| Citation: | Anders P. MØllER, Juan J. SOLER. 2012: A coevolutionary framework based on temporal and spatial ecology of host-parasite interactions: A missing link in studies of brood parasitism. Avian Research, 3(4): 259-273. DOI: 10.5122/cbirds.2012.0033 |
A central tenet of coevolutionary theory, including theory of the coevolutionary relationship between brood parasites and their hosts, is that temporal and spatial patterns may reveal important information about ecological and evolutionary dynamics. For instance, level of genetic structure of populations provides important information about the role of genetics and gene flow in determining local patterns of selection on hosts due to parasitism (i.e., egg rejection) and on parasites due to selection by hosts (i.e., egg mimicry). Furthermore, abiotic (i.e., climatic conditions) and biotic (phenotypic characteristics of animals) factors that also vary spatially may directly or indirectly affect populations of hosts and brood parasites and, therefore, their interaction. By reviewing the literature, we found considerable evidence for an effect of the spatially and temporally structured abiotic environment on the phenotype of both parasite and host eggs and the degree of mimicry. Moreover, we found examples suggesting that specific life history characteristics of hosts that vary geographically and/or temporally may affect the probability of initial colonization of a new host species and the direction and the speed of coevolution. We provide an exhaustive review of studies investigating temporal and spatial patterns of the interaction between brood parasites and their hosts. Such temporal and spatial trends in parasite and host traits are, together with genetic information on rejection and significant effects of gene flow, consistent with coevolutionary dynamics. However, gene flow and changes in the temporal and spatial patterns of abundance of both parasites and hosts may result in frequent cases of counter-intuitive relationships between the phenotype of the parasite and that of the host (i.e., poor or no mimicry), which may suggest limits to the degree of adaptation. We provide a list of scientific questions in need of further investigation, concluding that studies of brood parasites and their hosts may play a central role in testing the geographic theory of coevolution and several alternative hypotheses.
The central theme in ecology is to understand the distribution and the abundance of species, and simi-larly the main objective for studies of coevolution is to understand the distribution and the abundance of co-evolving species (Thompson, 1994, 2005). The so-called geographic mosaic theory of coevolution suggests that spatial and temporal variation in the intensity of the in-teraction between parasites and hosts arises from local coevolutionary dynamics and from gene flow among populations of parasites and hosts. Such interacting species vary in distribution and abundance over time, and this variance can be partitioned into effects of the abiotic environment and effects due to the biotic interaction between species, both at the local scale, but also elsewhere in the range of the interacting parties. While theoretical models have suggested that defense and offense will covary positively across gradients of productivity (Hochberg and van Baalen, 1998), the pre-dictions derived from the geographic mosaic theory of coevolution and alternative explanations are not easy to test with field data (Gomulkiewicz et al., 2000, 2007). The main reason is that information on spatial and temporal variation in the interaction is limited at best, with time series rarely lasting more than a decade. Such large-scale studies across temporal and spatial scales would allow a better understanding of patterns of co-evolutionary interactions in terms of adaptations of the parasite to its host and vice versa. Several model systems based on brood parasites and their hosts provide long-term data from multiple study sites, sometimes dating back in time for over two hundred years, and, therefore, they may provide unique empirical information for testing aspects of coevolutionary theory.
Brood parasites may not seem obvious candidate model systems suitable for studying parasite-host interactions. Brood parasites are large and hence relatively rare, not easy to catch and difficult to follow across generations. However, the mechanisms of parasitism and resistance in brood parasites and their hosts are well established, and both can be quantified relatively easily using standard experimental protocols that are much easier than quantifying parasitism by microorganisms or the immune responses of most host systems. Moreover, the specificity of host defenses and poor parasite counter-tactics allow researchers to readily identify focal adaptations and counter-adaptations that are caused by selection due to the two interacting parties. These effects cannot easily be confounded by selection due to other parasites sharing a given host, as is the case for most 'real' parasites (Rothstein, 1990). This provides enormous advantages derived from using the brood parasite-host model system when exploring general patterns of coevolutionary hypotheses dealing with spatial and temporal variation. In addition, studying multiple sites over time provides the added benefit that counterintuitive findings in a single year or a single site may be explained by the geographic dynamics of coevolution rather than constituting a specific case of apparent maladaptation (Thompson, 1994, 2005; Takasu, 2003).
Most of the almost 3000 papers dealing with parasitic cuckoos, cowbirds and other parasites listed on the Web of Science are studies based on research in a single location in a single year or a couple of years. Less than 20 studies have investigated the interactions between brood parasites and their hosts in multiple locations, and a similar number of studies have investigated the same interaction at different times. As an example, the study of the dynamics of coevolutionary interactions between the Magpie (Pica pica) and the Great Spotted Cuckoo (Clamator glandarius) was the first to investigate the effects of the dynamic interactions between hosts and parasites in a focal study area, but also due to gene flow among study sites (Soler et al., 2001b). This unique study has only been cited 26 times the last 11 years. When it comes to studies of interactions in dif-ferent sites and at different times, we are only aware of a single model system of the Magpie and the Great Spotted Cuckoo (Soler et al., 1999b) although long-term data in museum and private collections may eventually provide the basis for similar analyses. The small number of studies investigating the spatial ecology of host-parasite interactions related to brood parasites is surprising because brood parasites provide more information on the spatial ecology of coevolutionary interactions than most other interactions.
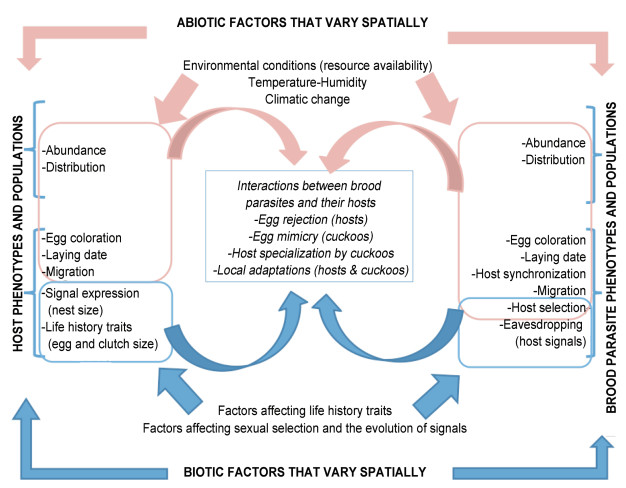
Here we briefly review the literature on the spatial ecology of brood parasite-host interactions by first investigating the effects of the abiotic environment, the effects of interactions with other phenotypic traits than those directly involved in coevolution, and finally investigating the spatial dynamics of interactions between brood parasites and their hosts. Figure 1 provides a conceptual framework for understanding these different aspects of coevolution. A full list of abiotic and biotic effects and their statistical magnitude is provided in Table 1. We finally provide a list of additional research questions in need of investigation. Differences in parasitism rate, anti-parasite defense of hosts or counter-defenses of parasites over time may be due to phenotypic plasticity, coevolution, gene flow among populations, or even a direct influence of a change in the environment. Below, we describe available information on the importance of such factors explaining host and parasite phenotypes and their geographical and temporal variation.
| Variable | Summary of effects | Comments | Test statistics | Reference |
| Abiotic environment | ||||
| Temporal pattern of mimicry in Reed Warbler | No significant effect due to rainfall | Avilés et al. (2006) | ||
| Temporal pattern of egg color in Reed Warbler | A significant effect of rainfall on one component of host egg color | F = 6.23, df = 1, 458, p = 0.012 | Avilés et al. (2007) | |
| Temporal pattern of egg color in Reed Warbler | A significant effect of rainfall on one component of Cuckoo egg color | F = 5.21, df = 1, 457, p = 0.02 | Avilés et al. (2007) | |
| Temporal pattern of mimicry in Reed Warbler and Cuckoo | A significant effect of rainfall on one component of egg color matching | F = 4.81, df = 1, 459, p = 0.03 | Avilés et al. (2007) | |
| Temporal pattern of mimicry in Reed Warbler | A significant effect of rainfall on spottiness of egg color | F = 4.87, df = 1, 458, p = 0.03 | Avilés et al. (2007) | |
| Temporal change in host use is linked to migratory phenology of Cuckoos and hosts | A significant advancement in arrival date is linked to a climate warming | χ2 = 9.27, df = 1, p = 0.0023 | Møller et al. (2011c) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in host egg phenotype were related to differences in spring climatic conditions | Temperature: Mantel r = -0.64, p = 0.01; Rainfall: Mantel r = -0.87, p = 0.001 | Avilés et al. (2012) | |
| Biotic environment | ||||
| Nest size evolution due to sexual selection and brood parasitism | Large nests signal phenotypic quality in magpies, and Great Spotted Cuckoos prefer hosts with large nests. However, in parasitized populations nest size has become small to prevent detection by cuckoos | Using parasitized nests: F = 24.87, df = 1, 308, p < 0.0001; Excluding parasitized nests: F = 19.67, df = 1, 268, p < 0.0001 | Soler et al. (1999a) | |
| Life history of host and brood parasitism | Increase in clutch size and decrease in egg volume in the presence of parasitism | F = 10.39, df = 1, 280, p = 0.0014; F = 10.53, df = 1, 262, p = 0.0013 | Soler et al. (2001a) | |
| Life history of host and brood parasitism | Clutch size was positively related to rejection rate, while egg size was negatively related to rejection rate | Mantel r = 0.33, p = 0.014; Mantel r = -0.38, p = 0.048 | Soler et al. (2001a) | |
| Coevolutionary interactions | ||||
| (a) Temporal patterns | ||||
| Temporal pattern of parasitism | Decrease in parasitism rate | χ2 = 47.1, df = 4, p < 0.001 | Brooke et al. (1998) | |
| Temporal pattern of rejection | Decrease in rejection rate | χ2 = 47.1, df = 4, p < 0.001 | Brooke et al. (1998) | |
| Temporal pattern of rejection | Increase in rejection from 13.8% to 89.3% in 16 years | χ2 = 49.15, df = 1, p < 0.0001 | Robert and Sorci (1999) | |
| Temporal pattern of parasitism rate | Temporal increase in parasitism rate of Azure-winged Magpie | No test statistics reported | Nakamura (1990) | |
| Temporal pattern in mimicry | Improved match with host eggs over time | Match due to host choice | F = 6.73, df = 1, 747, p = 0.018 | Avilés et al. (2006) |
| Temporal trend in parasitism and rejection rate | Temporal increase in rejection rate over time | F = 6.07, df = 1, 31, p = 0.02 | Soler et al. (1994) | |
| Temporal trend in parasitism rate | Temporal increase in parasitized nests with cuckoo eggs laid by more than one female | F = 8.15, df = 2, 30, p = 0.017 | Soler et al. (1994) | |
| Temporal trend in parasitism rate | Temporal increase in parasitized nests with more than one cuckoo egg | F = 4.22, df = 2, 123, p = 0.017 | Soler et al. (1994) | |
| Rate of parasitism and density of cuckoos | Temporal increase in parasitized nests with increasing abundance of cuckoos | F = 18.16, df = 1, 31, p = 0.0002 | Soler et al. (1994) | |
| Ejection rate and abundance of cuckoos | No significant increase in ejectio rate with abundance of cuckoos | F = 3.66, df = 1, 28, p = 0.07 | Soler et al. (1994) | |
| Ejection rate decreases in the absence of cuckoo parasitism | Village Weaverbirds (Ploceus cucullatus) show increasing variance in introduced populations | Significant differences in between individual variation in Hispaniola (F = 3.83, df = 111, 143, p < 0.001, F = 5.81, df = 105, 153, p < 0.00001, F = 18.5, df = 3, 433, p < 0.001, F = 11.8, df = 111, 143, p < 0.001, F = 35.0, df = 3, 499, p < 0.001, F = 4.51, df = 105, 153, p < 0.00001, F = 7.5, df = 3, 431, p < 0.001, U = 8, 575, p < 0.001) and Mauritius (F = 3.26, df =106, 51, p = 0.00003, F = 18.5, df = 3, 433, p < 0.10, F = 3.26, df = 106, 51, p < 0.10, U = 3, 724, p < 0.10). More variable clutches in introduced populations in Hispaniola (F = 16.8, df = 3, 431, p < 0.0001, F = 19.9, df =143, 111, p < 0.001, F = 15.8, df = 3, 431, p < 0.001, F = 11.8, df = 143, 111, p < 0.001, U = 9.514, p < 0.001, U = 9, 665, p < 0.001, U = 8, 681, p < 0.001, U = 9, 657, p < 0.001) and Mauritius (F = 1.93, df = 51, 121, p < 0.05, F = 4.59, df = 51, 121, p < 0.05, U = 3, 624, p < 0.05, U = 3, 499, p < 0.10, U = 3, 532, p < 0.10) | Lahti (2005) | |
| Temporal pattern of parasitism rate by Brownheaded Cowbird | Acadian Flycatcher (Empidonax virescens), Indigo Bunting (Passerina cyanea), Northern Cardinal (Cardinalis cardinalis) | Significant decline in parasitism rate and parasite intensity, although no test statistics reported | Cox et al. (2012) | |
| Parasitism rate | Decrease in parasitism rate by Rufous-bush Robin | Kendall tau = −1.00, p = 0.008 | Soler et al. (2012) | |
| Ejection rate | Decrease in rejection rate by Rufous-bush Robin | Kendall tau = −0.98, p = 0.001 | Soler et al. (2012) | |
| (b) Spatial patterns | ||||
| Spatial pattern of rejection | Higher level of rejection by village weavers in African range than in introduced range | No test statistic reported | Cruz and Wiley (1989) | |
| Spatial differences in parasitism and anti-parasite behavior | Differences in egg rejection of cuckoo eggs among sites differ in relation to duration of sympatry | G = 31.47, df = 2, p < 0.001 | Soler and Møller (1990), Soler (1990) | |
| Spatial differences in parasitism and anti-parasite behavior | Differences in egg rejection of Cowbird eggs among sites differ in relation to duration of sympatry | Difference in rejection between two sites | No test statistics reported | Briskie et al. (1992) |
| Spatial differences in parasitism and anti-parasite behavior | Differences in aggressive behavior towards Cowbird model in relation to duration of sympatry | Difference in aggressive behavior by host between two sites | No test statistics reported | Briskie et al.(1992) |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | Differences in rejection rates among three populations | Azumino vs Nagano χ2 = 1.38, df = 1, p = 0.17); Azumino vs Noveyama (χ2 = 12.0, df = 1, p < 0.001); Nagano vs Noveyama (χ2 = 18.1, df = 1, p < 0.001) No test statistics reported for differences in parasitism rates | Nakamura et al. (1998) |
| Spatial differences in parasitism | Lower parasitism rates in small marginal populations of Reed Warblers | t = −3.46, df = 18, p < 0.005 | Lindholm(1999) | |
| Spatial differences in antiparasite behavior | Lower rejection rates in small marginal populations of Reed Warblers | G = 18.9, df = 1, p < 0.0001, G = 2.5 df = 1, p = 0.11, G = 38.3, df = 1, p < 0.0001, G = 12.2, df = 1, p < 0.001, G = 0.2, df = 1, p = 0.6, G = 3.6, df = 1, p = 0.06, G = 0.2, df = 1, p = 0.6, G = 0.6, df = 1, p = 0.50 | Lindholm(1999) | |
| Spatial differences in antiparasite behavior | Lower responses in populations with less parasitism | H = 25, 6, p < 0.0001, H = 15.3, p = 0.0008, G = 2.2, p = 0.33, G = 10.0, p = 0.0067, G = 0.4, p = 0.81, G = 0.9, p = 0.64 | Lindholm(2000) | |
| Spatial differences in parasitism and anti-parasite behavior | Comparison of upland and marsh habitats | No test statistics reported | Strausberger(2001) | |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | Difference in rejection between two populations | Fisher's exact test, two tailed, p = 0.023; No test statistics reported for differences in parasitism rates | Moskát et al.(2002) |
| Spatial differences in parasitism rate | Differences in parasitism rate of Contopus flycatchers | No test statistics reported | Underwood et al. (2004) | |
| Frequency of parasitism | Local spatial variation in parasitism rate related to density of Cowbirds | r = 0.79, p = 0.02 | Jensen and Cully (2005) | |
| Frequency of parasitism | Cowbird parasitism increased with farm and house density | No test statistics reported | Tewksbury et al. (2006) | |
| Host density and cuckoo parasitism | Parasitism only occurs in high density populations of hosts above a certain threshold | χ2 = 4.37, df = 1, p = 0.037 | Stokke et al.(2007) | |
| Spatial variation in cowbird parasitism | Increase in rate of parasitism with abundance of Cowbirds | F = 97.29, df = 1, 14, p < 0.001 | Jewell and Arcese (2008) | |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | No difference in rejection rates of three populations not associated with parasitism rates | Fisher's exact test, two tailed, p > 0.53; No test statistics reported for differences in parasitism rates | Moskát et al.(2008) |
| Spatial heterogeneity in distribution and host races | Host races mainly occur in hosts with a homogeneous spatial distribution | t = 2.49, df = 63, p = 0.016 | Soler et al.(2009) | |
| Spatial patterns of population density and host races | Host races mainly occur in hosts with a high density | t = 2.35, df = 63, p = 0.22 | Soler et al.(2009) | |
| Rejection rate and parasitism rate in Reed Warbler populations | Increased rejection rate with increasing parasitism rate | Mantel r = 0.281, p = 0.007 | Stokke et al.(2008) | |
| Local adaptation of phenotype of cuckoo eggs to Reed Warbler host | Cuckoo eggs did not match eggs of local hosts, and matching was lower in populations with more different host species | Local adaptation: F = 1.24, df = 1, 3260, p = 0.26 No. host species: Mantel r = 0.55, p = 0.013 |
Avilés et al.(2011) | |
| Local adaptation of eggs of three hosts races of Cuckoos to three sympatric species of hosts | Eggs of host races of Cuckoo matched those of the host in terms of size, spotting pattern and egg color | size: χ2 = 520.8, df = 2, p < 0.0001 spotting: F = 4.46, df = 2, 303, p = 0.012 chroma: F = 3.27, df = 2, 94, p = 0.04 | Antonov et al.(2010) | |
| Tolerance of Cuckoo parasitism and rates of parasitism among sites | Tolerance increased with rate of parasitism | t = 4.65, df = 7, p = 0.0023 | Soler et al.(2011) | |
| Distribution of color pattern in the chaffinch becomes increasingly dissimilar to the color distribution of the Brambling towards the core area of breeding of the Cuckoo host race | Spatial variation in distribution of egg color | Chaffinch: UK and Fennoscandia: latitude: F = 43.1, df = 1, p = 0.0001, longitude: F = 32.6, df =1, p = 0.0001, Fennoscandia: latitude: F = 16.5, df = 1, p = 0.0001, longitude: F = 8.31, df = 1, p = 0.0022, Sweden and Denmark: latitude: F = 17.1, df = 1, p = 0.0001, longitude: F = 10.8, df = 1, p = 0.0004. Brambling: latitude: F = 1.70, df = 1, p = 0.18, longitude: F = 0.42, df = 1, p = 0.59. Cuckoo: latitude: F = 1.03, df = 1, p = 0.33, longitude: F = 0.57, df = 1, p = 0.54) | Vikan et al.(2011) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in egg rejection were related to differences in egg matching | Mantel r = −0.73, p = 0.003, Mantel r = −0.62, p = 0.016, Mantel r = 0.80, p = 0.007 | Avilés et al.(2012) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in host egg phenotype were related to differences in parasitism rate | Mantel r = 0.56, p = 0.01 | Avilés et al.(2012) | |
| Differences in aggressive behavior among sites | Aggressive behavior by hosts is more intense at sites with more Cuckoos | F = 10.5, df = 1, 30, p = 0.003, F = 9.2, df = 1, 30, p = 0.005, z = 2.35, p = 0.018, F = 10.2, df = 1, 29, p = 0.003 | Langmore et al. (2012) | |
| Differences in rejection rate and micro-satellite marker | Differences in rejection rate of Cuckoo eggs by magpies depend on allele size in a micro-satellite marker | Mantel r = 0.88, p < 0.001 | MartínGálvez et al.(2006) | |
| Differences in rejection rate and parasitism rate | Higher rejection rate in populations with higher parasitism rate | Mantel r = 0.62, p = 0.006 | MartínGálvez et al.(2007) | |
| Differences in allele size and parasitism rate | Higher frequency of allele in populations with higher parasitism rate | Mantel r = 0.68, p = 0.002 | MartínGálvez et al.(2007) | |
| Rejection behavior and gene flow | Rejection rate depends on gene flow from other populations of hosts | Mantel r = 0.64, p = 0.016 | Soler et al.(1999b) | |
| Parasitism and rejection of cuckoo eggs in relation to coevolutionary interactions within the focal study area and in neighboring study areas | Positive relationship between rejection rate and parasitism among populations, even after controlling for gene flow among populations | Mantel r = 0.37, p = 0.011 | Soler et al.(2001b) | |
Parasite eggs often match the phenotype of host eggs, and the basis for such matching is the pigments deposited in the eggshell and the structural color derived from its microstructure. Because individuals differ in egg color, and because eggs have more elaborate color than is required for camouflage, we can assume that females differ in their ability to produce brightly colored eggs, although the level of repeatability in eggshell coloration within clutches is moderate and within- and between-season repeatability appears to be low in at least one Common Cuckoo host (Honza et al., 2012). Some of this variation is environmental and genetic as in chickens. The environmental component of variability in egg coloration is likely mediated by variation in availability of food that may be closely related with weather conditions. Thus, weather may affect the phenotype of both parasite and host eggs and, in the case of both species exploiting similar resources (i.e. they are affected by similar climatic conditions), variation in environmental conditions may predict temporal and spatial covariation between hosts and parasites with respect to the appearance of their eggs.
Avilés et al. (2006) explored this possibility, but found no temporal change in mimicry between the Common Cuckoo (Cuculus canorus) and the Reed Warbler (Acrocephalus scirpaceus) due to rainfall during 24 years. In contrast, when exploring the effect of weather on egg color pattern of reed warblers, Avilés et al. (2007) found significant effects of rainfall on one of three components of egg color, while a marginal effect of temperature was detected for another egg color component. The effect of rainfall, but not that of temperature was also detected in the Cuckoo (Avilés et al., 2007). Finally, matching between cuckoo and host eggs in blue-greenness and spottiness was better in springs with a higher rainfall, suggesting that environmental factors may impact on coevolutionary interactions. Furthermore, when exploring geographic variation, Avilés et al. (2012) showed that between-population differences in phenotype of Reed Warbler eggs were re-lated to differences in spring climatic conditions (temperature and rainfall). These results highlight that for a proper understanding of coevolutionary systems it is important to consider temporal and geographical variation in environmental factors that may affect variation in defensive traits (in this case mimicry of cuckoo eggs), but that are not the consequence of antagonistic coevo-lutionary processes.
A different component of the abiotic environment is the current trend in temperature and to a certain extent also trends in precipitation and wind. These changes may have profound effects on brood parasites and their hosts. Climate change would equally affect hosts and parasites if they shared the same environment and the same resources. However, that is unlikely to be the case since for instance most cuckoo species winter in different areas than their hosts, and they have different requirements for migration and reproduction. These asymmetric effects of climatic change on brood parasites and their hosts may have profound consequences for coevolutionary systems that would only be correctly interpreted when considering geographical and/or temporal variation in host and brood parasite traits. In accordance with the importance of such asymmetric effects of climate change on hosts and brood parasites and, therefore, on the coevolutionary relationship, Møller et al. (2011c) showed that resident and short distance migratory host species of the cuckoo responded strongly to climate change by advancing their spring arrival date, while the cuckoo did not, causing a temporal mismatch in arrival time, while that was not the case for long distance migratory hosts. This resulted in a rapid decline in cuckoo host races exploiting resident and short distance migratory hosts that avoided parasitism by breeding ever earlier than the cuckoo. However, the decline in use of resident and short distance migratory host races has only occurred in countries with an increase in spring temperature, but not in countries without climatic warming. Thus, because the effects of climate warming are spatially heterogeneous with some European countries experiencing next to no change in temperature during the last decades, while others have shown dramatic increases during spring and summer, the detected effects on populations experiencing a warming climate may also affect the entire metapopulation of European cuckoos.
Host phenotype may evolve in response to natural and sexual selection outside the direct context of host-parasite interactions, and coevolution may only secondarily impact on evolution of the host. Accumulating evidence suggests that size or other characteristics of birds' nests reflects phenotypic quality of builders, including traits that may affect reproductive success of brood parasites (i.e., parental abilities). Consequently, parasites may eavesdrop on host traits that reveal phenotypic quality relevant for raising parasite offspring (Parejo and Avilés, 2007). Furthermore, we know that nest site selection is related to resource availability, which is not randomly distributed, and that only individuals able to defend high quality territories will eventually breed in such places. Thus, brood parasites that select high quality host individuals for parasitism should enjoy superior reproductive success, implying that parasitism is not randomly distributed within host populations (Grim, 2002), again highlighting the importance of considering the geographic distribution of hosts (and of parasites) for understanding the coevolutionary processes. For instance, large nests in Magpies reflect phenotypic quality because nest size integrates information on working ability, construction prowess and perhaps even cognitive ability of the nest builder. Great Spotted Cuckoos prefer hosts with large nests (Soler et al., 1995), because magpie hosts with large nests are better at raising cuckoo offspring, imposing selection for smaller nest size in sympatric populations of Magpies and cuckoos. Interestingly, when exploring variation in nest size and parasitism across Magpie populations, Soler et al. (1999a) detected that Magpie nests were smaller in the most intensely parasitized host populations, apparently due to a change in the balance between natural and sexual selection.
Brood parasitism may also affect the evolution of host life history traits that are adjusted to resource availability. For instance, it is known that birds adjust clutch and egg size to resource availability (e.g., De Neve et al., 2004), and that brood parasites modify effective clutch size either because of removal or breakage of host eggs during the parasitism act. In the case of hosts parasitized by evictor brood parasites (i.e., species with parasitic nestlings that remove all host offspring from nests), those that were able to detect and eject parasitic eggs from their nests should have smaller clutch size than the optimal according to estimated resource availability. Thus, increasing clutch size in relation to probability of brood parasitism would be advantageous for these species of hosts. Furthermore, for host species parasitized by non-evictor cuckoos an increase in clutch size may be advantageous because it would counteract egg breakage by cuckoos and increase the probability that some host nestlings survive together with the parasitic nestlings. Again, resource availability, but also selection due to parasitism are not randomly distributed within a host (meta)-population (Hauber et al., 2001; Hoover et al., 2006). Thus taking geographic variation in such characteristics into account is essential for understanding the hypothetical effects of brood parasitism on the evolution of life history trait of their hosts. Soler et al. (2001a) argued that since Magpie hosts suffer less from brood parasitism when producing a large clutch, and more eggs can be laid when eggs are smaller, Magpie populations that are exposed to cuckoo parasitism should have evolved larger clutches and smaller eggs than allopatric magpie populations. That was indeed what was observed in a study of 15 European host populations for which information on both geographic and genetic distance was available. These patterns were significantly related to rejection rate of model eggs, further suggesting a direct link between parasitism and host life history. Larger clutch size may in fact be a trait that allows the evolution of magpie tolerance to brood parasitism. A recent study detected geographic variation in tolerance covarying with parasitism rate and hence intensity to selection due to parasitism (Soler et al., 2011).
Rates of brood parasitism and anti-parasite behavior by avian hosts are repeatable across spatial scales. Indeed, Møller et al. (2011c) showed for the Common Cuckoo that the frequency of parasitism of different host species was highly consistent for paired samples from 1800 –1940 and 1941–2011. Likewise, Davies and Brooke (1989) and Moksnes et al. (1991) showed that rejection rates for the same species tested in the U.K. and Norway were similar. These findings may suggest that we should expect similarity in coevolutionary interactions across spatial scales, although the fact that these correlations only explained a moderate amount of variance may suggest that there is scope for other factors playing an additional role such as differences in the composition of the host race community in the two countries and the colder and more variable climate in Norway compared to the U.K.
We emphasize that it is important to use information from geographically and temporally separated populations (spatial ecology) to reach well supported conclusions about coevolutionary interactions. Exploring temporal variation allows detection of the evolution of mimicry through host selection, while exploration of geographic variation allows detection of a change in mimicry in relation to selection due to hosts. One of the first studies investigating such spatial relationships between brood parasites and the behavior of their hosts was by Soler and Møller (1990), who showed that ejection rate of model cuckoo eggs increased from a population in allopatry over a population of presumed recent sympatry to a population of ancient sympatry between the Magpie and the Great Spotted Cuckoo. Although Zuñiga and Redondo (1992) questioned these findings, subsequent studies have largely confirmed the spatial and temporal patterns of this interaction between Great Spotted Cuckoos and the Magpie host (Soler et al., 1994, 1999b; Martín-Gálvez et al., 2006, 2007), emphasizing a role of genetics through local adaptation and differential gene flow (Soler et al., 1999b). A number of subsequent studies have shown spatial variation in parasitism and egg ejection by hosts (e.g. Cruz and Wiley, 1989; Briskie et al., 1992; Lindholm, 1999, 2000). Avilés et al. (2006) found an increase in degree of mimicry between the Common Cuckoo and the Reed Warbler in a 24 years study. However, this increase in mimicry was not due to a change in egg phenotype of the host, but rather caused by an improvement in host choice. Avilés et al. (2012) showed for the Reed Warbler and the Common Cuckoo that between population differences in egg rejection were related to differences in egg matching between host and parasite and that between population differences in host egg phenotype were related to differences in parasitism rate.
The strength of coevolutionary relationships may depend on characteristics of host and parasite populations that just as population density vary geographically and may be heterogeneously distributed. Parasites may only be able to establish a viable local population if hosts are sufficiently abundant. Lindholm(1999, 2000) and Stokke et al. (2007) showed that parasitism rate was only possible in host populations of Reed Warblers that had a minimum threshold population density. In a companion study Stokke et al. (2008) showed that differences in rejection rate among Reed Warbler populations were mainly predicted by differences in parasitism rate among populations. Such effects of host density are equally important determinants of host-parasite interactions on evolutionary time scales. Host abundance and distribution may affect parasite specialization and speciation because parasite populations can only be maintained for sufficiently long time to allow evolution of specialized host races when parasites populations do not go extinct due to stochastic variation in population size of the host (see Norton and Carpenter, 1998). Based on this argument, Soler et al. (2009) hypothesized that cuckoos would be better able to specialize on specific hosts if hosts were abundant and evenly distributed in space. Accordingly, they found that specific host races of the cuckoo with eggs that matched those of the host have evolved in hosts with the highest density and the most homogeneous spatial distribution. This finding is paralleled by relationships between spatial heterogeneity in distribution and host-parasite interactions in particular (Møller et al., 2011b) and ecology in general (Møller et al., 2010).
A hallmark of coevolution is that patterns of parasitism and anti-parasite behavior covary resulting in local adaptation as reflected by intricate patterns of egg mimicry. Antonov et al. (2010) showed for three sympatric host races of the cuckoo that they were adapted locally to their own host species, with little evidence of migration between hosts or parasites laying eggs in the nests of different hosts. However, interactions between host races may arise because of competition for limiting host numbers. Møller et al. (2011a) showed evidence of such local spatial interactions between different host races of the Cuckoo because host races were temporally isolated through differences in breeding time, but also by differences in breeding habitat. Although these patterns held across different host races of the Cuckoo, there may be exceptions to this general pattern. Avilés et al. (2011) showed that local Cuckoos were no better at matching the eggs of their Reed Warbler hosts than any random population of Reed Warblers. However, the degree of mimicry was poor in Reed Warbler populations where more different host races of the Cuckoo coexisted, suggesting that Cuckoos may choose alternative hosts when easily accessible individuals at the right stage of egg laying among their primary host are unavailable. All these examples and results emphasize the variety of coevolutionary outcomes of the interaction between brood parasites and their hosts. These outcomes likely depend on geographic and temporal variation in environmental conditions (i.e., abiotic factors) affecting one or both parties of the interactions, but they may also depend on phenotypic traits of hosts and parasites that may be spatially structured and, therefore, differentially affected by environmental and temporal changes.
The spatial ecology of host-parasite interactions is partially determined by genes for defensive traits, but also by gene flow among populations of hosts. Therefore, it is important to take the meta-population struc ture of hosts and parasites into account when investigating coevolutionary dynamics because immigrants and emigrants may change status (i.e. from adaptive to non-adaptive) depending on their new population of reproduction. A resistant individual may loose its fitness advantage by migrating from a population of susceptible hosts to a population of resistant individuals. In contrast, such an individual will gain from entering a population of naïve hosts that are currently being heavily parasitized. Coevolution is based on the assumption that both parasitism and anti-parasite reactions of hosts have a genetic basis, and that the coevolved parasite and host traits show signs of such coevolution. As an example of such anti-parasite behavior Martín-Gálvez et al. (2006) showed that differences in rejection rate of Great Spotted Cuckoo eggs depended on allele size of a micro-satellite in the local population of Magpie hosts. In addition, egg rejection rate and frequency of a resistance allele covaried with probability of parasitism between study plots (Martín-Gálvez et al., 2007). This provides strong evidence of one side of the coevolutionary coin having a partially genetic basis.
However, coevolution is not only dependent on local interactions between parasites and hosts because both parasite and host traits may derive from populations other than the focal population due to differential gene flow among populations. Within this line of reasoning, Soler et al. (1999b) proposed that because the antiparasite behavior of Magpie hosts in a focal population may depend on selection due to local parasitism, but also due to immigration of hosts and parasites from elsewhere, studies of coevolution must take such gene flow into account. In addition, gene flow may differentially be skewed towards adaptive hosts (or parasites) because they have higher reproductive success in parasitized populations giving rise to what has been termed coevolutionary 'hot spots' (Thompson, 1994). Such hot spots contrast with 'cold spots' apparently without any current coevolutionary interaction. Attempting to determine whether coevolution is going on at the metapopulation level of Magpies and Great Spotted Cuckoos and whether specific populations constitute hot-spots of coevolution, Soler et al. (2001b) analyzed the relationship between parasitism and rejection of cuckoo eggs in 15 European populations while also considering gene flow from neighboring populations. In accordance with expectations for an ongoing coevolutionary process they found that rejection of model cuckoo eggs depended on the local rate of parasitism, although this rejection rate was modified by gene flow from other populations. However, the positive relationship between rejection rate and parasitism remained significantly positive even after controlling statistically for rejecter gene flow. Moskát et al. (2008) have likewise shown effects of gene flow on egg rejection rates in Great Reed Warblers. Thus, these results provide evidence of the existence of hot spots of coevolution from which individuals of defensive-adaptive phenotypes migrate to neighboring populations. This and other conclusions presented above were possible by considering the metapopulation structure of parasites and hosts when investigating their coevolutionary interactions.
We have provided an exhaustive review of the literature on brood parasitism in relation to abiotic and biotic environmental conditions and the temporal and spatial coevolutionary interactions between brood parasites and their hosts. We have reported 51 effects in Table 1 with these mainly being restricted to Common Cuckoo, Great Spotted Cuckoo and Brown-headed Cowbird (Molothrus ater). It is also noteworthy that most of these effects derived from the efforts of only three research groups. This emphasizes how much can be achieved with a concerted effort. We strongly believe that continued research along these lines, incorporating modern molecular and genomics tools will allow to pinpoint the underlying genetic mechanisms, as already shown by the study of a micro-satellite allele and rejection behavior in the Magpie (Martín-Gálvez et al., 2006, 2007).
An important take-home message from this review is that far from everything that can be observed in brood parasites and the responses of their hosts is due to coevolution. For example, Moksnes and Røskaft (1995) showed in a study of more than 12400 cuckoo eggs that 56.5% of these eggs were poor mimics of the eggs of the host. In a similar vein, Avilés et al. (2011) showed that Cuckoos were not locally adapted to the local phenotype of its Reed Warbler host, and that changes in phenotype may be due to change in environmental conditions rather than the cause of coevolution (Avilés et al., 2006, 2007, 2012). Thus, there are good reasons for contrasting predictions based on coevolutionary theory with predictions derived from alterantive hypotheses such as climate change, or even the hypothesis that there are limits to the extent of adaptation. Brood parasites may make errors because they cannot readily find the most suitable host due to gene flow, man-made changes in the extent and distribution of habitats, or changes in timing of reproduction of hosts due to effects of climate change (Møller et al., 2011c). Differentiating hypothetical effects of coevolution from alternative hypotheses explaining host and parasite phenotypic traits are possible by either adopting a metapopulation approach (i.e., spatial variation and gene flow) or by adopting studies of ecological genetics that extend for a few generations (i.e., temporal variation).
Spatially varying abiotic and biotic factors may affect characteristics of hosts and parasites that directly or indirectly would affect the interactions between brood parasites and their hosts and therefore coevolution (Fig. 1). Examples presented in this review suggest that not only environmental conditions, but also life-history and sexually selected traits of host and parasites may be of central importance when explaining coevolutionary patterns. Coloration of eggs is costly in terms of allocation of pigments with antioxidant properties to eggs (Moreno and Osorno, 2003; Hargitai et al., 2010; Soler et al., 2012). Thus, environmental conditions are likely to affect egg coloration in both hosts and parasites. Cuckoos and other brood parasites are likely to experience higher levels of pigment limitation than their hosts due to the large clutch sizes of parasites. This may affect the coevolutionary relationship through effects on the evolution of egg mimicry (Soler et al., 2012). Cuckoos may also take advantage of the information that egg pigmentation may reveal on host phenotypic quality by selecting hosts accordingly (Soler et al., 2012), which would also affect the interaction between hosts and brood parasites. Recent analyses of these possibilities in a comparative framework suggest that this may indeed be the case (Soler et al., 2012). The effects of biotic and abiotic factors on life history traits such as clutch size and egg size are likely to affect the coevolutionary process (Fig. 1), not only because it is directly related to reproductive investment by hosts and parasites, but also because this may be the actual traits that allow defensive tolerance of brood parasites by hosts as suggested for metapopulations of Spanish Magpies (Soler et al., 2011).
There are many aspects of coevolutionary interactions that can be analyzed using existing tools, resources and databases. We are convinced that long-term field studies may provide additional pieces to the coevolutionary puzzle. However, temporal trends in rates of parasitism and the distribution of different host races can readily be investigated using existing extensive collections of cuckoo eggs currently being recorded by B. G. Stokke and co-workers. This database will also allow determination of the geographic range of host races and changes in range size over time. Finally, this database will allow analyses of temporal and spatial patterns of mimicry for different host races of the cuckoo.
An understanding of the coevolutionary process requires exploration of the coevolutionary relationship between brood parasites and their hosts from a perspective of spatial (and temporal) ecology. Importantly, this approach will also allow investigation of generally important ecological and evolutionary questions about spatial and temporal variation in the strength of antagonistic interactions when diffuse coevolution is more likely. The temporal and spatial distribution of brood parasites and hosts and their characteristics can be explored by using museum collections, which is generally not possible for other coevolutionary interactions due to lack of significant collections. Moreover, sequencing of the entire genome of the Common Cuckoo is now finished, and annotation of the genome using information from the Chicken (Gallus gallus) and the Zebra Finch (Taeniopygia guttata) will provide an important genomics resource that may for instance allow identification of genes related to life history, and defensive traits (i.e., genetic markers of rejection of parasite eggs, Martín-Gálvez et al. (2006)). DNA samples from museum collections of hosts and parasites will open up a series of studies of genomics of hosts across large spatial and temporal scales.
W. Liang kindly invited us to contribute this review. We thank C. Moskát for helpful comments on this manu-script.
|
Avilés JM, Stokke BG, Moksnes A, Røskaft E, Møller AP. 2007. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuck-oo Cuculus canorus. Behav Ecol Sociobiol, 61:475–485.
|
|
Avilés JM, Vikan JR, Fossøy F, Antonov A, Moksnes A, Røskaft E, Shykoff JA, Møller AP, Stokke BG. 2012. Egg phenotype matching by cuckoos in relation to discrimination by hosts and climatic conditions. Proc R Soc Lond B, 279:1967–1976.
|
|
Moksnes A, Røskaft E, Braa AT. 1991. Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk, 108:348–354.
|
|
Møller AP, Saino N, Adamik P, Ambrosini R, Antonov A, Campobello D, Stokke BG, Fossøy F, Lehikoinen E, Martín-Vivaldi M, Moksnes A, Moskat C, Røskaft E, Rubolini D, Schulze-Hagen K, Soler M, Shykoff JA. 2011c. Rapid change in host use of the common cuckoo Cuculus canorus linked to climate change. Proc R Soc Lond B, 278:733–738.
|
|
Nakamura H, Kubota S, Suzuki R. 1998. Coevolution between the common cuckoo and its major hosts in Japan. In: Rothstein SI, Robinson SK (eds) Parasitic Birds and Their Hosts: Studies in Coevolution. Oxford University Press, New York, pp 94–112.
|
|
Soler JJ, Martín-Gálvez D, Martínez JG, Soler M, Canestrari D, Abad-Gomez JM, Møller AP. 2011. Evolution of tolerance by magpies to brood parasitism by great spotted cuckoos. Proc R Soc Lond B, 278:2047–2052.
|
|
Takasu F. 2003. Co-evolutionary dynamics of egg appearance in avian brood parasitism. Evol Ecol Res, 5:345–362.
|
|
Thompson JN. 1994. The Coevolutionary Process. University of Chicago Press, Chicago.
|
|
Thompson JN. 2005. The Geographic Mosaic of Coevolution, The University of Chicago Press, Chicago.
|
| Variable | Summary of effects | Comments | Test statistics | Reference |
| Abiotic environment | ||||
| Temporal pattern of mimicry in Reed Warbler | No significant effect due to rainfall | Avilés et al. (2006) | ||
| Temporal pattern of egg color in Reed Warbler | A significant effect of rainfall on one component of host egg color | F = 6.23, df = 1, 458, p = 0.012 | Avilés et al. (2007) | |
| Temporal pattern of egg color in Reed Warbler | A significant effect of rainfall on one component of Cuckoo egg color | F = 5.21, df = 1, 457, p = 0.02 | Avilés et al. (2007) | |
| Temporal pattern of mimicry in Reed Warbler and Cuckoo | A significant effect of rainfall on one component of egg color matching | F = 4.81, df = 1, 459, p = 0.03 | Avilés et al. (2007) | |
| Temporal pattern of mimicry in Reed Warbler | A significant effect of rainfall on spottiness of egg color | F = 4.87, df = 1, 458, p = 0.03 | Avilés et al. (2007) | |
| Temporal change in host use is linked to migratory phenology of Cuckoos and hosts | A significant advancement in arrival date is linked to a climate warming | χ2 = 9.27, df = 1, p = 0.0023 | Møller et al. (2011c) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in host egg phenotype were related to differences in spring climatic conditions | Temperature: Mantel r = -0.64, p = 0.01; Rainfall: Mantel r = -0.87, p = 0.001 | Avilés et al. (2012) | |
| Biotic environment | ||||
| Nest size evolution due to sexual selection and brood parasitism | Large nests signal phenotypic quality in magpies, and Great Spotted Cuckoos prefer hosts with large nests. However, in parasitized populations nest size has become small to prevent detection by cuckoos | Using parasitized nests: F = 24.87, df = 1, 308, p < 0.0001; Excluding parasitized nests: F = 19.67, df = 1, 268, p < 0.0001 | Soler et al. (1999a) | |
| Life history of host and brood parasitism | Increase in clutch size and decrease in egg volume in the presence of parasitism | F = 10.39, df = 1, 280, p = 0.0014; F = 10.53, df = 1, 262, p = 0.0013 | Soler et al. (2001a) | |
| Life history of host and brood parasitism | Clutch size was positively related to rejection rate, while egg size was negatively related to rejection rate | Mantel r = 0.33, p = 0.014; Mantel r = -0.38, p = 0.048 | Soler et al. (2001a) | |
| Coevolutionary interactions | ||||
| (a) Temporal patterns | ||||
| Temporal pattern of parasitism | Decrease in parasitism rate | χ2 = 47.1, df = 4, p < 0.001 | Brooke et al. (1998) | |
| Temporal pattern of rejection | Decrease in rejection rate | χ2 = 47.1, df = 4, p < 0.001 | Brooke et al. (1998) | |
| Temporal pattern of rejection | Increase in rejection from 13.8% to 89.3% in 16 years | χ2 = 49.15, df = 1, p < 0.0001 | Robert and Sorci (1999) | |
| Temporal pattern of parasitism rate | Temporal increase in parasitism rate of Azure-winged Magpie | No test statistics reported | Nakamura (1990) | |
| Temporal pattern in mimicry | Improved match with host eggs over time | Match due to host choice | F = 6.73, df = 1, 747, p = 0.018 | Avilés et al. (2006) |
| Temporal trend in parasitism and rejection rate | Temporal increase in rejection rate over time | F = 6.07, df = 1, 31, p = 0.02 | Soler et al. (1994) | |
| Temporal trend in parasitism rate | Temporal increase in parasitized nests with cuckoo eggs laid by more than one female | F = 8.15, df = 2, 30, p = 0.017 | Soler et al. (1994) | |
| Temporal trend in parasitism rate | Temporal increase in parasitized nests with more than one cuckoo egg | F = 4.22, df = 2, 123, p = 0.017 | Soler et al. (1994) | |
| Rate of parasitism and density of cuckoos | Temporal increase in parasitized nests with increasing abundance of cuckoos | F = 18.16, df = 1, 31, p = 0.0002 | Soler et al. (1994) | |
| Ejection rate and abundance of cuckoos | No significant increase in ejectio rate with abundance of cuckoos | F = 3.66, df = 1, 28, p = 0.07 | Soler et al. (1994) | |
| Ejection rate decreases in the absence of cuckoo parasitism | Village Weaverbirds (Ploceus cucullatus) show increasing variance in introduced populations | Significant differences in between individual variation in Hispaniola (F = 3.83, df = 111, 143, p < 0.001, F = 5.81, df = 105, 153, p < 0.00001, F = 18.5, df = 3, 433, p < 0.001, F = 11.8, df = 111, 143, p < 0.001, F = 35.0, df = 3, 499, p < 0.001, F = 4.51, df = 105, 153, p < 0.00001, F = 7.5, df = 3, 431, p < 0.001, U = 8, 575, p < 0.001) and Mauritius (F = 3.26, df =106, 51, p = 0.00003, F = 18.5, df = 3, 433, p < 0.10, F = 3.26, df = 106, 51, p < 0.10, U = 3, 724, p < 0.10). More variable clutches in introduced populations in Hispaniola (F = 16.8, df = 3, 431, p < 0.0001, F = 19.9, df =143, 111, p < 0.001, F = 15.8, df = 3, 431, p < 0.001, F = 11.8, df = 143, 111, p < 0.001, U = 9.514, p < 0.001, U = 9, 665, p < 0.001, U = 8, 681, p < 0.001, U = 9, 657, p < 0.001) and Mauritius (F = 1.93, df = 51, 121, p < 0.05, F = 4.59, df = 51, 121, p < 0.05, U = 3, 624, p < 0.05, U = 3, 499, p < 0.10, U = 3, 532, p < 0.10) | Lahti (2005) | |
| Temporal pattern of parasitism rate by Brownheaded Cowbird | Acadian Flycatcher (Empidonax virescens), Indigo Bunting (Passerina cyanea), Northern Cardinal (Cardinalis cardinalis) | Significant decline in parasitism rate and parasite intensity, although no test statistics reported | Cox et al. (2012) | |
| Parasitism rate | Decrease in parasitism rate by Rufous-bush Robin | Kendall tau = −1.00, p = 0.008 | Soler et al. (2012) | |
| Ejection rate | Decrease in rejection rate by Rufous-bush Robin | Kendall tau = −0.98, p = 0.001 | Soler et al. (2012) | |
| (b) Spatial patterns | ||||
| Spatial pattern of rejection | Higher level of rejection by village weavers in African range than in introduced range | No test statistic reported | Cruz and Wiley (1989) | |
| Spatial differences in parasitism and anti-parasite behavior | Differences in egg rejection of cuckoo eggs among sites differ in relation to duration of sympatry | G = 31.47, df = 2, p < 0.001 | Soler and Møller (1990), Soler (1990) | |
| Spatial differences in parasitism and anti-parasite behavior | Differences in egg rejection of Cowbird eggs among sites differ in relation to duration of sympatry | Difference in rejection between two sites | No test statistics reported | Briskie et al. (1992) |
| Spatial differences in parasitism and anti-parasite behavior | Differences in aggressive behavior towards Cowbird model in relation to duration of sympatry | Difference in aggressive behavior by host between two sites | No test statistics reported | Briskie et al.(1992) |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | Differences in rejection rates among three populations | Azumino vs Nagano χ2 = 1.38, df = 1, p = 0.17); Azumino vs Noveyama (χ2 = 12.0, df = 1, p < 0.001); Nagano vs Noveyama (χ2 = 18.1, df = 1, p < 0.001) No test statistics reported for differences in parasitism rates | Nakamura et al. (1998) |
| Spatial differences in parasitism | Lower parasitism rates in small marginal populations of Reed Warblers | t = −3.46, df = 18, p < 0.005 | Lindholm(1999) | |
| Spatial differences in antiparasite behavior | Lower rejection rates in small marginal populations of Reed Warblers | G = 18.9, df = 1, p < 0.0001, G = 2.5 df = 1, p = 0.11, G = 38.3, df = 1, p < 0.0001, G = 12.2, df = 1, p < 0.001, G = 0.2, df = 1, p = 0.6, G = 3.6, df = 1, p = 0.06, G = 0.2, df = 1, p = 0.6, G = 0.6, df = 1, p = 0.50 | Lindholm(1999) | |
| Spatial differences in antiparasite behavior | Lower responses in populations with less parasitism | H = 25, 6, p < 0.0001, H = 15.3, p = 0.0008, G = 2.2, p = 0.33, G = 10.0, p = 0.0067, G = 0.4, p = 0.81, G = 0.9, p = 0.64 | Lindholm(2000) | |
| Spatial differences in parasitism and anti-parasite behavior | Comparison of upland and marsh habitats | No test statistics reported | Strausberger(2001) | |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | Difference in rejection between two populations | Fisher's exact test, two tailed, p = 0.023; No test statistics reported for differences in parasitism rates | Moskát et al.(2002) |
| Spatial differences in parasitism rate | Differences in parasitism rate of Contopus flycatchers | No test statistics reported | Underwood et al. (2004) | |
| Frequency of parasitism | Local spatial variation in parasitism rate related to density of Cowbirds | r = 0.79, p = 0.02 | Jensen and Cully (2005) | |
| Frequency of parasitism | Cowbird parasitism increased with farm and house density | No test statistics reported | Tewksbury et al. (2006) | |
| Host density and cuckoo parasitism | Parasitism only occurs in high density populations of hosts above a certain threshold | χ2 = 4.37, df = 1, p = 0.037 | Stokke et al.(2007) | |
| Spatial variation in cowbird parasitism | Increase in rate of parasitism with abundance of Cowbirds | F = 97.29, df = 1, 14, p < 0.001 | Jewell and Arcese (2008) | |
| Spatial heterogeneity in parasitism rate | Differences in parasitism rate and rejection rate | No difference in rejection rates of three populations not associated with parasitism rates | Fisher's exact test, two tailed, p > 0.53; No test statistics reported for differences in parasitism rates | Moskát et al.(2008) |
| Spatial heterogeneity in distribution and host races | Host races mainly occur in hosts with a homogeneous spatial distribution | t = 2.49, df = 63, p = 0.016 | Soler et al.(2009) | |
| Spatial patterns of population density and host races | Host races mainly occur in hosts with a high density | t = 2.35, df = 63, p = 0.22 | Soler et al.(2009) | |
| Rejection rate and parasitism rate in Reed Warbler populations | Increased rejection rate with increasing parasitism rate | Mantel r = 0.281, p = 0.007 | Stokke et al.(2008) | |
| Local adaptation of phenotype of cuckoo eggs to Reed Warbler host | Cuckoo eggs did not match eggs of local hosts, and matching was lower in populations with more different host species | Local adaptation: F = 1.24, df = 1, 3260, p = 0.26 No. host species: Mantel r = 0.55, p = 0.013 |
Avilés et al.(2011) | |
| Local adaptation of eggs of three hosts races of Cuckoos to three sympatric species of hosts | Eggs of host races of Cuckoo matched those of the host in terms of size, spotting pattern and egg color | size: χ2 = 520.8, df = 2, p < 0.0001 spotting: F = 4.46, df = 2, 303, p = 0.012 chroma: F = 3.27, df = 2, 94, p = 0.04 | Antonov et al.(2010) | |
| Tolerance of Cuckoo parasitism and rates of parasitism among sites | Tolerance increased with rate of parasitism | t = 4.65, df = 7, p = 0.0023 | Soler et al.(2011) | |
| Distribution of color pattern in the chaffinch becomes increasingly dissimilar to the color distribution of the Brambling towards the core area of breeding of the Cuckoo host race | Spatial variation in distribution of egg color | Chaffinch: UK and Fennoscandia: latitude: F = 43.1, df = 1, p = 0.0001, longitude: F = 32.6, df =1, p = 0.0001, Fennoscandia: latitude: F = 16.5, df = 1, p = 0.0001, longitude: F = 8.31, df = 1, p = 0.0022, Sweden and Denmark: latitude: F = 17.1, df = 1, p = 0.0001, longitude: F = 10.8, df = 1, p = 0.0004. Brambling: latitude: F = 1.70, df = 1, p = 0.18, longitude: F = 0.42, df = 1, p = 0.59. Cuckoo: latitude: F = 1.03, df = 1, p = 0.33, longitude: F = 0.57, df = 1, p = 0.54) | Vikan et al.(2011) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in egg rejection were related to differences in egg matching | Mantel r = −0.73, p = 0.003, Mantel r = −0.62, p = 0.016, Mantel r = 0.80, p = 0.007 | Avilés et al.(2012) | |
| Phenotype matching between eggs of Cuckoo and parasites among Reed Warbler populations | Between population differences in host egg phenotype were related to differences in parasitism rate | Mantel r = 0.56, p = 0.01 | Avilés et al.(2012) | |
| Differences in aggressive behavior among sites | Aggressive behavior by hosts is more intense at sites with more Cuckoos | F = 10.5, df = 1, 30, p = 0.003, F = 9.2, df = 1, 30, p = 0.005, z = 2.35, p = 0.018, F = 10.2, df = 1, 29, p = 0.003 | Langmore et al. (2012) | |
| Differences in rejection rate and micro-satellite marker | Differences in rejection rate of Cuckoo eggs by magpies depend on allele size in a micro-satellite marker | Mantel r = 0.88, p < 0.001 | MartínGálvez et al.(2006) | |
| Differences in rejection rate and parasitism rate | Higher rejection rate in populations with higher parasitism rate | Mantel r = 0.62, p = 0.006 | MartínGálvez et al.(2007) | |
| Differences in allele size and parasitism rate | Higher frequency of allele in populations with higher parasitism rate | Mantel r = 0.68, p = 0.002 | MartínGálvez et al.(2007) | |
| Rejection behavior and gene flow | Rejection rate depends on gene flow from other populations of hosts | Mantel r = 0.64, p = 0.016 | Soler et al.(1999b) | |
| Parasitism and rejection of cuckoo eggs in relation to coevolutionary interactions within the focal study area and in neighboring study areas | Positive relationship between rejection rate and parasitism among populations, even after controlling for gene flow among populations | Mantel r = 0.37, p = 0.011 | Soler et al.(2001b) | |