| Citation: | Xianglong Xu, Jiahu Jiang, Yu Lei, Chao Wang, Baoping Qing, Changqing Ding. 2022: Using stable isotope to compare the habitat use and trophic level between the new and old breeding range of wild Crested Ibis in the early breeding season. Avian Research, 13(1): 100007. DOI: 10.1016/j.avrs.2022.100007 |
The concept of foraging niche provides an insight into habitat use and dietary information of animals. Knowing intraspecific variation in foraging niche and trophic level is critical to the understanding of the species response to environmental changes during the process of range expansion, as well as the habitat management for conservation of threatened species. Using stable isotopic values of eggshell membranes (δ13C and δ15N), we examined whether there are differences in habitat use, trophic level, foraging niche width between the new and old breeding habitats (plains vs. mountains) of wild Crested Ibis (Nipponia nippon) in the early breeding season. Crested Ibis exhibited high variability in both δ13C and δ15N values, δ13C and δ15N varied as a function of habitat types. Birds breeding in plains had significantly higher δ13C but lower δ15N values compared to the birds breeding in mountains. Higher δ15N suggested that individuals in mountains consumed a great proportion of higher trophic level prey species in the early breeding season. Moreover, the isotopic niches were distinctly different in positions and showed small overlap between the two habitat types. The niche width was wider in old habitat than in the expanded new habitat. Our results demonstrated that the wild Crested Ibis had a high intraspecific variation in habitat uses and trophic levels in the early breeding season, and they could be divided into mountain and plain groups based on their different foraging niches. The difference in δ15N and niche width revealed that high trophic level food resources might be insufficient in plains. These findings stressed the importance of protecting foraging grounds in mountains and the necessity of improving foraging grounds in plains during winter and spring. Our study highlights the feasibility of investigating intraspecific variation in foraging ecology of birds through non-invasive isotopes of eggshell membranes. Understanding foraging niche variation gives us an insight into the food resource diversity in local areas and provides important information regarding particular foraging habitats that require protection.
Foraging behavior is an important component in animal life history (Stearns, 1992). The quality of food resources obtained from the environment is a major determinant of individuals' fitness-related traits, such as survival and reproduction (Heiss et al., 2009; Meyrier et al., 2017; Weimerskirch, 2018; Francesiaz et al., 2020). Therefore, knowing dietary information has important implications for understanding the ecology of species and for the conservation and management of the focal animals (Bolnick et al., 2003; Becker et al., 2006; Kowalczyk et al., 2014; Lei et al., 2021).
Among the concepts of niches, foraging niche is essential to understand the adaption of animals when they expand to new habitats (Francesiaz et al., 2020). Most interspecific studies demonstrated the shift in distribution ranges of species was related to their foraging niches (see review by MacLean et al., 2017). For example, Rader et al. (2017) found that the elevation ranges of 12 species of Cinclodes were positively correlated with their foraging niches. Similarly, in North American birds, diet breadth also had a positive effect on the shift in their wintering ranges (Angert et al., 2011). These results suggested that expanding individuals or populations might add new food items to their diet and form a generalist forager with greater foraging niche (Buckley, 2012), and such generalists were expected to be more successful in responding to environmental changes than specialists (Jeschke and Strayer, 2006). For example, in Little Penguin (Eudyptula minor), birds with a higher dietary diversity and the consumption of prey with higher trophic level are more likely to have greater reproductive success (Kowalczyk et al., 2014). However, other studies found opposite patterns that bird species with narrower diet breadths exhibited greater range expansion because they might track their preferred food resources (Brommer, 2008; Auer and King, 2014). The universality of these findings is not yet clear, but additional studies on the variation in foraging niche at both individual and population levels during the process of range expansion, such as the individual variation of foraging strategies, have been beneficial to enhance our integral comprehension towards the mechanism of how species response to environmental changes. (Skórka et al., 2009; Balasubramaniam and Rotenberry, 2016).
The Crested Ibis (Nipponia nippon) is now categorized as an "Endangered" (EN) bird species in the IUCN Red List of threatened species (BirdLife International, 2018). Since seven birds were rediscovered in 1981 in a mountain village of Yang County, Shaanxi Province, China (Liu, 1981), the wild population of the Crested Ibis has been recuperated to more than 2600 individuals through diligent and continuous conservation efforts (Wang et al., 2014, 2020). In this growth process, the breeding ranges of wild Crested Ibis have spread from the mountains to plains, and from Yang County to Chenggu County of Shaanxi Province. The ibises use the surrounding areas of nesting sites for feeding (Ding, 2004), within a maximum foraging range of 3 km during breeding season (Hu, 2016). Hence the mountains and plains represent both the breeding and the feeding habitats. Previous studies discovered that the reproduction of Crested Ibis differed between two breeding ranges: nests in plains have higher reproductive success than those in the mountains (Song, 2018; Wang et al., 2020). However, the underlying mechanism leading to this disparity remains largely unknown. We believe that such variation might primarily relates to the differences in dietary resources between the two feeding habitats. However, mainly due to the difficulty of obtaining information on food types and their richness, diet and foraging behavior has always been the bottleneck problem for study and conservation of the Crested Ibis. Traditional methods, such as direct observation, fecal and stomach dissection analysis, demand substantial time and manpower, require extensive taxonomic knowledge, identify only an insignificant portion of prey items, or conflict with the non-invasive sampling requirements of endangered species studies (Owen, 1975; Galimberti et al., 2016). While DNA barcoding, seems to be an accurate and convenient technique to detect diet from dropping samples (Jedlicka et al., 2013; Kress et al., 2015), it cannot be applied to the studies of certain species when the dropping samples would be hard-won.
In contrast, stable isotope analysis (SIA) is able to overcome the above limitations and is increasingly employed in studies of foraging ecology of animals (Fisk et al., 2002; Han et al., 2019; Milano et al., 2021). SIA relies on an assumption that the isotopic signatures of consumers originate from the preys they eat; these isotopic markers therefore can be used to infer the dietary information of an organism (see review by Inger and Bearhop, 2008). Carbon and nitrogen isotopes are most frequently used in foraging ecology studies. δ13C is determined by primary production and can be used to infer foraging location and habitat, while δ15N is enriched with trophic level in a stepwise manner and therefore can be used to estimate trophic levels (Bearhop et al., 2002, 2006). For the same species, different tissues have different element assimilation rates and reflect diet at varying timescales (such as plasma: 1–7 days, feather: entire period during feather growth) (Hobson and Clark, 1992; Bearhop et al., 2002, 2003). Eggshell membranes are synthesized approximately 3–5 days before the egg is laid (Hobson, 1995). Oppel et al. (2009) found no evidence for systematic isotopic changes between complete egg membranes and remaining (hatched or depredated) membranes, or between different incubation stages. Thus, stable isotopic analysis of eggshell membranes appears to provide a potentially non-invasive way to investigate dietary information in the early breeding season (during egg formation) for endangered bird species.
In this study, our objective was to evaluate the variability of dietary information of wild Crested Ibis in the early breeding season, to investigate if there are differences in habitat use and trophic level between the old and new breeding ranges of ibis, using δ13C and δ15N values of eggshell membrane samples. We also tested the prediction that birds in the expanded new habitat (plains) should have a wider foraging niche width than birds in the old habitat (mountains). Tackling these problems helps breaking through the existing bottleneck of sampling methods, and therefore assists the dietary study of wild Crested Ibis by providing information on the food resource diversity of different breeding habitats. This study also delves into analyzing the driving factors behind the shift in breeding ranges and life history traits of Crested Ibis.
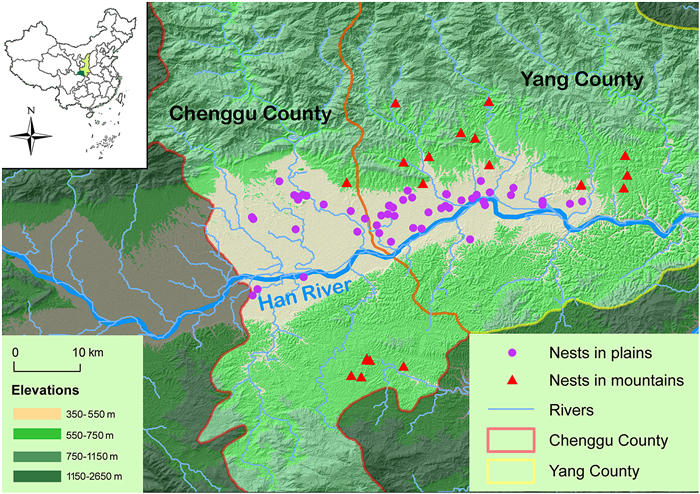
Our study area includes the Shaanxi Hanzhong Crested Ibis National Nature Reserve and surrounding areas (32°45′–33°43′ N; 107°03′−108°33′ E), mainly in Yang County and Chenggu County, Shaanxi Province, central China, with elevation rangeing from 430 m to 1100 m. This area is a basin surrounded by the Qingling Mountains to the north and Daba Mountains to the south, with the Han River plains in center (Fig. 1). Presently, the wild Crested Ibis mainly distributes in the plains and middle-lower elevation mountain areas. Plains are covered by vast stretches of croplands, while mountains have relatively small and fragmented patches of cropland only in valleys. Furthermore, the farming methods between mountains and plains are different, and the cropland can be mainly divided into winter-flooded paddy fields and fields with dryland farming. In mountains, a number of winter-flooded paddy fields are kept naturally flooded until they are planted in May, which play significant role in foraging and nest-site selection for the Crested Ibis. In plains, however, most of the croplands are planted with dryland farming (such as wheat, rape) in winter and the next spring. When crops are harvested in May, these fields are immediately irrigated and artificially flooded to transplant rice seedlings. Other types of land cover include forest, shrub, grass, ponds, reservoirs, building land and bare land in both mountains and plains.
The Crested Ibis is a resident bird. They breed from early March to June, nest in tall trees (such as Pinus spp., Ulmus pumila), and show strong nest-site fidelity (Ding, 2004; Song et al., 2019). Female birds usually lay one clutch of one to four eggs (Ding, 2004). Incubation starts with the first egg being laid, and both parents incubate eggs for around 28 days before nestlings' hatch (Ding, 2004). Ibises feed near their nesting sites as they, mainly forage in paddies and rivers, sometimes in grasslands, drylands and reservoirs in the mountain valleys and plains, to consume various aquatic organisms. The dominated preys include loaches, swamp eels, batrachia, field snails, and other aquatic insects in wintering and breeding season. Other recorded dietary items include insects, such as Coleoptera and Diptera (Ding, 2004).
Between March to June in 2021, most breeding nests of Crested Ibis were located by checking previously used nests (we have accumulated historical data of each previously used nest through collaborative work with the staff of the nature reserve) and searching new nests in the study area. We check all nest sites to record reproductive status of the birds on a regular basis. In this process, we collected all remaining eggshells (including hatched or abandoned eggs) under nest trees, screened out those that belong to different eggs, and stored them seperately with marking of the nest where they came from. In the event a nest was depredated or blown off by wind, we would also search and collect remaining eggshells. The nests of the Crested Ibis are usually fairly dispersed, which ensures that the eggshells from one nest could not be mixed with the ones from other nests. Additionally, some unfertilized or unhatched eggs were collected from nests in banding process. Most membranes were air dried in the field and have separated from eggshell. For complete eggs, or unsegregated membranes with shells, we soaked them with boiling water for 15 min (Gloutney and Hobson, 1998), then separated the membranes from shells. All samples were stored at −20 ℃ till further analysis.
In laboratory, we cleaned all eggshell membranes with small brushes in deionized water, dried them to a constant weight at 60 ℃ for 48 h, ground them into powder, and then packed the powder into tin cups (around 0.15 mg) for analysis. Stable isotope analyses were conducted at the Institute of the Quality Standard and Testing Technology for Agro-Products of Chinese Academy of Agricultural Sciences. Carbon and nitrogen isotope ratios were detected using elemental analyzer (Flash 2000) and IRMS (Delta V, Thermo), and expressed as δ13C ‰ and δ15N ‰ according to the following equation:
|
δX=[(Rsample /Rstandard )−1]×1000, |
where X represents 13C or 15N, R represents the ratio of 13C/12C or 15N/14N, Rstandard is the ratio of international references materials Vienna PeeDee Belemnite (V-PDB) for 13C, and atmospheric N2 for 15N. The average measurement errors of replicate runs were ±0.15‰ for 13C and 15N.
We distinguished two breeding habitats based on the local landforms and landscape characteristics of nesting sites: (a) plains (mean elevation: 487 m, range: 440–520 m) corresponding to the new breeding range with open and mostly flat landscape, where cropland is the dominant type of land cover; (b) mountains (mean elevation: 612 m, range from 550–1100 m) corresponding to the old breeding range covered by thick forests, while the valleys have original paddy fields.
We first used the generalized linear mixed model (GLMM) with normal error structure to examine δ13C and δ15N variation between the two habitat types, and we also included the first colonizing time of the nesting site as an indicator of dispersal time to evaluate the variation of stable isotope values during breeding habitat expansions. Because Oppel et al. (2009) showed the linear effect of δ15N between complete and hatched egg membranes is not good, the eggshell condition (unhatched vs. hatched) was also included as a fixed effect. Nest ID was included as a random factor to control for the repeated sampling from the same nest. We then designated individual isotopic niche width as a proxy of foraging niche width (Bearhop et al., 2004; Sheppard et al., 2018). Isotopic niches between habitat types were quantified by standard ellipse areas calculated with a Bayesian method in R package SIBER (Jackson et al., 2011). We presented both the corrected standard ellipses for small sample size (SEAc) and Bayesian standard ellipse areas (SEAB), where SEAc represents the core isotopic niche and is more suitable for graphical description of niche variation for each group, while SEAB is unbiased Bayesian estimates of core isotopic niches (presented as modes and 95% credibility intervals) (Mills et al., 2021). SEAc and SEAB were both used to compare the trophic niche width between groups. We also reported the convex hull area as a metric of total area (TA) of isotopic niche size for all samples in each group. Finally, we calculated SEAc overlap between two groups to determine whether foraging niche differentiated.
All analyses were performed in R Version 3.5.3 (R Core Team, 2019), isotopic values were presented as mean ± standard deviation, and significant level was set at P ≤ 0.05.
In the breeding season, there was no significant difference in laying date between birds in mountains and plains (Independent-sample t-test, t = −0.26, df = 6.79, P = 0.81). A total of 105 eggshell membrane samples from 81 clutches were analyzed, and the results indicated that the Crested Ibis exhibited high variability in both isotopic signatures. δ13C values ranged from −26.48‰ to −18.99‰, and δ15N values ranged from 6.79‰ to 17.26‰. The mean standard deviation of different eggshell membranes from the same clutch was 0.42‰ (range: 0.01–2.23‰, n = 19) for δ13C and 0.55‰ (range: 0.05–1.25‰, n = 19) for δ15N. The variation of all eggshell membranes from different clutches (δ13C: 1.37‰, δ15N: 1.80‰, n = 81) was higher than the variation of the same clutch.
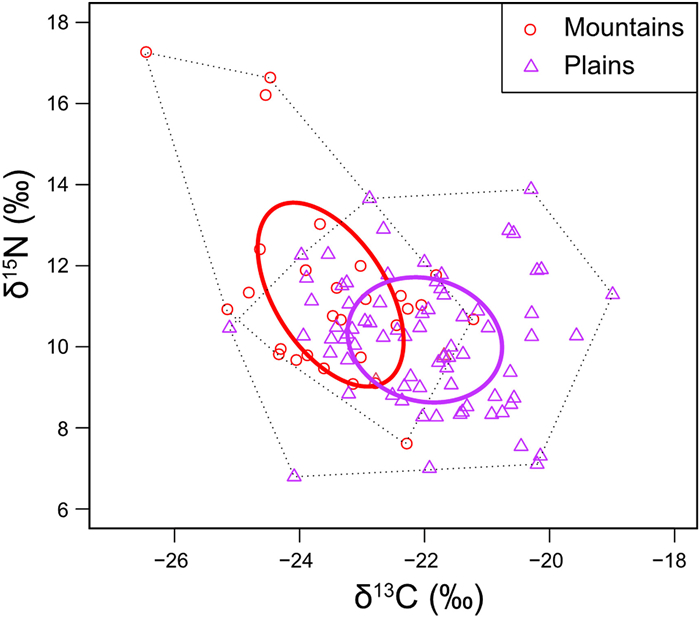
During the early breeding season, δ13C of eggshell membrane samples for individuals from mountains ranged from −26.48‰ to −21.24‰ (n = 28) and for individuals from plains ranged from −25.12‰ to −18.99‰ (n = 77). δ15N of eggshell membrane samples for individuals from mountains ranged from 7.61‰ to 17.26‰ and for individuals from plains ranged from 6.79‰ to 13.88‰. The results of GLMM showed that both δ13C and δ15N varied significantly between mountains and plains (Table 1): individuals from plains had significantly higher δ13C, but lower δ15N values compared to individuals from mountains (Table 2, Fig. 2). The eggshell condition had no effects on stable isotope values (Table 1), and our study demonstrates no correlation between the δ13C and δ15N variations and the dispersal time (Table 1).
| Term | δ13C (‰) | δ15N (‰) |
| Intercept | −5.65 (−209.59; 198.58) | −85.53 (−389.70; 218.55) |
| Habitat−plains | 1.48 (0.91; 2.06) | −1.08 (−1.93; −0.24) |
| Eggshell-unhatched | −0.49 (−1.27; 0.29) | 0.92 (−0.12; 1.94) |
| Dispersal time | −0.01 (−0.11; 0.09) | 0.05 (−0.10; 0.20) |
| Random effects | Variance (SD) | Variance (SD) |
| Nest ID | 0.60 (0.78) | 2.43 (1.56) |
| Residual | 0.88 (0.94) | 0.59 (0.77) |
| Isotope | Breeding habitats | |
| Mountains | Plains | |
| δ13C (‰) | −23.50 ± 1.14 | −22.00 ± 1.23 |
| δ15N (‰) | 11.29 ± 2.22 | 10.17 ± 1.53 |
| TA (‰2) | 23.61 | 32.15 |
| SEAc (‰2) | 7.16 | 5.95 |
| SEAB (‰2) | 6.78 (4.66, 9.89) | 5.85 (4.64, 7.25) |
| SEAc overlap (‰2) | 1.63 | |
Standard ellipse area corrected for small sample size (SEAc) of eggshell membrane samples showed that the isotopic niche width varied between the two breeding habitats. The isotopic niche of individuals from mountains was slightly larger than the niche of individuals from plains (Table 2, Fig. 3), which was consistent with the results of Bayesian standard ellipse areas (SEAB) (Table 2, Fig. 4). The isotopic niche showed a small overlap between two habitat types (SEAc overlap = 1.63, Table 2, Fig. 3).
Carbon and nitrogen isotope values have been proven to be effective measures of habitat and resource use of animals (Post, 2002; Newsome et al., 2007). Our study showed a high variation in both δ13C and δ15N values of eggshell membranes in Crested Ibis, and such variations were mainly contributed by nest differences (among clutches). This result was consistent with the high variety in foraging habitats and prey items of Crested Ibis in the breeding season (Ding, 2004), demonstrating that Crested Ibis is a generalist forager from the isotopic perspective. Within clutches, there were also relatively small differences in both δ13C and δ15N values among eggs, and such variations possibly resulted from females foraging on different locations and prey species in different stages during egg formation (Oppel et al., 2009), given that Crested Ibis generally lay one egg every two days, laying four eggs usually takes 8–9 days (Ding, 2004).
Our study indicated that the isotopic values of eggshell membranes were significantly different between mountain and plain habitats. Stable-carbon isotope ratios show less enrichment with trophic levels, and its variation mainly originated from differences in primary production of the locations (Marshall et al., 2007; Inger and Bearhop, 2008). In our case, with the increasing population size of the Crested Ibis since its rediscovery in the mountains in 1981, from 2004 onwards, the population rapidly dispersed from the old breeding range (mountains) to plains (Wang et al., 2020). According to previous marking-rediscovery records, ibises usually feed in the fields near their nests during breeding season, that is, mountain birds feed on mountains and vice versa (Ding, 2004). Indeed, the changes of δ13C from mountains to plains presented by this study demonstrated that the wild Crested Ibis could be divided into mountain and plain groups based on their foraging habitats in breeding season. This result will lay the foundation for future studies on habitat-related life history variation of the wild Crested Ibis.
As for δ15N, individuals from mountains had significantly higher δ15N values, which indicated that birds in mountains might consume a higher proportion of higher trophic level prey species during the early breeding season. The reason for such differences in resource use is possibly related to the shift of farming method and land use during winter and spring in our study area. In mountain valleys, people retain a number of winter-flooded paddy fields (paddy fields with a naturally flooded fallow period from November to early May), which provides ideal foraging grounds to obtain high trophic level aquatic preys such as loaches (δ15N of loaches in plains: 11.04 ± 3.49‰, n = 5, unpublished data) and eels during egg formation period (mainly in March and April) of the Crested Ibis. In plains, however, most of the fields are covered by wheats and rapes approaching matured in winter and spring, and ibis could not forage on these fields, thus they have to search relatively lower trophic level prey items such as insects, aquatic insects and snails (δ15N: 8.15 ± 1.74‰, n = 6, unpublished data) in grassland and rivers. Hence, the original winter-flooded paddy fields in mountain valleys are better feeding habitat for ibis, and the nature reserve has also always intentionally retained a certain amount of winter-flooded paddy fields in mountains. Our results also indicated that it is necessary and urgent to improve the quality of foraging grounds in plains for Crested Ibis during winter and spring, given that most individuals have dispersed from mountains to plain habitats in recent years. Additionally, Schmidt et al. (2003) found δ15N increased with body size in fish, squid and crustaceans, but whether the prey items in mountains are averagely larger than those in plains, and whether the higher δ15N of mountain birds is a result of consuming larger-size prey individuals, still need to be clarified in future studies.
Food quality and abundance are two concerns in foraging ecology (Wright et al., 1998), and a trade-off may exist between them (Litzow et al., 2004; Francesiaz, 2020). Indeed, in plains, when crops are harvested, fields are artificially flooded and then ploughed in May, there is a wide range of paddy fields providing abundant aquatic foods for Crested Ibis, and this time period corresponds to the peak of food requirements for nestlings in rearing process. Although mountain paddy fields are always flooded, such relatively smaller feeding area provides limited food resources. We believe that the shift in food abundance resulted from the transition of land use in plains may partly explain why individuals breeding in plains have higher reproductive success than those breeding in mountains (Song, 2018; Wang et al., 2020), despite the mountain birds consume higher trophic level prey species in the early breeding season (egg formation period). In future studies, we will compare the δ15N of food species among habitats and different breeding stages to further verify these results, which will provide for more detailed references for the conservation of the Crested Ibis.
Our results also indicated no influence on either δ13C or δ15N by the eggshell condition, which is consistent with the results of Oppel et al. (2009), suggesting that isotopic values of hatched eggshell could be used to detect dietary information during egg formation period (Kowalczyk et al., 2014). We also did not find that the isotopes varied as a function of dispersal time and female age (all samples were collected in 2021, hence this dispersal time can also represent the age of individuals). Indeed, Crested Ibis are resident and are royal to their nesting sites, and the foraging range of adults is relatively stable and small according to GPS/GSM transmitters data (Hu, 2016). Hence the habitat use and trophic level rarely change with dispersal time and individual age.
Contrary to our prediction related to the variation of niche width, the isotopic niches between the two habitat types showed a small overlap that was wider in old habitat than in the expanded new habitat. This is because the isotopic niche reflects habitat and resource use (Newsome et al., 2007; Rader et al., 2017). As mentioned above, mountain birds probably feed on more diverse prey items given that flooded paddy fields may have multiple aquatic food resources compared to drylands in plains during the early breeding season. Other two possible explanations are: (1) the wider niche breadth may suggest mountain birds have larger foraging ranges than plain birds. This hypothesis can be tested by tracking individuals with GPS device in the future. (2) Birds with greater range of expansion exhibit narrower diet breadth because they may track their preferred food resources (Brommer, 2008; Auer and King, 2014). In this study, we think that the narrower niche breadth in the expanded habitat is probably not caused by ibises tracking their preferred food resources or change their foraging strategies, but is instead mainly related to the resource diversity between the two breeding ranges: mountain paddy fields could provide more abundant food diversity during egg formation. However, the results of this study only focused on the early breeding season, the changes of diet breadth regarding birds expanding to new habitat during incubation, rearing period and non-breeding season remain undiscussed for opportunities of researches. Understanding the change patterns more comprehensively would generously benefit the conservation management of bird diversity.
Differences in δ15N values may reflect not only the variation in foraging diet composition, but also the changes in baseline δ15N in the environment (Becker and Beissinger, 2006; Marshall et al., 2019). This effect did exist, but we believed that the changes in isotopic values presented in our study were not due to the changes in baseline for two reasons. First, there was no significant difference in δ15N between Yang County and Chenggu County (unpublished data), suggesting that the changes in baseline δ15N would be limited in these two adjacent counties. Second, our preliminary study on main prey species collected from plains showed that the variations in δ15N of different prey species from the same site were greater than that of the same prey species from different sites (unpublished data). These reasons demonstrated that the changes in δ15N values between two the habitat types were mostly caused by the changes in diet.
Our research rendered the novel and insightful finding of the high intraspecific variation in habitat use and trophic levels of wild Crested Ibis in the early breeding season. Our results indicate that Crested Ibis could be divided into mountain and plain groups based on their distinctly different foraging niches, individuals from mountains consume a great proportion of higher trophic level prey species according to δ15N values, and birds in the old habitats have a relatively wider isotopic niche width than birds that expanded to the new habitat. These results are possibly related to the shift of local tillage methods and land use during winter and spring. Our study highlights that we can obtain information of foraging niche variation as well as food resource diversity in local areas using non-invasive sampling method by implementing the stable isotope analysis of eggshell membranes of Crested Ibis. Our successful application of the stable isotope analysis in studying foraging niche and trophic level of Crested Ibis can provide significant references for the habitat recovery and conservation management of other endangered species.
CD and XX conceived the study. XX, JJ, YL, CW and BQ contributed to the data collection; XX conducted the experiments and analyzed the data; CD and XX led the writing and revision of the manuscript. All authors substantially contributed to the drafts and gave final approval for publication.
The authors declare that they have no competing interests.
Our sincere appreciation goes to Changming Li, Wenbin Duan, Jiaqi Yan and Shan Yan for their assistance with fieldwork., We are also grateful to Shaanxi Hanzhong Crested Ibis National Nature Reserve for their support to carry out this study. This project was funded by the National Natural Science Foundation of China (No. 31772483, 31900371) and the Biodiversity Survey, Monitoring and Assessment Project of Ministry of Ecology and Environment, China (No. 2019HB2096001006).
|
Brommer JE. Extent of recent polewards range margin shifts in Finnish birds depends on their body mass and feeding ecology. Ornis Fennica. 2008;85: 109-117.
|
|
Ding C. Research on the crested ibis. Shanghai: Shanghai Scientific and Technological Educational Publishing House. 2004.
|
|
Gloutney ML, Hobson KA. Field preservation techniques for the analysis of stable-carbon and nitrogen isotope ratios in eggs. J Field Ornithol 1998;69: 223-227.
|
|
Hu C. The home range and dispersal ecology of Crested Ibis (Nipponia nippon). PhD thesis, Beijing Forestry University, 2016.
|
|
Lei W, Masero JA, Dingle C, Liu Y, Chai Z, Zhu B, Peng H, Zhang Z, Piersma T. The value of coastal saltpans for migratory shorebirds: conservation insights from a stable isotope approach based on feeding guild and body size. Anim. Conserv. 2021;1-13.
|
|
Liu Y. Rediscovery of crested ibis Nipponia nippon in Qinling Mountain. Chinese Journal of Zoology. 1981;27: 237.
|
|
Marshall HH, Inger R, Jackson AL, McDonald RA, Thompson FJ, Cant MA. Stable isotopes are quantitative indicators of trophic niche. Ecol let. 2019;22: 1990-2.
|
|
Song Z. Sex allocation pattern and reproduction strategy in the wild population of Crested Ibis (Nipponia nippon). PhD thesis, Beijing Forestry University, 2018.
|
|
Stearns SC. The evolution of life histories. Oxford, UK: Oxford University Press, 1992.
|
|
Wang C, Liu D, Qing B, Ding H, Cui Y, Ye Y, Lu J, Yan L, Ke L, Ding C. The current population and distribution of wild crested ibis Nipponia nippon. Chinese Journal of Zoology. 2014;49: 666-671.
|
| Term | δ13C (‰) | δ15N (‰) |
| Intercept | −5.65 (−209.59; 198.58) | −85.53 (−389.70; 218.55) |
| Habitat−plains | 1.48 (0.91; 2.06) | −1.08 (−1.93; −0.24) |
| Eggshell-unhatched | −0.49 (−1.27; 0.29) | 0.92 (−0.12; 1.94) |
| Dispersal time | −0.01 (−0.11; 0.09) | 0.05 (−0.10; 0.20) |
| Random effects | Variance (SD) | Variance (SD) |
| Nest ID | 0.60 (0.78) | 2.43 (1.56) |
| Residual | 0.88 (0.94) | 0.59 (0.77) |
| Isotope | Breeding habitats | |
| Mountains | Plains | |
| δ13C (‰) | −23.50 ± 1.14 | −22.00 ± 1.23 |
| δ15N (‰) | 11.29 ± 2.22 | 10.17 ± 1.53 |
| TA (‰2) | 23.61 | 32.15 |
| SEAc (‰2) | 7.16 | 5.95 |
| SEAB (‰2) | 6.78 (4.66, 9.89) | 5.85 (4.64, 7.25) |
| SEAc overlap (‰2) | 1.63 | |