| Citation: | Mariya V. SIVAY, Nikita Y. SILKO, Kirill A. SHARSHOV, Aleksander V. PROKUDIN, Laixing LI, Min YANG, Sheng CAO, Aleksander M. SHESTOPALOV. 2011: The role of wild goose (Anser) populations of Russia and the Tibet Plateau in the spread of the avian influenza virus. Avian Research, 2(3): 140-146. DOI: 10.5122/cbirds.2011.0022 |
Wild birds of the orders Anseriformes and Charadriiformes represent a natural reservoir of low pathogenic avian influenza (LPAI) viruses (family Orthomyxoviridae). Wild geese (order Anseriformes) relating to waterfowls undertake extensive migration flights reaching thousands of kilometers. Isolation of the avian influenza virus (AIV) from wild geese is quite low or absent. The aims of this study are to monitor the AIV in different wild goose species, nesting on Russian territory and the Tibet Plateau and to analyze the derived data for the purpose of determining the role of these wild bird species in spreading pathogens. In our study 3245 samples from nine wild goose species in nine regions of Russia and on the territory of the Tibet Plateau (the Xizang Autonomous Region) were tested and no AIV were detected. Our study shows the non-essential role of wild geese in the spread of the AIV over long distances and reaches the conclusion that geese are probably not natural reservoirs for the primary viruses. However, further inquiry of AIV in wild goose populations is required. Studies of wild geese and AIV ecology will allow us to obtain more information about pathogen-host relationships and to make arrangements for the maintenance of wild goose populations.
Wild birds of the orders Anseriformes and Charadriiformes represent a natural reservoir of low pathogenic avian influenza (LPAI) viruses (family Orthomyxoviridae) (Webster et al., 1992). LPAI viruses in wild birds recognize a limited number of cell types, primarily those infected in the gastrointestinal system. Infection typically occurs following ingestion of viruses shed in feces, while virus-contaminated water likely serves as an important 'vector' by facilitating fecal-oral transmission of LPAI viruses in wild birds associated with water. Wild birds undertake migration flights over long distances, spreading different variants of avian influenza viruses (AIVs) (Boyce et al., 2009).
Wild geese (order Anseriformes), related to waterfowls, undertake extensive migration flights. Eleven wild goose species inhabit Russian territory — the Brant Goose (Branta bernicla), the Barnacle Goose (B. leucopsis), the Red-breasted Goose (Rufibrenta ruficollis), the White-fronted Goose (A. albifrons), the Graylag Goose (A. ans), the Swan Goose (A. cygnoides), the Lesser White-fronted Goose (A. erythropus), the Bean Goose (A. fabalis), the Bar-headed Goose (A. indicus), the Snow Goose (A. caerulescens) and the Emperor Goose (Chen canagica), most of which winter in Europe and Central Asia, including China (Gladkova and Mikheev, 1970). Some of these wild goose populations nest in north-western Siberia, including the Taimyr Peninsula. Migration flights of birds aim for this territory from all continents.
Some wild goose species inhabit Central Asia and the Bar-headed Goose is the most widely occurring species in this territory. Qinghai Lake and many other lakes are important breeding sites and the Yarlung Zangbo River valley is the largest wintering site for the Bar-headed Goose found on the Tibet Plateau. From September, they migrate southwards to valleys mostly in Yunnan and secondly to the Guizhou Plateau. Some even venture as far south as the Indian Himalayas. All return northward to their breeding lakes around April of the following year (Chen et al., 2005).
Circulation of the AIV in wild birds of the order Anseriformes has been the focus of many studies, but wild ducks play a more important role in this process (Guan et al., 2007). Experimental infections of various wild ducks species have shown an important role of these birds as a long-distance vector in the spread of the AIV. Consequently, these studies indicate that some duck species are prime candidates for being long-distance vectors of pathogens because it is the only species to show abundant virus excretion without clinical or pathologic evidence of debilitating diseases (Keawcharoen et al., 2008). The rate of isolation of the AIV in wild ducks varies from 0% to 14.3%. A number of studies have shown the rate of isolation of AIV in wild geese to vary from 0% to 3.5% (Slemons et al., 1991; Fouchier et al., 2003; Chen et al., 2006; Olsen et al., 2006b; Komar and Olsen, 2008; Lindh et al., 2008; Kou et al., 2009; Fereidouni et al., 2010; Germundsson et al., 2010). A four-year monitoring of the AIV in Iran showed an absence of the virus in wild goose samples. However, AIV antibodies were detected (Fereidouni et al., 2010). A study in Finland showed the presence of the virus in cloacal swabs (1.7%) and AIV antibodies in sera (1.6%) collected from the Bean Goose and the presence of the virus in cloacal swabs (3.5%) collected from the Graylag Goose (Lindh et al., 2008). Monitoring of the AIV H5N1 subtype (2004–2007) in China showed AIV arate of isolation of 1.93% in the Bar-headed Goose and a 2.10% rate in the Lesser White-fronted Goose (Kou et al., 2009). Global studies of AIV isolation from wild birds by Olsen et al. (2006) showed that eight wild goose species were investigated. The viruses were detected in the Canada Goose (Branta canadensis), the Graylag Goose and the White-fronted Goose, with isolation rates of 0.8%, 1.1% and 2.2% respectively. Results of a serological study by Winkler et al. (1972) of the Canada Goose sera showed AIV antibodies present in 4.7% of the samples.
However, the prevalence of the AIV depends on many factors: season, sample collection sites, bird species and sample analysis methods (Parmley et al., 2005; Krauss, 2007; Ip, 2008). Differences in feeding behavior and diet could also account for the differences in prevalence between bird families and species. While dabbling ducks feed on water surfaces, geese and certain swan species graze in pastures and agricultural fields. This could potentially lead to a less efficient transmission and subsequently lead to a lower prevalence of the influenza A virus in these geese and swan species as observed in northern Europe. The lower overall prevalence in geese and the limited number of HA subtypes identified in influenza A viruses isolated from geese is in agreement with this hypothesis (Munster and Fouchier, 2009). Low pathogenic avian influenza (LPAI) viruses can be found in a large number of other bird species, but it is unclear in which of these species influenza viruses are endemic and in which the virus is a temporary pathogen. Species in which influenza viruses are endemic share the same habitat at least part of the year with other species in which influenza viruses are frequently detected, including geese, swans, rails, petrels and cormorants. In these and in other birds, prevalence of the influenza virus seems to be lower than in dabbling ducks, but it should be noted that studies on these species are limited and it is possible that peak prevalence has been missed because of its seasonal nature or location (Olsen et al., 2006a).
Wild geese are frequently subjected to variants of high pathogenic epizootic viruses. For instance, the outbreak of the HPAI H5N1 subtype occurred in the spring of 2005 at Qinghai Lake. More than 6000 dead birds were found (Neumann et al., 2010); 90% of the dead birds were Bar-headed Geese, as well as some Brown-headed Gulls (Larus brunnicephalus), Great Black-headed Gulls (L. ichthyaetus) and Great Cormorants (Phalacrocorax carbo). According to a study by Chen et al. (2006), severe hyperemia and edema in the brain, hemorrhage, necrosis in the pancreas and severe cloudy swelling of the kidneys were observed in the Bar-headed Geese, Great Black-headed Gulls, Brown-headed Gulls and Great Cormorants, examined immediately after their death. Histological analysis of organ samples from two moribund Bar-headed Geese revealed typical non-suppurative encephalitis. This single epizootic caused an estimated 10% decrease of the global population of Bar-headed geese, highlighting its potentially devastating effect on vulnerable wildlife (Olsen et al., 2006a). This fact indicates not only the pandemic and epizootic risk of the HPAI H5N1 subtype among hosts of the pathogen, but also shows the risk to unbalanced ecosystems and bio-resources. Several experiments about susceptibility of wild geese to the influenza virus have been carried out. Experimental infections of different wild goose species (the Bar-headed Goose, the Cackling Goose (Branta hutchinsii), the Canada Goose and the Snow Goose) with the HPAI H5N1 subtype showed high susceptibility of birds to this pathogen — high rates of mortality and the appearance of neurological signs of disease (Zhou et al., 2006; Pasick et al., 2007; Brown and Stallknecht, 2008).
As per one of the main hypotheses, wild migration waterfowls serve as a long-distance vector for the distribution of the HPIA virus. HPAI outbreaks were detected in the Asian part of Russia (south-western Siberia) in the summer of 2005. By the autumn of 2006 the virus had spread to the European part of Russia, to several European countries and to Africa. However, the role of wild goose species in the distribution of AIV is not clear.
The aim of this study was to monitor the AIV in different wild goose species, nesting in the territory of Russia and the Tibet Plateau, as well as analyzing the data derived in our study of the role of these wild bird species in spreading the pathogen.
Biological samples (cloacal swabs, feces and blood sera) were collected from wild geese entrapped, shot or living free during the hunt. Hunting permits were obtained. The samples were collected from 2005 to 2009 in nine regions of Russia (the Nigney, Saratov, Novosibirsk, Omsk, Altay, Krasnoyarsk regions, the Kamchatka and Chukotka peninsulas and the Primorye Region) and on the territory of the Tibet Plateau (the Xizang Autonomous Region) at sites of mass waterbird congestion during nesting and in wintering areas.
Samples were tested for influenza viruses by inoculation into the allantoic cavity of 10-day-old embryonating specific pathogen free (SPF) chicken eggs, according to standard procedures (WHO, 2002).
Viral RNAs were isolated from feces and cloacal swabs by trisol-chloroform methods (http://molbiol.edu.ru/protocol/15_10.html). Reverse transcription (RT) reactions were made with the RevertAidTM M-MuLV Reverse Transcriptase (Fermentas, Lithuania) and the PrimeScript™ RT Reagent Kit (TaKaRa, China) as specified by the manufacturer. A random Hexamer Primer was used for RT.
The cDNAs of the AIV were detected by Real-Time PCR with Taq DNA polymerase (Medigen Laboratory, Russia) and a Premix Ex Taq™ (TaKaRa, Japan). Taqman probes (5′-TCGAAACGTACGTTCTCTCTATC-3′, 5′-FAM-TCAGGCCCCCTCAAAGCCGA-Q-3′, 5′-TGTCTTCAGCCATTCCATGAG-3′), specific for the viral M gen, were used.
Blood sera were tested by hemagglutination inhibition (HI) tests with 0.5% chicken red blood cells and a panel of antisera against avian HAs of the H1, H3, H5 and H7 subtypes — A/New Coledonia/22/99/H1N1, A/Mallard/Primorye/2007/H3N8, A/Common gull/Chany/2006/H5N1, A/Goose/Krasnoozerskoye/627/05/H5N1, A/duck/Tuva/01/06/H5N1, A/Anas platyrhynchos/ChanyLake/9/03/H5N3, A/Duck/Ukraine/63/H3N2, antiserum GP-1 (H7N1) and A/RT/NJ/65/85/H7N1.
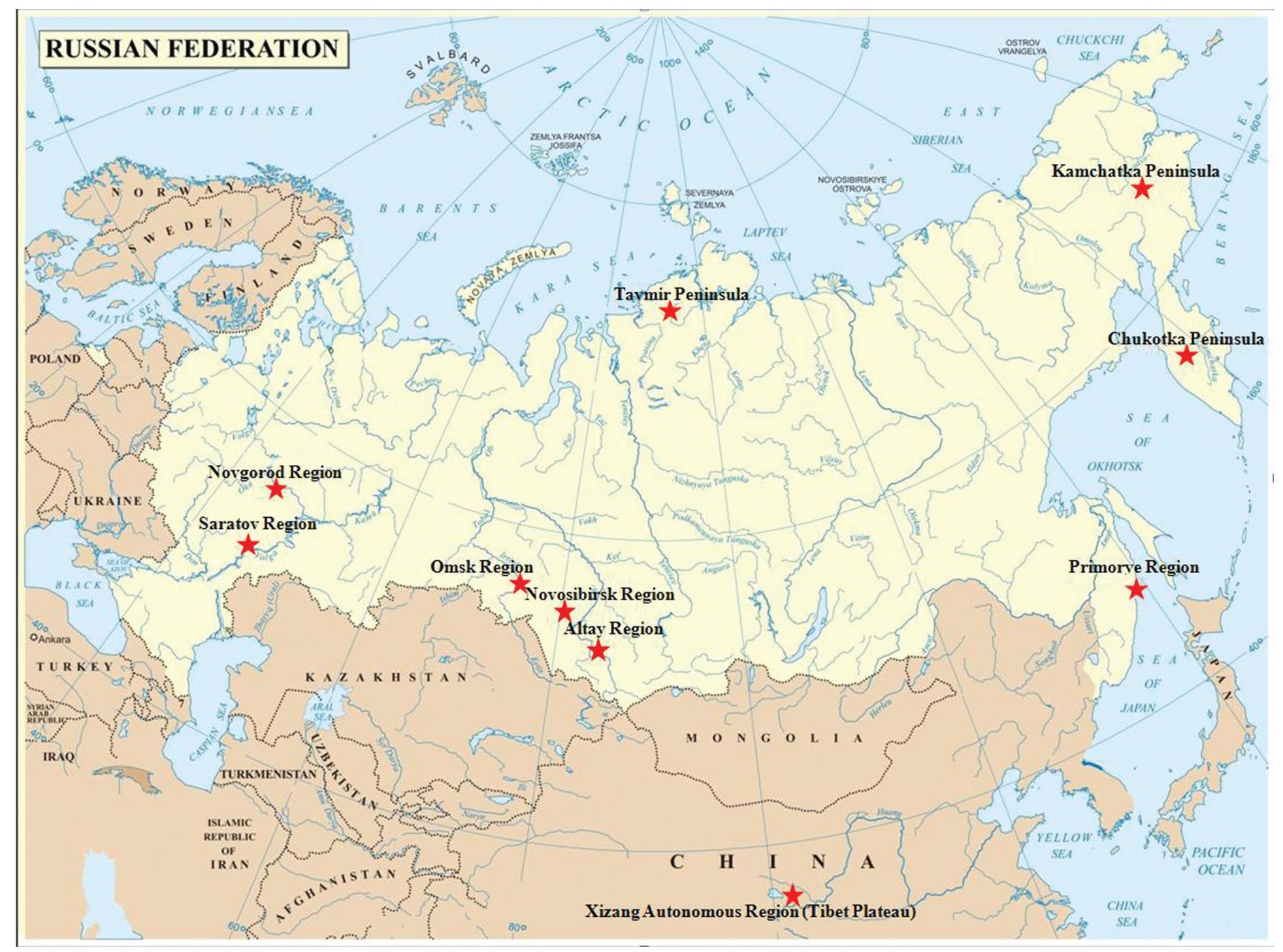
We analyzed 3245 samples from nine wild goose species — Bean Goose, White-fronted Goose, Graylag Goose, Lesser White-fronted Goose, Brant Goose, Red-breasted Goose, Snow Gooose, Bar-headed Goose and other goose species. Samples were collected in nine regions of Russia (the Omsk, Saratov and Novgorod regions, the Kamchatka Peninsula, the Novosibirsk Region, the Chukotka and Taimyr peninsulas and the Primorye and Altay regions) and on the territory of Qinghai Province (northern China) (Fig. 1).
Samples from the White-fronted Goose were collected in the Omsk Region (n = 8), the Saratov Region (n = 6), the Novgorod Region (n = 169), the Kamchatka Peninsula (n = 8), the Novosibirsk Region (n = 3), the Chukotka Peninsula (n = 72) and the Taimyr Peninsula (n = 987). Samples from the Bean Goose were collected in the Primorye Region (n = 5), the Chukotka Peninsula (n = 15) and the Taimyr Peninsula (n = 39). Eighteen samples from the Graylag Goose were collected in the Novosibirsk Region and four in the Altay Region. Samples from the Brant Goose were collected in the Primorye Region (n = 1) and Taimyr Peninsula (n = 256). Samples from the Lesser White-fronted Goose, the Red-breasted Goose and other goose species were collected in the Taimyr Peninsula (n = 6, 9 and 46 respectively) and from the Snow Goose in Altay (n = 1). All 1600 samples from the Bar-headed Goose were collected in Qinghai Province (Table 1).
| Samples collection regions | Wild goose species | ||||||||
| Bean Goose | White-fronted Goose | Graylag Goose | Lesser White-fronted Goose | Brant Goose | Red-breasted Goose | Snow Goose | Bar-headed Goose | Other goose species | |
| Omsk Region | 8 | ||||||||
| Novosibirsk Region | 3 | 18 | |||||||
| Saratov Region | 6 | ||||||||
| Novgorod Region | 169 | ||||||||
| Kamchatka Peninsula | 8 | ||||||||
| Taimyr Peninsula | 39 | 978 | 6 | 256 | 9 | 46 | |||
| Primorye Region | 5 | 1 | |||||||
| Altay Region | 4 | ||||||||
| Chukotka Peninsula | 15 | 72 | 1 | ||||||
| Qinghai Province | 1600 | ||||||||
| Total | 3245 | ||||||||
Samples from Russia were used for a virologic study by inoculation of embryonated 10-day-old chicken eggs. No AIVs were detected. Simultaneously, these samples were tested by Real-time PCR and again, no cDNAs of AIVs were detected.
Blood sera were collected from 208 birds out of the 3245 samples — 1 sample from the Bean Goose, 107 samples from the White-fronted Geese, 2 samples from the Red-breasted Geese and 98 samples from the Brant Geese nestling in the Krasnoyarsk Region (Taimyr Peninsula). Serological investigation of 296 serum samples was provided by a HI assay with A/common gull/Chany/P/2006 H5N1 antigen. The rates of seroprevalence against AIV H5N1 were 53.5% for 2007 and 90.4% for 2008. However, the composition of bird species in the two sampling periods was different.
Validation of a serological assay for detection of AIV-specific antibodies in wild birds is still under discussion (De Marco et al., 2003; Fouchier et al., 2003). Only limited information about the sensitivity of the HI assay in these species is available. Thus, more information is required to clarify the role of different bird species in maintaining avian influenza infection.
In our study, the overall seroprevalence (H5) based on 296 serum samples from different species was more than 50%. These results emphasize the importance of goose species for the epidemiology of AIV in nature. Thus, although the sample size for geese was small, the rates of detected prevalence are important for epidemiological studies.
Samples from Qinghai Province were tested by Real-Time PCR. Out of 1600 samples, no AIV were detected.
According to other data (Brown, 2006; Guan et al., 2007; Boyce et al., 2009; Fereidouni et al., 2010), wild geese along with wild ducks play an important role in long-distance AIV distribution due to migration flights.
In our study 3245 samples from nine wild goose species were tested and no AIV were detected. Sample collection from wild geese presented some difficulties, but we managed to collect a representative sample for AIV detection. Samples were collected in roosting and nestling areas of wild geese. Most of the samples from wild geese in Russia were collected on the Taimyr Peninsula — a major site for bird populations from all over the world during breeding and molting periods. Some bird species fly continental routes from Europe, the Mediterranean, Iran, Africa, India and China, while other bird species fly by sea routes from west to east. By far, the largest concentration of migrating birds were observed from the south-west, the south and the south-east. The White-fronted Goose, the Red-breasted Goose and ducks migrate over the Kazakhstan steppes and the southern Siberia taiga and from the Baltic Sea coast (Borzgov, 1978). The Brant Goose migrates from the Barents Sea along the coast. Wild geese from the Taimyr Peninsula winter in the Mediterranean, northern Africa, India, Korea, China, Japan, southern and northern Europe. Migration connections of wild birds from Russia with birds from distant areas lead to close ecological relationships of the regions and can serve as favorable factors for AIV distribution by migrating wild birds. However, our findings showed a lack of AIV in wild goose populations on Russian territory. Possibly, these results could be explained by the resistance of wild geese to the pathogen or elimination of sick birds in wintering areas. The AIV evolution in host populations is aimed at optimal ratios of the involved individuals. Hence, the situation for geese might be different in contrast to other species of wild birds (Lebarbenchon et al., 2010). Probably, the prevalence of AIV in wild goose populations is less than that in duck populations. Our data confirmed this fact.
Investigation of the Bar-headed Goose population in the Tibet Plateau also showed a similar lack of the AIV. Qinghai Lake is the roosting site of the Bar-headed Goose and serves as an inauspicious site for the HPAI H5N1 subtype. Ecological relationships of this region with Mongolia, Kazakhstan, south-western Siberia and the Russian Far East indicate the important role of the region in the spread of the AIV. This statement confirms that the HPAI H5N1 virus subtypes detected in these regions are closely related.
The presence of AIV antibodies in wild geese is probably evidence of earlier AIV circulation in wild goose populations. H5 AIV antibodies were not detected in blood samples which could be evidence of resistance of wild geese to this pathogen. Probably, infection is caused by a higher dose of the virus that was shown in studies by experimental infection of different wild goose species (Winkler et al., 1972; Zhou, 2006; Pasick et al., 2007; Brown and Stallknecht, 2008). In this case, explicit clinical signs of the disease or fatal outcomes, which prevent migration flights, were observed.
The non-essential role of wild geese in spreading the AIV over long distances was discovered in our study. As well, we found that wild geese are probably not a primary natural reservoir of the virus. However, further inquiry of the AIV in wild goose populations is required. Studies of wild geese and AIV ecology will allow us to obtain more information about pathogen-host relationships and to make arrangements for the maintenance of wild goose populations.
We thank Dr. Aleksander K. Urlov, Head of the Laboratory of the Bird Ecology Institute of Animal Ecology and Systematics of the Siberian Branch of the Russian Academy of Science; workers of the Biological Station on Chany Lake of the Institute of Animal Ecology and Systematics of the Siberian Branch of the Russian Academy of Science; workers of the administration for guarding, controlling and applying the key regulations of exploitation of objects of natural origin and habitat in the Novosibirsk Region. This work was supported by the Russian Government (Government Project #11.519.11.2014) and the Bio Industry Initiative (BII) USA (ISTC#3436).
| Samples collection regions | Wild goose species | ||||||||
| Bean Goose | White-fronted Goose | Graylag Goose | Lesser White-fronted Goose | Brant Goose | Red-breasted Goose | Snow Goose | Bar-headed Goose | Other goose species | |
| Omsk Region | 8 | ||||||||
| Novosibirsk Region | 3 | 18 | |||||||
| Saratov Region | 6 | ||||||||
| Novgorod Region | 169 | ||||||||
| Kamchatka Peninsula | 8 | ||||||||
| Taimyr Peninsula | 39 | 978 | 6 | 256 | 9 | 46 | |||
| Primorye Region | 5 | 1 | |||||||
| Altay Region | 4 | ||||||||
| Chukotka Peninsula | 15 | 72 | 1 | ||||||
| Qinghai Province | 1600 | ||||||||
| Total | 3245 | ||||||||