| Citation: | Xuan Peng, Limin Wang, Chenchen Shao, Dongming Li. 2025: Avian acoustic communication: Understanding of peripheral and central neural systems with ecological adaptations. Avian Research, 16(1): 100248. DOI: 10.1016/j.avrs.2025.100248 |
Avian vocal communication represents one of the most intricate forms of animal language, playing a critical role in behavioral interactions. Both peripheral and central auditory-vocal pathways are essential for precisely integrating acoustic signals, ensuring effective communication. Like humans, songbirds exhibit vocal learning behaviors supported by complex neural mechanisms. However, unlike most mammals, songbirds possess the remarkable ability to regenerate damaged auditory cells. These capabilities offer unique opportunities to explore how birds adjust their vocal behavior and auditory processing in response to dynamic environmental conditions. Recent studies have advanced our understanding of the plasticity of avian vocal communication system, yet the vocal diversity and neurophysiological mechanisms underlying vocalization and hearing have often been examined independently. A comprehensive overview of how these systems interact and adapt in birds remains lacking. To address this gap, this review synthesizes the peripheral and central features of avian vocalization and hearing, while also exploring the mechanisms that drive the remarkable plasticity of these systems. Furthermore, it explores seasonal variations in bird vocalization and hearing and adaptations to environmental noise, focusing on how hormonal, neural, and ecological factors together shape vocal behavior and auditory sensitivity. Avian vocal communication systems present an exceptional model for studying the integration of peripheral and central vocal-auditory pathways and their adaptive responses to ever-changing environments. This review underscores the dynamic interactions between avian vocal communication systems and environmental stimuli, offering new insights into broader principles of sensory processing, and neuroplasticity.
In natural environments, vocal communication plays a crucial role in the behavioral ecology of animals, serving essential functions in mate attraction, territorial defense, and social interaction (Webster and Podos, 2018; Yadav et al., 2024). Among these animals, birds are notable for their sophisticated vocalizations, which form a key part of the “acoustic niche” in various ecosystems worldwide (Catchpole and Slater, 2003). Effective vocal communication requires accurate production and precise perception of acoustic signals. These processes are mediated by integrated vocal communication systems involving peripheral and central auditory pathways (Woolley and Moore, 2011; Elie et al., 2020).
Notably, certain bird species—including songbirds, parrots, and hummingbirds—exhibit vocal learning, a specialized mechanism that allows them to imitate sounds (Jarvis et al., 2000; Cahill et al., 2021). This capacity is fundamental to developing complex vocalizations and has attracted substantial research attention due to its striking parallels with human speech (Catchpole and Slater, 2003; Pfenning et al., 2014; Vernes et al., 2021). Among these species, songbirds display vocalizations that are not only widespread but also characterized by remarkable diversity and complexity, making them one of the most intricate forms of animal communication (Podos et al., 2004; Brenowitz and Beecher, 2005; Sainburg et al., 2019). Unlike mammals, birds possess the remarkable ability to regenerate damaged auditory cells, making them exceptional models for studying neuroplasticity and its responses to acoustic stress (Marean et al., 1993; Woolley et al., 2001; Warchol, 2011; Rubel et al., 2013; Sato et al., 2024). This regenerative capability offers an exciting opportunity to explore how birds’ nervous systems respond to challenges such as environmental noise and other acoustic stressors.
Recent studies have increasingly focused on the plasticity of the vocal communication system, advancing our understanding of how birds adjust their vocal behavior and auditory processing in response to dynamic environmental conditions (Nieder and Mooney, 2020; Derryberry and Luther, 2021; Duque and Carruth, 2022; Engel et al., 2024). The adaptability of birds’ vocal and auditory systems is influenced by a complex interplay of developmental, hormonal, and neural factors (Harding, 2004; Louder et al., 2019; Sato et al., 2024), allowing them to maintain effective communication despite the challenges posed by habitat changes, seasonal cycles, and noise pollution (Catchpole and Slater, 2003; Sisneros et al., 2004; Luther and Derryberry, 2012; Deoniziak and Osiejuk, 2019). The vocal and auditory systems are intricately connected, including the peripheral organs responsible for sound production and reception and the sophisticated neural circuits involved in sound processing. Both systems exhibit remarkable plasticity, adjusting to environmental demands and seasonal variations (Sisneros et al., 2004). However, while the vocal diversity and neurophysiological mechanisms behind vocalization and hearing have been explored independently, a comprehensive overview of how these systems interact and adapt in birds is still lacking. This review aims to fill this gap by summarizing the key features of avian vocalization and hearing, discussing the influence of environmental and hormonal factors on vocal behavior and hearing sensitivity, and highlighting the neural mechanisms underlying the remarkable plasticity of the avian vocal communication system.
Bird vocalizations are highly diverse and serve essential functions in communication, including territory defense, mate attraction, coordination of group behaviors, alarm calls, and parent-offspring interactions. These vocalizations are typically categorized into calls and songs (Marler and Slabbekoorn, 2004; Rose et al., 2022). Calls are short and simple signals, often consisting of a single syllable with a basic frequency pattern. They are produced by both sexes throughout the year and serve a range of functions such as attracting mates, defending territories, signaling hunger by nestlings, warning of predators, assisting in food finding, and maintaining group cohesion (Marler and Slabbekoorn, 2004). In contrast, songs are longer vocalizations, lasting from several seconds to minutes, that are acoustically complex and consist of hierarchically organized syllables (Ivanitskii and Marova, 2022). Songs are generally produced by songbirds during the breeding season and play a crucial role in mate attraction and territorial defense (Eens et al., 1991; Forstmeier and Balsby, 2002). Some species, such as Budgerigars (Melopsittacus undulatus), can mimic human speech, and their songs are structured with consonant- and vowel-like segments (Mann et al., 2021). Similarly, Grey Catbirds (Dumetella carolinensis) exhibit extensive syllable repertoires, suggesting a generative system for syllable creation (Kroodsma et al., 1997). Consequently, songbirds serve as an excellent model for studying vocal communication, as their vocal behavior shares key similarities with human speech (Sainburg et al., 2019; Vernes et al., 2021).
The frequency range of avian vocalizations varies significantly across species, reflecting their ecological needs, social behaviors, and evolutionary adaptations. Typically, the vocalization frequency of most birds ranges between 1 and 4 kHz (Nowicki, 1997). However, some species produce complex songs with a broader frequency range, reaching down to 23 Hz and up to 10 kHz (Mack and Jones, 2003; Duque et al., 2020). Such adaptations enable effective long-distance signal transmission, particularly in acoustically challenging environments (Hardt and Benedict, 2021). On the other hand, species like doves produce low-frequency calls that travel efficiently through complex environments (Guo et al., 2016). Vocalization frequency is also influenced by body size and the structure of the vocal apparatus (Fletcher, 2004; Riede et al., 2006). Larger birds tend to produce lower-frequency sounds due to the physical constraints of their syrinx, which can generate sounds that travel farther than those of smaller birds (Marten et al., 1977; Friis et al., 2021; Mikula et al., 2021). Additionally, habitat characteristics play a significant role in shaping vocalization frequency: birds in open spaces tend to favor higher frequencies to minimize interference from wind, while forest-dwelling birds often produce lower frequencies to reduce signal degradation caused by reflections and absorption in dense foliage (Morton, 1975). Furthermore, increasing vocal amplitude can enhance signal transmission by reducing the masking effects of environmental noise (Nemeth et al., 2013).
During the breeding season, various aspects of song parameters are often used as indicators of individual quality. For example, song complexity, measured by the number of syllables and singing rate in male Zebra Finches (Taeniopygia guttata), has been linked to overall health and fitness (Nowicki and Searcy, 2004). In Swamp Sparrows (Melospiza georgiana) and White-crowned Sparrows (Zonotrichia leucophrys), females assess male quality by evaluating the trill rate and frequency bandwidth of their songs (Ballentine et al., 2004; Phillips and Derryberry, 2017). Alarm calls produced by both males and females can also be recognized by sympatric heterospecifics, facilitating both conspecific and interspecific social information exchange within mixed-species groups (Keen et al., 2020). Overall, avian vocalizations exhibit considerable diversity in structure and function, reflecting a complex interplay of ecological, social, and physiological factors.
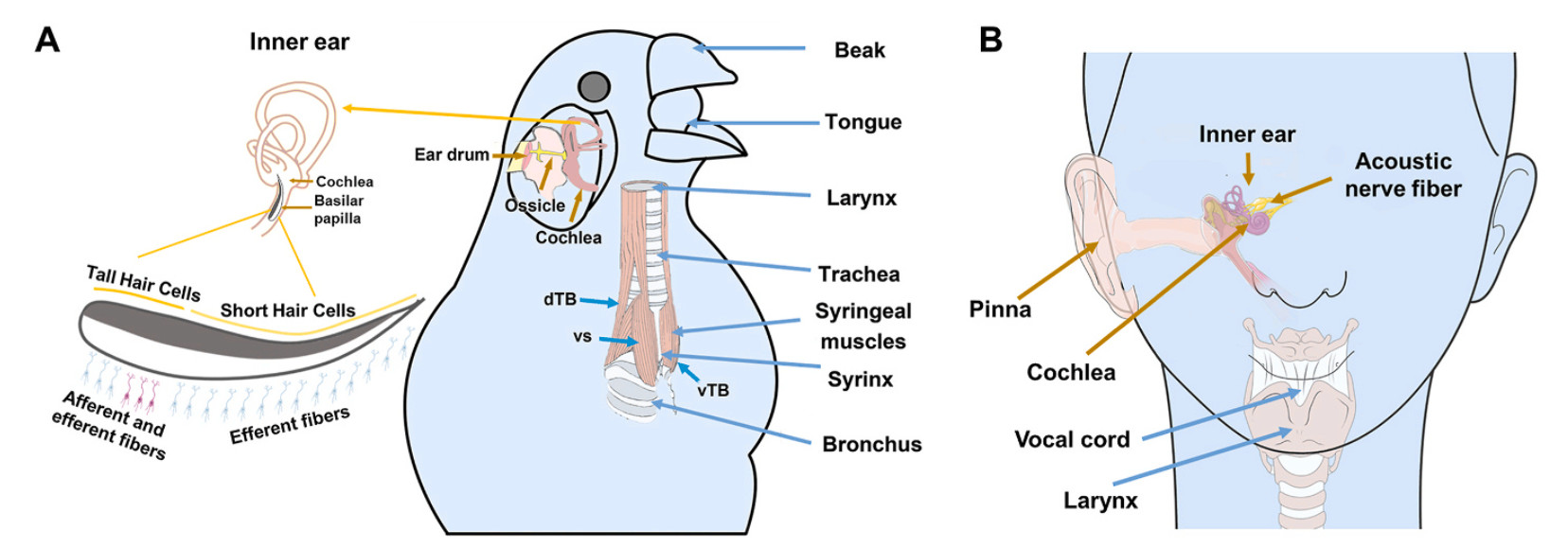
In contrast to non-avian vertebrates, where the larynx is the primary vocal organ for sound production (Tecumseh Fitch and Reby, 2001), birds produce sounds using the syrinx, located at the junction of the trachea and bronchi (Fig. 1; Goller, 2022). Although birds possess both a larynx and a syrinx (Kingsley et al., 2018), the syrinx is the specialized organ for phonation. In songbirds, the syrinx is controlled by four to six bilaterally and ipsilaterally innervated syringeal muscles (Düring et al., 2013). The dorsal syringeal muscle (ds) and ventral syringeal muscle (vs) are the largest, with the ventral muscles playing a critical role in controlling tension in the sound-generating labia (Goller and Suthers, 1996; Alonso et al., 2014; Döppler et al., 2018). The dorsal and ventral tracheobronchial muscles (dTB and vTB) move the lateral labium, affecting adduction and abduction, thereby modulating the sound produced (Goller and Riede, 2013). Moreover, vocalizations are further modified by the upper vocal tract, where various mechanisms, including beak and tongue movements and tracheal length adjustments, contribute to shaping the acoustic filter characteristics (Faiβ et al., 2022). In contrast, non-songbirds generally possess a simpler syrinx with fewer intrinsic muscles. This anatomical simplicity limits their vocal versatility compared to songbirds (Elemans et al., 2008).
Birds exhibit a wide range of hearing frequency ranges essential for their survival and communication (Table 1). Hearing sensitivity and frequency range varies significantly across species, depending on their ecological lifestyle (Dooling et al., 2000). Some birds can detect frequencies as high as 15 kHz, which is still lower than the upper hearing limits observed in mammals (Wever et al., 1969). Notably, avian hearing sensitivity tends to be greatest within the frequency range of their vocalizations, which helps birds detect calls even in noisy environments (Henry et al., 2016). For instance, songbirds, which often produce high-frequency vocalizations, exhibit enhanced sensitivity to these frequencies, while owls are particularly sensitive to lower frequencies, which are crucial for locating prey (Takahashi, 2010; Duque et al., 2020).
| Order | Family | Species | Auditory frequency range | Reference |
| Sphenisciformes | Spheniscidae | African Penguin (Spheniscus demersus) | 100 Hz–15 kHz | Wever et al. (1969) |
| Anseriformes | Anatidae | Mallard Duck (Anas platyrhynchos) | 16 Hz–9 kHz | Hill (2017) |
| Anseriformes | Anatidae | Canvasback (Aythya valisineria) | 190 Hz–5.2 kHz | Edwards (1943) |
| Galliformes | Numididae | Helmeted Guineafowl (Numida meleagris) | 2 Hz–8 kHz | Heffner et al. (2024) |
| Galliformes | Phasianidae | Indian Peafowl (Pavo cristatus) | 4 Hz–7.065 kHz | Heffner et al. (2020) |
| Galliformes | Phasianidae | Chicken (Gallus gallus domesticus) | 9.1 Hz–7.2 kHz | Hill et al. (2014) |
| Galliformes | Phasianidae | Japanese Quail (Coturnix japonica) | 16 Hz–8 kHz | Strawn and Hill (2020) |
| Galliformes | Phasianidae | Common Pheasant (Phasianus colchicus) | 250 Hz–10.5 kHz | Stewart (1955) |
| Charadriiformes | Laridae | Black-headed Gull (Chroicocephalus ridibundus) | 100 Hz–10 kHz | Beuter and Weiss (1986) |
| Columbiformes | Columbidae | Domestic Pigeon (Columba livia) | 2 Hz–8 kHz | Heffner et al. (2013) |
| Columbiformes | Columbidae | Common Pigeon (Columba livia) | 200 Hz–7.5 kHz | Brand and Kellogg, 1939 |
| Strigiformes | Strigidae | Great Horned Owl (Bubo virginianus) | 60 Hz–7 kHz | Edwards (1943) |
| Strigiformes | Strigidae | Northern Saw-whet Owl (Aegolius acadicus) | 700 Hz–8.6 kHz | Beatini et al. (2018) |
| Strigiformes | Tytonidae | Western Barn Owl (Tyto alba) | 1 kHz–10 kHz | Krumm et al. (2017) |
| Psittaciformes | Psittaculidae | Budgerigar (Melopsittacus undulatus) | 77 Hz–7.6 kHz | Heffner et al. (2016) |
| Apodiformes | Trochilidae | Chimborazo Hillstar (Oreotrochilus chimborazo) | >10 kHz | Duque et al. (2020) |
| Passeriformes | Calcariidae | Snow Bunting (Plectrophenax nivalis) | 400 Hz–7.2 kHz | Edwards (1943) |
| Passeriformes | Alaudidae | Shore Lark (Eremophila alpestris) | 350 Hz–7.6 kHz | Edwards (1943) |
| Passeriformes | Corvidae | Blue Jay (Cyanocitta cristata) | ~7.8 kHz | Cohen et al. (1978) |
| Passeriformes | Fringillidae | Atlantic Canary (Serinus canaria) | 250 Hz–9 kHz | Dooling et al. (1971) |
| Passeriformes | Fringillidae | House Finch (Haemorhous mexicanus) | ~7.2 kHz | Dooling et al. (1978) |
| Passeriformes | Icteridae | Red-winged Blackbird (Agelaius phoeniceus) | ~9.6 kHz | Hienz et al. (1977) |
| Passeriformes | Icteridae | Brown-headed Cowbird (Molothrus ater) | ~9.7 kHz | Hienz et al. (1977) |
| Passeriformes | Passerellidae | Field Sparrow (Spizella pusilla) | ~11 kHz | Dooling et al. (1979) |
| Passeriformes | Passeridae | House Sparrow (Passer domesticus) | 675 Hz–11.5 kHz | Brand and Kellogg, 1939 |
| Passeriformes | Sturnidae | Common Starling (Sturnus vulgaris) | 700 Hz–14 kHz | Brand and Kellogg, 1939 |
In birds, the basilar papilla, located in the tubular cochlear duct of the inner ear, is the primary auditory organ and is functionally analogous to the mammalian cochlea (Fig. 1; Fischer, 1994; Basch et al., 2016). The hair cells of the basilar papilla are sensory receptors interspersed with supporting cells (McPherson, 2018). These hair cells are responsible for mechanoelectrical transduction, which converts sound vibrations into neural signals conveyed along the auditory nerve to the brainstem, where central auditory processing begins (Hudspeth et al., 2000).
The hair cells of the basilar papilla are classified into two types: tall and short. Tall hair cells are positioned in the fibrocartilaginous plate above the auditory ganglion, while short hair cells are located on the epithelium above the basilar membrane, extending to the inferior fibrocartilaginous plate (Janesick et al., 2021). The relative numbers of tall and short hair cells vary along the tonotopic gradient (Hirokawa, 1978). These hair cells exhibit distinct cytomorphologies, notably in the number of stereocilia within their hair bundles. Tall hair cells are primarily innervated by afferent fibers, as well as small bouton efferent fibers, whereas short hair cells are predominantly innervated by efferent fibers, which are characterized by prominent presynaptic cups (Peng and Ricci, 2011). It is known that tall hair cells are more specialized for sensing low-frequency signals, while short hair cells are more attuned to high-frequency signals (Xia et al., 2016). Furthermore, the health of these hair cells is critical to hearing sensitivity. Damage to these cells can result in temporary or permanent hearing loss, significantly affecting auditory function (Sato et al., 2024).
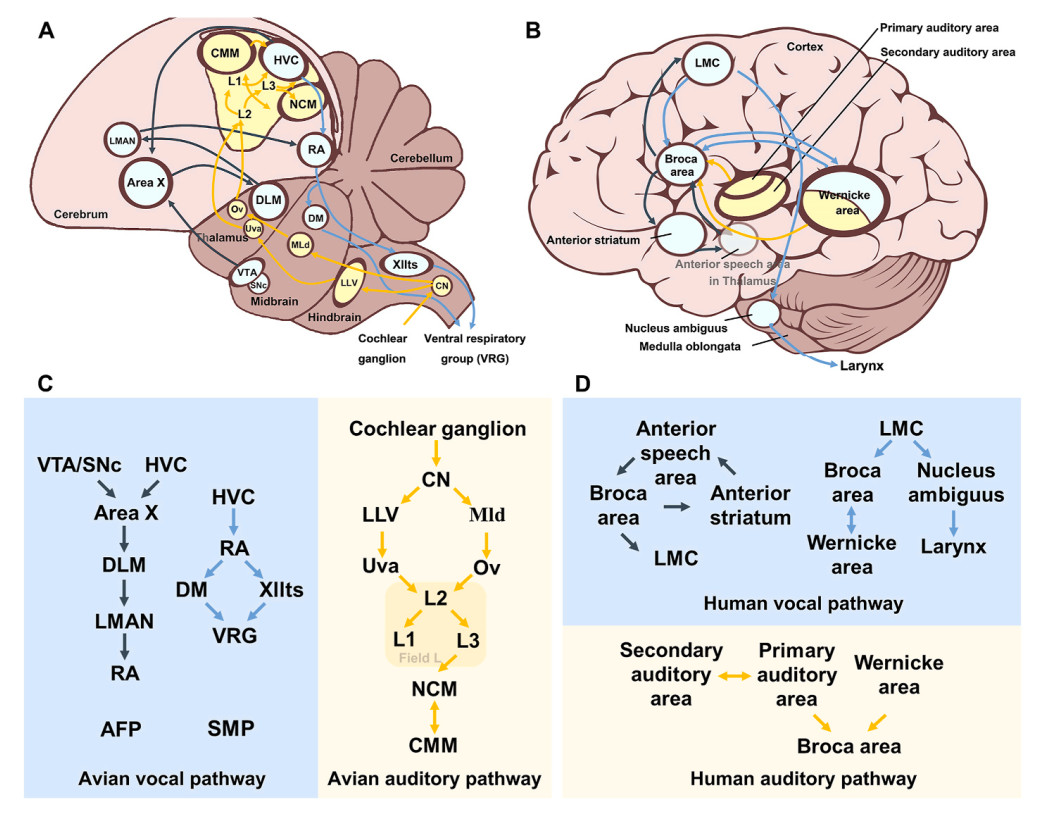
The neuronal connectivity of vocal systems in birds varies greatly across taxa. Non-songbirds generally lack the specialized forebrain circuits indispensable for complex vocal learning and production, except for independently evolved vocal learners such as parrots and hummingbirds (Ball, 1994; Colquitt et al., 2021). In songbirds, the vocal system comprises interconnected brain nuclei in the forebrain, striatum, thalamus, and brainstem (Fig. 2; Zhang et al., 2023). This system can be broadly divided into two major pathways: the anterior forebrain pathway (AFP) and the song motor pathway (SMP). The AFP is primarily involved in the acquisition and maintenance of vocalizations, whereas the SMP plays a key role in producing vocalizations, functioning as the primary motor pathway responsible for generating songs (Roberts and Mooney, 2013). Prior to vocalization, neurons in both the AFP and SMP exhibit increased activity, which persists even after deafening, suggesting that these pathways are motor-driven in origin (Schmidt, 2003). The AFP initiates song production through phasic activity in the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) of the midbrain, which together provide the initial “start signal” for song initiation (Yanagihara et al., 2021). Neurons in the VTA/SNc project to the cerebral song system nuclei, including the HVC (a proper noun), play a critical role in controlling song properties (Gale and Perkel, 2006; Yanagihara et al., 2021). Signals from HVC are transmitted to Area X, a nucleus that provides inhibitory input to the medial nucleus of the dorsolateral thalamus (DLM). The DLM, in turn, sends excitatory projections to the lateral portion of the magnocellular nucleus of the anterior nidopallium (LMAN), a nucleus essential for song variability and plasticity (Warren et al., 2011). The output of the AFP is subsequently directed to the robust nucleus of the arcopallium (RA), which acts as the primary target of LMAN and is instrumental in controlling motor aspects of song production (Warren et al., 2011).
The SMP, originating in HVC, directly projects to RA. Axons from RA terminate on motor neurons in the tracheosyringeal part of the hypoglossal motor nucleus (XIIts), which control the syringeal muscles responsible for sound production, and on respiratory premotor neurons in the ventral respiratory group (VRG), which regulate the respiratory muscles for breathing (Schmidt et al., 2012; Adam et al., 2021). RA axons also project to the dorsomedial intercollicular nucleus (DM) in the midbrain, which generates calls (Mooney, 2009). Within the SMP, both HVC and RA play critical roles in controlling song timing and spectral properties (Hahnloser et al., 2002; Lynch et al., 2016). Neurons within the RA contribute to encoding the features of songs, although they do not appear to influence the temporal structure of the song itself (Miller et al., 2017). Before songbirds begin singing, neurons in the HVC-RA pathway exhibit precise spiking patterns tightly timed within the song sequence. Specific neurons in HVC respond selectively to particular combinations of song elements, highlighting the HVC's role in generating song sequences (Nishikawa et al., 2008). Meanwhile, individual RA neurons can fire up to 10 times per motif, in contrast to HVC-RA neurons, suggesting that information from HVC is translated into more specific signals that correlate with the acoustic features of the song (Yip et al., 2012).
The AFP also plays an instructive role during song development in juvenile songbirds and in song plasticity in adults (Tachibana et al., 2022). The output nucleus LMAN is vital for introducing song variations during juvenile learning and adult singing. Lesions in LMAN result in less variable and more crystallized songs, with reduced or absent context-dependent changes in variability, especially after deafening (Bottjer et al., 1984; Kao and Brainard, 2006). Notably, spike timing variability and burst frequency in LMAN correlate with sensorimotor learning and singing variability (Kao et al., 2008; Chung and Bottjer, 2022). For example, male Zebra Finches modify the structure of their songs depending on the social context (Woolley and Doupe, 2008). When singing to females, their songs become more directed and stereotyped, a change reflected in altered neural activity within the AFP. This ability to adapt songs based on social context is crucial for mate attraction and social interaction. Furthermore, preferential responsiveness to species-specific songs is found throughout the song system, including in the hypoglossal motor nucleus, which innervates the syrinx. This selectivity allows songbirds to distinguish between potential mates and rivals (Solis et al., 2000). It also contributes to the learning and refinement of their own songs, which is essential for their social integration within the species.
Avian vocal signals are initially detected by the inner hair cells of the cochlea, which transmit auditory information to the cochlear ganglion cells. These ganglion cells project to the cochlear nucleus (CN), which then relays the auditory signals to the brain via two primary pathways (Fig. 2; Theunissen et al., 2004). One pathway runs through the ventral portion of the lateral lemniscus (LLV) and the thalamic nucleus Uvaformis (Uva) of the hindbrain, while the other travels through the dorsal lateral nucleus of the mesencephalon (MLd), reaching the thalamic nucleus ovoidalis (Ov) and ultimately terminating in the Field L complex. The Field L complex is considered the primary auditory cortex of birds and consists of the thalamo-recipient subregion (L2) along with secondary subregions (L1 and L3), which are interconnected with L2 and located in the caudomedial forebrain (Pytte et al., 2010). Neurons in L2 and L3 project directly to the caudomedial nidopallium (NCM), which reciprocally connects with the caudolateral mesopallium (CMM) (Pinaud and Terleph, 2008). NCM also receives direct input from the shell of the Ov (Vates et al., 1996), and CMM has interconnections with the lateral mesopallium (CLM) (Bolhuis et al., 2010).
The avian nucleus magnocellularis (NM), homologous to the mammalian anteroventral cochlear nucleus (AVCN), is a key component of the sound localization system. Neurons in the NM receive excitatory input from auditory nerve fibers (ANFs) and transmit temporal information by generating spikes at specific phases of sound waves (Al-Yaari et al., 2020). In both NM and the nucleus angularis of the brainstem, auditory spatial information is processed, specifically through two distinct mechanisms: interaural level differences (ILDs) and interaural time differences (ITDs), which together form the auditory spatial receptive fields (aSRFs) in the external nucleus of the inferior colliculus (ICx) of the inferior colliculus (IC) (Fischer and Peña, 2011; Maldarelli et al., 2022).
The neural circuits involved in auditory processing are highly conserved among amniotic animals (Grothe et al., 2004; Tang et al., 2012). For instance, the avian Field L complex is homologous to the primary auditory cortex in the mammalian superior temporal gyrus, and the projection regions of Field L (NCM and CMM) are analogous to the auditory association cortex in mammals, particularly the belt and parabelt regions (Bolhuis et al., 2010). The NCM, a central structure in the avian auditory system, is primarily responsible for processing complex auditory stimuli, much like the mammalian auditory association cortex (Kang et al., 2025). NCM plays a pivotal role in forming and storing auditory memories, especially during song learning in juvenile songbirds. For example, in adult Zebra Finches, neurons in NCM retain long-term memory of tutor songs from the birds’ early development (Katic et al., 2022). Neuronal activation in NCM in response to the tutor song correlates with the number of song elements learned (Terpstra et al., 2004), and bilateral neurotoxic lesions of the NCM impair recognition of tutor songs while not affecting their ability to discriminate between calls (Gobes and Bolhuis, 2007). This highlights the critical function of NCM in preserving and recalling song memories.
Furthermore, NCM is involved in the real-time processing of auditory feedback during singing, helping maintain song fidelity. When song pitch is experimentally distorted, an intact NCM is necessary for motor recovery, suggesting that it is involved in storing and recalling the specific features of a bird's own song (BOS) (Canopoli et al., 2014). NCM neurons respond more robustly to conspecific songs than to heterospecific songs or other complex auditory stimuli, underscoring the region's role in song discrimination (Mello et al., 2004; Schroeder and Remage-Healey, 2021). In addition to its role in song learning and memory, NCM plays a key role in mate choice. Lesions to NCM result in a decreased preference for specific males, affecting female songbirds' song evaluation and mate selection (Lawley et al., 2022). Recent studies also suggest that NCM is involved in auditory scene analysis (ASA), with specific populations of neurons that can tolerate high levels of background noise and selectively respond to target signals (Fernández-Vargas et al., 2024).
The CMM is another important auditory region that plays a fundamental role in auditory signal transmission and song selectivity. CMM receives input from the lateral mesopallium (CLM) and exhibits greater selectivity for song components, while also being more variable in its responses to repeated song elements (Jeanne et al., 2011; Lynch et al., 2018). This suggests that the CMM is crucial for processing sound recognition, especially in the context of song learning and selection. Although the latencies and spiking patterns in response to song stimuli differ between NCM and CMM, both regions share many functional similarities in auditory processing (Inda et al., 2020). During the learning phase, both NCM and CMM in juvenile male Zebra Finches show increased neuronal activation, as evidenced by the expression of the immediate early gene product ZENK (an acronym for zif-268, egr-1, ngfi-a, and krox-24), particularly in the context of the bird's own song (Scully et al., 2017).
Songbirds learn to sing by listening to and memorizing tutor songs during two main phases: the sensory phase and the sensorimotor phase. Juveniles listen to and memorize one or more tutor songs in the memorization phase. In the sensorimotor phase, they use auditory feedback to compare their own songs with the memorized models, refining their vocalizations through thousands of repetitions (Tschida and Mooney, 2012). This process shares parallels with human speech learning, both at the behavioral, neural, and genetic levels (Fig. 2; Kuebrich and Sober, 2015), and provides valuable insights into the mechanisms of vocal learning in humans. The homologous components of the auditory and song-control circuits in songbird and mammalian brains make songbirds an ideal model for studying the neural mechanisms underlying vocal learning, with potential implications for understanding human language acquisition and treating language disorders.
The song system, consisting of the AFP and SMP, is intricately connected with the auditory system, ensuring efficient transmission and processing of vocal information. Specific neurons in both the HVC and auditory regions exhibit similar patterns of activity when songbirds either sing their songs or passively listen to them (Prather et al., 2008; Prather, 2013). In the HVC, there are two types of glutamatergic projection neurons (PNs); one population projects to the striatal song nucleus Area X (HVCX), and the other projects to the robust nucleus of the arcopallium (HVCRA). Additionally, local interneurons (HVCInt) inhibit HVCX cells, contributing to regulating neural activity within the HVC (Mooney and Prather, 2005). These PNs receive auditory inputs, providing a critical link between auditory perception and vocal control (Rosen and Mooney, 2006). Upon playback of BOS, both HVCX and HVCRA neurons exhibit bursts of action potentials. However, these two PN types display distinct subthreshold responses, likely reflecting their specific roles in processing and modifying auditory information before it is transmitted to downstream nuclei such as Area X and RA (Rosen and Mooney, 2006). This suggests the critical role of the HVC in integrating auditory input and shaping it for precise vocal production.
The nucleus interface of the nidopallium (NIf) serves as a major source of auditory excitatory input to all types of HVC PNs. It receives input from the CMM nucleus Avalanche (Av) (Akutagawa and Konishi, 2010), thus forming a feedback loop between the auditory forebrain and the song system (Lewandowski et al., 2013). Additionally, higher auditory regions, notably the CLM, provide secondary input to NIf (Shaevitz and Theunissen, 2007). The thalamic nucleus Uvaeformis also contributes by indirectly linking the brainstem nucleus LLV (ventral lateral lemniscus) to NIf and HVC (Danish et al., 2017). Notably, the firing patterns evoked by BOS in HVC PNs are sparser compared to those in NIf PNs, with HVC PNs showing more exclusive responses to BOS than NIf neurons (Coleman and Mooney, 2004). This difference highlights these regions' distinct roles in auditory processing and vocal control, with HVC being especially crucial for the representation of self-generated vocal sounds.
Moreover, there is an indirect connection between auditory inputs and dopaminergic neurons in VTA, which are associated with motivation and courtship behavior (Las and Fee, 2008). The CMM also projects to the vocal motor area RA, facilitating the timing and coordination of vocal production, especially during courtship (Louder et al., 2019). This interplay between vocal motor and auditory systems is necessary for effective songbird communication. For instance, in species like Plain-tailed Wrens (Pheugopedius euophrys) and White-browed Sparrow Weavers (Plocepasser mahali), the timing of sound production is tightly coordinated with a partner to produce vocal duets during the breeding season (Voigt et al., 2006; Fortune et al., 2011). This highlights the essential role of both auditory and song production networks in social communication.
Despite the strong connection between the song system and the auditory system, the cognitive systems responsible for vocal production and auditory recognition are largely subserved by distinct brain regions. For example, lesions to the NCM in adult male Zebra Finches impair recognition of the tutor song but do not affect song production, suggesting that the NCM is critical for auditory memory and recognition but not for vocal motor control (Gobes and Bolhuis, 2007). Conversely, lesions to the HVC in starlings disrupt song production but do not impair song recognition, further supporting the idea that the NCM and HVC are functionally dissociated (Gentner et al., 2000). This dissociation is consistent with the concept of “double dissociation, ” where lesions to the auditory system (NCM) and the song system (HVC) produce distinct deficits in song processing.
Unlike the specialized regions of NCM and CMM, neurons in midbrain auditory areas respond to a wide variety of sound stimuli, including conspecific and heterospecific songs, different tones, noise, and synthetic sounds mimicking songs (Woolley and Casseday, 2005; Schneider and Woolley, 2009; Schumacher and Woolley, 2009). These areas are involved in general auditory processing and sound discrimination, which is essential for sound localization functions. Sound localization, which helps avoid predators, detect prey, and identify potential mates, is processed early in the auditory pathway, particularly in the ICx region of the inferior colliculus (Maldarelli et al., 2022).
Interestingly, although the hippocampus lies outside the classical auditory pathway, it shows activation in response to conspecific songs in adult female Zebra Finches but less activation when they hear heterospecific songs (Bailey et al., 2002). This suggests that the hippocampus may be involved in the processing and storing auditory memories related to song recognition. Noise-induced hearing loss (NIHL) reduces auditory input to the central nervous system, impairing hippocampal function (Manohar et al., 2022). Exposure to intense noise can also disrupt place-specific firing patterns in hippocampal neurons, further highlighting the role of the hippocampus in auditory memory and spatial processing (Kraus et al., 2010).
Among the factors that influence neuroplasticity in vocal and auditory processing, adult neurogenesis—the generation of new neurons during adulthood—is one of the most striking features observed in songbirds (Barnea and Pravosudov, 2011). Adult neurogenesis is observed to occur in specific brain regions such as the HVC, Area X, and NCM, contributing to the functional adaptability of the songbird brain by integrating into existing neural circuits (Barnea and Pravosudov, 2011). Adult neurogenesis in songbirds is not uniform across these regions, with different neuronal populations exhibiting varying capacities for renewal. For instance, the HVCRA population is highly regenerative, whereas the HVCX and HVCInt populations show minimal neuronal turnover (Scotto-Lomassese et al., 2007). Similarly, the density of newly matured neurons in the medial NCM is significantly greater than in the lateral NCM, highlighting the unique role of neurogenesis in brain regions critical for song production and auditory memory (Pytte et al., 2010).
The amount and quality of song produced by a songbird play a significant role in regulating the addition of neurons within the HVC-RA circuit. Increased singing activity promotes the survival of newly generated neurons in the HVC, particularly within the HVCRA PNs population, through the upregulation of brain-derived neurotrophic factor (BDNF), a molecule critical for neuron incorporation and survival (Fig. 3; Li et al., 2000). For instance, in male canaries, the amount of song produced correlates with the number of new neurons incorporated into the HVC (Li et al., 2000; Alvarez-Borda and Nottebohm, 2002). Song quality is equally important: higher-quality songs result in more stable retention of new neurons, while poor-quality songs are associated with pruning or loss of these neurons (Wilbrecht and Kirn, 2004). Open-ended learners, such as canaries, who continue to learn new songs or song elements throughout their lives exhibit relatively stable turnover of HVCRA neurons (Alvarez-Borda and Nottebohm, 2002). In contrast, closed-ended learners, including Zebra Finches and Bengalese Finches (Lonchura striata), which acquire their songs primarily during early development, show a significant decline in the recruitment of new HVCRA neurons as they age (Wang et al., 2002; Beecher and Brenowitz, 2005). The electrical activity of target neurons in the RA also influences the addition of new HVCRA projection neurons. For example, when RA activity is experimentally inhibited in breeding white-crowned sparrows, the number of newborn neurons in the HVC decreases by 56% (Larson et al., 2013). This finding underscores the critical role of RA in modulating adult neurogenesis. Together, these observations indicate that both ongoing song learning and song production, or the lack thereof, directly impact neurogenesis and neural plasticity in the HVC-RA circuit.
Adult neurogenesis in the HVC is influenced not only by the songbird's own behavior but also by external auditory stimuli and social environment. For example, when adult birds are deafened, their song quality deteriorates, and the structure of their songs becomes more unstable due to the lack of sensory feedback (Pytte et al., 2012). Interestingly, birds with more newly incorporated HVC-RA neurons are better able to maintain the stability and stereotypy of their songs despite the absence of auditory input. This is thought to be due to the compensatory role of new neurons, which help stabilize song production by offsetting the loss of sensory feedback (Pytte et al., 2012). Similarly, when song structure is disrupted artificially—such as through Botox injections into the syrinx muscles to impair vocalization—birds with higher levels of new HVC neurons show more robust song recovery, indicating a link between neurogenesis in the HVC-RA circuit and song plasticity (Pytte et al., 2011).
Auditory feedback also plays an essential role in maintaining neurogenesis in auditory regions. For instance, auditory deprivation, such as the removal of cochlea in adult Zebra Finches, significantly decreases new neuron incorporation in the medial NCM (Pytte et al., 2010). Additionally, experiments involving unilateral denervation of the syrinx by cutting one of the tracheosyringeal nerves (nXIIts) have shown altered lateralization of new neurons in the NCM. This phenomenon is believed to arise from mismatches between the bird's expected and actual acoustic feedback during singing, further underscoring the impact of auditory input on neurogenesis (Aronowitz et al., 2021).
The social environment is another critical factor influencing adult neurogenesis (Fig. 3). Songbirds housed in complex, mixed-sex social groups exhibit greater neurogenesis in the HVC, Area X, and NCM compared to those housed in isolation or with unfamiliar mates (Lipkind et al., 2002; Barnea et al., 2006; Shevchouk et al., 2017). This suggests that social interactions and dynamic auditory environments promote the survival and incorporation of new neurons into both vocal and auditory circuits, as these settings impose higher cognitive and behavioral demands. Learning new songs, forming auditory memories, and adjusting vocalizations to respond to social cues likely drive this enhancement of neurogenesis.
The mechanisms underlying neuronal replacement in the songbird brain remain less understood. Under normal conditions, newborn neurons can replace older ones without learning new information. This process likely serves to maintain a healthy neuronal population, supporting neural plasticity and the ability to store existing auditory and vocal information. Interestingly, the retention of newly incorporated neurons is stronger when new song learning occurs. In contrast, a stable social environment that reinforces the retention of familiar songs tends to promote the preservation of older neurons (Lipkind et al., 2002). This dynamic balance between integrating new neurons for learning and maintaining older neurons for established memories highlights the remarkable plasticity and adaptability of the avian brain, particularly in those regions involved in vocalization and auditory processing.
Avian vocalization and hearing, i.e., the capability of sound production and processing, are regulated by a complex interplay of hormonal and neural factors (Fig. 3). Within the vocal system, the interaction among neurotransmitters, sexual hormones and neurotrophins can contribute to neuron growth and survival (Brenowitz, 2013). During singing, the dopamine (DA) secretion increases in songbirds (Sasaki et al., 2006). DA plays a crucial role in the regulation of learning and maintaining song patterns through modulating excitability and synaptic transmission in spiny neurons of the basal ganglia, particularly Area X (Ding and Perkel, 2002; Ding et al., 2003; Leblois et al., 2010). Lesions in dopaminergic inputs to the basal ganglia significantly impair vocal learning without affecting vocal performance quality (Hoffmann et al., 2016; Saravanan et al., 2019).
Elevated testosterone (T) levels promote the survival of adult-born neurons in the HVC by increasing the expression of BDNF, a key molecule for neuronal growth and integration (Brenowitz, 2013). In male canaries, T increases the number of perineuronal nets (PNN) in the HVC, RA, and Area X, supporting neuronal stability and integration (Cornez et al., 2020). Moreover, the enzyme aromatase converts T into estradiol (E2), resulting in the concurrent presence of both androgenic and estrogenic signals in the HVC. These two hormones work synergistically to promote endothelial cell division and angiogenesis in the HVC by inducing the localized expression of vascular endothelial growth factor (VEGF). The resulting expansion of the microvascular network in the HVC leads to the secretion of BDNF, which further supports the recruitment and integration of newly generated neurons into the existing vocal control circuits (Chen et al., 2013). These hormonal interactions ultimately facilitate the acquisition of purposeful and adaptive song production.
In addition to the trophic effects of androgens on neuronal generation in the HVC, estrogen, specifically neuroestrogen (locally synthesized E2), plays a critical role in regulating auditory processing in songbirds. Neuroestrogen can directly influence the function of neurons involved in central auditory processing, thereby shaping auditory-based behaviors (Sanford et al., 2010; Tremere and Pinaud, 2011; de Bournonville et al., 2020). Both male and female songbirds experience increased E2 synthesis within the NCM, a key region involved in auditory memory and song discrimination in response to social and auditory stimuli. This increase in E2 is rapid and localized specifically in the NCM, suggesting a specialized role for this hormone in auditory processing, particularly related to song perception (Remage-Healey et al., 2008; Jeong et al., 2011; Yoder and Vicario, 2012). Interestingly, this rise in E2 in the NCM does not occur in other auditory regions of the forebrain, highlighting the unique role of the NCM in processing auditory signals related to song.
The presence of estrogen receptors and aromatase within the NCM further supports the role of estrogen in regulating auditory function (Yoder and Vicario, 2012). Estrogen receptors are highly enriched in this region, unlike other auditory forebrain areas (de Bournonville et al., 2020). For example, when T levels increase, acute song-driven increases in neuroestrogen can be observed in the left hemisphere of the NCM (de Bournonville et al., 2020). Moreover, other forms of estrogen, such as estrone and estriol, may also contribute to the rapid regulation of auditory functions, and their effects may depend on the rise in T levels observed in response to song stimuli (de Bournonville et al., 2020). This suggests that the dynamic interaction between testosterone and estrogen in the NCM may modulate song perception and memory and the neural mechanisms underlying song discrimination.
Overall, the neurogenesis that supports vocal learning and auditory processing is modulated by various factors, including T, E2, and neuroendocrinological pathways. These factors promote the survival and integration of new neurons into key brain regions such as the HVC and NCM, facilitating the acquisition of accurate vocal patterns and auditory memory. Besides, these new neurons are crucial for learning new song motor patterns and maintaining stable song production, especially during the breeding season when accurate vocal performance is vital for communication and mate attraction (Brenowitz and Larson, 2015). In this context, the survival of new neurons can link to their contribution to song accuracy, reinforcing the relationship between vocal learning and neural plasticity.
The avian vocal communication system exhibits remarkable plasticity, allowing songbirds to adapt their vocal behavior and auditory processing to a variety of changing social, environmental, and physiological conditions (Snell-Rood, 2012). Many songbirds, in particular, demonstrate a high degree of vocal plasticity, learning their songs through social interaction during critical periods of development. Juvenile songbirds listen to conspecifics, imitate their vocalizations, and refine their songs based on auditory feedback. This capacity for vocal learning enables birds to modify their songs, adjust to different acoustic environments, and respond to seasonal and environmental changes (Table 2).
| Influential factor | Specific influential factor | Main consequences | Order | Family | Species | Reference |
| Habitat alteration | Different altitudes | Change in song structure | Passeriformes | Paridae | Mountain Chickadee (Poecile gambeli) | Branch and Pravosudov (2015) |
| Different altitudes | Various in performance of birdsong | Passeriformes | Meliphagidae | Honeyeater Family | Hay et al. (2024) | |
| Geographical isolation | Formation of dialects | Passeriformes | Fringillidae | Red Crossbill (Loxia curvirostra) | Hynes and Miller (2014) | |
| High vegetation density | Slower bird song with altered song divergence | Passeriformes | Troglodytidae | Grey-breasted Wood-wren (Henicorhina leucophrys) | Dingle et al. (2008) | |
| Increasing in vegetation | Increased minimum frequency and decreased bandwidth | Passeriformes | Passerellidae | Chipping Sparrow (Spizella passerina) | Job et al. (2016) | |
| Rainforest environment | Increased frequency range and altered song note delivery rate | Passeriformes | Pycnonotidae | Little Greenbul (Andropadus virens) | Slabbekoorn and Smith (2002) | |
| Migration distances | Reduced variety of shared song types | Passeriformes | Passerellidae | Golden-crowned Sparrow (Zonotrichia atricapilla) | Shizuka et al. (2016) | |
| Long time of geographically isolation | A loss of the terminal syllable | Passeriformes | Parulidae | Hermit Warbler (Setophaga occidentalis) | Janes and Ryker (2013) | |
| Environmental noise | Oceanic noise | Higher dominant frequency | Piciformes | Lybiidae | Yellow-fronted Tinkerbird (Pogoniulus chrysoconus) | Sebastianelli et al. (2020) |
| Oceanic noise | Reduced frequency and lower amplitude | Charadriiformes | Alcidae | Atlantic Puffin (Fratercula arctica) | Mooney et al. (2019) | |
| Oceanic noise | Higher amplitude | Passeriformes | Passerellidae | White-crowned Sparrow (Zonotrichia leucophrys) | Reed et al. (2022) | |
| Oceanic noise | Higher amplitude | Passeriformes | Paradoxornithidae | Wrentit (Chamaea fasciata) | Reed et al. (2022) | |
| Rain noise | Decreased singing behavior | Strigiformes | Strigidae | Tawny Owl (Strix aluco) | Lengagne and Slater (2002) | |
| Wind noise | Higher calling rates | Sphenisciformes | Spheniscidae | King Penguin (Aptenodytes patagonicus) | Lengagne et al. (1999) | |
| Waterfall noise | Greater signal redundancy | Passeriformes | Fringillidae | Chaffinch (Fringilla coelebs) | Brumm and Slater (2006) | |
| Natural and anthropogenic noise | Longer syllables and extended song duration | Carinatae | Troglodytidae | Pacific Wren (Troglodytes pacificus) | Gough et al. (2014) | |
| Anthropogenic noise | Higher frequencies and reduced song performance | Passeriformes | Passerellidae | White-crowned Sparrow (Zonotrichia leucophyrs) | Moseley et al. (2018) | |
| Traffic noise | Longer duration, lower introductory and peak frequencies, and greater variability in syllable types in bird songs | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Courter et al. (2020) | |
| Urban noise | More complex bird song | Passeriformes | Turdidae | Song Thrush (Turdus philomelos) | Deoniziak and Osiejuk (2019) | |
| Urban noise | Singing early than those in semi-natural habitats | Passeriformes | Turdidae | Common Blackbird (Turdus merula) | Nordt and Klenke (2013) | |
| Urban noise | Louder, higher-pitched songs | Passeriformes | Turdidae | Eastern Bluebird (Sialia sialis) | Kight and Swaddle (2015) | |
| Urban noise | Increased song amplitude | Passeriformes | Passeridae | House Sparrow (Passer domesticus) | Grimes et al. (2024) | |
| Urban noise | Increased song amplitude | Passeriformes | Fringillidae | House Finch (Haemorhous mexicanus) | Grimes et al. (2024) | |
| Urban noise | Extended songs, longer intervals, and slower syllable rates. | Passeriformes | Muscicapidae | Oriental Magpie-robin (Copsychus saularis) | Hill et al. (2018) | |
| Urban noise | Higher minimum frequency | Passeriformes | Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Derryberry et al. (2016) | |
| Loss of urban noise | Lower minimum frequency and increased bandwidth | Passeriformes | Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Derryberry et al. (2020) |
Plasticity within the vocal communication system is closely tied to the avian life cycle, particularly the seasonal changes that influence vocal behavior and auditory processing (Fig. 3). In wild birds, singing behavior shows clear seasonal variations. During the spring, male songbirds produce highly stereotyped songs at a high rate to attract females and repel other males. Female songbirds often participate in duets with their mates, engaging in pair bonding, reproductive synchronization, and territorial defense (Mountjoy and Lemon, 1991; Catchpole and Slater, 2003; Alger et al., 2016). This increase in vocal activity is coupled with hormonal changes, such as elevated levels of T and estrogen, during the breeding season. These hormones modulate vocal and auditory neuron activity, enhancing the precision of song generation and auditory discrimination (Caras et al., 2012).
T levels rise early in the breeding season and are known to regulate the morphology and neurophysiology of song-control nuclei, such as the HVC and RA (Thompson et al., 2012; Heberden, 2017). As these brain areas grow in volume and experience increased neuron replacement, birds are better equipped for song learning, memory, and performance. After the breeding season, T levels decline, leading to a reduction in the size of these brain areas. This neural shrinkage is accompanied by decreased vocal activity as the songbird no longer needs to produce stereotyped songs for mate attraction or territorial defense (DeVoogd and Nottebohm, 1981). As a result, the costs of maintaining song circuits are minimized, with no ecological penalty for the degradation of song behavior or neural structures (Larson et al., 2014).
In addition to T, elevated estrogen levels during the breeding season are crucial in enhancing auditory processing. Estrogen has been shown to increase the sensitivity of auditory neurons and improve signal-to-noise processing in the auditory forebrain, including areas such as the NCM, CMM, and Ov (Sisneros et al., 2004; Caras et al., 2012; Yoder and Vicario, 2012). The enhanced neuronal responsiveness and synaptic strength in these regions enable birds to better detect, recognize, and discriminate species-specific vocalizations amidst the noise of the surrounding environment.
Beyond the central auditory system, peripheral auditory organs also exhibit seasonal plasticity. During energetically demanding periods such as breeding or migration, the structure and function of sensory hair cells in the cochlea enhance auditory sensitivity, allowing birds to respond more effectively to acoustic signals (Lucas et al., 2002). This adaptive change is particularly important during times when communication signals need to be clearly distinguished from background noise, such as during mate attraction or territorial disputes.
Therefore, seasonal plasticity in the vocal communication system enables birds to dynamically adjust both vocal production and auditory processing in response to varying environmental demands, life history stages, and social contexts. This highlights the intricate interactions between neural, hormonal, and ecological factors that shape avian communication strategies. The ability of birds to modify their acoustic behavior according to their physiological state and environmental conditions underscores the remarkable flexibility of their vocal communication system.
Animals inhabiting closed, forested environments typically vocalize at lower frequencies, while those in open, grassy habitats tend to use higher frequencies to optimize their vocalizations for effective transmission (Morton, 1975; Wiley and Richards, 1978). Among the various environmental stimuli birds face, whether anthropogenic or natural, noise is one of the most significant factors influencing communication efficiency (Table 2). Birds adjust their songs to cope with environmental noise by modifying pitch, amplitude, or duration, a phenomenon known as “vocal adjustment, ” which is crucial for effective signal transmission (Slabbekoorn and Peet, 2003; Catchpole and Slater, 2003; McMullen et al., 2014). In urban environments with high noise levels, such as vehicular traffic and machinery, which produce low-frequency, high-energy sounds, these noises can mask the frequencies commonly used in bird songs (Dooling and Popper, 2016).
One primary response to noisy environments is the Lombard effect, where birds sing at higher amplitudes to compensate for background noise (Kunc et al., 2022). Additionally, birds may reduce the bandwidth of their song frequency to concentrate within the range that is most sensitive to their conspecifics, a strategy observed in species such as White-crowned Sparrows, Lazuli Buntings (Passerina amoena), and Song Sparrows (Melospiza melodia) as noise levels increase (Gentry et al., 2017; Gentry and Luther, 2019). In some cases, songbirds also shorten the duration of their songs without altering syllable rate, eliminating superfluous elements and retaining only those critical for recognition (Reed et al., 2022). In marine environments where coastal noise is prevalent, birds such as the Puffin (Fratercula arctica) vocalize at lower frequencies and amplitudes to ensure long-distance signal transmission (Mooney et al., 2019).
Generally, sensitive ears are crucial for maximizing hearing sensitivity and ensuring efficient auditory signal transmission. The hair cells in the inner ear are delicate and vulnerable to continuous mechanical stress from the environment. In mammalian models, prolonged sound exposure leads to a significant loss of hair cells, particularly along the basilar membrane (Sato et al., 2024). In chicks, studies by Cotanche and Dopyera (1990) have demonstrated that noise-induced damage begins after 4 h of exposure, first as localized expansion of supporting cell surfaces near the inferior edge of the basilar papilla. Hair cell expulsion becomes evident after 12 h, followed by further collapse of supporting cells and expulsion of hair cells after 24–48 h. This damage primarily affects short hair cells in the abneural part of the avian basilar membrane (Smolders, 1999).
Unlike mammals, however, birds can regenerate their hair cells after damage. In chicks, substantial hair cell recovery is observed shortly after noise exposure (Cotanche, 1987; Husbands et al., 1999). Hair cell regeneration involves both transdifferentiation and self-repair: supporting cells and hyaline cells in the inferior region of the basilar papilla act as precursor populations, dividing mitotically to produce new hair cells. These cells form protrusions toward the basement membrane, where synaptic specializations occur. Over time, reinnervation and morphological maturation allow these cells to revert to typical hair cell morphology and synapse formation (Rubel et al., 2013; Sato et al., 2024).
In addition to affecting vocal and auditory functions, noise also influences the central nervous system. A fMRI study shows that responses in the cluster NCM are reduced as noise levels increase in the song stimulus (Boumans et al., 2008). However, when bird songs are masked by synthetic noise, some neurons in NCM exhibit strong resilience, with a higher concentration of noise-invariant neurons in the ventral regions (Moore et al., 2013). This suggests that noise significantly impacts auditory processing regions in the brain. Furthermore, NIHL cannot be fully explained by damage to peripheral auditory nerve fibers alone; it also affects higher auditory processing areas. In mammals, repeated noise exposure is a major risk factor for NIHL, resulting in neuronal loss in areas such as the inferior colliculus (IC), the medial geniculate body (MGB), and the primary auditory cortex (AI) within a week of exposure (Sekiya et al., 2012; Frohlich et al., 2017). As the primary sensory gateway to the cerebral cortex, the thalamus is involved in this process, and noise-induced apoptosis in the AI may be a consequence of complex interactions within the auditory pathway. Experimental evidence also suggests that noise exposure increases levels of stress hormones, which mediate inflammatory and oxidative stress (OS) pathways, leading to endothelial and neuronal dysfunction (Manukyan, 2022).
The vocal communication system exemplifies remarkable flexibility and adaptability, enabling songbirds to dynamically adjust their vocalizations and auditory processing to meet various environmental, social, and physiological demands. Seasonal plasticity allows birds to optimize their communication during critical life stages such as breeding and migration by modifying song production and auditory sensitivity. These adaptations are driven by complex interactions among hormonal, neural, and ecological factors, showcasing the dynamic nature of avian communication. Additionally, the ability of birds to adjust vocalization frequency, amplitude, and duration, along with auditory processing, in response to environmental noise demonstrates the robustness and resilience of this system. These findings offer critical insights into how birds cope with environmental changes, contributing to the broader understanding of animal communication and neuroplasticity.
Despite significant progress, several questions remain unanswered, highlighting the need for further research. Exploring the molecular mechanisms underlying neural plasticity in response to hormonal fluctuations could deepen our understanding of the long-term adaptability of the song-control brain regions. Additionally, the effects of anthropogenic noise on bird populations require more focused investigation, particularly in the context of global environmental changes that increasingly alter natural habitats. Understanding how birds mitigate the challenges posed by noise pollution will be vital for informing conservation strategies and protecting vulnerable species. Future research should also examine the role of sensory feedback and auditory learning in shaping vocal development and modification, especially in species with complex vocal behaviors. Investigating genetic and environmental factors influencing vocal learning and auditory plasticity will not only shed light on avian communication systems but also provide broader insights into the principles of neuroplasticity applicable across taxa. Advanced tools, such as neural imaging, bioacoustics, and artificial intelligence, offer promising avenues to enhance our understanding of how birds process and adapt to complex acoustic signals in dynamic environments. Ultimately, the continued study of avian communication systems offers valuable insights into animal behavior and the broader field of neuroethology, with implications for understanding sensory processing and plasticity in other species, including humans.
Xuan Peng: Writing – original draft. Limin Wang: Writing – review & editing. Chenchen Shao: Investigation. Dongming Li: Writing – review & editing.
Not applicable.
The authors declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
|
Beuter, K.J., Weiss, R., 1986. Properties of the auditory system in birds and the effectiveness of acoustic scaring signals. In: Proceedings of the International Bird Strike Committee, Copenhagen, Denmark, pp. 60–73.
|
|
Brand, A.R., Kellogg, P.P., 1939. Auditory responses of starlings, English sparrows, and domestic pigeons. Wilson Bull. 38–41.
|
|
Catchpole, C.K., Slater, P.J., 2003. Bird Song: Biological Themes and Variations. Cambridge University Press, Cambridge.
|
|
Cohen, S.M., Stebbins, W.C., Moody, D.B., 1978. Audibility thresholds of the blue jay. Auk 95, 563–568.
|
|
Cornez, G., Shevchouk, O.T., Ghorbanpoor, S., Ball, G.F., Cornil, C.A., Balthazart, J., 2020. Testosterone stimulates perineuronal nets development around parvalbumin cells in the adult canary brain in parallel with song crystallization. Horm. Beyond Behav. 119, 104643.
|
|
de Bournonville, C., McGrath, A., Remage-Healey, L., 2020. Testosterone synthesis in the female songbird brain. Horm. Beyond Behav. 121, 104716.
|
|
Dooling, R.J., Lohr, B., Dent, M.L., 2000. Hearing in Birds and Reptiles. Springer, New York.
|
|
Elie, J.E., Hoffmann, S., Dunning, J.L., Coleman, M.J., Fortune, E.S., Prather, J.F., 2020. From perception to action: the role of auditory input in shaping vocal communication and social behaviors in birds. Brain Behav. Evol. 94, 51–60.
|
|
Faiß, M., Riede, T., Goller, F., 2022. Tonality over a broad frequency range is linked to vocal learning in birds. Proc. Biol. Sci. 289, 20220792.
|
|
Fernández-Vargas, M., Macedo-Lima, M., Remage-Healey, L., 2024. Acute aromatase inhibition impairs neural and behavioral auditory scene analysis in zebra finches. eNeuro 11, 423.
|
|
Fischer, F.P., 1994. General pattern and morphological specializations of the avian cochlea. Scanning Microsc. 8, 18.
|
|
Forstmeier, W., Balsby, T.J., 2002. Why mated dusky warblers sing so much: territory guarding and male quality announcement. Behaviour, 89–111.
|
|
Grothe, B., Carr, C.E., Casseday, J.H., Fritzsch, B., Köppl, C., 2004. The evolution of central pathways and their neural processing patterns. Evolution of the Vertebrate Auditory System, pp. 289–359.
|
|
Hay, E.M., McGee, M.D., White, C.R., Chown, S.L., 2024. Body size shapes song in honeyeaters. Proc. Biol. Sci. 291, 20240339.
|
|
Henry, K.S., Gall, M.D., Vélez, A., Lucas, J.R., 2016. Avian auditory processing at four different scales: variation among species, seasons, sexes, and individuals. Psychological Mechanisms in Animal Communication, pp. 17–55.
|
|
Janesick, A., Scheibinger, M., Benkafadar, N., Kirti, S., Ellwanger, D.C., Heller, S., 2021. Cell-type identity of the avian cochlea. Cell Rep. 34.
|
|
Job, J.R., Kohler, S.L., Gill, S.A., 2016. Song adjustments by an open habitat bird to anthropogenic noise, urban structure, and vegetation. Behav. Ecol. 27, 1734–1744.
|
|
Kang, H., Dos Santos, E.B., Kojima, S., 2025. Neural sensitivity to frequency changes in song structure in a high-order auditory area reflects tutor song memory in adult songbirds. Brain Struct. Funct. 230, 1–9.
|
|
Las, L., Fee, M., 2008. Recordings of striatal-projecting neurons in the ventral tegmental area (VTA) of the juvenile zebra finch during song learning. In: Society for Neuroscience Annual Meeting Abstracts.
|
|
Manohar, S., Chen, G.D., Ding, D., Liu, L., Wang, J., Chen, Y.C., et al., 2022. Unexpected consequences of noise-induced hearing loss: impaired hippocampal neurogenesis, memory, and stress. Front. Integr. Neurosci 16, 871223.
|
|
Marler, P.R., Slabbekoorn, H., 2004. Nature’s Music: the Science of Birdsong. Elsevier.
|
|
Mountjoy, D.J., Lemon, R.E., 1991. Song as an attractant for male and female European starlings, and the influence of song complexity on their response. Behav. Ecol. Sociobiol. 28, 97–100.
|
|
Nowicki, S., 1997. Bird acoustics. In: Crocker, M.J. (Ed.), Encyclopedia of Acoustics. John Wiley Sons, pp. 1813–1817.
|
|
Peng, A.W., Ricci, A.J., 2011. Somatic motility and hair bundle mechanics, are both necessary for cochlear amplification? Hear. Res. 273, 109–122.
|
|
Saravanan, V., Hoffmann, L.A., Jacob, A.L., Berman, G.J., Sober, S.J., 2019. Dopamine depletion affects vocal acoustics and disrupts sensorimotor adaptation in songbirds. eNeuro 6, 190.
|
|
Schneider, D.M., Woolley, S.M., 2009. Stimulus-dependent receptive field dynamics are driven by subthreshold excitation and natural stimulus statistics. Society for Neuroscience Annual Meeting Abstracts.
|
|
Schumacher, J.W., Woolley, S.M., 2009. Encoding properties of midbrain neurons in awake and anesthetized songbirds. Society for Neuroscience Annual Meeting Abstracts.
|
|
Slabbekoorn, H., Smith, T.B., 2002. Habitat‐dependent song divergence in the little greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution 56, 1849–1858.
|
|
Stewart, P.A., 1955. An audibility curve for two ring-necked pheasants. Ohio. J. Sci. 55, 122–125.
|
|
Voigt, C., Leitner, S., Gahr, M., 2006. Repertoire and structure of duet and solo songs in cooperatively breeding white-browed sparrow weavers. Behaviour, 159–182.
|
|
Webster, M.S., Podos, J., 2018. Acoustic communication. In: Morrison, M.L., Rodewald, A.D., Voelker, G., Colon, M.R., Prather, J.F. (Eds.), Ornithology: Foundation, Analysis, and Application. Johns Hopkins University Press, pp. 409–436.
|
|
Yadav, S., Rab, S., Wan, M., Yadav, D., Singh, V.R., 2024. Sound communication in nature. In: Garg, N., Gautam, C., Rab, S., Wan, M., Agarwal, R., Yadav, S. (Eds.), Handbook of Vibroacoustics, Noise and Harshness. Springer Nature Singapore, Singapore, pp. 951–976.
|
| Order | Family | Species | Auditory frequency range | Reference |
| Sphenisciformes | Spheniscidae | African Penguin (Spheniscus demersus) | 100 Hz–15 kHz | Wever et al. (1969) |
| Anseriformes | Anatidae | Mallard Duck (Anas platyrhynchos) | 16 Hz–9 kHz | Hill (2017) |
| Anseriformes | Anatidae | Canvasback (Aythya valisineria) | 190 Hz–5.2 kHz | Edwards (1943) |
| Galliformes | Numididae | Helmeted Guineafowl (Numida meleagris) | 2 Hz–8 kHz | Heffner et al. (2024) |
| Galliformes | Phasianidae | Indian Peafowl (Pavo cristatus) | 4 Hz–7.065 kHz | Heffner et al. (2020) |
| Galliformes | Phasianidae | Chicken (Gallus gallus domesticus) | 9.1 Hz–7.2 kHz | Hill et al. (2014) |
| Galliformes | Phasianidae | Japanese Quail (Coturnix japonica) | 16 Hz–8 kHz | Strawn and Hill (2020) |
| Galliformes | Phasianidae | Common Pheasant (Phasianus colchicus) | 250 Hz–10.5 kHz | Stewart (1955) |
| Charadriiformes | Laridae | Black-headed Gull (Chroicocephalus ridibundus) | 100 Hz–10 kHz | Beuter and Weiss (1986) |
| Columbiformes | Columbidae | Domestic Pigeon (Columba livia) | 2 Hz–8 kHz | Heffner et al. (2013) |
| Columbiformes | Columbidae | Common Pigeon (Columba livia) | 200 Hz–7.5 kHz | Brand and Kellogg, 1939 |
| Strigiformes | Strigidae | Great Horned Owl (Bubo virginianus) | 60 Hz–7 kHz | Edwards (1943) |
| Strigiformes | Strigidae | Northern Saw-whet Owl (Aegolius acadicus) | 700 Hz–8.6 kHz | Beatini et al. (2018) |
| Strigiformes | Tytonidae | Western Barn Owl (Tyto alba) | 1 kHz–10 kHz | Krumm et al. (2017) |
| Psittaciformes | Psittaculidae | Budgerigar (Melopsittacus undulatus) | 77 Hz–7.6 kHz | Heffner et al. (2016) |
| Apodiformes | Trochilidae | Chimborazo Hillstar (Oreotrochilus chimborazo) | >10 kHz | Duque et al. (2020) |
| Passeriformes | Calcariidae | Snow Bunting (Plectrophenax nivalis) | 400 Hz–7.2 kHz | Edwards (1943) |
| Passeriformes | Alaudidae | Shore Lark (Eremophila alpestris) | 350 Hz–7.6 kHz | Edwards (1943) |
| Passeriformes | Corvidae | Blue Jay (Cyanocitta cristata) | ~7.8 kHz | Cohen et al. (1978) |
| Passeriformes | Fringillidae | Atlantic Canary (Serinus canaria) | 250 Hz–9 kHz | Dooling et al. (1971) |
| Passeriformes | Fringillidae | House Finch (Haemorhous mexicanus) | ~7.2 kHz | Dooling et al. (1978) |
| Passeriformes | Icteridae | Red-winged Blackbird (Agelaius phoeniceus) | ~9.6 kHz | Hienz et al. (1977) |
| Passeriformes | Icteridae | Brown-headed Cowbird (Molothrus ater) | ~9.7 kHz | Hienz et al. (1977) |
| Passeriformes | Passerellidae | Field Sparrow (Spizella pusilla) | ~11 kHz | Dooling et al. (1979) |
| Passeriformes | Passeridae | House Sparrow (Passer domesticus) | 675 Hz–11.5 kHz | Brand and Kellogg, 1939 |
| Passeriformes | Sturnidae | Common Starling (Sturnus vulgaris) | 700 Hz–14 kHz | Brand and Kellogg, 1939 |
| Influential factor | Specific influential factor | Main consequences | Order | Family | Species | Reference |
| Habitat alteration | Different altitudes | Change in song structure | Passeriformes | Paridae | Mountain Chickadee (Poecile gambeli) | Branch and Pravosudov (2015) |
| Different altitudes | Various in performance of birdsong | Passeriformes | Meliphagidae | Honeyeater Family | Hay et al. (2024) | |
| Geographical isolation | Formation of dialects | Passeriformes | Fringillidae | Red Crossbill (Loxia curvirostra) | Hynes and Miller (2014) | |
| High vegetation density | Slower bird song with altered song divergence | Passeriformes | Troglodytidae | Grey-breasted Wood-wren (Henicorhina leucophrys) | Dingle et al. (2008) | |
| Increasing in vegetation | Increased minimum frequency and decreased bandwidth | Passeriformes | Passerellidae | Chipping Sparrow (Spizella passerina) | Job et al. (2016) | |
| Rainforest environment | Increased frequency range and altered song note delivery rate | Passeriformes | Pycnonotidae | Little Greenbul (Andropadus virens) | Slabbekoorn and Smith (2002) | |
| Migration distances | Reduced variety of shared song types | Passeriformes | Passerellidae | Golden-crowned Sparrow (Zonotrichia atricapilla) | Shizuka et al. (2016) | |
| Long time of geographically isolation | A loss of the terminal syllable | Passeriformes | Parulidae | Hermit Warbler (Setophaga occidentalis) | Janes and Ryker (2013) | |
| Environmental noise | Oceanic noise | Higher dominant frequency | Piciformes | Lybiidae | Yellow-fronted Tinkerbird (Pogoniulus chrysoconus) | Sebastianelli et al. (2020) |
| Oceanic noise | Reduced frequency and lower amplitude | Charadriiformes | Alcidae | Atlantic Puffin (Fratercula arctica) | Mooney et al. (2019) | |
| Oceanic noise | Higher amplitude | Passeriformes | Passerellidae | White-crowned Sparrow (Zonotrichia leucophrys) | Reed et al. (2022) | |
| Oceanic noise | Higher amplitude | Passeriformes | Paradoxornithidae | Wrentit (Chamaea fasciata) | Reed et al. (2022) | |
| Rain noise | Decreased singing behavior | Strigiformes | Strigidae | Tawny Owl (Strix aluco) | Lengagne and Slater (2002) | |
| Wind noise | Higher calling rates | Sphenisciformes | Spheniscidae | King Penguin (Aptenodytes patagonicus) | Lengagne et al. (1999) | |
| Waterfall noise | Greater signal redundancy | Passeriformes | Fringillidae | Chaffinch (Fringilla coelebs) | Brumm and Slater (2006) | |
| Natural and anthropogenic noise | Longer syllables and extended song duration | Carinatae | Troglodytidae | Pacific Wren (Troglodytes pacificus) | Gough et al. (2014) | |
| Anthropogenic noise | Higher frequencies and reduced song performance | Passeriformes | Passerellidae | White-crowned Sparrow (Zonotrichia leucophyrs) | Moseley et al. (2018) | |
| Traffic noise | Longer duration, lower introductory and peak frequencies, and greater variability in syllable types in bird songs | Passeriformes | Paridae | Black-capped Chickadee (Poecile atricapillus) | Courter et al. (2020) | |
| Urban noise | More complex bird song | Passeriformes | Turdidae | Song Thrush (Turdus philomelos) | Deoniziak and Osiejuk (2019) | |
| Urban noise | Singing early than those in semi-natural habitats | Passeriformes | Turdidae | Common Blackbird (Turdus merula) | Nordt and Klenke (2013) | |
| Urban noise | Louder, higher-pitched songs | Passeriformes | Turdidae | Eastern Bluebird (Sialia sialis) | Kight and Swaddle (2015) | |
| Urban noise | Increased song amplitude | Passeriformes | Passeridae | House Sparrow (Passer domesticus) | Grimes et al. (2024) | |
| Urban noise | Increased song amplitude | Passeriformes | Fringillidae | House Finch (Haemorhous mexicanus) | Grimes et al. (2024) | |
| Urban noise | Extended songs, longer intervals, and slower syllable rates. | Passeriformes | Muscicapidae | Oriental Magpie-robin (Copsychus saularis) | Hill et al. (2018) | |
| Urban noise | Higher minimum frequency | Passeriformes | Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Derryberry et al. (2016) | |
| Loss of urban noise | Lower minimum frequency and increased bandwidth | Passeriformes | Emberizidae | White-crowned Sparrow (Zonotrichia leucophrys) | Derryberry et al. (2020) |