| Citation: | Xue Wang, Guang Zhu, Haohao Ma, Yi Wu, Wenwen Zhang, Yong Zhang, Chunlin Li, Willem F. de Boer. 2022: Bird communities’ responses to human-modified landscapes in the southern Anhui Mountainous Area. Avian Research, 13(1): 100006. DOI: 10.1016/j.avrs.2022.100006 |
Conversion of natural environments to human-modified landscapes is continuing at an unprecedented rate, exerting fundamental influences on global biodiversity. Understanding how wildlife communities respond to landscape modifications is critical to improve biodiversity conservation in human-dominated landscapes. In this study, we surveyed bird communities in three common habitats (i.e., farmland, village, and forest) in the southern Anhui Mountainous Area during summer (August 2019) and winter (December 2020). The diversity metrics and species compositions of the avian communities were compared among the habitats, and the effects of land use composition in these habitats were tested. During the field surveys, we recorded 7599 birds of 120 species along 45 line transects of 1 km in length. The land use compositions differed among habitats, and land use diversity was the highest in villages and lowest in forests. The species richness and bird abundance in the two human-dominated habitats (i.e., farmland and village) were higher than those in forest in both seasons. Bird species composition also differed across habitat types in both seasons. Bird species feeding on vertebrates, fish and carrion, and species feeding on plants and seeds were mainly found in habitats with less construction lands and lower land use diversity, while omnivorous species and species feeding on fruits and nectar or on invertebrates were less affected by these two variables. The indicator species analysis showed that most species associated with forest feed on invertebrates, while species feeding on plants and seeds were more correlated with farmland and village. The results indicated that the conversion of natural habitats to human-dominated landscapes has pronounced impacts on bird communities in the study area. Human-dominated habitats harboured more avian species that deserve conservation attention. Meanwhile, bird conservations should not be relaxed in forests because there were more than 20 species that had a high specificity for forests.
The Earth's biomes are experiencing an unprecedented decline of biodiversity, and are increasingly characterized as “anthropogenic biomes”, restructuring the species composition in these biomes (Coetzee and Chown, 2016; Aguirre-Gutiérrez et al., 2020). It is widely accepted that these changes are a consequence of human activities (Dirzo et al., 2014; Jacobson et al., 2019), and that conversion of wild habitats to human-dominated landscapes is a key driver (Gaston et al., 2003; Asefa et al., 2017). Due to human activities (e.g. deforestation, agricultural expansion, construction of roads), natural habitats are modified, fragmented or lost and are replaced by anthropogenic landscapes, changing species communities (DeClerck et al., 2010; Allen et al., 2019; Maseko et al., 2020). The conversion of natural environments to human-modified landscapes is continuing at an unprecedented rate (Cushman, 2006; Maseko et al., 2020). Therefore, there is an urgency to better understand how species respond to landscape modifications, in order to inform wildlife conservation activities in these human-dominated landscapes.
As an important part of biodiversity, birds provide diverse ecosystem services, contributing to resilience and stability of ecosystems (Şekercioğlu et al., 2004; Whelan et al., 2015). For instances, seed-dispersing frugivores and pollinating nectarivores are recognized as transmitters of genetic material from one plant/habitat to another (Şekercioğlu, 2006). Insectivorous birds contribute to controlling crop pests and thereby increase the production of crops (Şekercioğlu, 2006; García et al., 2018; Michel et al., 2020). In addition, because birds are easily observed and are highly sensitive to environmental changes, they are often used as bioindicators (Morrison, 1986; Watson et al., 2005). Environmental changes often result in shifts in species composition and diversity in avian communities (Chapman and Reich, 2007; Coetzee and Chown, 2016). Therefore, understanding how birds respond to modification of natural habitats can provide insights to the maintenance of ecosystem resilience (Şekercioğlu, 2006).
Birds respond differentially to landscape modifications, depending on species-specific ecological traits and differences in modifications (Asefa et al., 2017; Matuoka et al., 2020). Habitat generalists can survive in various environments and are widely found in human-dominated landscapes (Bonier et al., 2007; Aronson et al., 2016). Some specialists are also attracted by human landscapes, such as seed-feeding birds and some large-bodied raptors commonly found in agricultural lands with open views and abundant cereals (Benton et al., 2003; Bain et al., 2020). However, species sensitive to human disturbances are vulnerable to human-induced landscape conversions (Bonier et al., 2007). For example, insectivorous birds are often the first taxa to disappear from human-modified habitats, and are therefore frequently identified as sentinels of habitat quality changes (Şekercioğlu et al., 2002; Stratford and Stouffer, 2015; Jarrett et al., 2021). The effects of anthropogenic landscape changes on bird communities also differ with the intensity of these modifications. As proposed by the intermediate disturbance hypothesis, species diversity would be higher where the intensity of disturbance is moderate (Connell, 1978). The intermediate disturbance hypothesis might also apply to landscape modifications, as habitat conversions at intermediate levels may increase environmental heterogeneity, and therefore provide more habitats for more diverse species communities (Chapman and Reich, 2007). However, in intensively modified environments, species diversity often falls due to landscape homogenization and habitat loss (Asefa et al., 2017).
The southern Anhui Mountainous Area is identified as a prioritized area for biodiversity conservation in China, as it features a rich biodiversity of both flora and fauna (Li, 2016). The vegetation in this region is dominated by subtropical evergreen broadleaved forests, with evergreen-deciduous broadleaved mixed forests at lower elevations (Wu and Gu, 2017). The diverse vegetation types provide various habitats of high quality for a wide range of invertebrate and vertebrate species. Among them, birds are an abundant taxon living there along with humans for centuries (Wu and Gu, 2017). Over the last decades, the conversion of natural forests to semi-natural and artificial landscapes has been intensifying due to increasing human activities in the region (Jin and Wu, 2002). However, natural forests still dominate the regional landscape, with interspersed patches of human-created land uses, such as roads, farmlands, and human settlements with small gardens (Wu and Gu, 2017). Therefore, the level of human disturbances to the natural ecosystem might be intermediate in this area, so that the area offers a model system to test the intermediate disturbance hypothesis.
In this study, we surveyed bird communities in two common human-modified habitats (farmland and village) and one natural habitat, i.e. forest, in summer and winter to quantify the impacts of habitat conversion in human-dominated landscapes on bird communities in the southern Anhui Mountainous Area. Diversity metrics and species compositions of avian communities were compared among the habitat types, and the effects of land use composition were assessed. We expected that: (1) anthropogenic habitats would have more birds and higher species diversity than forests given that the landscape modification is moderate in the area, and (2) avian species compositions would differ between anthropogenic habitats and forests, with anthropogenic habitats supporting less insectivorous birds, due to their high sensitivity to land use changes, but more carnivorous and seed-feeding species as these resources are more common in anthropogenic habitats, and visibility for predators is higher.
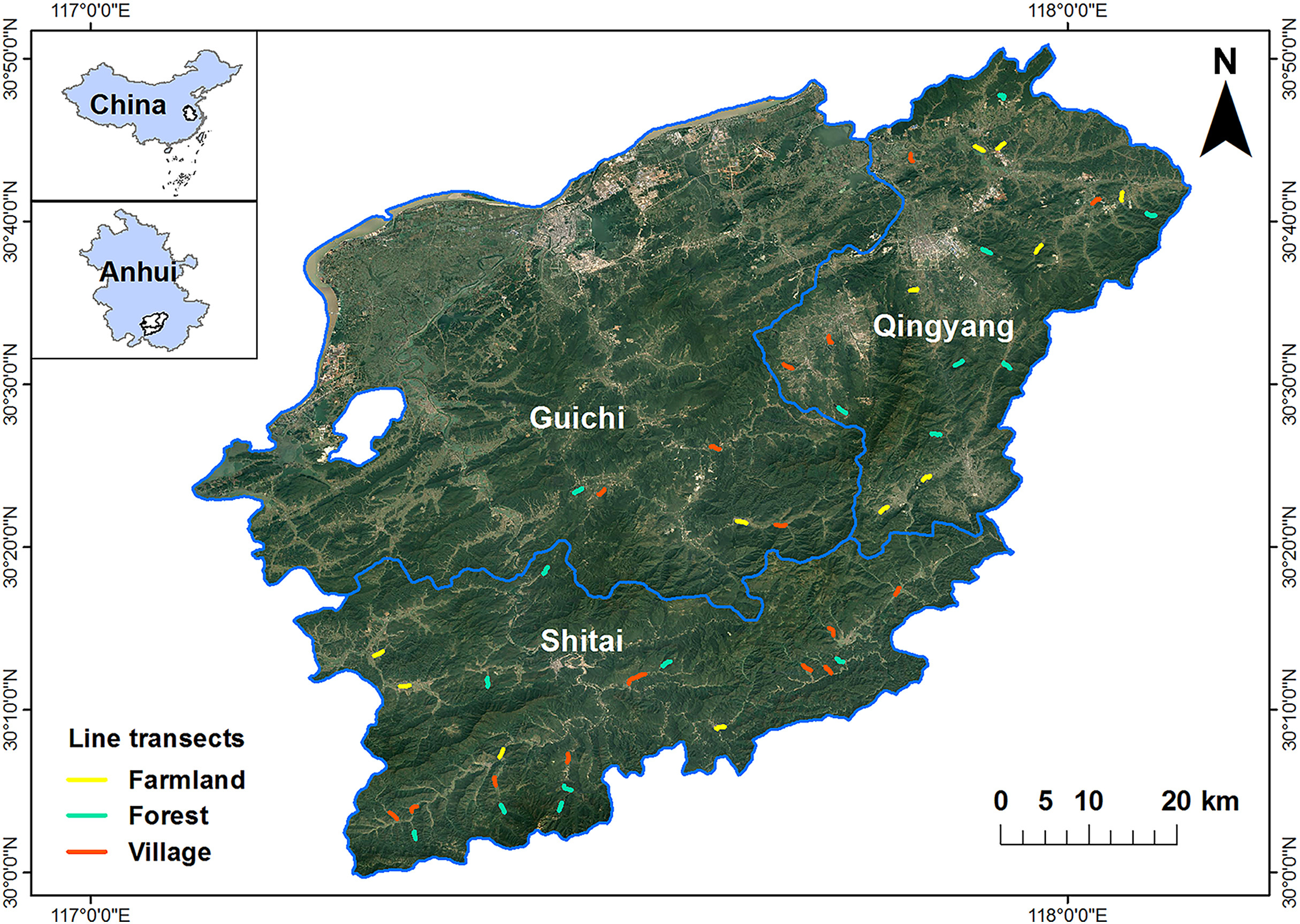
We conducted the study in the southern Anhui Mountainous Area including Qingyang County, Shitai County and Guichi District (29°33ʹ–30°51ʹ N, 116°38ʹ–108°05ʹ E; Fig. 1). The elevation across the study area ranges from −4 m to 1730 m. The region has a typical subtropical monsoon climate, which is mild and rainy, with a mean annual rainfall of 1400–2200 mm and mean temperature of 26–33 °C in summer and 2–10 °C in winter. The vegetation in this region is mainly characterized by mixed forests with dense tree coverage and local villages with relatively low population density are scattered in the forest landscape. The inhabitants of the villages are mainly engaged in traditionally agricultural activities, mainly planting rice, rape, tea plants and various Chinese medicinal herbs (Wang et al., 2021). As a consequence of economic development and population growth, this region has been transformed to a mix of natural and artificial landscape through on-going land use changes, including deforestation, agricultural intensification, and extension of residential areas.
According to prior knowledge, we defined one natural (i.e., forest) and two anthropogenic habitat types (i.e., village and farmland), each dominated by their corresponding land use. We conducted bird surveys along 15 fixed 1-km line transects (Bibby et al., 2002) in each habitat. We used high-resolution images of Google Earth to measure cover of the different land use types in a 100-m buffer zone along each side of the transect, which were verified during field surveys (Rime et al., 2020). The four identified land use types included construction lands (buildings and roads), farmlands (mainly growing rice, rape and tea plants), woodlands (coniferous forest, broad-leaved forest, and mixed forest), and water bodies. The composition of the land uses in the transects did not change during our surveys. For each transect, we calculated the Shannon-Wiener index as a proxy of the land use type diversity (LD):
| LD=−∑ni=1pilogpi |
where pi is the proportional area of land use type i and n is the total number of land use types. Although the dominant land use type in buffer zones around villages was similar to that around farmlands, we did not combine the two habitat types because the former had linear populated areas (i.e., villages) along which we placed our transect lines, while there was only a small area of scattered construction lands in farmlands.
To test our preliminary habitat classification, we used hierarchical cluster analysis to re-classify the 45 line transects into the 3 habitat types based on the Euclidean distance matrix of the differences in land use compositions. Specifically, we placed one transect that was originally classified as village to forests, 3 village transects to farmlands, and 6 farmland transects to villages, resulting in 16 transects in forests, 17 in villages, and 12 in farmlands (Fig. S1). The following analyses were all based on these re-classified habitat types.
Bird surveys were carried out by the same experienced observers during 4 h from dawn and 3 h before sunset in good weather in the dry winter (December 2020) and wet summer (August 2019). During the surveys, the observers walked along each transect at a constant speed of approximately 2.0 km/h and used binoculars (Swarovski 10 × 42 WB) to identify birds. We recorded all birds heard or seen within a 50-m radius (Martin et al., 2017) while excluded those flying over (Pringle et al., 2019). The bird taxonomy and nomenclature followed MacKinnon et al. (2000).
As a preliminary analysis, species accumulation curves were calculated to evaluate the sampling effort. The curve of each habitat flattened out, indicating that our sampling efforts were acceptable (Fig. S2). In addition, our goal was to compare bird community compositions among different habitats rather than to quantify absolute bird densities in each habitat. Therefore, we used direct bird count data, without correcting for detectability, in the following analyses. For each transect, we calculated species richness (i.e., number of species), abundance of each species, Shannon-Wiener diversity index (Shannon and Weaver, 1998) and Pielou evenness index (Pielou, 1966). According to Wilman et al. (2014), species were categorized into five foraging guilds based on diet composition: Omnivore, Invertebrate (feeding on invertebrates), VertFishScav (feeding on vertebrates, fish and carrion), PlantSeed (feeding on plant and seeds), or FruiNect (feeding on fruits and nectar).
Because the land use data failed to fit the assumption of normal distribution and variance equality, Kruskal-Wallis H-tests were used to test for differences in land use composition and land use diversity among the three habitat types. Pair-wise comparisons were performed using the Wilcoxon rank-sum test with Bonferroni corrections.
A two-way ANOVA followed by Tukey's post hoc tests was used to test the effects of season, habitat type, and their interaction on the Shannon-Wiener index, which followed a normally distribution. The non-parametric version of a two-way ANOVA (the Scheirer-Ray-Hare test) was used to test the same effects on species abundance, richness, Pielou evenness index, and the abundance of each foraging guilds, which all deviated from normal distributions. The intergroup comparisons were performed using the Wilcoxon rank-sum test with Bonferroni corrections.
To assess whether bird species compositions were significantly different between habitat types, we conducted a multi-response permutation procedure (MRPP; McCune et al., 2002). The MRPP is a group of distance-based statistical tests, and tests whether there are significant differences among two or more groups of sampling units. Based on randomizations, the MRPP compares the within-group compositional dissimilarity with those between random collections of sampling units from the entire population. In our study, MRPP tests were based on Bray-Curtis distance matrices, and was run for 999 permutations. Indicator species analysis was used to identify indicator species in the three habitat types, i.e., species whose relative frequencies and abundances were particularly high in one type of habitat (de Cáceres and Legendre, 2009). An indicator value (IndVal) was calculated for each species and significance was tested using a random reallocation process with 999 iterations.
We used non-metric multidimensional scaling (NMDS) to graphically display differences in bird community composition across habitat types, and to explore the responses of the species in different foraging guilds to environmental variables (i.e. the area of different land use types in the 100-m buffer zone of each transect: area of woodlands; area of water bodies; area of farmlands; area of construction lands; land use type diversity). NMDS is an indirect gradient analysis which produces an ordination based on a distance or dissimilarity matrix (Minchin, 1987). The NMDS diagram was constructed based on Bray-Curtis distances, using the species abundance matrix. In the NMDS ordination diagram, communities with similar species compositions are plotted closer. The environmental variables were fitted to the NMDS diagram using the envfit function. We used the Pearson correlation coefficients to evaluate the correlations between the NMDS axis scores and each environmental variable, and used 999 permutations to assess the significance of the correlation coefficients.
Because avian communities may be more similar if they are spatially closer, we tested our dataset for spatial autocorrelation. We used a Mantel's test to examine the Pearson correlation between the Bray-Curtis distance matrix of the bird communities and the geographic distance matrix, and tested for an association using a randomization procedure (999 iterations; McCune et al., 2002).
All statistical analyses were performed with R version 4.0.1 (R Core Team, 2020). The package vegan was used to perform the NMDS, MRPP and Mantel's test, and the package indicspecies was used to perform the indicator species analysis. Data are shown as mean ± standard difference (SD).
Land use compositions differed significantly among habitat types. Land use type diversity was highest in villages and lowest in forests (Table 1).
| Land use type | Farmlands | Villages | Forests | ||||||||||||||||
| Area of woodlands (ha) | 1.75 ± 1.82c | 6.31 ± 2.53b | 19.91 ± 2.83a | ||||||||||||||||
| Area of water bodies (ha) | 0.48 ± 0.55ab | 0.84 ± 0.92a | 0.65 ± 1.73b | ||||||||||||||||
| Area of farmlands (ha) | 19.27 ± 2.51a | 11.28 ± 1.81b | 1.39 ± 1.93c | ||||||||||||||||
| Area of construction lands (ha) | 1.08 ± 1.24b | 4.01 ± 3.49a | 0.37 ± 1.28c | ||||||||||||||||
| Land use type diversity | 0.47 ± 0.26b | 1.01 ± 0.12a | 0.29 ± 0.31b | ||||||||||||||||
| Columns with same superscript letters indicate no significant differences between habitat types (Kruskal-Wallis H-tests followed by a post-hoc Wilcoxon rank sum). | |||||||||||||||||||
In total, we recorded 7599 birds of 120 species belonging to 32 families and 11 orders during the two field surveys, including 82 passerine species and 38 non-passerine species (Table 2; Table S1). There were 39 species which were recorded in all 3 habitat types, while 37 species were only recorded in one habitat type (15 in forests, 10 in villages, and 12 in farmlands). Among the recorded species, 2 were listed as Vulnerable (VU) on the IUCN Red List, i.e. Collared Crow (Corvus torquatus) and Rustic Bunting (Emberiza rustica), and 13 were listed as Class II Key Protected Wild Animal Species in China (Table S1).
| Habitat types | Family richness | Species richness | Number of individuals | ||||||||||||||||||||||||
| Summer | Winter | Total | Summer | Winter | |||||||||||||||||||||||
| Villages | 26 | 50 (4) | 72 (6) | 86 (10) | 1720 | 1458 | |||||||||||||||||||||
| Farmlands | 25 | 56 (3) | 56 (3) | 81 (5) | 1025 | 1398 | |||||||||||||||||||||
| Forests | 23 | 53 (2) | 52 (3) | 71 (5) | 838 | 1160 | |||||||||||||||||||||
| Total | 32 | 85 (6) | 91 (8) | 120 (13) | 3583 | 4016 | |||||||||||||||||||||
| The numbers of species listed as Class II Key Protected Wild Animal Species in China are displayed in parentheses. | |||||||||||||||||||||||||||
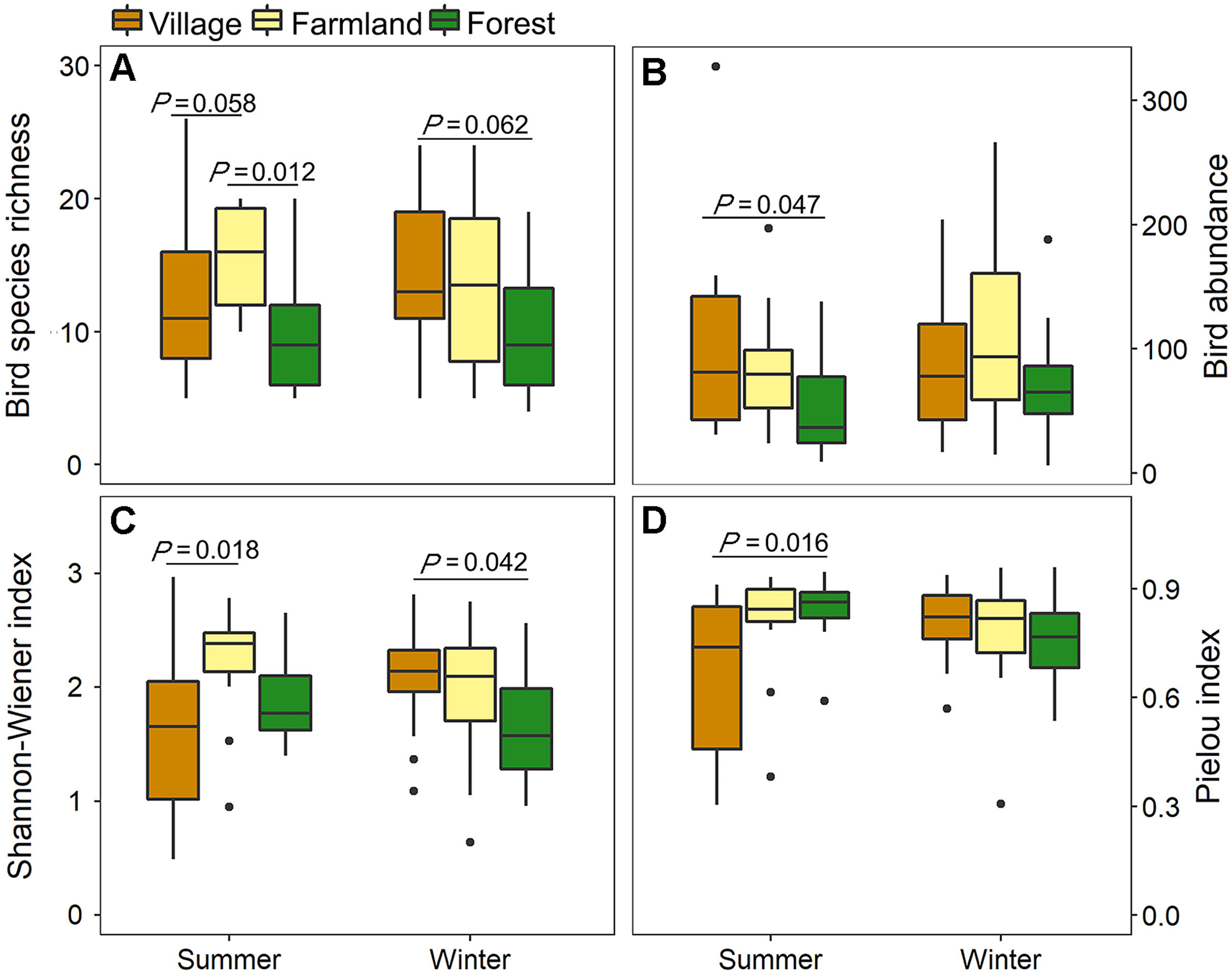
Bird species richness and abundance differed among habitat types (species richness: H2,84 = 11.45, P = 0.003; abundance: H2,84 = 6.82, P = 0.033), but were not affected by season (species richness: H1,84 = 0.03, P = 0.859; abundance: H1,84 = 0.65, P = 0.420) and the interaction between season and habitat type (species richness: H2,84 = 3.70, P = 0.157; abundance: H2,84 = 1.59, P = 0.452). Compared with farmlands and villages, species richness and bird abundance in forests was lowest in both seasons (Fig. 2).
The Shannon diversity index and Pielou evenness index were both influenced by the interaction between season and habitat type (Shannon: F2,84 = 4.85, P = 0.010; Pielou: H2,84 = 8.39, P = 0.015). The main effect of season was no longer significant in the models (Shannon: F1,84 = 0.04, P = 0.836; Pielou: H1,84 = 0.70, P = 0.404), but the Shannon diversity index slightly differed among habitat types (F2,84 = 2.48, P = 0.089). In summer, the Shannon diversity index was highest in farmlands and lowest in villages, while in winter, the index was highest in villages and lowest in forests. The Pielou evenness index was highest in forests and lowest in villages in summer, but was similar among habitat types in winter (Fig. 2).
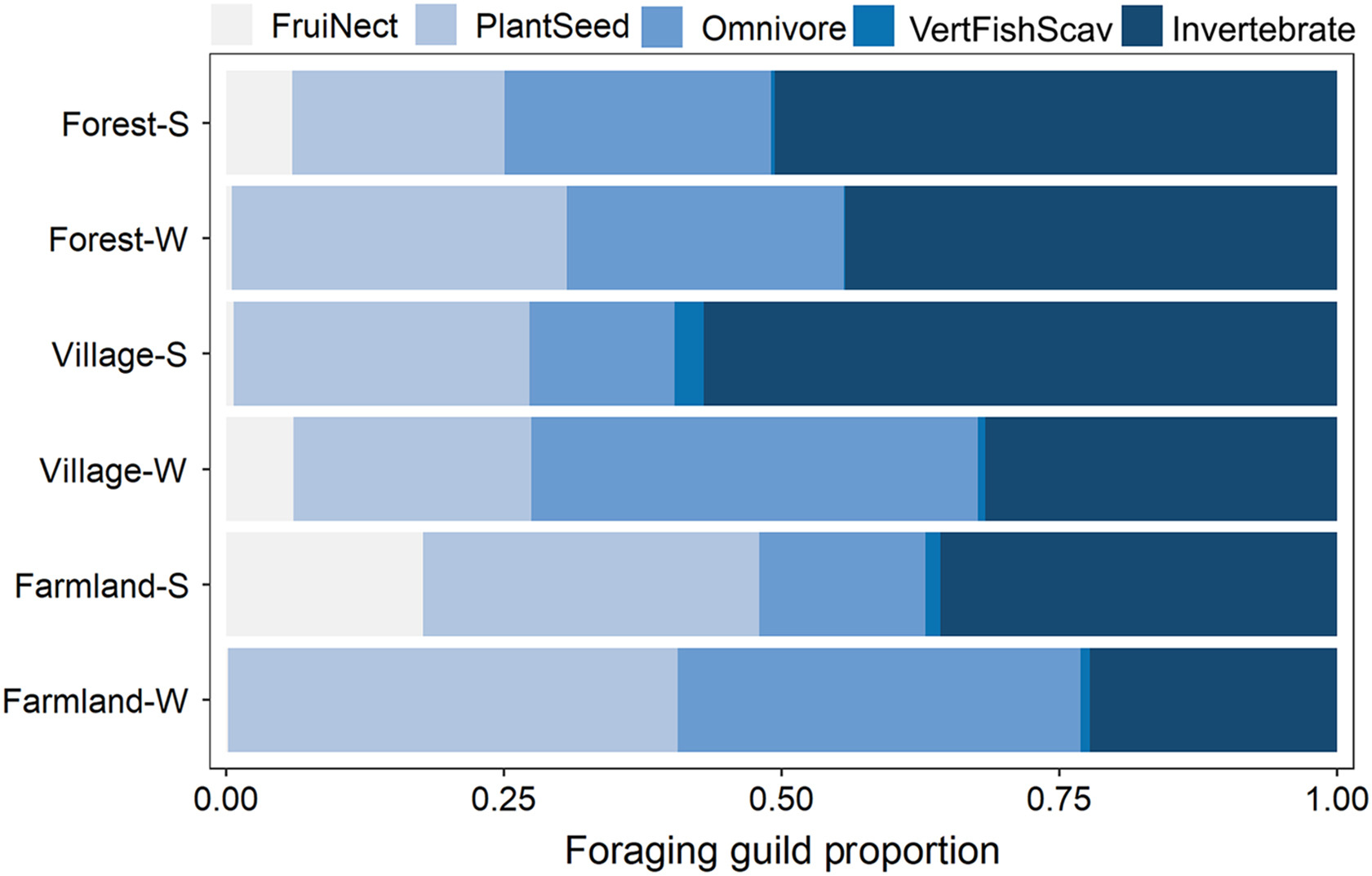
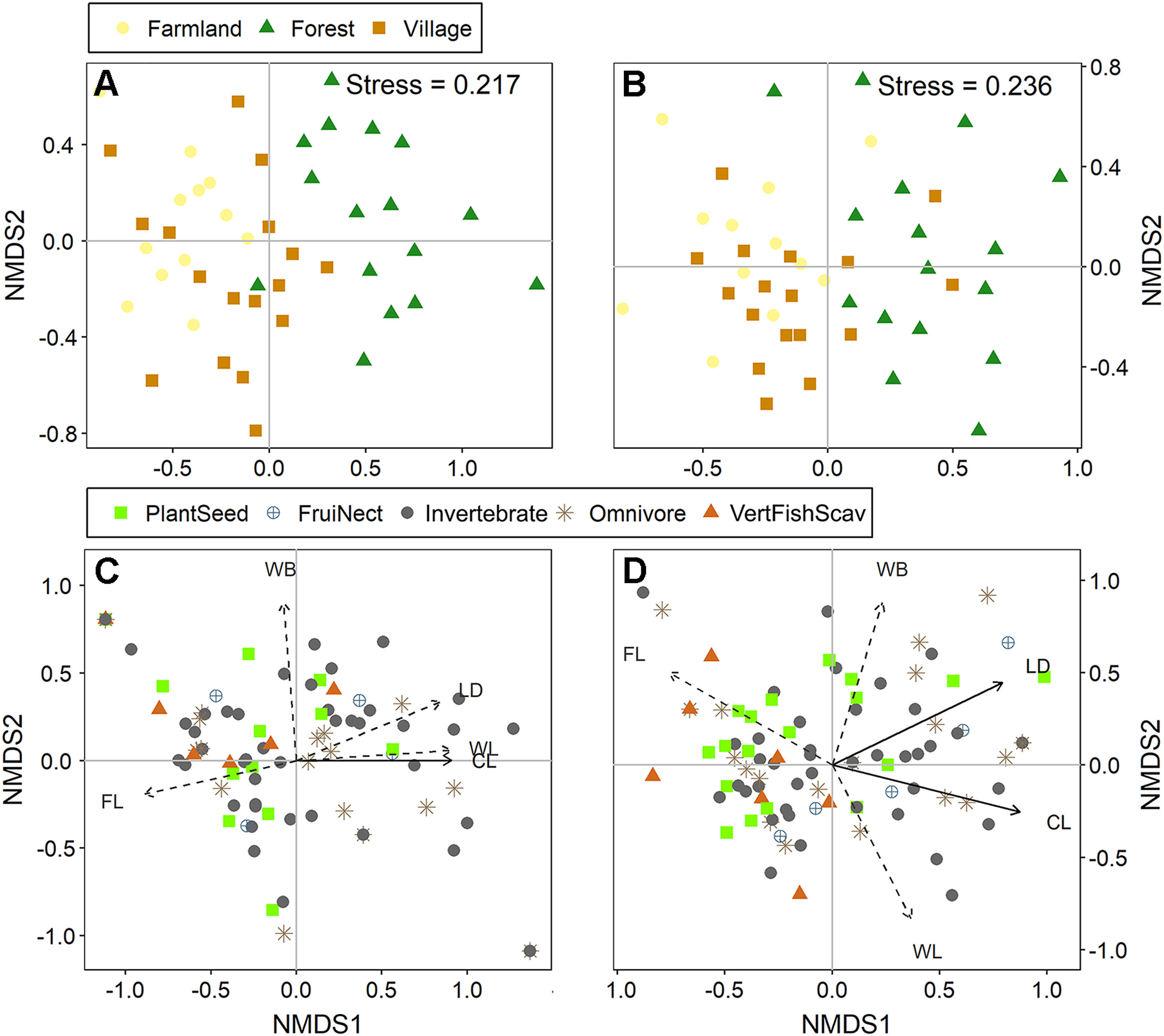
The effect of spatial autocorrelation was not significant (summer: rM = 0.01, P = 0.313; winter: rM = 0.03, P = 0.215). The MRPP results showed that avian species compositions differed among habitat types in both summer and winter (Table 3; Fig. 3). Except for FruiNects, which were affected by the interaction between season and habitat type (F2,84 = 7.60, P = 0.022), the other foraging guilds were mainly affected by main factors. The abundance of FruiNects was slightly higher in villages in winter (forest: P = 0.048; farmland: P = 0.111), while it exhibited no significant difference among habitat types in summer. The abundance of PlantSeeds was affected by habitat type (H2,84 = 9.51, P = 0.008), as farmlands had the highest abundance compared to the other two habitat types (forest: P = 0.003; village: P = 0.041). Forests had fewest VertFishScavs (H2,84 = 11.77, P = 0.003) among habitat types (farmland: P = 0.002; village: P = 0.001). The abundance of omnivores was not affected by habitat type (H2,84 = 0.40, P = 0.821), but higher values were reported in winter than in summer (H1,84 = 4.76, P = 0.029). Invertebrate feeding birds were neither influenced by season (F1,84 = 1.33, P = 0.248) nor by habitat type (F2,84 = 2.10, P = 0.351).
| Pairwise comparisons | A-value | P-value | ||||||||||||
| Summer | Winter | Summer | Winter | |||||||||||
| Forest vs farmland | 0.069 | 0.045 | 0.001 | 0.001 | ||||||||||
| Forest vs village | 0.066 | 0.025 | 0.001 | 0.001 | ||||||||||
| Farmland vs village | 0.027 | 0.015 | 0.017 | 0.005 | ||||||||||
| A-values were used to evaluate differences within and between groups, with A > 0 indicating that the difference between groups is larger than that within groups. P-values < 0.05 are significant. | ||||||||||||||
The indicator species analysis revealed that 19 species in summer and 14 in winter were significantly associated with specific habitat types (Table S2). Most species significantly associated with forest were feeding on invertebrates, while PlantSeeds were more significantly correlated with farmland and village. On the NMDS ordination diagram, clear groupings of the bird communities by habitat types were observed, specifically between the forests and the other two habitats (Fig. 4A and B). Among the measured land use variables, area of construction land and land use type diversity were best correlated to the variation in species compositions. The area of construction land was significantly linked to the NMDS axes in both seasons (summer: r2 = 0.22, P = 0.012; winter: r2 = 0.26, P = 0.004). Land use type diversity was correlated to the NMDS axes in winter (winter: r2 = 0.16, P = 0.025). VertFishScavs and PlantSeeds were mainly found in habitats with a small area of construction land and low land use type diversity. Comparatively, Omnivores, FruiNects and Invertebrate-feeding species were less affected by these two variables (Fig. 4C and D).
We found that bird abundance, species richness and diversity were higher in anthropogenic habitats (farmlands and villages) than natural habitats (forests) in both summer and winter; only the Shannon-Wiener index was lower in villages in summer due to the exceptional high abundance of Red-rumped Swallow (Cecropis daurica). This pattern is not only consistent with our expectations, but also consistent with the findings from previous studies (Chapman and Reich, 2007; Catterall et al., 2010; Coetzee and Chown, 2016; Asefa et al., 2017). Spatial distribution of avian diversity is influenced by multiple environmental factors, one of which is food resource diversity and availability (Ferger et al., 2014). In the forest landscape like our study area, the scattered anthropogenic habitats (e.g. villages and farmlands) can provide additional food for birds that have higher environmental plasticity, and can thus find food in both natural and anthropogenic habitats (Oro et al., 2013). Specifically, birds in farmlands use the abundant cereal crops, soil invertebrates and weed seeds, while those in and around villages can get food from fruit trees, vegetable fields, and leftovers around houses. In addition to supporting more species, the anthropogenic habitats deserve a higher conservation status also because there were many threatened species using these habitats. Across the whole study period, a total of 13 national key protected wild animal species were recorded, 12 of which were found in the human-dominated habitats (10 in villages, and 5 in farmlands).
Additionally, previous work has shown that more heterogenous vegetation can support higher diversity and abundance of avian communities, likely by offering more diverse nesting resources and broader niche spaces (MacArthur and MacArthur, 1961; Kissling et al., 2008; Basnet et al., 2016). The forest vegetation in our study area was dominated by subtropical evergreen broadleaved forests with woody plants and dense canopies. There were more diverse vegetation types with lower canopy cover in farmlands and villages, such as shrubs, grasslands, tea gardens, vegetable fields, which all can provide various habitats and different food resources for birds. Some studies found the opposite pattern, i.e. lower species richness and diversity of avian communities in anthropogenic habitats compared with natural habitats (Pringle et al., 2019; Şekercioğlu et al., 2019; Piano et al., 2020). The main reason for the contrasting patterns might be the difference in the extent of the landscape modifications. In these studies, the natural land use types were completely converted to anthropogenic landscapes that were intensively utilized. However, the landscape modifications in our anthropogenic habitats were moderate, leaving some natural or semi-natural land use types in these anthropogenic landscapes.
As predicted, the higher avian diversity in the anthropogenic habitats could be in line with the expectations of the intermediate disturbance hypothesis, which implies that intermediate disturbances maintain high biodiversity by creating varied and favourable conditions for diverse species (Connell, 1978). Although the intermediate disturbance hypothesis remains under debate (Fox, 2013; Sheil and Burslem, 2013), it has been supported by theoretical and empirical studies on a range of taxa (Molino and Sabatier, 2001; Liu et al., 2019; Swart et al., 2019). For example, Mayor et al. (2012) found that vascular plant species richness showed a unimodal relationship with human disturbance, reaching a peak at intermediate disturbance. Desrochers et al. (2011) found that conversion of <50% of natural areas to human-dominated land uses increased bird species richness, but conversion of >50% led to species losses. The intermediate disturbance hypothesis has found wide applications in biodiversity conservation, where intermediate disturbance levels should not be exceeded to enhance species diversity (Svensson et al., 2007). The level of human disturbances to the natural ecosystem might be intermediate in the southern Anhui Mountainous Area, which at present is still dominated by natural mixed forests with interspersed patches of human-created land uses (Wu and Gu, 2017). The local sparsely populated villages are typically small, and surrounded by farmlands and forests (Wang and Wang, 2015). The farmlands with scattered bushes and shrubs are also surrounded by forests, and only traditional agricultural practices are used (Li, 2011). The moderate level of human disturbances in the anthropogenic habitats might contribute to maintain this high avian species diversity and richness. However, an increasing human disturbance should be avoided to reduce potential negative effects on certain bird species, and possible homogenization of avian communities (Coetzee and Chown, 2016; Liang et al., 2019).
Apart from the diversity metrics discussed above, avian species composition differed between the human-modified habitats and natural forest habitat, especially in the guild feeding on vertebrates, fish and carrion and the guild feeding on plants and seeds. The differences in land use patterns and the associated environmental conditions may explain the compositional differences. Different guilds have various resource requirements and environmental tolerance thresholds, and therefore differ in their spatial distributions (Ding et al., 2019). We found more VertFishScavs, including waterbirds and raptors, in the anthropogenic habitats where there were large areas of construction land and farmlands. There were also many shallow water bodies, such as rice paddies, irrigation ditches and ponds, in the anthropogenic habitats, and waterbirds could prey on vertebrates and invertebrates in these small pools and channels. The open landscape in the anthropogenic habitats also allowed raptors and other carnivore species to efficiently detect and catch prey. Species feeding on plants and seeds showed a strong association with farmlands and villages where they could forage on abundant crops and weed seeds. The phenology of plants (i.e. flowering and fruiting) determines the resources available to animals (Pothasin et al., 2016). In this study, only five species of FruiNects were recorded, and we speculated that this was because both summer and winter were not optimal seasons for flowering and fruiting in our study area, which resulted in a lower resource availability for FruiNects. However, there were many fruits on trees in villages in early winter, which could explain why the number of FruiNects was slightly higher in villages in winter. We found no difference in the abundance of omnivorous species among habitat types, as these species are generalists that have a broad range of diets, and can adapt to various habitats (Basnet et al., 2016). However, we found more omnivorous birds in winter, which might be attributed to the fact that some species (such as Pycnonotus sinensis, Fringilla montifringilla and Parus major) in this guild often aggregate in winter resulting in higher detection probability. Previous studies have found that forest insectivores have high habitat specificity, and are more confined to forest interior than other forest passerine guilds (Şekercioğlu et al., 2002; Stratford and Stouffer, 2015). In our study, although the abundance of invertebrate-feeding birds did not differ among habitats, the indicator species analysis revealed that most species that were significantly associated with forests were from this guild of invertebrate feeders (Table S2). In addition to differences in species compositions among habitats, the indicator species analysis also showed seasonal differences. Previous studies also found that the species – habitat relationship was not consistent across seasons, but was influenced by seasonal fluctuations in resource availability (Yabuhara et al., 2019).
Bird abundance, species richness and diversity were higher in human-dominated habitats (i.e., farmland and village) than natural habitats (i.e., forest) in the southern Anhui Mountainous Area, which is consistent with the predictions from the intermediate disturbance hypothesis. Apparently, many bird species have adapted to these moderate human-induced landscape modifications in the region. The higher diversity metrics justify the value of the anthropogenic landscapes for the conservation of birds. Particular efforts should be made to control potential negative effects of various human interferences present in these anthropogenic landscapes. Despite the high conservation value of these human-dominated habitats, conservation efforts in the forests should not be loosened, because there were still many species (e.g., many insectivorous species) that had higher specificity for this natural habitat, as well as some species that persisted only in natural habitat and rarely occurred in modified habitats. Furthermore, given the increasingly intensifying human-induced landscape modifications and global changes, we suggest continuously monitoring the bird communities and their responses to the ongoing environmental changes, to improve management and conservation plans for avian communities in this southern Anhui Mountainous Area.
CL conceived the study. XW, GZ, HM, YW, WZ and YZ collected the data. XW and CL performed the analyses. XW wrote the first draft of the paper. CL and WB revised the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
We thank Weiming Zou, Xiangrong Song, Weiqiang Li and the staff of the Anhui Guniujiang National Nature Reserve for their help during the field surveys. This work was supported by the National Natural Science Foundation of China (grant numbers 31970500 and 31770571), the Excellent Youth Project of the Anhui Natural Science Foundation (2108085Y09) and the Biodiversity Investigation, Observation and Assessment Program (2019–2023) of Ministry of Ecology and Environment of China. The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2022.100006.
|
Allen DC, Bateman HL, Warren PS, Albuquerque FS, Arnett-Romero S, Harding B. Long-term effects of land-use change on bird communities depend on spatial scale and land-use type. Ecosphere. 2019;10:e02952
|
|
Bibby, C., Burgess, N., Hill, D., Mustoe, S., 2002. Bird Census Techniques, second ed. Academic Press, London.
|
|
Jin X, Wu H. Wild animal resources and its management in the south of Anhui Province. J Huaibei Norm Univ. 2002;23:51-54 (In Chinese)
|
|
Li, J., 2016. Prioritized Areas for Land Area Biodiversity Conservation in china. Science Press, Beijing (In Chinese).
|
|
Li S. A study of modern rural development modes and mechanism in Anhui mountainous areas. J Chizhou College. 2011;25:1-5 (In Chinese)
|
|
MacKinnon, J.R., Phillipps, K., He, F., 2000. A Field Guide to the Birds of China. Oxford University Press, New York.
|
|
McCune, B., Grace, J.B., Urban, D.L., 2002. Analysis of Ecological Communities. MjM Software Design, Gleneden Beach.
|
|
Minchin, P.R., 1987. An evaluation of the relative robustness of techniques for ecological ordination. In: Prentice, I.C., van der Maarel, E. (Eds.), Theory and Models in Vegetation Science. Springer Netherlands, Dordrecht, pp. 89–107.
|
|
Morrison, M.L., 1986. Bird populations as indicators of environmental change. In: Johnston, R.F. (Ed.), Current Ornithology. Springer US, Boston, MA, pp. 429–451.
|
|
Shannon, C.E., Weaver, W., 1998. The Mathematical Theory of Communication. University of Illinois Press, Urbana.
|
|
Wang H, Wang F. Research on the difference of village community in Anhui. J Anhui Agr Sci. 2015;43:280-282 (In Chinese)
|
|
Wu, H., Gu, C., 2017. A Guide to the Birds of Anhui. Anhui Normal University Press, Anhui (In Chinese).
|
| Land use type | Farmlands | Villages | Forests | ||||||||||||||||
| Area of woodlands (ha) | 1.75 ± 1.82c | 6.31 ± 2.53b | 19.91 ± 2.83a | ||||||||||||||||
| Area of water bodies (ha) | 0.48 ± 0.55ab | 0.84 ± 0.92a | 0.65 ± 1.73b | ||||||||||||||||
| Area of farmlands (ha) | 19.27 ± 2.51a | 11.28 ± 1.81b | 1.39 ± 1.93c | ||||||||||||||||
| Area of construction lands (ha) | 1.08 ± 1.24b | 4.01 ± 3.49a | 0.37 ± 1.28c | ||||||||||||||||
| Land use type diversity | 0.47 ± 0.26b | 1.01 ± 0.12a | 0.29 ± 0.31b | ||||||||||||||||
| Columns with same superscript letters indicate no significant differences between habitat types (Kruskal-Wallis H-tests followed by a post-hoc Wilcoxon rank sum). | |||||||||||||||||||
| Habitat types | Family richness | Species richness | Number of individuals | ||||||||||||||||||||||||
| Summer | Winter | Total | Summer | Winter | |||||||||||||||||||||||
| Villages | 26 | 50 (4) | 72 (6) | 86 (10) | 1720 | 1458 | |||||||||||||||||||||
| Farmlands | 25 | 56 (3) | 56 (3) | 81 (5) | 1025 | 1398 | |||||||||||||||||||||
| Forests | 23 | 53 (2) | 52 (3) | 71 (5) | 838 | 1160 | |||||||||||||||||||||
| Total | 32 | 85 (6) | 91 (8) | 120 (13) | 3583 | 4016 | |||||||||||||||||||||
| The numbers of species listed as Class II Key Protected Wild Animal Species in China are displayed in parentheses. | |||||||||||||||||||||||||||
| Pairwise comparisons | A-value | P-value | ||||||||||||
| Summer | Winter | Summer | Winter | |||||||||||
| Forest vs farmland | 0.069 | 0.045 | 0.001 | 0.001 | ||||||||||
| Forest vs village | 0.066 | 0.025 | 0.001 | 0.001 | ||||||||||
| Farmland vs village | 0.027 | 0.015 | 0.017 | 0.005 | ||||||||||
| A-values were used to evaluate differences within and between groups, with A > 0 indicating that the difference between groups is larger than that within groups. P-values < 0.05 are significant. | ||||||||||||||