| Citation: | Rose THOROGOOD, Nicholas B. DAVIES. 2013: Hawk mimicry and the evolution of polymorphic cuckoos. Avian Research, 4(1): 39-50. DOI: 10.5122/cbirds.2013.0002 |
The resemblance of some parasitic cuckoos to Accipiter hawks has been known since ancient times. Recent experiments show that the hawk-like features of Common Cuckoos (Cuculus canorus) facilitate access to Reed Warbler (Acrocephalus scirpaceus) host nests. However, social information alerts hosts to see through the cuckoo's mimetic disguise. In turn, this has promoted the evolution of a cuckoo polymorphism to thwart host recognition. Here we show by comparative analyses that parasitic cuckoos with hawk-like features (yellow eyes, barred underparts, yellow legs) are more likely to be polymorphic (29% of species) than those without (8% of species). Phylogenetic analyses confirm correlated evolution of hawk-like features and cuckoo polymorphism. We suggest that mimicry dynamics are particularly likely to promote the evolution of various guises in parasitic cuckoos to beat host defences.
The uncanny resemblance of Old World cuckoos to Accipiter hawks has been remarked upon since ancient times, with the metamorphosis of cuckoos to Accipiter hawks and vice versa prevalent in old Norse, Greek and Chinese mythology (Lai, 1998). Most Accipiter hawks have barred underparts, yellow feet, and yellow eyes or yellow eye ring (del Hoyo et al., 1994), and some cuckoo species share these features (Voipio, 1953; Payne, 1967; Johnsgard, 1997). However, it was not until Wallace (1889) that this similarity was suggested to be a mimetic defence. Wallace suggested that a cuckoo mimics hawks for protection from the hawks themselves, and indeed, cuckoos suffer less from hawk-predation than expected by chance (Møller et al., 2012). However, recent work has demonstrated that this disguise can also be another feature in the arms race between brood parasite and host (Davies and Welbergen, 2008; Welbergen and Davies, 2011). Reed Warblers (Acrocephalus scirpaceus), a common host in Europe, are much more likely to mob a Common Cuckoo (Cuculus canorus) model if its barred underparts are covered (Welbergen and Davies, 2011), and tits (Paridae) who are naïve to cuckoo parasitism are as afraid of Common Cuckoos as sparrowhawks (Accipiter nisus), unless the cuckoo's barring is obscured (Davies and Welbergen, 2008). Of course, barring is unlikely to be the only salient cue that causes confusion (Trnka et al., 2012), with the yellow eye ring, legs, and flight behavior all probably part of the disguise. But, for Reed Warblers at least (cf. Trnka and Prokop, 2012), we now know that these hawk-like features are an effective disguise that facilitates access to host nests because of the risk to hosts of incorrect identification of a dangerous predator.
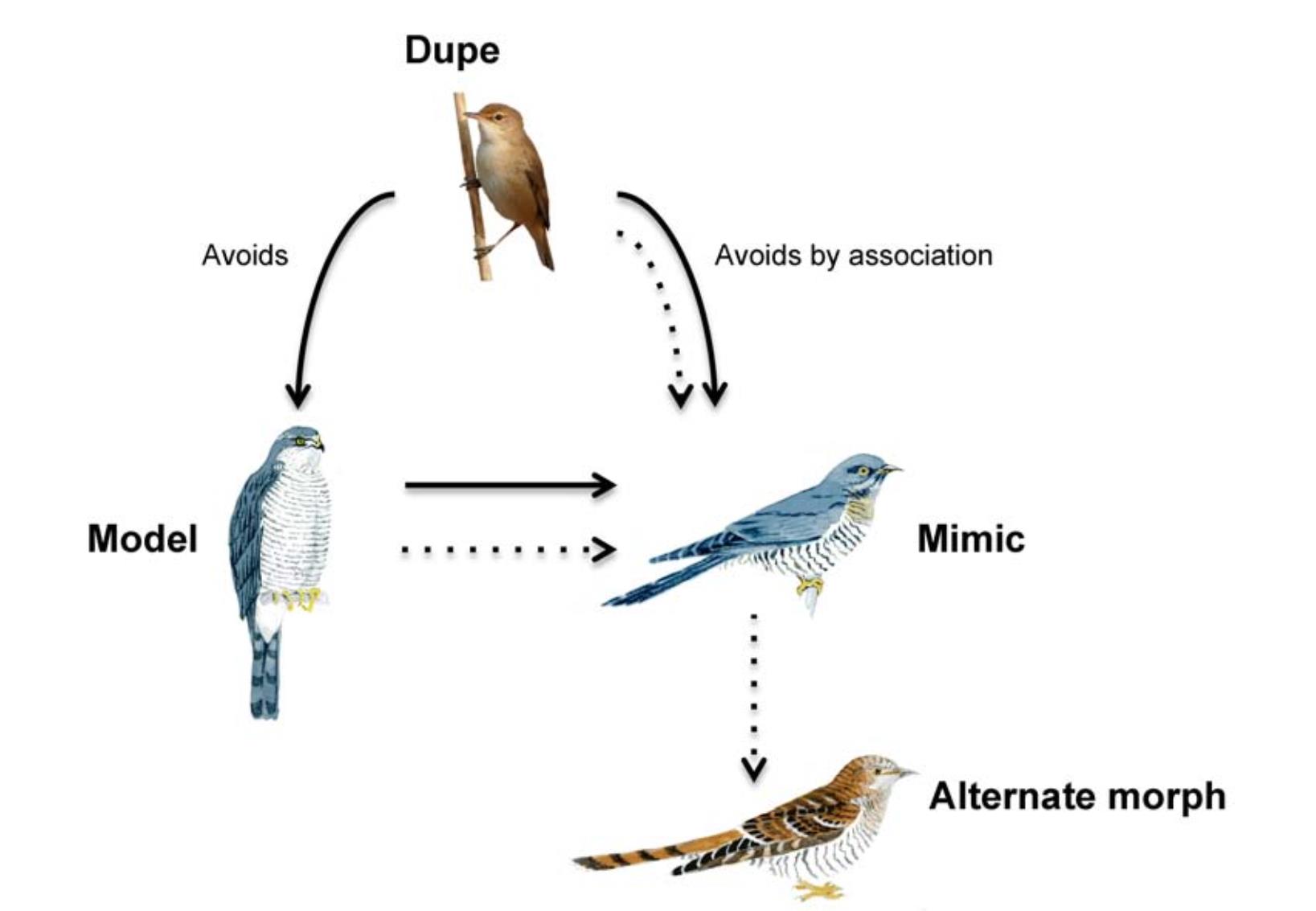
Appearing similar to a harmful hawk to gain access to Reed Warblers' nests is best thought of as an example of Batesian mimicry (Fig. 1): a species that could be safely attacked uses the disguise of a harmful model to avoid interactions with a dupe. According to definitions developed largely with predators and prey in mind (Ruxton et al., 2004), a dupe avoids a Batesian mimic, even though the interaction would be harmless, because the mimic has copied the signals or cues given by a dangerous model that elicit innate or learned fear in the dupe. Thus, the cuckoo, which can be safely attacked by hosts, is a "parasite in wolf's clothing" (Welbergen and Davies, 2011). This contrasts with aggressive mimicry, when an opponent resembles a non-threatening, or inviting, model to gain access to prey or hosts (Wickler, 1968). A cuckoo that gained access to host nests by mimicking a harmless dove, for example, would be an aggressive mimic. Unlike many Batesian mimics (e.g. tasty butterflies that mimic distasteful models), cuckoos are not harmless to duped hosts; they are predators of eggs and chicks (Gärtner, 1981) and for many hosts the loss of reproductive investment that comes from raising a parasite can severely impact their fitness (reviewed in Welbergen and Davies, 2011). However, while mistaking a cuckoo for a hawk could result in the loss of the Reed Warbler's reproductive success that season (unless it later recognizes and correctly rejects an imposter's egg), mistakenly approaching a hawk could be deadly (Newton, 1986). It is not the cost of the mimic to the host that defines mimicry, but the cost of incorrect identification of the model (Pasteur, 1982; Pough, 1988). Therefore, when hosts are duped by the cuckoo's hawk-like appearance, it occurs because of the model's deterrence.
A cuckoo's disguise is not only useful for deterring mobbing. The sight of a cuckoo at the nest can make hosts more likely to reject foreign eggs (Davies and Brooke, 1988; Moksnes et al., 1993), but only when cuckoos are common (Brooke et al., 1998). This means that as cuckoos increase in the environment, information about their disguise will spread and alert multiple lines of host defences (Davies and Welbergen, 2009; Campobello and Sealy, 2011; Thorogood and Davies, 2012). Environmental changes may also influence the success of the cuckoo's mimicry. For example, sparrowhawk numbers may vary as prey availability alters, increasing or decreasing the risk of parasitism relative to the risk of predation. Therefore, mimetic defences may become less successful if models become more rare or less dangerous, or if dupes evolve new behaviors or acquire more information that allow improved recognition. How might cuckoos fight back against a loss of mimicry's effectiveness?
Theory and models suggest that when mimetic defences become less effective, alternative forms may be selected if they escape the dupe's recognition (Turner, 1978; Vane-Wright, 1979; Holen and Johnstone, 2004). These alternate forms may then evolve to become mimics of other models, and/or vary in frequency depending on the success of the primary morph. Analysis of evolutionary pathways in Papilionid butterflies provides good evidence to support this hypothesis; here it seems that mimicry promotes the evolution of polymorphic species (Kunte, 2009). Polymorphisms may evolve whether a mimic is Batesian or aggressive, but models show that different forms are more likely to evolve in Batesian mimic species (Holen and Johnstone, 2004). Alternatively, multiple forms can evolve in non-mimetic species simply because rare forms are more likely to evade detection (apostatic selection, Clarke, 1969; Endler, 1981; Bond, 2007).
Although rare in birds (3.5% of species, Roulin, 2004), polymorphisms are common among birds of prey, including the hawks with which some cuckoos share features (11 of 46 Accipiter hawk species are polymorphic, Ferguson-Lees and Christie, 2001). Polymorphisms in these birds may sometimes be adaptive (Roulin, 2004) as they are often linked to alternative strategies within species (ecological niches, mating strategies, crypsis, and avoidance of prey or predators; Fowlie and Krüger, 2003; Galeotti et al., 2003). Many cuckoo species also come in multiple plumages and most of these species are parasitic (Payne, 1967; Galeotti et al., 2003; Payne, 2005, see Results below). Previous hypotheses have focused on alternate cuckoo forms being selected via apostatic selection (Payne, 1967; Galeotti et al., 2003). Alternatively, polymorphisms may be more likely to evolve in parasitic cuckoos if the effectiveness of hawk mimicry declines (Fig. 1). Common Cuckoos come in two colour morphs: a grey morph that resembles sparrowhawks (and is the more commonly studied form), and a rufous morph (Voipio, 1953; Honza et al., 2006). Our recent experimental work has shown that when Reed Warblers are alerted by social information to the presence of either morph, then the alternate morph is more likely to slip past host defences (Thorogood and Davies, 2012). We suggested, therefore, that the Common Cuckoo's polymorphism is maintained by frequency-dependent selection.
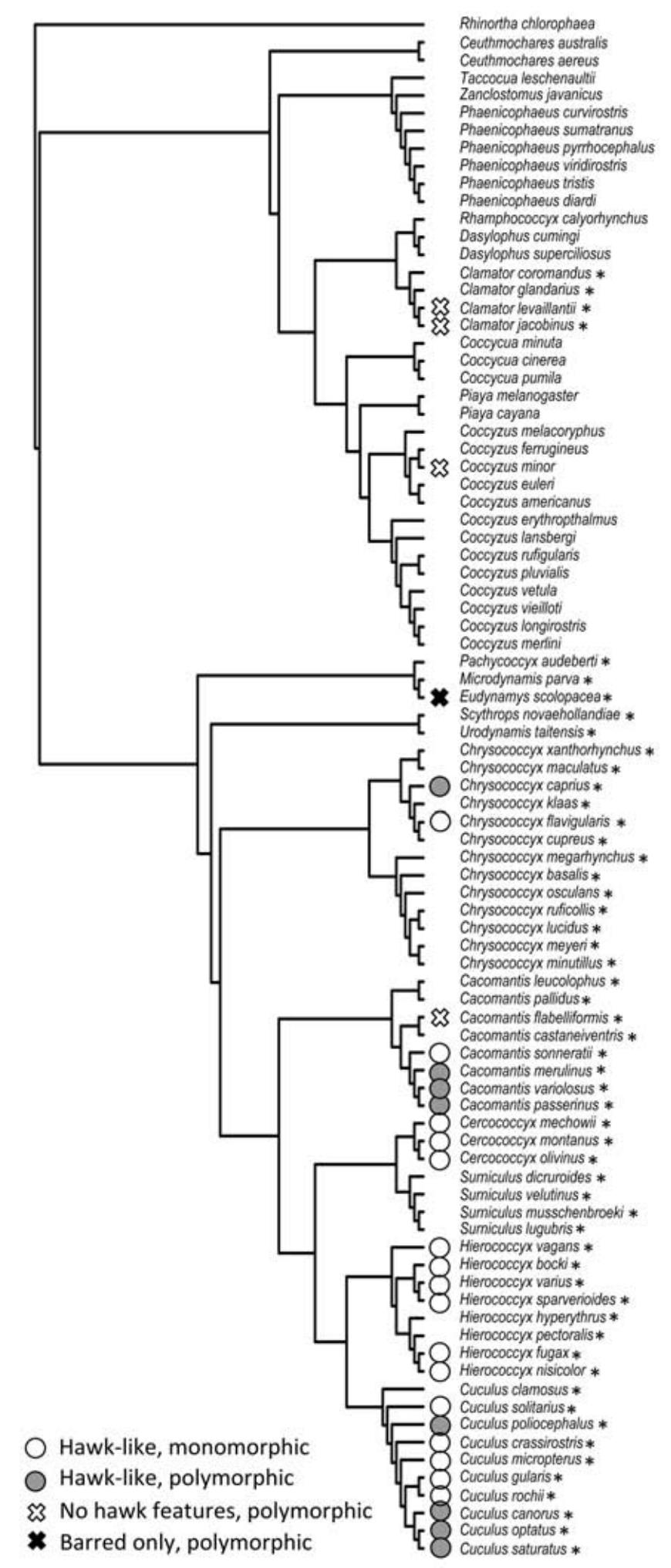
Here we use a comparative approach to look across cuckoo species and ask if cuckoos with hawk-like features are more likely to be polymorphic. In this analysis we mapped the presence of three hawk-like features (barred plumage, yellow eye ring, and yellow feet) on to the cuckoo phylogeny of Sorenson and Payne (2005) and scored each species as mono- or polymorphic. Barred plumage in cuckoos could represent convergent evolution for crypsis to avoid host detection rather than hawk mimicry (Krüger et al., 2007), but the behavioral responses of hosts and non-hosts with cuckoo and sparrowhawk mounts suggest that barring is part of the suite of cues required to elicit fearful responses towards cuckoos (Davies and Welbergen, 2008; Welbergen and Davies, 2011; Trnka et al., 2012). Bright yellow features are unlikely to aid crypsis. Without behavioral tests we cannot be sure that hawk-like features are used as mimetic defences by all species scored as such (Grim, 2005), however this should only make our analyses more conservative. We predict that if polymorphisms are linked to hawk-mimicry, then a greater proportion of species exhibiting hawk-like features will be polymorphic than species without hawk features. However, if polymorphisms are only a result of apostatic selection, then we predict that polymorphisms will be just as likely to arise in cuckoo species that do not show hawklike features.
We collected data from species descriptions (Payne, 2005) for each of the 141 species of cuckoos listed in Sorenson and Payne's (2005) phylogeny. All variables were treated as binary: (1) barring was recorded present if it occurred on the breast or belly, (2) legs and feet were scored as yellow if they were described as such, and (3) eye colour was scored as yellow if either the iris or eye ring included yellow in the description. Plumage morphs were assigned as polymorphic if species were recorded showing different plumage forms across their range (sexually dimorphic plumage was not included), but insufficient data exists to include range size in analyses. For these analyses we only considered variation in adult plumage as our hypothesis relates to protection from hosts during breeding. We took a conservative approach and did not restrict polymorphisms only to where multiple forms co-occur because of the dynamic nature of host-parasite interactions — different forms could have arisen because of mimicry but are now specialized on different hosts or in different habitats (Voipio, 1953). Our approach relied on human-based categories of plumage morphs. Avian vision differs from our own, and so our gross classification of morphs may have under-estimated the true range of plumage variation present in some species (Seddon et al., 2010; Burns and Schultz, 2012; Stoddard, 2012). Our data were compared against datasets used in two previous studies of plumage in cuckoos (Galeotti et al., 2003; Krüger et al., 2007) and concordance was almost complete.
To assess whether plumage polymorphisms have evolved together or independently from hawk-mimicry, we used the DISCRETE module of BAYESTRAITS (Pagel, 1999; Pagel and Meade, 2006) with the phylogeny of Sorenson and Payne (2005). DISCRETE reconstructs the evolution of binary traits on a phylogenetic tree by assessing the frequency of transitions between the various combinations of the two traits, either linked or where they occur at random. The log likelihoods of these two models can then be compared using a likelihood ratio (LR) test, with the model with the significantly greater likelihood being the one that best explains the current pattern of traits on a tree. Differences in the rates of transition between states can be assessed by restricting rates of evolution to be equal for two transitions of interest, and then comparing the log likelihood of this model with that of an unrestricted model. We performed separate analyses with either ultrametric branch lengths taken from the phylogeny, or with equal branch lengths to account for uncertainty about the mode of evolution (Grafen, 1989) and estimated transitions using maximum likelihood methods (25 optimization attempts per model). Significance for LR tests was assessed against a chi-squared distribution with four degrees of freedom.
Of the 141 species of cuckoos that we included in our analysis (Fig. 2, only the Cuculinae are shown for ease of presentation), 16 exhibited at least two different plumage types (11.3 %), and all but four of these were parasitic cuckoos (Coccyzus minor, Centropus ateralbus, Centropus senegalensis, and Centropus viridis were polymorphic but nesting cuckoos). Polymorphic cuckoos were largely restricted to those from the Cuculinae, except for the three Centropus spp.: 4/83 non-parasitic cuckoos (4.8%) are polymorphic compared to 12/58 parasitic species (20.7%). Similarly, all of the 24 species showing barred plumage with yellow eyes and/or feet were Cuculinae (17%). Of these hawk-like cuckoos, 8 of the 24 were polymorphic (33.3%), so species with hawk features are more likely to be polymorphic than those without (8/117, 6.8%, two-tailed Fisher's exact test: p = 0.001).
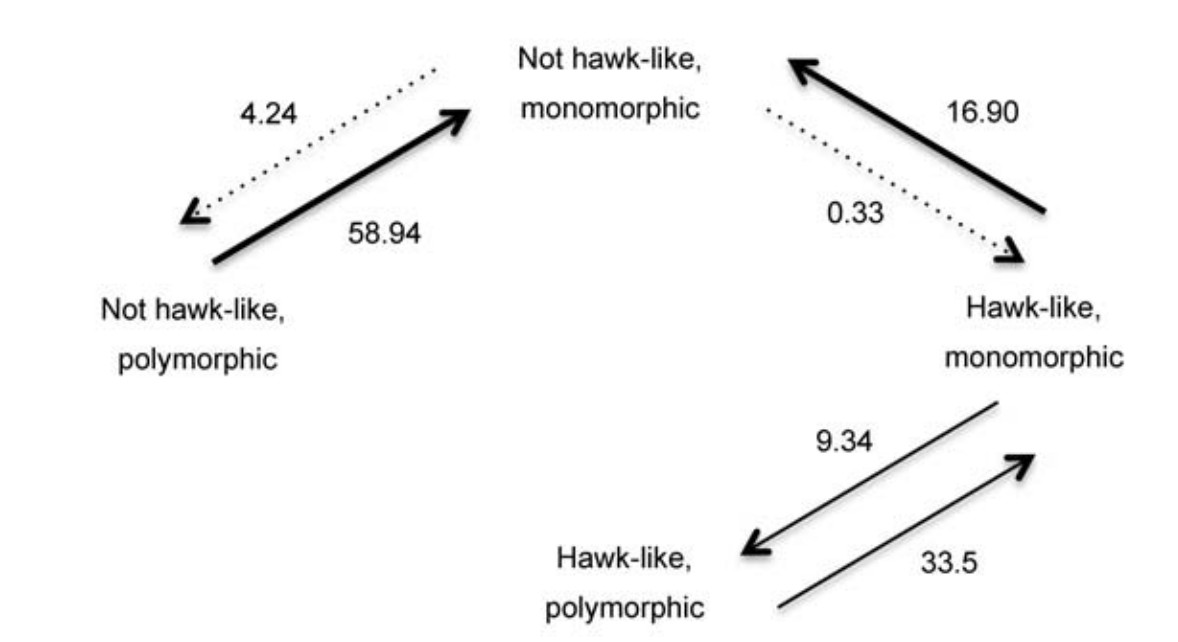
When we took phylogeny into account, we found that for each trait there were clear differences in the rates of evolution between states. Looking first at each trait separately, species lost hawk-like features more quickly than they gained them (LR = 20.68, df = 1, p < 0.001), and polymorphic species became monomorphic more quickly than they evolved alternate forms (LR = 14.05, df = 1, p < 0.001, results were similar when branch lengths were equal). When we tested for correlated evolution between these two traits, we found some evidence that the evolution of hawk features and polymorphisms are linked in cuckoos (Fig. 3, ultrametric tree: LR = 7.74, df = 4, p = 0.040; equal branch lengths: LR = 9.54, df = 4, p = 0.020). Species that did not look similar to hawks lost polymorphisms significantly faster than they evolved (LR = 10.19, df = 1, p = 0.0008). This transition was slower for hawk-like species, although it was not significantly different to the rate at which polymorphisms were acquired (LR = 1.18, df = 1, p = 0.20). Interestingly, hawk features were acquired more slowly than they were lost by monomorphic species (LR = 8.34, df = 1, p = 0.002), and never acquired after a polymorphism evolved (Fig. 3). Polymorphic, hawk-like species never lost their hawk features. These patterns of correlated evolution were only found if we included the additional hawk features of yellow eyes and/or yellow feet (21/24 species identified as 'hawk-like' had both yellow eyes and feet: 1 species had yellow eyes but not feet, and 2 species with yellow feet did not have yellow eyes). If we restricted our analyses to plumage barring only, a dependent model of evolution was no better than an independent one (ultrametric tree: LR = 4.01, df = 4, p = 0.14; equal branch lengths: LR = 4.09, df = 4, p = 0.13).
Our results show that there is not one explanation for the evolution of polymorphisms among cuckoo species as both hawk features and polymorphisms can sometimes arise independently of the other. However, polymorphisms were lost more quickly than they were gained in lineages without hawk features, but not in those that appear similar to hawks. Furthermore, extant hawk-like cuckoos were more likely to be polymorphic than those without hawk features. Together, this suggests that both apostatic selection and mimicry dynamics may underlie polymorphisms in cuckoos.
Of course, polymorphisms in cuckoos may sometimes also be a neutral or non-adaptive trait (Galeotti et al., 2003). However, we found that for cuckoos unlike hawks, the rate for losing a polymorphism was much greater than the rate at which it evolved (for hawk-like cuckoos the opposite was true). Polymorphisms may be lost because: (1) one morph has a selective advantage over another so that it gradually disappears, (2) random genetic processes mean that the genetic variation responsible for plumage morphs is exhausted, or (3) color morphs may speciate, leading to two differently colored descendent species (Roulin, 2004). Our results suggest that the most stable state for cuckoos unlike hawks is to appear monomorphic, and thus polymorphisms, when they do occur, are unlikely to be neutral for cuckoo species. Field experiments are needed to determine whether apostatic selection is responsible for maintaining polymorphisms in species without hawk-features or, if like birds of prey, they arise as a result of large population sizes (Fowlie and Krüger, 2003), and are maintained via sexual (Fowlie and Krüger, 2003) or disruptive selection (Galeotti et al., 2003). Although less frequent, polymorphisms also occur in non-parasitic cuckoos, and all but one occur in the Centropodinae (4 species overall, Fig. 2). It would be fascinating to look within this group to ascertain why some are polymorphic while their close relatives are not, and whether their reversal in sex roles is important (Andersson, 1995).
We found that hawk features are limited to five genera of parasitic Old World cuckoos (Cuculus, Hierococcyx, Cercococcyx, Cacomantis, Chrysococcyx), and that 60% of all barred cuckoo species also had yellow eyes and/or feet. Previous analyses of only barred plumage in cuckoos have found this to be a derived trait that most likely arose after the evolution of parasitism (Krüger et al., 2007). Analyses with all avian families have also shown that sexual selection may play a role in the evolution of barred plumage (Gluckman and Cardoso, 2010), but this does not seem to be the case for cuckoos (Krüger et al., 2007). Our results show that barred plumage alone does not lead to the evolution of polymorphisms, suggesting that yellow hawk-like features play an additional role (Trnka et al., 2012).
Hawk mimicry is a useful deterrent that aids parasitism, at least for Common Cuckoos and their Reed Warbler hosts (Davies and Welbergen, 2008; Welbergen and Davies, 2009, 2011; Thorogood and Davies, 2012). So why are hawk features not more widespread amongst cuckoo species? Indeed, why do other groups of brood parasites (e.g. cowbirds) not also take advantage of hawk mimicry? Great Reed Warblers (Acrocephalus arundinaceus) are another frequent host of the Common Cuckoo, but they do not seem to be deterred by the cuckoo's disguise (Trnka and Prokop, 2012). Instead, Great Reed Warblers will attack any threat at their nest, even harmless doves (Honza et al., 2010). This shows that hawk mimicry may not be an effective disguise against all hosts, or that some hosts may have learned to see past this disguise. Furthermore, hawk-like features may not always benefit cuckoos, but entail costs. Swallows (Hirundo rustica) and bushtits (Psaltriparus minimus) mob cuckoos in a similar manner to raptors (Lyon and Gilbert, in press), so hawk-like features might lead to increased aggression throughout the year.
Although not hawk-like, adults of other cuckoo species may also use mimicry to their advantage. Among the most derived genera of cuckoos, it is notable that Surniculus species lack hawk features. Commonly known as drongo-cuckoos because of their close resemblance to the unrelated drongo, it was previously thought that this was a case of host mimicry (Payne, 1967). However, we now know that drongo-cuckoos primarily parasitise small babblers rather than drongos (Johnsgard, 1997; Payne, 2005), and that this genus too may use mimicry of aggressive models to intimidate or distract hosts (Johnsgard, 1997). Alternatively, adults of parasitic species unrelated to the Cuculidae might use aggressive mimicry to fool their hosts. Honeyguides (Prodotiscus regulus) and Cuckoo Finches (Anomalospiza imberbis) share visual similarities with innocuous species, and flocking with these and their hosts may help to disguise their threat (Payne, 1967). Field experiments are needed to determine the role of this apparent mimicry in these groups.
Comparative analyses exploring patterns of evolution rely on the assumption that extant species represent diversity randomly. However, if hosts evolve behaviors that allow better discrimination (e.g. through social learning) and defeat mimetic defences, then the species that represent the tips of our phylogeny's branches may not be representative of the evolutionary past. When mimetic defences are beaten and alternate forms arise, species may maintain the original mimetic form. Alternatively, they may revert to monomorphism by losing either the mimetic or alternate form (VaneWright, 1979). These changes in host discrimination and cuckoo appearance may well be microevolutionary, and hence not visible in our phylogeny (Kunte, 2009). Therefore, to best understand how mimicry dynamics might explain parasitic cuckoo polymorphisms, we need to know more of host discrimination across the cuckoo phylogeny. By looking at the distribution of hawk features and polymorphisms across the cuckoo phylogeny, our results suggest that the relationship between Reed Warbler dupes, sparrowhawks, and the multiple forms of hawk-like Common Cuckoos may not be unique to this brood parasite. Future research should explore similarities and differences between hawk-like and not hawk-like cuckoos by asking if some hosts are more easily fooled by hawk disguises, if some use different strategies for parasitism (e.g. distraction tactics by Clamator cuckoos (Soler and Soler, 2000)), or if some cuckoos simply have no suitable models to copy.
We thank all participants of the symposium for their informative discussions and providing the inspiration for this paper. In particular, we thank Mary Caswell Stoddard and Martin Stevens for stimulating debate, three anonymous reviewers' helpful comments and Wei Liang, Eivin Røskaft and their committee for organizing the symposium and facilitating these discussions. This research was funded by the Natural Environment Research Council.
| Species | Barred plumage | Yellow feet or legs | Yellow eye or ring | Hawk-like | Polymorphic |
| Cuculus saturatus | 1 | 1 | 1 | 1 | 1 |
| Cuculus optatus | 1 | 1 | 1 | 1 | 1 |
| Cuculus canorus | 1 | 1 | 1 | 1 | 1 |
| Cuculus rochii | 1 | 1 | 1 | 1 | 0 |
| Cuculus gularis | 1 | 1 | 1 | 1 | 0 |
| Cuculus micropterus | 1 | 1 | 1 | 1 | 0 |
| Cuculus crassirostris | 1 | 1 | 1 | 1 | 0 |
| Cuculus poliocephalus | 1 | 1 | 1 | 1 | 1 |
| Cuculus solitarius | 1 | 1 | 1 | 1 | 0 |
| Cuculus clamosus | 1 | 0 | 0 | 0 | 0 |
| Hierococcyx nisicolor | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx fugax | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx pectoralis | 0 | 1 | 1 | 0 | 0 |
| Hierococcyx hyperythrus | 0 | 1 | 1 | 0 | 0 |
| Hierococcyx sparverioides | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx varius | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx bocki | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx vagans | 1 | 1 | 1 | 1 | 0 |
| Surniculus lugubris | 0 | 0 | 0 | 0 | 0 |
| Surniculus musschenbroeki | 0 | 0 | 0 | 0 | 0 |
| Surniculus velutinus | 0 | 0 | 0 | 0 | 0 |
| Surniculus dicruroides | 0 | 0 | 0 | 0 | 0 |
| Cercococcyx olivinus | 1 | 1 | 0 | 1 | 0 |
| Cercococcyx montanus | 1 | 1 | 1 | 1 | 0 |
| Cercococcyx mechowii | 1 | 1 | 1 | 1 | 0 |
| Cacomantis passerinus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis variolosus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis merulinus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis sonneratii | 1 | 0 | 1 | 1 | 0 |
| Cacomantis castaneiventris | 0 | 1 | 1 | 0 | 0 |
| Cacomantis flabelliformis | 0 | 1 | 1 | 0 | 1 |
| Cacomantis pallidus | 0 | 0 | 1 | 0 | 0 |
| Cacomantis leucolophus | 0 | 0 | 0 | 0 | 0 |
| Chrysococcyx minutillus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx meyeri | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx lucidus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx ruficollis | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx osculans | 0 | 0 | 0 | 0 | 0 |
| Chrysococcyx basalis | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx megarhynchus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx cupreus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx flavigularis | 1 | 1 | 1 | 1 | 0 |
| Chrysococcyx klaas | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx caprius | 1 | 0 | 1 | 1 | 1 |
| Chrysococcyx maculatus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx xanthorhynchus | 1 | 0 | 0 | 0 | 0 |
| Urodynamis taitensis | 0 | 0 | 0 | 0 | 0 |
| Scythrops novaehollandiae | 1 | 0 | 0 | 0 | 0 |
| Eudynamys scolopacea | 1 | 0 | 0 | 0 | 1 |
| Microdynamis parva | 0 | 0 | 0 | 0 | 0 |
| Pachycoccyx audeberti | 0 | 0 | 0 | 0 | 0 |
| Coccyzus merlini | 0 | 0 | 0 | 0 | 0 |
| Coccyzus longirostris | 0 | 0 | 0 | 0 | 0 |
| Coccyzus vieilloti | 0 | 0 | 0 | 0 | 0 |
| Coccyzus vetula | 0 | 0 | 0 | 0 | 0 |
| Coccyzus pluvialis | 0 | 0 | 0 | 0 | 0 |
| Coccyzus rufigularis | 0 | 0 | 0 | 0 | 0 |
| Coccyzus lansbergi | 0 | 0 | 1 | 0 | 0 |
| Coccyzus erythropthalmus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus americanus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus euleri | 0 | 0 | 0 | 0 | 0 |
| Coccyzus minor | 0 | 0 | 1 | 0 | 1 |
| Coccyzus ferrugineus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus melacoryphus | 0 | 0 | 1 | 0 | 0 |
| Piaya cayana | 0 | 0 | 0 | 0 | 0 |
| Piaya melanogaster | 0 | 0 | 0 | 0 | 0 |
| Coccycua pumila | 0 | 0 | 0 | 0 | 0 |
| Coccycua cinerea | 0 | 0 | 0 | 0 | 0 |
| Coccycua minuta | 0 | 0 | 0 | 0 | 0 |
| Clamator jacobinus | 0 | 0 | 0 | 0 | 1 |
| Clamator levaillantii | 0 | 0 | 0 | 0 | 1 |
| Clamator glandarius | 0 | 0 | 0 | 0 | 0 |
| Clamator coromandus | 0 | 0 | 0 | 0 | 0 |
| Dasylophus superciliosus | 0 | 1 | 1 | 0 | 0 |
| Dasylophus cumingi | 0 | 0 | 0 | 0 | 0 |
| Rhamphococcyx calyorhynchus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus diardi | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus tristis | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus viridirostris | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus pyrrhocephalus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus sumatranus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus curvirostris | 0 | 1 | 1 | 0 | 0 |
| Zanclostomus javanicus | 0 | 0 | 0 | 0 | 0 |
| Taccocua leschenaultii | 0 | 0 | 0 | 0 | 0 |
| Ceuthmochares aereus | 0 | 0 | 1 | 0 | 0 |
| Ceuthmochares australis | 0 | 0 | 1 | 0 | 0 |
| Rhinortha chlorophaea | 0 | 0 | 0 | 0 | 0 |
| Centropus phasianinus | 0 | 0 | 0 | 0 | 0 |
| Centropus bernsteini | 0 | 0 | 0 | 0 | 0 |
| Centropus violaceus | 0 | 0 | 1 | 0 | 0 |
| Centropus bengalensis | 0 | 0 | 0 | 0 | 0 |
| Centropus viridis | 0 | 0 | 0 | 0 | 1 |
| Centropus grillii | 0 | 0 | 0 | 0 | 0 |
| Centropus goliath | 0 | 0 | 0 | 0 | 0 |
| Centropus toulou | 0 | 0 | 0 | 0 | 0 |
| Centropus sinensis | 0 | 0 | 0 | 0 | 0 |
| Centropus andamanensis | 0 | 0 | 0 | 0 | 0 |
| Centropus nigrorufus | 0 | 0 | 0 | 0 | 0 |
| Centropus cupreicaudus | 0 | 0 | 0 | 0 | 0 |
| Centropus superciliosus | 0 | 0 | 0 | 0 | 0 |
| Centropus monachus | 0 | 0 | 0 | 0 | 0 |
| Centropus senegalensis | 0 | 0 | 0 | 0 | 1 |
| Centropus leucogaster | 0 | 0 | 0 | 0 | 0 |
| Centropus anselli | 0 | 0 | 0 | 0 | 0 |
| Centropus c celebensis | 0 | 0 | 0 | 0 | 0 |
| Centropus steerii | 0 | 0 | 0 | 0 | 0 |
| Centropus rectunguis | 0 | 0 | 0 | 0 | 0 |
| Centropus melanops | 0 | 0 | 0 | 0 | 0 |
| Centropus chlororhynchos | 0 | 0 | 0 | 0 | 0 |
| Centropus unirufus | 0 | 0 | 1 | 0 | 0 |
| Centropus menbeki | 0 | 0 | 0 | 0 | 0 |
| Centropus chalybeus | 0 | 0 | 1 | 0 | 0 |
| Centropus ateralbus | 0 | 0 | 0 | 0 | 1 |
| Centropus milo | 0 | 0 | 0 | 0 | 0 |
| Coua serriana | 0 | 0 | 0 | 0 | 0 |
| Coua delalandei | 0 | 0 | 1 | 0 | 0 |
| Coua gigas | 0 | 0 | 0 | 0 | 0 |
| Coua coquereli | 0 | 0 | 0 | 0 | 0 |
| Coua cursor | 0 | 0 | 0 | 0 | 0 |
| Coua reynaudii | 0 | 0 | 0 | 0 | 0 |
| Coua ruficeps | 0 | 0 | 0 | 0 | 0 |
| Coua caerulea | 0 | 0 | 0 | 0 | 0 |
| Coua verreauxi | 0 | 0 | 0 | 0 | 0 |
| Coua cristata | 0 | 0 | 0 | 0 | 0 |
| Carpococcyx renauldi | 0 | 0 | 0 | 0 | 0 |
| Carpococcyx radiatus | 1 | 0 | 0 | 0 | 0 |
| Carpococcyx viridis | 1 | 0 | 0 | 0 | 0 |
| Neomorphus geoffroyi | 0 | 0 | 0 | 0 | 0 |
| Neomorphus radiolosus | 1 | 0 | 0 | 0 | 0 |
| Neomorphus rufipennis | 0 | 0 | 0 | 0 | 0 |
| Neomorphus pucheranii | 0 | 0 | 0 | 0 | 0 |
| Geococcyx californianus | 0 | 0 | 0 | 0 | 0 |
| Geococcyx velox | 0 | 0 | 0 | 0 | 0 |
| Morococcyx ertyhropygus | 0 | 0 | 0 | 0 | 0 |
| Dromococcyx phasianellus | 0 | 0 | 0 | 0 | 0 |
| Dromococcyx pavoninus | 0 | 0 | 1 | 0 | 0 |
| Tapera naevia | 0 | 0 | 1 | 0 | 0 |
| Crotophaga ani | 0 | 0 | 1 | 0 | 0 |
| Crotophaga sulcirostris | 0 | 0 | 0 | 0 | 0 |
| Crotophaga major | 0 | 0 | 0 | 0 | 0 |
|
del Hoyo J, Elliott A, Sargatal J. 1994. Handbook of the Birds of the World, Vol. 2. New World Vultures to Guinea Fowl. Lynx Edition, Barcelona, Spain. Endler J. 1981. An overview of the relationships between mimicry and crypsis. Biol J Linn Soc, 16: 25–31.
|
|
Ferguson-Lees J, Christie DA. 2001. Raptors of the World. Christopher Helm, London, U.K.
|
|
Gärtner K. 1981. Das Wegnehmen von Wirtsvogeleiern durch den Kuckuck Cuculus canorus. Ornithologische Mitteilungen, 33: 115–131.
|
|
Grafen A. 1989. The phylogenetic regression. Philos T Roy Soc B, 326: 119–157.
|
|
Grim T. 2005. Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol J Linn Soc, 84: 69–78.
|
|
Honza M, Procházka P, Šicha V, Požgayová, M. 2010. Nest defence in a cuckoo host: great reed warblers risk themselves equally for their own and parasitic chicks. Behaviour, 147, 741–756.
|
|
Johnsgard PA. 1997. The Avian Brood Parasites: Deception at the Nest. Oxford University Press, Oxford, U.K.
|
|
Lyon BE, Gilbert GS. In press. Unparasitized species mob and alarm call to cuckoos: implications for sparrowhawk mimicry by brood parasitic cuckoos. Wilson J Ornithol.
|
|
Newton I. 1986. The Sparrowhawk. T & AD Poyser, London, U.K.
|
|
Payne RB. 2005. The Cuckoos. Oxford University Press, Oxford, U.K.
|
|
Roulin A. 2004. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol Rev, 79: 815–848.
|
|
Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding Attack: the Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press, New York.
|
|
Sorenson MD, Payne RB. 2005. A molecular genetic analysis of the cuckoo phylogeny. In: Payne RB (ed) The Cuckoos. Oxford University Press, Oxford, U.K. pp 68–94.
|
|
Voipio P. 1953. The hepaticus variety and the juvenile plumage types of the cuckoo. Ornis Fennica, 30: 99–117.
|
|
Wallace AR. 1889. Darwinism: An Exposition of the Theory of Natural Selection with Some of Its Applications. Macmillan, London, U.K.
|
|
Wickler W. 1968. Mimicry in Plants and Animals. World University Library, London, U.K.
|
| 1. | Axelle Scoizec, Eric Niqueux, Audrey Schmitz, et al. New Patterns for Highly Pathogenic Avian Influenza and Adjustment of Prevention, Control and Surveillance Strategies: The Example of France. Viruses, 2024, 16(1): 101. DOI:10.3390/v16010101 |
| 2. | Yuting Xu, Ling Tang, Xiaojun Gu, et al. Characterization of avian influenza A (H4N2) viruses isolated from wild birds in Shanghai during 2019 to 2021. Poultry Science, 2023, 102(10): 102948. DOI:10.1016/j.psj.2023.102948 |
| 3. | N. M. Faustova, S. S. Petlitskaya, I. N. Ampilogova, et al. Neuraminidase Inhibitors: Development and Validation of a Procedure for <i>In Vitro</i> Determination of the Inhibitory Effect. Bulletin of the Scientific Centre for Expert Evaluation of Medicinal Products. Regulatory Research and Medicine Evaluation, 2023, 13(1): 60. DOI:10.30895/1991-2919-2022-387 |
| 4. | Christos S. Zerefos, Stavros Solomos, John Kapsomenakis, et al. Lessons learned and questions raised during and post-COVID-19 anthropopause period in relation to the environment and climate. Environment, Development and Sustainability, 2021, 23(7): 10623. DOI:10.1007/s10668-020-01075-4 |
| Species | Barred plumage | Yellow feet or legs | Yellow eye or ring | Hawk-like | Polymorphic |
| Cuculus saturatus | 1 | 1 | 1 | 1 | 1 |
| Cuculus optatus | 1 | 1 | 1 | 1 | 1 |
| Cuculus canorus | 1 | 1 | 1 | 1 | 1 |
| Cuculus rochii | 1 | 1 | 1 | 1 | 0 |
| Cuculus gularis | 1 | 1 | 1 | 1 | 0 |
| Cuculus micropterus | 1 | 1 | 1 | 1 | 0 |
| Cuculus crassirostris | 1 | 1 | 1 | 1 | 0 |
| Cuculus poliocephalus | 1 | 1 | 1 | 1 | 1 |
| Cuculus solitarius | 1 | 1 | 1 | 1 | 0 |
| Cuculus clamosus | 1 | 0 | 0 | 0 | 0 |
| Hierococcyx nisicolor | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx fugax | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx pectoralis | 0 | 1 | 1 | 0 | 0 |
| Hierococcyx hyperythrus | 0 | 1 | 1 | 0 | 0 |
| Hierococcyx sparverioides | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx varius | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx bocki | 1 | 1 | 1 | 1 | 0 |
| Hierococcyx vagans | 1 | 1 | 1 | 1 | 0 |
| Surniculus lugubris | 0 | 0 | 0 | 0 | 0 |
| Surniculus musschenbroeki | 0 | 0 | 0 | 0 | 0 |
| Surniculus velutinus | 0 | 0 | 0 | 0 | 0 |
| Surniculus dicruroides | 0 | 0 | 0 | 0 | 0 |
| Cercococcyx olivinus | 1 | 1 | 0 | 1 | 0 |
| Cercococcyx montanus | 1 | 1 | 1 | 1 | 0 |
| Cercococcyx mechowii | 1 | 1 | 1 | 1 | 0 |
| Cacomantis passerinus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis variolosus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis merulinus | 1 | 1 | 1 | 1 | 1 |
| Cacomantis sonneratii | 1 | 0 | 1 | 1 | 0 |
| Cacomantis castaneiventris | 0 | 1 | 1 | 0 | 0 |
| Cacomantis flabelliformis | 0 | 1 | 1 | 0 | 1 |
| Cacomantis pallidus | 0 | 0 | 1 | 0 | 0 |
| Cacomantis leucolophus | 0 | 0 | 0 | 0 | 0 |
| Chrysococcyx minutillus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx meyeri | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx lucidus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx ruficollis | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx osculans | 0 | 0 | 0 | 0 | 0 |
| Chrysococcyx basalis | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx megarhynchus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx cupreus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx flavigularis | 1 | 1 | 1 | 1 | 0 |
| Chrysococcyx klaas | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx caprius | 1 | 0 | 1 | 1 | 1 |
| Chrysococcyx maculatus | 1 | 0 | 0 | 0 | 0 |
| Chrysococcyx xanthorhynchus | 1 | 0 | 0 | 0 | 0 |
| Urodynamis taitensis | 0 | 0 | 0 | 0 | 0 |
| Scythrops novaehollandiae | 1 | 0 | 0 | 0 | 0 |
| Eudynamys scolopacea | 1 | 0 | 0 | 0 | 1 |
| Microdynamis parva | 0 | 0 | 0 | 0 | 0 |
| Pachycoccyx audeberti | 0 | 0 | 0 | 0 | 0 |
| Coccyzus merlini | 0 | 0 | 0 | 0 | 0 |
| Coccyzus longirostris | 0 | 0 | 0 | 0 | 0 |
| Coccyzus vieilloti | 0 | 0 | 0 | 0 | 0 |
| Coccyzus vetula | 0 | 0 | 0 | 0 | 0 |
| Coccyzus pluvialis | 0 | 0 | 0 | 0 | 0 |
| Coccyzus rufigularis | 0 | 0 | 0 | 0 | 0 |
| Coccyzus lansbergi | 0 | 0 | 1 | 0 | 0 |
| Coccyzus erythropthalmus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus americanus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus euleri | 0 | 0 | 0 | 0 | 0 |
| Coccyzus minor | 0 | 0 | 1 | 0 | 1 |
| Coccyzus ferrugineus | 0 | 0 | 1 | 0 | 0 |
| Coccyzus melacoryphus | 0 | 0 | 1 | 0 | 0 |
| Piaya cayana | 0 | 0 | 0 | 0 | 0 |
| Piaya melanogaster | 0 | 0 | 0 | 0 | 0 |
| Coccycua pumila | 0 | 0 | 0 | 0 | 0 |
| Coccycua cinerea | 0 | 0 | 0 | 0 | 0 |
| Coccycua minuta | 0 | 0 | 0 | 0 | 0 |
| Clamator jacobinus | 0 | 0 | 0 | 0 | 1 |
| Clamator levaillantii | 0 | 0 | 0 | 0 | 1 |
| Clamator glandarius | 0 | 0 | 0 | 0 | 0 |
| Clamator coromandus | 0 | 0 | 0 | 0 | 0 |
| Dasylophus superciliosus | 0 | 1 | 1 | 0 | 0 |
| Dasylophus cumingi | 0 | 0 | 0 | 0 | 0 |
| Rhamphococcyx calyorhynchus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus diardi | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus tristis | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus viridirostris | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus pyrrhocephalus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus sumatranus | 0 | 0 | 0 | 0 | 0 |
| Phaenicophaeus curvirostris | 0 | 1 | 1 | 0 | 0 |
| Zanclostomus javanicus | 0 | 0 | 0 | 0 | 0 |
| Taccocua leschenaultii | 0 | 0 | 0 | 0 | 0 |
| Ceuthmochares aereus | 0 | 0 | 1 | 0 | 0 |
| Ceuthmochares australis | 0 | 0 | 1 | 0 | 0 |
| Rhinortha chlorophaea | 0 | 0 | 0 | 0 | 0 |
| Centropus phasianinus | 0 | 0 | 0 | 0 | 0 |
| Centropus bernsteini | 0 | 0 | 0 | 0 | 0 |
| Centropus violaceus | 0 | 0 | 1 | 0 | 0 |
| Centropus bengalensis | 0 | 0 | 0 | 0 | 0 |
| Centropus viridis | 0 | 0 | 0 | 0 | 1 |
| Centropus grillii | 0 | 0 | 0 | 0 | 0 |
| Centropus goliath | 0 | 0 | 0 | 0 | 0 |
| Centropus toulou | 0 | 0 | 0 | 0 | 0 |
| Centropus sinensis | 0 | 0 | 0 | 0 | 0 |
| Centropus andamanensis | 0 | 0 | 0 | 0 | 0 |
| Centropus nigrorufus | 0 | 0 | 0 | 0 | 0 |
| Centropus cupreicaudus | 0 | 0 | 0 | 0 | 0 |
| Centropus superciliosus | 0 | 0 | 0 | 0 | 0 |
| Centropus monachus | 0 | 0 | 0 | 0 | 0 |
| Centropus senegalensis | 0 | 0 | 0 | 0 | 1 |
| Centropus leucogaster | 0 | 0 | 0 | 0 | 0 |
| Centropus anselli | 0 | 0 | 0 | 0 | 0 |
| Centropus c celebensis | 0 | 0 | 0 | 0 | 0 |
| Centropus steerii | 0 | 0 | 0 | 0 | 0 |
| Centropus rectunguis | 0 | 0 | 0 | 0 | 0 |
| Centropus melanops | 0 | 0 | 0 | 0 | 0 |
| Centropus chlororhynchos | 0 | 0 | 0 | 0 | 0 |
| Centropus unirufus | 0 | 0 | 1 | 0 | 0 |
| Centropus menbeki | 0 | 0 | 0 | 0 | 0 |
| Centropus chalybeus | 0 | 0 | 1 | 0 | 0 |
| Centropus ateralbus | 0 | 0 | 0 | 0 | 1 |
| Centropus milo | 0 | 0 | 0 | 0 | 0 |
| Coua serriana | 0 | 0 | 0 | 0 | 0 |
| Coua delalandei | 0 | 0 | 1 | 0 | 0 |
| Coua gigas | 0 | 0 | 0 | 0 | 0 |
| Coua coquereli | 0 | 0 | 0 | 0 | 0 |
| Coua cursor | 0 | 0 | 0 | 0 | 0 |
| Coua reynaudii | 0 | 0 | 0 | 0 | 0 |
| Coua ruficeps | 0 | 0 | 0 | 0 | 0 |
| Coua caerulea | 0 | 0 | 0 | 0 | 0 |
| Coua verreauxi | 0 | 0 | 0 | 0 | 0 |
| Coua cristata | 0 | 0 | 0 | 0 | 0 |
| Carpococcyx renauldi | 0 | 0 | 0 | 0 | 0 |
| Carpococcyx radiatus | 1 | 0 | 0 | 0 | 0 |
| Carpococcyx viridis | 1 | 0 | 0 | 0 | 0 |
| Neomorphus geoffroyi | 0 | 0 | 0 | 0 | 0 |
| Neomorphus radiolosus | 1 | 0 | 0 | 0 | 0 |
| Neomorphus rufipennis | 0 | 0 | 0 | 0 | 0 |
| Neomorphus pucheranii | 0 | 0 | 0 | 0 | 0 |
| Geococcyx californianus | 0 | 0 | 0 | 0 | 0 |
| Geococcyx velox | 0 | 0 | 0 | 0 | 0 |
| Morococcyx ertyhropygus | 0 | 0 | 0 | 0 | 0 |
| Dromococcyx phasianellus | 0 | 0 | 0 | 0 | 0 |
| Dromococcyx pavoninus | 0 | 0 | 1 | 0 | 0 |
| Tapera naevia | 0 | 0 | 1 | 0 | 0 |
| Crotophaga ani | 0 | 0 | 1 | 0 | 0 |
| Crotophaga sulcirostris | 0 | 0 | 0 | 0 | 0 |
| Crotophaga major | 0 | 0 | 0 | 0 | 0 |