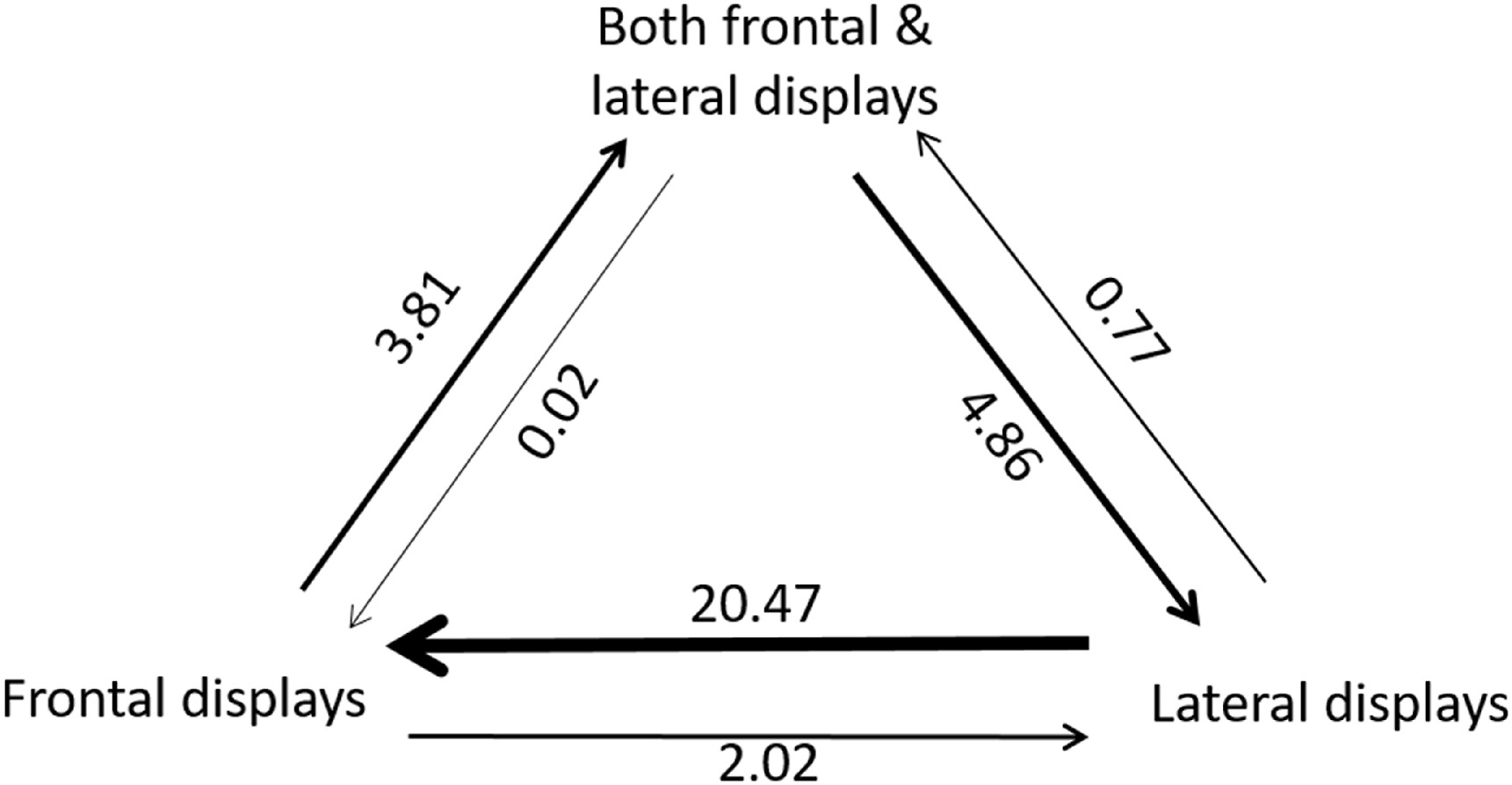
Similarly, we found that the analysis of transition rates between these three states showed that the ‘ARD’ model is the best fit model with the highest likelihood value (−54.6) compared with the ‘ER’ (−60.9, likelihood ratio test, p = 0.03) and ‘SYM’ models (−59.6, likelihood ratio test, p = 0.02). The analysis of transition rates between states suggests that transitions from ‘frontal displays’ to ‘lateral displays’ occurred at a much lower rate (average: 2.0) than the reverse (average: 20.5, Fig. 3). Some transitions occurred from ‘both frontal and lateral displays’ to ‘lateral displays’ (average: 4.9) but not from ‘both frontal and lateral displays’ to ‘frontal displays’ (Fig. 3). Furthermore, relatively few transitions occurred from ‘frontal displays’ to ‘both frontal and lateral displays’ (average: 3.8, Fig. 3).
The courtship display is a fundamental aspect of the breeding life history of Galliformes. With 131 gallinaceous species classified for their courtship displays, including members of all families of Galliformes, we provide the first view of the origin and subsequent transitions of courtship displays in this avian group. Our results revealed that courtship displays in Galliformes might have evolved from a rather short and straightforward display including both frontal and lateral elements (i.e., ‘both frontal and lateral displays’). This pattern seems particularly apparent for the family of Phasianidae: species having either elaborate and sophisticated ‘frontal displays’ or ‘lateral displays’ evolved from the ancestral state of ‘both frontal and lateral displays’ (Fig. 2). Furthermore, our results from transition rate analysis showed that transition occurred from the state of ‘lateral displays’ to ‘frontal displays’ much more often than the other way around during the evolutionary history (average number of transitions: 20.47 vs. 2.02, Fig. 3). Here, we discuss the implications of these results and highlight some areas of particular interest and possible avenues for further research.
The analysis reconstructing ancestral states of courtship displays at the root of the phylogeny supports the ‘both frontal and lateral displays’ first scenario in Galliformes (Fig. 2). This analysis was based on well-established phylogenetic relationships of Galliformes (i.e., trees used in this study, Fig. 2), at least at the family level (Jetz et al., 2012). According to phylogenetic work, megapodes (Megapodiidae) are thought to have diverged first, followed by curassows (Cracidae) (Pereira and Baker, 2006). The New World quails (Odontophoridae) and guineafowl (Numididae) split off later, although the order of this is uncertain (Armstrong et al., 2001). All phylogenies suggest that the remaining taxa all belong to a single clade referred to as the Phasianidae (Kimball et al., 1999; Dyke et al., 2003), although it contains groups previously thought to be separate families such as the turkeys (Meleagridae) and grouse (Tetraonidae) (Dimcheff et al., 2002). In accordance with these phylogenetic relationships, our data showed that families at the base of Galliformes fall with the category of ‘both frontal and lateral displays’ (one species of Megapodiidae, 18 species of Odontophoridae, and one species of Numididae; Fig. 2). Furthermore, courtship displays from these ‘basal’ families are rather elemental, usually including only a few simple actions within a short period (e.g., Alectura lathami, Acryllium vulturinum) compared with much more complicated and sophisticated courtship displays from the family Phasianidae (e.g., Tragopan caboti, Centrocercus urophasianus). Therefore, we here propose that the trait of courtship displays in Galliformes originated from a state of displays with both simple frontal and lateral elements. Then, this ancestral state evolved towards two extremes, either to ‘frontal displays’ or ‘lateral displays’, but with more complicated and elaborate display components. In this process, sexual selection might be the primary driving force, which calls for future research work. Several members of the Galliformes with their courtship displays are very well studied and provide classic examples of sexual selection (Petrie et al., 1991; Petrie, 1992, 1994; Zuk et al., 1992; Mateos and Carranza, 1997; Hagelin, 2002). This makes it appealing to carry out comparative studies of these birds in terms of their life history characteristics, including mating behaviour under the framework of sexual selection.
Although our ancestral state analysis showed that the origin of courtship displays is the category of ‘both frontal and lateral displays’, transition rate analysis between these three states further revealed more details of the evolutionary history of this trait. Overall, the highest transition rate occurred from the state of ‘lateral displays’ to the state of ‘frontal displays’ (average transitions: 20.5, Fig. 3). However, there were much fewer transitions from the state of ‘frontal displays’ to the state of ‘lateral displays’ (average transitions: 2.0, Fig. 3). This pattern might suggest that there was direct selection towards more ‘frontal displays’ in Galliformes across an evolutionary time scale. Indeed, in the family of Phasianidae in which the clade diverged recently on the phylogeny (Kimball et al., 1999; Dyke et al., 2003), most species (including groups of turkeys and grouses) typically have ‘frontal courtship displays’. Furthermore, ‘frontal courtship displays’ in this family are usually more complicated and sophisticated with longer display times. Some of these species are known as ‘lekking’ species (e.g., Centrocercus urophasianus, Tetrao tetrix) (Gibson et al., 1991; Baines, 1996). A ‘lek’ is an aggregation of males gathered to engage in competitive displays and courtship rituals, to entice visiting females who are surveying prospective partners to mate with (Fiske et al., 1998). Males of ‘lekking’ species are characterized by highly developed and spectacular mating displays for attracting females. With strong female mate choice (i.e., intense sperm competition under sexual selection), the variation in mating success is quite large in ‘lekking’ mating systems: only a small percentage of males in the whole population can mate with females, and males provide neither paternal care nor resources such as nesting or foraging sites (Mackenzie et al., 1995). In short, such elaborate courtship displays of ‘lekking’ species intrigued a puzzle known as the ‘lekking paradox’: strong sexual selection by females for specific male traits ought to erode genetic diversity by Fisherian runaway (i.e. a positive loop between the female mate choice for the male trait and the resulted male extraordinary trait), but the diversity is maintained, and runaway does not occur (Beehler and Foster, 1988; Thery, 1992; Jiguet and Bretagnolle, 2006; Duraes et al., 2008). Many attempts have been made to explain it away, but the paradox remains. Therefore, studying courtship displays of these ‘lekking’ species under sexual selection theory to solve the ‘lekking paradox’ provides an avenue for further research.
As phylogenies are hypotheses about evolutionary relationships (Reynolds et al., 2002), the results and patterns of comparative studies based on phylogenetic relationships should be interpreted with caution. Despite the fact that we used courtship displays of 131 gallinaceous species in this study to investigate the origin and subsequent transitions of this trait in Galliformes, some limitations need to be considered. At best, data of 131 species from five families used in this study are representative of all species of Galliformes, and our results are complementary to various kinds of data from different sources that differ in quality. At worst, the inferences (the origin and subsequent transitions of courtship displays in Galliformes) might be biased. We still missed the courtship displays of more than 160 species; moreover, the courtship displays of some species we have used in this study might only be partially recorded. However, although some patterns would change as more information becomes available, we believe that the overall direction of our results is probably correct. It is worth noting that this kind of uncertainty is not unique to comparative studies using phylogenetic information; there are errors around all statistical estimates, including those derived from experimental studies.
Studies on Galliformes have demonstrated remarkable achievements since 1990, but life history information on many galliform species is still lacking, which has hindered conservation efforts and effectiveness. We showed that courtship displays in this avian group evolved from an origin of rather short and straightforward displays, including both frontal and lateral elements. This original state then evolved towards two extremes, either ‘frontal displays’ or ‘lateral displays’, with more complicated and elaborate display components. Moreover, subsequent transitions occurred from the state of ‘lateral displays’ to ‘frontal displays’ much more often than the other way around during the evolutionary history, indicating a positive selection of ‘frontal displays’.
DW designed the study; DW, XG and XR collected the data; DW analyzed the data; DW, XG, XR and GS wrote the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
We thank Prof. Dr. Damien Farine for providing courtship display data of Vulturine Guineafowl. We thank the two reviewers who provided constructive comments for our manuscript. D.W. was supported by the CAS pioneer hundred talents program. X.G. and G.S. were supported by the National Science and Technology Major Project (No. 2018ZX10101004). X.R. was supported by the National Natural Science Foundation of China (No. 31800320), the Joint Fund of the Natural Science Foundation of Hainan Province (No. 320RC506), and the Scientific Research start-up Fund of Hainan University (No. KYQD (ZR) 20057).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100008.
|
Baines, D., 1996. Seasonal variation in lek attendance and lekking behaviour by male black grouse Tetrao tetrix. Ibis 138, 177-180
|
|
Bastock, M., 1967. Physiology of courtship and mating behaviour. Adv. Reprod. Physiol. 2, 9
|
|
Darwin, C., 1871. The Descent of Man and Selection in Relation to Sex. Murray, London.
|
|
del Hoyo, J., Elliott, A., Sargatal, J., 1994. Handbook of the Birds of the World. Lynx Edicions, Barcelona.
|
|
del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., Juana, A.E.D., 2020. Birds of the World. Cornell Lab of Ornithology, Ithaca, NY, USA.
|
|
Fuller, R.A., Garson, P.J., 2000. Pheasants: Status Survey and Conservation Action Plan 2000‒2004. IUCN, Gland.
|
|
Liao, W., Hu, J., 2010. A review of the ecological traits of Arborophila rufipectus. J. Mianyang Normal Univ. 2, 67-71
|
|
Prum, R.O.J.E., 1990. Phylogenetic analysis of the evolution of display behavior in the Neotropical manakins (Aves: Pipridae). Ethology 84, 202-231
|
|
Team, R.D.C., 2021. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
|
| Family | Species | Displays | Source |
| Megapodiidae | Alectura lathami | Both | BOW video |
| Megapodiidae | Leipoa ocellata | Frontal | Youtube |
| Megapodiidae | Macrocephalon maleo | Frontal | Youtube |
| Cracidae | Ortalis vetula | Frontal | Youtube |
| Cracidae | Ortalis garrula | Frontal | Youtube |
| Cracidae | Ortalis canicollis | Frontal | Youtube |
| Cracidae | Penelope albipennis | Frontal | BOW description |
| Cracidae | Penelope obscura | Frontal | Literature |
| Cracidae | Pipile pipile | Frontal | Youtube |
| Cracidae | Pipile jacutinga | Frontal | Literature |
| Cracidae | Penelopina nigra | Frontal | Youtube |
| Cracidae | Mitu salvini | Frontal | Youtube |
| Cracidae | Mitu mitu | Frontal | Youtube |
| Cracidae | Crax rubra | Frontal | Youtube |
| Cracidae | Crax alector | Frontal | BOW description |
| Cracidae | Crax fasciolata | Frontal | BOW video |
| Numididae | Acryllium vulturinum | Both | Personal video |
| Odontophoridae | Oreortyx pictus | Lateral | BOW description |
| Odontophoridae | Colinus virginianus | Lateral | Literature |
| Odontophoridae | Callipepla squamata | Both | Literature |
| Odontophoridae | Callipepla californica | Both | BOW description |
| Odontophoridae | Callipepla gambelii | Both | BOW description |
| Odontophoridae | Odontophorus gujanensis | Both | Literature |
| Odontophoridae | Odontophorus capueira | Both | Literature |
| Odontophoridae | Odontophorus melanotis | Both | Literature |
| Odontophoridae | Odontophorus atrifrons | Both | Literature |
| Odontophoridae | Odontophorus erythrops | Both | Literature |
| Odontophoridae | Odontophorus hyperythrus | Both | Literature |
| Odontophoridae | Odontophorus melanonotus | Both | Literature |
| Odontophoridae | Odontophorus speciosus | Both | Literature |
| Odontophoridae | Odontophorus dialeucos | Both | Literature |
| Odontophoridae | Odontophorus strophium | Both | Literature |
| Odontophoridae | Odontophorus columbianus | Both | Literature |
| Odontophoridae | Odontophorus leucolaemus | Both | Literature |
| Odontophoridae | Odontophorus balliviani | Both | Literature |
| Odontophoridae | Odontophorus stellatus | Both | Literature |
| Odontophoridae | Odontophorus guttatus | Both | Literature |
| Phasianidae | Arborophila rufipectus | Lateral | Literature |
| Phasianidae | Rheinardia ocellata | Both | Youtube |
| Phasianidae | Argusianus argus | Both | Youtube/Literature |
| Phasianidae | Pavo cristatus | Frontal | BOW video |
| Phasianidae | Pavo muticus | Frontal | Youtube |
| Phasianidae | Afropavo congensis | Frontal | Youtube |
| Phasianidae | Haematortyx sanguiniceps | Frontal | Youtube |
| Phasianidae | Galloperdix spadicea | Frontal | Youtube |
| Phasianidae | Galloperdix lunulata | Frontal | Youtube |
| Phasianidae | Polyplectron napoleonis | Lateral | BOW video |
| Phasianidae | Polyplectron malacense | Lateral | BOW video |
| Phasianidae | Polyplectron schleiermacheri | Lateral | Youtube |
| Phasianidae | Polyplectron germaini | Lateral | Youtube |
| Phasianidae | Polyplectron inopinatum | Lateral | BOW video |
| Phasianidae | Polyplectron chalcurum | Lateral | Youtube |
| Phasianidae | Polyplectron bicalcaratum | Lateral | Youtube |
| Phasianidae | Ammoperdix griseogularis | Frontal | Youtube |
| Phasianidae | Ammoperdix heyi | Frontal | Youtube |
| Phasianidae | Synoicus chinensis | Both | Youtube |
| Phasianidae | Coturnix japonica | Frontal | Literature |
| Phasianidae | Coturnix coturnix | Frontal | Literature |
| Phasianidae | Coturnix delegorguei | Frontal | Literature |
| Phasianidae | Coturnix coromandelica | Frontal | Literature |
| Phasianidae | Coturnix pectoralis | Frontal | Literature |
| Phasianidae | Alectoris graeca | Frontal | Youtube |
| Phasianidae | Alectoris chukar | Frontal | Youtube |
| Phasianidae | Alectoris philbyi | Frontal | Youtube |
| Phasianidae | Alectoris rufa | Frontal | Youtube |
| Phasianidae | Alectoris melanocephala | Frontal | Youtube |
| Phasianidae | Alectoris barbara | Frontal | Youtube |
| Phasianidae | Tetraogallus caucasicus | Frontal | Youtube |
| Phasianidae | Tetraogallus caspius | Frontal | Youtube |
| Phasianidae | Tetraogallus tibetanus | Frontal | BOW video |
| Phasianidae | Tetraogallus himalayensis | Frontal | BOW description |
| Phasianidae | Pternistis natalensis | Frontal | Youtube |
| Phasianidae | Pternistis harwoodi | Frontal | Literature |
| Phasianidae | Pternistis swainsonii | Both | BOW description |
| Phasianidae | Pternistis afer | Frontal | BOW video |
| Phasianidae | Francolinus francolinus | Frontal | Youtube |
| Phasianidae | Dendroperdix sephaena | Lateral | Youtube |
| Phasianidae | Bambusicola fytchii | Frontal | Youtube |
| Phasianidae | Bambusicola thoracicus | Frontal | Youtube |
| Phasianidae | Gallus gallus | Lateral | Youtube |
| Phasianidae | Peliperdix coqui | Lateral | Literature |
| Phasianidae | Ithaginis cruentus | Frontal | Baidu |
| Phasianidae | Lophophorus impejanus | Frontal | Youtube |
| Phasianidae | Lophophorus sclateri | Frontal | Youtube |
| Phasianidae | Lophophorus lhuysii | Frontal | Youtube |
| Phasianidae | Lerwa lerwa | Frontal | BOW description |
| Phasianidae | Tetraophasis szechenyii | Frontal | Baidu |
| Phasianidae | Tragopan melanocephalus | Frontal | Youtube |
| Phasianidae | Tragopan satyra | Frontal | Youtube |
| Phasianidae | Tragopan temminckii | Frontal | Youtube |
| Phasianidae | Tragopan caboti | Frontal | Youtube |
| Phasianidae | Syrmaticus reevesii | Lateral | Youtube/xinhuanet |
| Phasianidae | Syrmaticus soemmerringii | Frontal | Youtube |
| Phasianidae | Syrmaticus mikado | Frontal | Youtube |
| Phasianidae | Syrmaticus ellioti | Frontal | BOW video |
| Phasianidae | Syrmaticus humiae | Frontal | Youtube |
| Phasianidae | Chrysolophus pictus | Lateral | Youtube |
| Phasianidae | Chrysolophus amherstiae | Lateral | Youtube |
| Phasianidae | Phasianus colchicus | Frontal | Youtube |
| Phasianidae | Crossoptilon crossoptilon | Frontal | Baidu |
| Phasianidae | Crossoptilon mantchuricum | Frontal | Youtube |
| Phasianidae | Crossoptilon auritum | Frontal | Baidu |
| Phasianidae | Lophura nycthemera | Frontal | Youtube |
| Phasianidae | Lophura leucomelanos | Frontal | Youtube |
| Phasianidae | Lophura diardi | Frontal | Youtube |
| Phasianidae | Lophura bulweri | Lateral | Youtube |
| Phasianidae | Lophura swinhoii | Frontal | Youtube |
| Phasianidae | Lophura erythrophthalma | Lateral | Youtube |
| Phasianidae | Lophura ignita | Frontal | Youku |
| Phasianidae | Perdix perdix | Frontal | Youtube |
| Phasianidae | Pucrasia macrolopha | Frontal | BOW video |
| Phasianidae | Tetrao urogalloides | Frontal | Youtube |
| Phasianidae | Tetrao urogallus | Frontal | BOW description |
| Phasianidae | Tetrao tetrix | Frontal | Youtube |
| Phasianidae | Tetrao mlokosiewiczi | Frontal | Youtube |
| Phasianidae | Tetrastes bonasia | Frontal | Youtube |
| Phasianidae | Bonasa umbellus | Frontal | Youtube |
| Phasianidae | Centrocercus urophasianus | Frontal | Youtube |
| Phasianidae | Centrocercus minimus | Frontal | Youtube |
| Phasianidae | Falcipennis falcipennis | Frontal | Youtube |
| Phasianidae | Falcipennis canadensis | Frontal | Youtube |
| Phasianidae | Lagopus lagopus | Frontal | BOW description |
| Phasianidae | Lagopus muta | Frontal | BOW description |
| Phasianidae | Lagopus leucura | Frontal | BOW description |
| Phasianidae | Dendragapus obscurus | Frontal | Youtube |
| Phasianidae | Dendragapus fuliginosus | Frontal | Youtube |
| Phasianidae | Tympanuchus phasianellus | Frontal | Youtube |
| Phasianidae | Tympanuchus cupido | Frontal | Youtube |
| Phasianidae | Tympanuchus pallidicinctus | Frontal | Youtube |
| Phasianidae | Meleagris gallopavo | Frontal | Youtube |
| Phasianidae | Meleagris ocellata | Frontal | Youtube |