| Citation: | Ning LI, Wei ZHOU, Wei LI, Qing ZHANG, Xuerong WANG. 2010: Comparison of roosting habitat characteristics of two sympatric pheasants during springtime at Dazhong Mountain, southwestern China. Avian Research, 1(2): 132-140. DOI: 10.5122/cbirds.2010.0006 |
Hume's Pheasant (Syrmaticus humiae) and the Silver Pheasant (Lophura nycthemera) are two sympatric bird species at Dazhong Mountain of Yunnan Province, southwestern China. We investigated characteristics of roosting habitats of the two pheasants from February to April, 2004 in this area. Multiple statistics, Matryoshka and a habitat classification-tree were used to analyze the selection of roosting habitats of these pheasants. The results of the habitat classification-tree indicated that several separations occurred in their macro and micro roosting habitats in the study area. The two pheasants had similar crucial requirements for and selection of ecological roosting factors, which allow them to live in the same macrohabitat. Competition between these two pheasants was avoided by separation of spatial elements, such as roosting trees and topographic characteristics. For safety strategy, Hume's Pheasant adopted primarily a way of "uneasily found habitat cover plus easy escape", while the Silver Pheasant employed a unique way of "uneasily found habitat cover". For tactics of keeping warm, Hume's Pheasant selected mainly a method of "suitable vegetation supplemented with suitable topography", while the Silver Pheasant chose a unique manner of "suitable vegetation".
Similarities and differences for resource requirements are key factors affecting coexistence of sympatric species (Johnson, 2000). When resources are rich different species, completely overlapping in their niche, might be found in the same area (Zhang and Jiang, 1997). However, sympatric species might separate at least in one niche for coexistence if resources were to become scarce (Munday et al., 2001), resulting in coexisting species developing their own strategies for niche separation, such as spatial separation (May, 1973; Jenni, 1993).
Roosting strategies of birds show how they select and use spatial resources at night, including roosting behavior and roost selection (Cody, 1985). Birds, daily active, cannot be aware of potential dangerous situations, which lead them often to be exposed to dangers during nighttime because of poor visibility (Chamberlain et al., 2000). A suitable roosting habitat not only retains a desirable temperature for birds, but also protects them from predation (Cody, 1985). Therefore, the selection of a roosting habitat affects the fitness of birds (Cody, 1985; Elmore et al., 2004). So far, many studies reporting roosting site selection of rare pheasants, have focused primarily on roosting behavior and site selection. Some characteristics have been commonly recognized for avian roosting, i.e., birds prefer to stay in areas with steep terrain and high tree cover (Kelty and Lustick, 1977; Cody, 1985). However, there are obvious interspecific differences in tree species for roosting, height of perching branch and canopy of perching position (Ding et al., 2002; Jia et al., 2005; Shao and Hu, 2005; Jiang et al., 2006; Lu and Zheng, 2007). From the study of roosting habitat selection by Tetraonidae, it has been shown that the structure of trees, microhabitat of perching position and terrain characteristics are factors mainly affecting roosting habitat selection in avian species (Godfrey, 1970; Korhonen, 1980). But few of these studies compared the roosting strategies of different species in the same area.
Hume's Pheasant (Syrmaticus humiae), listed as globally near-threatened (Birdlife International, 2008) and the Silver Pheasant (Lophura nycthemera), which is not threatened, are found in sympatry in the Dazhong Mountain of Yunnan Province, southwestern China (Li et al., 2006). So far, no comparative analysis of roosting site of the sympatric pheasants has been described in detail. In this study, we investigated the night roosting habitat characteristics of Hume's Pheasant and Silver Pheasant and compared their roosting site strategies. Multiple statistics, Matryoshka and habitat classification-tree were used to analyze roosting habitat selection in the spring. We also discuss the mechanism how the pheasants choose roosting sites at night.
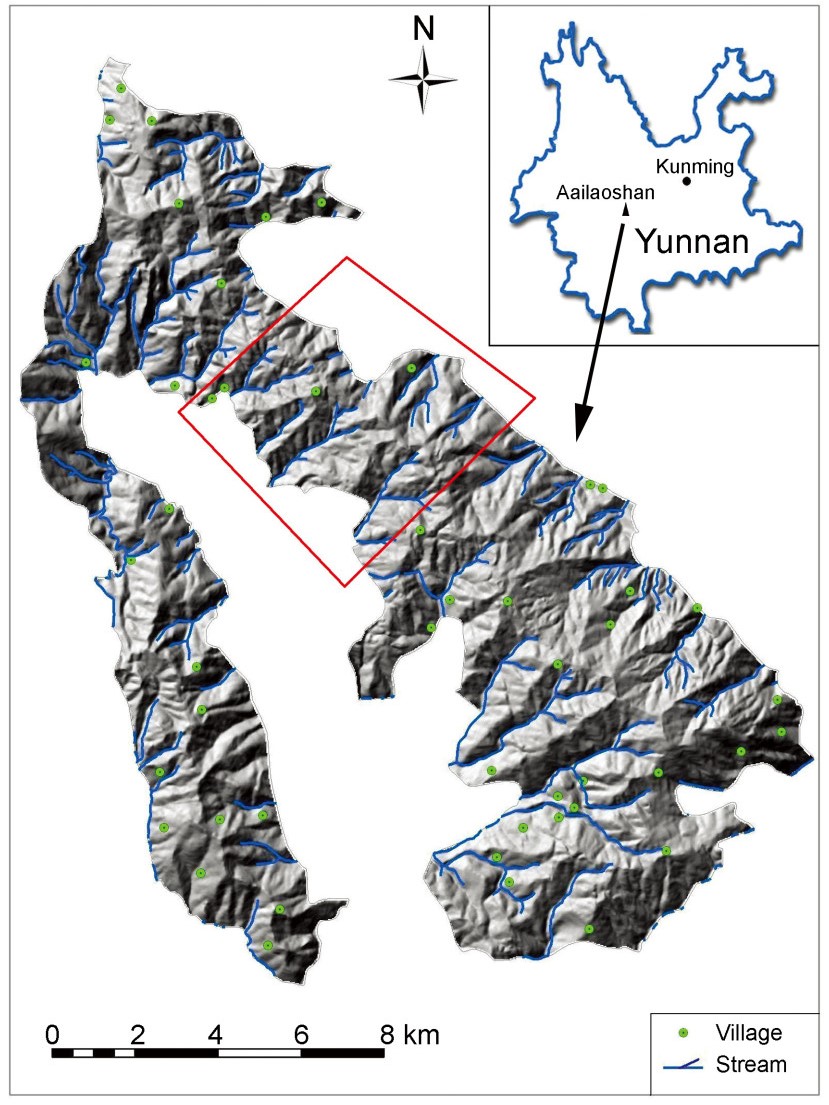
Dazhong Mountain (24°43′32″–25°01′10″N, 100°44′28″–100°57′42″E) is a part of the Ailaoshan National Nature Reserve, located in the southwestern part of Nanhua County, Chuxiong Prefecture in central Yunnan Province, China (Fig. 1). This area lies at the juncture of the central Yunnan Plateau, Hengduan mountains and the southern tip of the Qinghai-Tibetan Plateau, comprising mid-alpine mountains and valleys caused by age-old movements in the earth's crust. These upward movements of the earth led to modified soils and climate regimes which in turn have affected vegetation and species diversity and distribution. Pinus yunnanensis and scrub forests dominate in areas below 1500 m, semi-moisture broadleaf evergreen forests and deciduous broadleaf forests are found at elevations between 1500–2400 m and the vegetation above 2400 m comprises mid-alpine broadleaf evergreen and Pinus armandii forests (Wang, 2000).
Field data were collected from February to April, 2004 in the Dazhong Mountain.
Three transects (at elevations of 2400, 2450 and 2500 m), 4–6 km long (the transects were often beyond the boundary of the natural reserve) were established in the study area where Hume's Pheasant and Silver Pheasant occur in sympatry. Both pheasants often appeared near roosting sites at dawn and dusk (06:30–09:30 and 17:00–19:00 hours) (Li et al., 2006). When they came to roost or flew away from the roosting tree, a loud sound, "pu…pu…" caused by fanning their wings, could be heard and their roost could be identified easily. If these sounds could not be heard, we used flashlights to look directly for the pheasants and confirmed roosting trees at night, or indirectly identify the roosting trees by searching for faecal in the morning according to the amount and their freshness under the roosting trees. The faecal of the two pheasants could be distinguished by visual observations: the faecal of Hume's Pheasant is a cone-shaped black leptospira with white uric acid crystals at the larger ends; in comparison, that of the Silver Pheasant is blank and columnar with white uric acid crystals covering the surface. The faecal volume of the Silver Pheasant is larger than that of Hume's Pheasant.
Following the methods of Young et al. (1991), plots of 10 m × 10 m were established with roosting trees as centers. Twenty-two factors, referring to bird roost selection, were measured, given the instructions of Zheng (1995). These factors can be categorized into three groups:
1) Macro-habitat characteristics. Elevation (EL), aspect (AS), slope (SL), vegetation types (VT), distance to water (DSW) and distance to roads (DSR). EL, AS and SL were measured by compass and DSW and DSR by a measuring tape.
2) Vegetation characteristics. Canopy tree density (CTD), canopy tree cover (CTC), average height of canopy tree (AHCT), average diameter at breast height of canopy tree (ADBH), shrub density (SD), shrub cover (SC), average height of shrubs (AHS), herb cover (HC) and leaf litter cover (LLC).
3) Perch characteristics. Tree species (TS), tree height (TH), diameter at breast height (DBH), perch height (PH), obtained by tape measure, angle between perching branch and stock (APS), obtained by goniometer, cover over perch (COP), crown size (CS) (i.e., umbriferous crown area of roost tree, assumed to be elliptical or round).
A chi-square test and Ivlev's Resource Selection Index (RSI) were used to analyze the selection by the pheasants of two factors, i.e., roost tree species and vegetation type (Ivlev, 1961; Manly et al., 2002). Ivlev's Resource Selection Index is defined as:
|
Ei=(Ri−Ni)/(Ri+Ni) |
(1) |
where resource utilization (Ri) represents the actual frequency of utilization of resource i by animals (here referring to birds) in a given period, resource availability (Ni) represents the availability of resource i by birds and the resource selection index (Ei) indicates whether the bird selects the resource i. If Ei = 0, the birds have no preferential selection for resource i and is expressed as "0"; if Ei < 0, birds avoid resource i, expressed as "−"; if Ei > 0, the birds prefer to select resource i and is expressed as "+" (Ivlev, 1961).
The other 20 quantitative factors were analyzed by t-test to compare mean differences between the two species. Since the data should be normally distributed, slope and aspect have been transformed either by an arcsine or a logarithmic transformation before analysis (Manly et al., 2002). Principal component analysis (PCA), a multivariate technique that produces a simplified, reduced expression of the original data with complex relationships, has been widely applied in studies of wildlife habitats (Fowler et al., 1998). All quantitative variables were analyzed via PCA based on their correlation matrix with a varimax rotation to screen out the key factors in roosting habitat selection of Hume's Pheasant and Silver Pheasant.
All statistics were analyzed by SPSS 13.0 for windows.
A habitat classification-tree was constructed from the result of multiple statistics for roosting habitat and the theory of Matryoshka, who developed a system of habitat classification for a complete multi-scale habitat study. Habitat selection in birds can be divided in several levels, from macrohabitat to microhabitat (Hanski, 2006). This kind of system not only shows species requirements at each layer but also reveals which layer is destroyed. Simultaneously, the definition of habitat layer can be used for comparing the differences and similarities of congeneric species, which provides evidence to ascertain whether habitats overlap or are separated in any given layer.
Twenty roosting trees for each pheasant species were found in the field; in total forty utilization plots were established.
For vegetation type, both pheasants selected moist evergreen broadleaf forests in the middle-mountain as their roosting sites (Table 1). In the twenty trees, chosen by Hume's Pheasant for roosting, ten (50%) were oak species (Lithocarpus xylocarpus, L. truncates and L. cleistocarpus), five (25%) were Pinus armandi trees, two (10%) were Ternstroemia gymnanthera and the others (15%) were Lyonia ovalifolia, Gaultheria leucocarpa var. crenulata and Alnus nepalensis, in total eight tree species. For Silver Pheasant, twelve roosting trees (60%) were oak species (Lithocarpus xylocarpus, L. truncatus and L. echinophorus), two (10%) were Camellia oleifera and the other six (30%) were Castanopsis megaphylla, Lyonia ovalifolia, Rhododendron delarayi, Gaultheria forrestii var. forrestii, G. leucocarpa var. crenulata and Cerasus serrulate trees, i.e., ten species in total. Oak was the main tree species for roosting. There were no significant difference in the preference for oak as roosting tree between the two pheasants (χ2 = 0.4, df = 1, p > 0.05).
| Factor | i | Ni | Ri | Ei | Selective | |||||
| S | L | S | L | S | L | |||||
| Vegetation type | MEBF | 0.49 | 0.78 | 1.00 | 0.23 | 0.34 | + | + | ||
| DBF | 0.05 | 0.00 | 0.00 | −1.00 | −1.00 | − | − | |||
| PYF | 0.46 | 0.22 | 0.00 | −0.35 | −1.00 | − | − | |||
| Note: S, Syrmaticus humiae; L, Lophura nycthemera; DBF, deciduous broadleaf forest; MEBF, middle-mountain, moist evergreen broadleaf forest; PYF, Pinus yunnanensis forest; +, observed usage is significantly higher than expected; 0, observed usage is almost equal to expected; −, observed usage is significantly lower than expected; other abbreviations are the same as in Eq. (1). | ||||||||||
There were highly significant differences in the height of roosting trees, perch height and elevation (Table 2). The differences of diameter at breast height, canopy tree density, herb coverage and distance to water were significant (Table 2). Except for these seven habitat factors, there were no clear statistical differences in the other thirteen factors (Table 2).
| Habitat characteristics | Factor types | Roosting habitat (mean ± SE) | t-test (two-tailed) | |||
| S | L | t | p | |||
| Roost tree | DBH | 18.5±5.3 | 27.5±5.1 | −2.586 | 0.014* | |
| Crown size | 32.2±5.6 | 31.2±12.6 | 0.099 | 0.922 | ||
| Height | 8.1±1.5 | 11.3±1.5 | −3.100 | 0.004** | ||
| Roost branch | Height | 3.6±0.6 | 6.4±0.8 | −5.894 | 0.000** | |
| APS | 94.7±5.6 | 92.0±5.0 | 0.765 | 0.449 | ||
| COP | 79.3±5.9 | 76.5±7.5 | 0.624 | 0.537 | ||
| Tree layer | Density | 25.0±8.0 | 38.9±10.7 | −2.180 | 0.036* | |
| AHCT | 8.2±1.1 | 9.3±0.8 | −1.747 | 0.089 | ||
| ADBH | 11.4±2.0 | 12.6±1.9 | −0.876 | 0.387 | ||
| Cover | 60.7±6.3 | 66.8±5.2 | −1.557 | 0.128 | ||
| Shrub layer | Cover | 17.7±7.4 | 11.9±4.8 | 1.374 | 0.177 | |
| AHS | 1.5±0.3 | 1.7±0.2 | −1.216 | 0.231 | ||
| Density | 9.9±6.5 | 4.9±0.9 | 1.613 | 0.123 | ||
| Herb layer | LLC | 82.2±4.7 | 86.3±3.5 | −1.466 | 0.151 | |
| HC | 10.5±7.0 | 2.3±3.3 | 2.214 | 0.035* | ||
| Macrohabitat | DSR | 34.4±7.9 | 57.4±29.9 | −1.561 | 0.133 | |
| DSW | 39.3±11.2 | 72.5±23.8 | −2.642 | 0.014* | ||
| Aspect | 11.8±34.8 | 29.3±12.3 | −0.993 | 0.331 | ||
| Slope | 31.2±4.8 | 30.1±3.5 | 0.371 | 0.712 | ||
| Elevation | 2421.6±16.8 | 2465.3±23.3 | −3.189 | 0.003** | ||
| Note: S, Syrmaticus humiae; L, Lophura nycthemera; ADBH, average diameter at breast height of canopy trees; AHCT, average height of canopy trees; AHS, average height of shrubs; APS, angle between perching branch and stock; COP, cover over perch; DBH, diameter at breast height; DSR, distance to road; DSW, distance to water; HC, herb coverage; LLC, leaf litter coverage; * p < 0.05; ** p < 0.01; other abbreviations are the same as for Table 1. | ||||||
Given the results of PCA, the unique factors affecting only the roosting habitat of Hume's Pheasant were slope, aspect, angle between perch and stock, shrub density and distance to water. Elevation and canopy tree cover were unique factors only affecting the Silver Pheasant. Two factors, leaf litter coverage and distance to roads, had roughly the same effect on roosting of the two pheasants; the values of these factors were reversed. Furthermore, only five factors of roosting trees and shrubs that affected the roosting habitat of the two pheasants are ordered in the same sequence, while the sequence of the other eight factors contributed alternately (Table 3).
| Factor | Factor type | Factor loading | ||||||||||||||||
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |||||||||||||
| S | L | S | L | S | L | S | L | S | L | S | L | |||||||
| Roost tree | DBH | 0.92 | 0.84 | 0.02 | 0.11 | −0.09 | 0.01 | −0.08 | 0.15 | 0.21 | 0.14 | −0.02 | −0.24 | |||||
| Crown size | 0.89 | 0.82 | −0.01 | −0.26 | −0.09 | −0.02 | −0.07 | 0.24 | 0.21 | 0.16 | 0.14 | 0.11 | ||||||
| Height | 0.83 | 0.84 | −0.09 | 0.39 | −0.20 | 0.15 | −0.16 | 0.16 | 0.26 | −0.07 | −0.24 | 0.05 | ||||||
| Roost branch | Height | 0.64 | 0.40 | −0.07 | 0.54 | −0.37 | 0.62 | −0.26 | 0.00 | 0.19 | 0.07 | −0.09 | −0.21 | |||||
| APS | 0.00 | −0.16 | 0.83 | 0.00 | 0.10 | −0.15 | 0.22 | 0.11 | −0.12 | −0.01 | 0.15 | −0.20 | ||||||
| COP | 0.13 | −0.16 | 0.48 | −0.01 | −0.08 | 0.60 | −0.10 | 0.08 | 0.34 | 0.61 | 0.71 | 0.25 | ||||||
| Tree layer | Density | −0.27 | −0.07 | 0.17 | −0.55 | 0.80 | −0.12 | 0.05 | −0.22 | −0.42 | 0.73 | 0.04 | −0.15 | |||||
| AHCT | 0.27 | 0.17 | −0.06 | 0.92 | 0.14 | 0.04 | −0.05 | 0.21 | 0.89 | 0.02 | 0.09 | −0.22 | ||||||
| ADBH | 0.40 | −0.14 | −0.04 | 0.65 | −0.16 | 0.59 | 0.02 | 0.32 | 0.84 | 0.02 | 0.04 | 0.18 | ||||||
| Cover | 0.49 | 0.17 | 0.22 | −0.01 | 0.43 | 0.81 | −0.22 | 0.27 | −0.36 | −0.04 | 0.42 | −0.29 | ||||||
| Shrub layer | Cover | −0.18 | 0.30 | −0.06 | 0.02 | 0.07 | 0.21 | 0.94 | 0.89 | −0.11 | 0.02 | −0.02 | 0.01 | |||||
| AHS | −0.08 | 0.29 | 0.20 | 0.14 | 0.12 | 0.09 | 0.83 | 0.78 | 0.23 | 0.28 | 0.04 | −0.32 | ||||||
| Density | −0.12 | 0.56 | −0.51 | −0.11 | 0.04 | 0.36 | 0.75 | 0.33 | −0.22 | 0.13 | 0.07 | 0.12 | ||||||
| Herb layer | LLC | −0.22 | 0.00 | −0.22 | −0.75 | 0.83 | 0.06 | 0.14 | 0.19 | 0.14 | −0.05 | 0.04 | 0.18 | |||||
| HC | −0.02 | −0.61 | 0.13 | −0.15 | 0.00 | −0.13 | −0.12 | −0.16 | 0.12 | 0.33 | −0.88 | 0.55 | ||||||
| Macrohabitat | DSR | 0.08 | 0.02 | −0.82 | −0.17 | −0.13 | −0.08 | 0.15 | −0.08 | 0.10 | −0.09 | 0.04 | 0.92 | |||||
| DSW | −0.06 | −0.45 | 0.18 | −0.29 | 0.72 | −0.10 | 0.08 | −0.31 | 0.20 | −0.17 | −0.23 | 0.46 | ||||||
| Aspect | −0.34 | 0.43 | 0.27 | −0.40 | −0.11 | 0.51 | 0.03 | −0.33 | 0.17 | 0.41 | 0.74 | −0.19 | ||||||
| Slope | −0.03 | −0.23 | 0.70 | 0.22 | −0.47 | 0.26 | −0.17 | −0.37 | 0.14 | 0.32 | 0.28 | −0.41 | ||||||
| Elevation | 0.31 | −0.19 | −0.11 | 0.04 | 0.05 | 0.04 | 0.00 | −0.08 | −0.08 | 0.89 | −0.07 | 0.26 | ||||||
| Percentage of variance explained (%) | 18.1 | 18.6 | 13.3 | 15.1 | 12.8 | 11.8 | 12.2 | 11.6 | 11.9 | 11.4 | 11.3 | 10.9 | ||||||
| Cumulative percentage (%) | 18.1 | 18.6 | 31.4 | 33.7 | 44.2 | 45.5 | 56.3 | 57.1 | 68.2 | 68.5 | 79.5 | 79.4 | ||||||
| Abbreviations are the same as for Tables 1 and 2. | ||||||||||||||||||
Based on the results of RSI, t-tests and PCA, a habitat classification-tree for the two pheasants at Dazhong Mountain was established. There are several separations in the roosting habitat of the two pheasants, from macrohabitat to microhabitat (Table 4).
| Habitat factors | Classification feature of habitat |
| Macrohabitat | 1. MEBF ………………………………………………….. 2 |
| 2a. Slope 31.2, Aspect 11.8, DSW 39.3 m, DSR 34.4 m …………………………………………. S |
|
| 2b. Elevation 2465 m, DSR 57.4 m ………….. L | |
| Tree layer | 3. AHCT > 8.2 m, ADBH > 11.4 m …………… 4 |
| 4a. Density 25 tubes per m2 …………………… S | |
| 4b. Density 39 tubes per m2, Cover 66.8 … L | |
| Shrub layer | 5. Cover > 11.9, AHS > 1.5 ……………………… 6 |
| 6a. Density 10 tubes per m2 …………………… S | |
| 6b. Density 5 tubes per m2 …………………….. L | |
| Herb layer | 7a. HC 10.5, LLC big ……………………………….. S |
| 7b. HC 2.6, LLC small ………………………………. L | |
| Roost tree | 8. Oak, Crown size > 31.2 ……………………….. 9 |
| 9a. DBH 18.5 cm, Height 8.1 m ……………….. S | |
| 9b. DBH 27.5 cm, Height 11.3 m …………….. L | |
| Roosting branch | 10. COP > 76.5 ……………………………………… 11 |
| 11a. Height 3.6 m, APS 94.7 ………………….… S | |
| 11b. Height 6.4 m …………………………………… L | |
| Abbreviations are the same as for Tables 1 and 2. | |
The reason for the two pheasants roosting together was that they have the same crucial requirements and selection in habitat factors. In the spring, both Hume's Pheasant and the Silver Pheasant prefer roosts in moist evergreen broadleaf forests in the middle-mountain (Table 1) over other vegetation types. Deciduous broadleaf and Pinus yunnanensis forests in the Dazhong Mountain could not supply suitable coverage and temperatures for avian roosts, because dominant trees are sparse and crown closure is low. Therefore, these two pheasants simultaneously choose the same vegetation type and roosting trees in this area. The fact that birds favor trees which supply suitable shelter for safety and warmth as roosting place in their distribution area had been shown in previous studies (Ding et al., 2002; Jia et al., 2005; Shao and Hu, 2005; Jiang et al., 2006; Lu and Zheng, 2007).
Based on the results of t-tests, there were significant differences in tree diameters at breast height, height of trees and perch height (Table 2). The values of these three factors selected by the Silver Pheasant were much larger than those of Hume's Pheasant. These separations avoided their competition for roosting in the same tree; thus the optimal spatial use of roosting trees by the two species was established. Moreover, there are still some other separations of habitat factors, such as canopy tree density, herb coverage, elevation and distance to water (Table 3), which reflect the different responses of the two pheasants to the same vegetation structure and terrain. For example, the Silver Pheasant seems to prefer denser tree canopy, higher herb coverage, farther distance to water and higher elevation than Hume's Pheasant. Although Hume's Pheasant and the Silver Pheasant lived sympatrically, their ecological niches separated in spatial dimensions and the habitat classification-tree reflects this separation (Table 4). Therefore, the same macrohabitat can accommodate several species with similar niches.
The requirements of the two birds for safety differ in their roosting strategy. Safe shelters for roosts were composed of trees and shrubs of high density, perching position of high cover and terrain (Cody, 1985). Based on PCA, the position of roosting trees and shrub characteristics selected by the two pheasants were in the same or similar selection sequence; but characteristics of roosting branch selection in the two pheasants had different sequences. Slope was a unique factor only affecting Hume's Pheasant and tree canopy was unique only to the Silver Pheasant. The different requirements of pheasants for safety are reflected by selection of ecological factors and their order of priority (Table 3). Higher roosting branches and larger distances greatly decreases attacks from nocturnal animals (Prionailurus bengalensis, Mustela sibirica), which reflects an anti-predator strategy on a vertical spatial scale, used for roosting by both pheasants in Dazhong Mountain. However, the selection of other habitat factors reflects a difference in safety strategy of the two pheasants, i.e., slope was a unique factor only affecting Hume's Pheasant. The steeper the slope, the more chances for birds to escape by gliding. Therefore, the means for easy escape is one of the important factors affecting security of Hume's Pheasant. This fact was confirmed by the study of roost site selection in Chrysolophus pictus (Cong and Zeng, 2008). Tree canopy is a factor only affecting the Silver Pheasant. The higher the tree canopy, the smaller the danger for pheasants from predation. Hence, increasing the height of cover in the environment is still the main strategy for roost security of the Silver Pheasant. Briefly, for its safety strategy, Hume's Pheasant adopted primarily a way of "uneasily found habitat cover plus easy escape". The Silver Pheasant employed solely a way of "uneasily found habitat cover".
The requirement for optimum temperature cannot be neglected in the roosting strategy of the two birds. A suitable roosting temperature is maintained by trees and shrubs by avoiding wind and rain (Kelty and Lustick, 1977; Cody, 1985). According to our PCA, shrub characteristics selected by the two pheasants were in the same order of selection and tree factors alternately appeared in their selection order. Aspect was a unique factor affecting the roosting selection only in Hume's Pheasant (Table 3). The function of tree factors contributed the same effects to both pheasants, which might imply that tree factors are the most important for keeping warm when roosting. Shrub density contributed to maintaining roosting temperatures (Moore, 1945), so these factors were selected by both pheasants. Different slope aspects provided different macroclimates. The leeward aspect provides more suitable temperatures for roosting than the windward aspect during the night (Cody, 1985). The aspect selected by Hume's Pheasant was leewards. Aspect seems therefore an auxiliary factor for keeping warm, used by Hume's Pheasant except for vegetation. In brief, for the tactics of keeping warm, Hume's Pheasant selected mainly a method of suitable vegetation, supplemented by topography. The Silver Pheasant chose uniquely the manner of suitable vegetation.
We are specially thankful to the entire staff of Dazhong Mountain Station and the Nanhua Administration Bureau of the Ailaoshan National Nature Reserve, which supplied logistical support for the field investigation. Mr. D. Ji, a student of Southwest Forestry College, helped with the collection of some field data. This study was financed by the Wildlife Conservation Program of the State Forestry Administration of China in 2009.
|
Cody ML. 1985. Habitats Selection in Birds. Academic Press, London.
|
|
Ding P, Yang YW, Li Z, Jiang SR, Zhuge Y. 2002. Studies on the selection of roosting sites of Elliot's pheasant. J Zhejiang Univ Sci Edn, 29:564–568. (in Chinese with English abstract)
|
|
Fowler J, Cohen L, Jarvis P. 1998. Practical Statistics for Field Biology. 2nd edn. Open University Press, West Sussex.
|
|
Godfrey GA. 1970. Snow roosting behavior of immature ruffed grouse. Auk, 87:578–579.
|
|
Hanski I. 2006. The shrinking world: ecological consequences of habitat loss. International Ecology Institute, Nordbunte 23, 21385 oldenorf/uhe, Germany, 18–38.
|
|
Ivlev VS. 1961. Experimental Ecology of the Feeding of Fishes. Yale University Press, New Haven.
|
|
Jenni L. 1993. Structure of a brambling Fringilla montifringilla roost according to sex, age and body-mass. Ibis, 135:85–90.
|
|
Jia F, Wang N, Zheng GM. 2005. Analysis of roosting site characteristics of wintering Crossoptilion crossoptlion populations. Chinese J Ecol, 24(2):153–158. (in Chinese with English abstract)
|
|
Jiang AW, Zhou F, Lu Z, Han XJ, Sun RJ, Li XL. 2006. Roost-site Selection of Mrs Hume's Pheasant (Syrmaticus humiae) in Guangxi, China. Zool Res, 27(3):249–254. (in Chinese with English abstract)
|
|
Korhonen K. 1980. Microclimate in the snow burrows of willow grouse (Lagopus lagopus). Ann Zool Fen, 17:5–9.
|
|
Li W, Zhou W, Zhang XY, Cao M, Zhang RG. 2006. Spring foraging sites of three pheasants at Nanhua part in Ailaoshan National Nature Reserve. Zool Res, 27(5):495–504. (in Chinese with English abstract)
|
|
Lu X, Zheng GM. 2007. Dominance-dependent microroost use in flock-living Tibetan eared pheasants. Ardea, 95:225–234.
|
|
Manly BFJ, McDonald LL, Tomas DL, McDonald TL, Erickson WP. 2002. Resource selection by animals: statistical design and analysis for field studies. Kluwer Academic Press, London.
|
|
May RM. 1973. Stability and complexity in model ecosystems. Princeton University Press, Princeton.
|
|
Moore AD. 1945. Winter night habits of birds. Wilson Bull, 57:253–260.
|
|
Shao C, Hu YZ. 2005. Lophura nycthemere's choice of night roosts and their night-roosting behavior. J Zhejiang Forest Coll, 22(5):562–565. (in Chinese with English abstract)
|
|
Wang YK. 2000. Integrated Investigation Report on Dazhongshan Provincial Natural Reserve. Nanhua County. Nanhua: Nanhua Forest Bureau. (in Chinese)
|
|
Young L, Zheng GM, Zhang ZW. 1991. Winter movements and habitat use by Cabot's tragopan in Southeastern China. Ibis, 133(2):121–126.
|
|
Zhang DY, Jiang XH. 1997. A hypothesis for the origin and maintenance of within-community species diversity. Biodivers Sci, 5(3):161–167. (in Chinese with English abstract)
|
|
Zheng GM. 1995. Ornithology. Beijing Normal University Press, Beijing, pp 491–497. (in Chinese)
|
| Factor | i | Ni | Ri | Ei | Selective | |||||
| S | L | S | L | S | L | |||||
| Vegetation type | MEBF | 0.49 | 0.78 | 1.00 | 0.23 | 0.34 | + | + | ||
| DBF | 0.05 | 0.00 | 0.00 | −1.00 | −1.00 | − | − | |||
| PYF | 0.46 | 0.22 | 0.00 | −0.35 | −1.00 | − | − | |||
| Note: S, Syrmaticus humiae; L, Lophura nycthemera; DBF, deciduous broadleaf forest; MEBF, middle-mountain, moist evergreen broadleaf forest; PYF, Pinus yunnanensis forest; +, observed usage is significantly higher than expected; 0, observed usage is almost equal to expected; −, observed usage is significantly lower than expected; other abbreviations are the same as in Eq. (1). | ||||||||||
| Habitat characteristics | Factor types | Roosting habitat (mean ± SE) | t-test (two-tailed) | |||
| S | L | t | p | |||
| Roost tree | DBH | 18.5±5.3 | 27.5±5.1 | −2.586 | 0.014* | |
| Crown size | 32.2±5.6 | 31.2±12.6 | 0.099 | 0.922 | ||
| Height | 8.1±1.5 | 11.3±1.5 | −3.100 | 0.004** | ||
| Roost branch | Height | 3.6±0.6 | 6.4±0.8 | −5.894 | 0.000** | |
| APS | 94.7±5.6 | 92.0±5.0 | 0.765 | 0.449 | ||
| COP | 79.3±5.9 | 76.5±7.5 | 0.624 | 0.537 | ||
| Tree layer | Density | 25.0±8.0 | 38.9±10.7 | −2.180 | 0.036* | |
| AHCT | 8.2±1.1 | 9.3±0.8 | −1.747 | 0.089 | ||
| ADBH | 11.4±2.0 | 12.6±1.9 | −0.876 | 0.387 | ||
| Cover | 60.7±6.3 | 66.8±5.2 | −1.557 | 0.128 | ||
| Shrub layer | Cover | 17.7±7.4 | 11.9±4.8 | 1.374 | 0.177 | |
| AHS | 1.5±0.3 | 1.7±0.2 | −1.216 | 0.231 | ||
| Density | 9.9±6.5 | 4.9±0.9 | 1.613 | 0.123 | ||
| Herb layer | LLC | 82.2±4.7 | 86.3±3.5 | −1.466 | 0.151 | |
| HC | 10.5±7.0 | 2.3±3.3 | 2.214 | 0.035* | ||
| Macrohabitat | DSR | 34.4±7.9 | 57.4±29.9 | −1.561 | 0.133 | |
| DSW | 39.3±11.2 | 72.5±23.8 | −2.642 | 0.014* | ||
| Aspect | 11.8±34.8 | 29.3±12.3 | −0.993 | 0.331 | ||
| Slope | 31.2±4.8 | 30.1±3.5 | 0.371 | 0.712 | ||
| Elevation | 2421.6±16.8 | 2465.3±23.3 | −3.189 | 0.003** | ||
| Note: S, Syrmaticus humiae; L, Lophura nycthemera; ADBH, average diameter at breast height of canopy trees; AHCT, average height of canopy trees; AHS, average height of shrubs; APS, angle between perching branch and stock; COP, cover over perch; DBH, diameter at breast height; DSR, distance to road; DSW, distance to water; HC, herb coverage; LLC, leaf litter coverage; * p < 0.05; ** p < 0.01; other abbreviations are the same as for Table 1. | ||||||
| Factor | Factor type | Factor loading | ||||||||||||||||
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |||||||||||||
| S | L | S | L | S | L | S | L | S | L | S | L | |||||||
| Roost tree | DBH | 0.92 | 0.84 | 0.02 | 0.11 | −0.09 | 0.01 | −0.08 | 0.15 | 0.21 | 0.14 | −0.02 | −0.24 | |||||
| Crown size | 0.89 | 0.82 | −0.01 | −0.26 | −0.09 | −0.02 | −0.07 | 0.24 | 0.21 | 0.16 | 0.14 | 0.11 | ||||||
| Height | 0.83 | 0.84 | −0.09 | 0.39 | −0.20 | 0.15 | −0.16 | 0.16 | 0.26 | −0.07 | −0.24 | 0.05 | ||||||
| Roost branch | Height | 0.64 | 0.40 | −0.07 | 0.54 | −0.37 | 0.62 | −0.26 | 0.00 | 0.19 | 0.07 | −0.09 | −0.21 | |||||
| APS | 0.00 | −0.16 | 0.83 | 0.00 | 0.10 | −0.15 | 0.22 | 0.11 | −0.12 | −0.01 | 0.15 | −0.20 | ||||||
| COP | 0.13 | −0.16 | 0.48 | −0.01 | −0.08 | 0.60 | −0.10 | 0.08 | 0.34 | 0.61 | 0.71 | 0.25 | ||||||
| Tree layer | Density | −0.27 | −0.07 | 0.17 | −0.55 | 0.80 | −0.12 | 0.05 | −0.22 | −0.42 | 0.73 | 0.04 | −0.15 | |||||
| AHCT | 0.27 | 0.17 | −0.06 | 0.92 | 0.14 | 0.04 | −0.05 | 0.21 | 0.89 | 0.02 | 0.09 | −0.22 | ||||||
| ADBH | 0.40 | −0.14 | −0.04 | 0.65 | −0.16 | 0.59 | 0.02 | 0.32 | 0.84 | 0.02 | 0.04 | 0.18 | ||||||
| Cover | 0.49 | 0.17 | 0.22 | −0.01 | 0.43 | 0.81 | −0.22 | 0.27 | −0.36 | −0.04 | 0.42 | −0.29 | ||||||
| Shrub layer | Cover | −0.18 | 0.30 | −0.06 | 0.02 | 0.07 | 0.21 | 0.94 | 0.89 | −0.11 | 0.02 | −0.02 | 0.01 | |||||
| AHS | −0.08 | 0.29 | 0.20 | 0.14 | 0.12 | 0.09 | 0.83 | 0.78 | 0.23 | 0.28 | 0.04 | −0.32 | ||||||
| Density | −0.12 | 0.56 | −0.51 | −0.11 | 0.04 | 0.36 | 0.75 | 0.33 | −0.22 | 0.13 | 0.07 | 0.12 | ||||||
| Herb layer | LLC | −0.22 | 0.00 | −0.22 | −0.75 | 0.83 | 0.06 | 0.14 | 0.19 | 0.14 | −0.05 | 0.04 | 0.18 | |||||
| HC | −0.02 | −0.61 | 0.13 | −0.15 | 0.00 | −0.13 | −0.12 | −0.16 | 0.12 | 0.33 | −0.88 | 0.55 | ||||||
| Macrohabitat | DSR | 0.08 | 0.02 | −0.82 | −0.17 | −0.13 | −0.08 | 0.15 | −0.08 | 0.10 | −0.09 | 0.04 | 0.92 | |||||
| DSW | −0.06 | −0.45 | 0.18 | −0.29 | 0.72 | −0.10 | 0.08 | −0.31 | 0.20 | −0.17 | −0.23 | 0.46 | ||||||
| Aspect | −0.34 | 0.43 | 0.27 | −0.40 | −0.11 | 0.51 | 0.03 | −0.33 | 0.17 | 0.41 | 0.74 | −0.19 | ||||||
| Slope | −0.03 | −0.23 | 0.70 | 0.22 | −0.47 | 0.26 | −0.17 | −0.37 | 0.14 | 0.32 | 0.28 | −0.41 | ||||||
| Elevation | 0.31 | −0.19 | −0.11 | 0.04 | 0.05 | 0.04 | 0.00 | −0.08 | −0.08 | 0.89 | −0.07 | 0.26 | ||||||
| Percentage of variance explained (%) | 18.1 | 18.6 | 13.3 | 15.1 | 12.8 | 11.8 | 12.2 | 11.6 | 11.9 | 11.4 | 11.3 | 10.9 | ||||||
| Cumulative percentage (%) | 18.1 | 18.6 | 31.4 | 33.7 | 44.2 | 45.5 | 56.3 | 57.1 | 68.2 | 68.5 | 79.5 | 79.4 | ||||||
| Abbreviations are the same as for Tables 1 and 2. | ||||||||||||||||||
| Habitat factors | Classification feature of habitat |
| Macrohabitat | 1. MEBF ………………………………………………….. 2 |
| 2a. Slope 31.2, Aspect 11.8, DSW 39.3 m, DSR 34.4 m …………………………………………. S |
|
| 2b. Elevation 2465 m, DSR 57.4 m ………….. L | |
| Tree layer | 3. AHCT > 8.2 m, ADBH > 11.4 m …………… 4 |
| 4a. Density 25 tubes per m2 …………………… S | |
| 4b. Density 39 tubes per m2, Cover 66.8 … L | |
| Shrub layer | 5. Cover > 11.9, AHS > 1.5 ……………………… 6 |
| 6a. Density 10 tubes per m2 …………………… S | |
| 6b. Density 5 tubes per m2 …………………….. L | |
| Herb layer | 7a. HC 10.5, LLC big ……………………………….. S |
| 7b. HC 2.6, LLC small ………………………………. L | |
| Roost tree | 8. Oak, Crown size > 31.2 ……………………….. 9 |
| 9a. DBH 18.5 cm, Height 8.1 m ……………….. S | |
| 9b. DBH 27.5 cm, Height 11.3 m …………….. L | |
| Roosting branch | 10. COP > 76.5 ……………………………………… 11 |
| 11a. Height 3.6 m, APS 94.7 ………………….… S | |
| 11b. Height 6.4 m …………………………………… L | |
| Abbreviations are the same as for Tables 1 and 2. | |