| Citation: | Camilo Ernesto Espinosa, James Montoya Lerma, Hector Fabio Rivera-Gutierrez, Lorena Cruz-Bernate. 2025: Crown saturation and intrasexual dominance: Evidence of a negatively correlated handicap in male Saffron Finches. Avian Research, 16(1): 100241. DOI: 10.1016/j.avrs.2025.100241 |
Carotenoid-based plumage coloration may signal individuals’ overall body condition, influencing reproduction and survival of birds. In tropical species, little is known about the influence of color on social interactions and mate attraction. We evaluated the chromatic variation of 136 adult Saffron Finches (Sicalis flaveola) in Cali and Jamundí, Colombia. Our aim was to determine whether plumage coloration in this social, abundant, and widely distributed species is a signal used in mate choice and establishment of hierarchies. We predicted that there was intrasexual chromatic variation in crown and throat, and that individuals with higher saturation, regardless of sex, would be preferred by opposite sex and they would be dominant in intrasexual aggressive interactions, reflecting better condition. We quantified the reflectance of the crown and throat with visible and ultraviolet light (300–700 nm) subsequent to the molecular sex determination of each individual. Our results suggest that the chromatic variation in the crown and throat is explained by the perception of medium and long wavelengths in both sexes. Additionally, saturation is the color characteristic that best explains the chromatic variation. We formed duos of individuals based on chromatic contrast and conducted 23 mate choice experiments and 21 dominance experiments. The analysis of spectra and experiments revealed that dominance in males was associated with less saturated crowns. Our results reveal that in the Saffron Finches the “negatively correlated handicap” is a plausible hypothesis. Crown color appears to mediate dominance interactions, with less saturated males potentially taking greater risks to gain access to resources.
Birds are highly visually-oriented organisms, making plumage coloration a key trait for social communication and mate selection (Bennett and Théry, 2007; Martin and Osorio, 2008; Stoddard and Prum, 2011). Plumage coloration can either blend cryptically or stand out, depending on the observer’s perspective. For example, in male Blue Tits (Cyanistes caeruleus), individuals with more saturated white cheeks exhibit a higher load of Plasmodium parasites, while brighter cheeks are linked to higher body mass (Badás et al., 2018). Such color traits are influenced by both conspecifics and other species, reflecting unique coloration strategies shaped by environmental factors and perception (Hill and McGraw, 2006). Plumage coloration may also serve as an honest signal of physical condition, health, or behavior. In blacker male Pied Flycatchers (Ficedula hypoleuca), darker plumage correlates with a greater tendency to disperse into new environments and increased fearfulness toward predators (Camacho et al., 2018). Furthermore, the brightness of male Red-crested Cardinals (Paroaria coronata) has been positively associated with nest defense and reproductive success (Segura and Mahler, 2019). Intraspecific differences in plumage color can thus function as indicators of mate choice and social status, highlighting the crucial role of coloration in avian communication (Hill and McGraw, 2006).
Plumage coloration is often considered a result of sexual selection, with females choosing males based on specific plumage patterns that indicate higher overall health and quality (Candolin, 2003; Hill and McGraw, 2006). For example, redder House Finches (Haemorhous mexicanus) show better mitochondrial performance (Hill et al., 2019), better overall body condition (DePinto and McGraw, 2022), and greater participation in parental care (Hill, 1991). However, sexual selection pressures can also act on females (Amundsen, 2000), as seen in species such as the Rock Sparrow (Petronia petronia), where males prefer females with larger yellow breast patches (Griggio et al., 2009), or in Bluethroats (Luscinia s. svecica), where males prefer more colorful females (Amundsen et al., 1997). The evolution of color in birds is a complex process, intricately intertwined with conspecific male and female plumage traits. This relationship is influenced by a multitude of factors, including natural, social, and sexual selection pressures (Dale et al., 2015; Medina et al., 2017; Wang et al., 2022). For instance, female plumage ornamentation tends to be more prominent in larger species with equatorial breeding ranges and high levels of female-female competition. Conversely, a reduction in female ornamentation is associated with strong male-biased sexual selection (Dale et al., 2015). This indicates that evolutionary forces can operate differently between the sexes (Cooney et al., 2019), resulting in the same patch having different selective pressures for males and females. So, a color patch may signal different things about a bird’s status or attractiveness depending on the sex (Nolazco et al., 2023). For instance, in Purple-crowned Fairywrens (Malurus coronatus) the crown is a sign of intrasexual dominance in males, while in females less ornate, not associated with reproductive success and dominance (Nolazco et al., 2023). In Variable Seedeaters (Sporophila corvina) the reduced variation on dorsal patch coloration among females across subspecies probably is due to pressures by natural crypsis selection, while male coloration may be influenced by sexual selection (Ocampo et al., 2023).
In this context, the physiological origin of feather color also plays a crucial role in the evolution of color expression (Hill and McGraw, 2006). Most work on passerines has focused on coloration by pigments, particularly carotenoids and melanin (Badyaev and Hill, 2000). Yellow, orange, and red plumage coloration is primarily related to the intake of carotenoids, suggesting that an intraspecific color difference may be associated to the individuals’ ability to compete for and acquire carotenoid-rich resources (Hill, 2000; McGraw et al., 2006). Besides intake, the ability to metabolize carotenoids influences color expression (Weaver et al., 2018). For example, red ketocarotenoid-based coloration is linked to the mitochondrial energy metabolism, as suggested by the shared-pathway hypothesis, linking the individual’s intake of carotenoids to mitochondrial metabolism (Cantarero et al., 2020; Powers and Hill, 2021). Carotenoid plumage coloration has been associated with different traits that reflect the individual’s condition or reproductive success. For instance, in Great Tits (Parus major) the yellow breast brighteness is related with the corporal size (Hegyi et al., 2007), males House Finches with redder plumage exhibit better resistance to parasites compared to males with yellower plumage (Hill and Farmer, 2005). In Blue Tits nestlings, broods with higher mean body mass also exhibit higher brightness and UV chroma (García-Campa et al., 2023), while in Common Crossbills (Loxia curvirostra), survival almost doubled in red males compared to yellow males (Fernández-Eslava et al., 2022). Although carotenoids may be an important signal during the mate selection process (Badyaev and Hill, 2000), carotenoids can also be associated with antagonist behaviors, such that more conspicuous-colored individuals might show an increased level of dominance (Andersson, 1994). Experimentally, in Australian Gouldian Finches (Chloebia gouldiae), red-headed individuals of both sexes are dominant against a black- and yellow-headed in intrasexual dominance contests (Pryke and Griffith, 2006; Pryke, 2007).
This study aimed to determine whether the color of two plumage patches in Saffron Finches (Sicalis flaveola) influenced dominance and mate choice. The Saffron Finches have carotenoid-based coloration, where breast color signals dominance in S. f. brasiliensis, a tropical subspecies from Brazil (Araújo-Silva et al., 2022). Unlike the subspecies distributed in South American temperate zones, the tropical one does not present marked sexual dichromatism (Rising et al., 2011). Both male and female have orange coloration on the crown and throat (Hilty and Brown, 2001) but it is unknown if this coloration is associated to life-history traits and social interactions. We measured the crown and throat coloration and performed experimental studies on mate choice and dominance in Cali, Colombia. We expected some level of intrasexual variation in the crown and throat of Sicalis flaveola to be perceptible among conspecifics in the population of Cali. In addition, we predicted that in behavioral experiments individuals with higher saturation would be favored, being observed most of the time by opposite sex in the mate choice experiments, and would win in most of the aggressive interactions (supplant flight and physical contact) in intrasexual confrontations.
The research was carried out in the Universidad de Valle campus-Univalle, Cali (3°22'30″ N, 76°32'04″ W) and the urban area of Jamundí (3°15ʹ N, 76°33ʹ W) two areas of the department of Valle del Cauca, Colombia. Morphometric analysis and determination of chromatic variation were carried out on the Saffron Finch population from Univalle from March 2017 to October 2019. We carried out captures throughout the year, using 10 mist nets (12 m×2.5 m and 30 mm mesh eye) installed between 07:00 a.m. and 15:00 p.m. Additionally, as part of a long-term monitoring program for the socially monogamous Saffron Finch, we capture breeding pairs by manually triggering a door at the entrance of one of the 240 nest boxes at Univalle, once an individual is observed entering the nest. The individuals were taken to the Laboratory of Ornithology and Animal Behavior-OYCA, we took blood samples, recorded measurements (morphometric and plumage coloration reflectance), and banded the individuals with a unique combination of colors. Subsequently, we released all the banded individuals at their original capture sites.
We took the following 12 morphometric measurements from each adult that did not present molting and was captured with definitive plumage, the characteristic set of feathers that a mature bird acquires and maintains throughout its life: length, width and depth of the bill; commissure; nostrils; wing length; Kipp’s distance; tarsus length; hallux; tail length; full body length and body mass.
Sex was determined for 136 birds using the molecular method proposed by Ellegren (1996) and implemented for Saffron Finches by Espinosa et al. (2017) consisting of the amplification of CDH1 gene intron (chromo-helicase domain) located on the sex chromosomes of birds (W, Z). In brief, we gathered 10 μL of blood from the brachial vein by puncture, using a micropipette, and stored it in a microtube with Queen lysis buffer (Seutin et al., 1991). Subsequently, we extracted DNA using the ‘salting out’ protocol (Gustincich et al., 1989). For the DNA polymerase chain reaction (conventional PCR) the primers 2550F–2718R (Fridolfsson and Ellegren, 1999) were used. The amplified products were separated on 1% agarose gels. Birds were identified as females (WZ) if both CHD1W (450 bp) and CHD1Z (600 bp) fragments were amplified, while males (ZZ) were identified by the presence of only the CHD1Z (600 bp) fragment (Fridolfsson and Ellegren, 1999).
In a dark room, to eliminate any possible ambient light interference during measurements, we directly measured the reflectance on the plumage of each of the 136 captured birds using a flame spectrometer (Ocean Optics) with a detection range in the ultraviolet (300–400 nm) and visible wavelengths (400–700 nm) and a deuterium-tungsten halogen light (DH-2000-BAL) (Eaton, 2005). Measurements were taken at a 90° angle and at a distance of 6 mm from the plumage; this was achieved using a rigid, dark cover fitted to the end of the spectrometer’s fibre optic probe (Delhey, 2005). We recorded the reflectance spectrum of the crown and throat (at five and three different points, respectively), at first glance no appreciable differences in the patches were found among individuals. Reflectance spectra were averaged for each patch for each individual (Delhey, 2005). We chose the crown and throat because they are the most conspicuous plumage patches in the Saffron Finch and most likely to present chromatic variation, as occurs in other species with patches based on carotenoids (Delhey et al., 2017).
We analyzed four colorimetric variables: brightness, hue, saturation, and carotenoid chroma using the “pavo” package (Maia et al., 2019). We calculated the brightness as the mean of the reflectance at all wavelengths. We calculated the hue (λR50) as halfway between the Rmax and the Rmin. For saturation, we used λR50 as a segment divider and calculated with the following equation:
We quantified Saffron Finch color using the color vision model (Vorobyev et al., 1998) implemented in the R package “pavo” (Maia et al., 2019). The function vismodel (Vorobyev et al., 1998) was used to calculate quantum catches at each photoreceptor (Maia et al., 2019). In birds, the vision is tetrachromatic and the retinal cone cells are sensitive to long (L), medium (M), short (S), and UV-sensitive (UVS) wavelengths (Eaton, 2005). We used the Blue Tit visual system as a reference for the visual model, and the luminosity condition for open habitats since the ecology of the Saffron Finch was similar. Moreover Blue Tits, similarly to tanagers have the capacity to perceive the near UV wavelength (Casalía et al., 2020). The function colspace was used to convert relative quantum catches stimulation spectra into coordinates of the tetrahedral color space (Maia et al., 2019). We calculated the three colorimetric variables: Theta and Phi (hue), and r achieved (saturation) using the tetrahedral color space coordinates (Stoddard and Prum, 2008). Theta and Phi represent the latitude and longitude within the tetrahedron, and r achieved corresponds to the distance from the achromatic origin, weighted by the maximum distance that the vector could achieve, the greater the distance to the achromatic origin, the greater the intensity of the color (Stoddard and Prum, 2008). With the colspace function, the luminance (achromatic variable) was also calculated based on the stimulation of the double cones of Blue Tits (Maia et al., 2019).
For colorimetric variables we define brightness (luminance) as the amount of light reflected by an object, telling us how bright or dark a color is (Montgomerie, 2006). The huet is the property that allows us to distinguish a specific color (red, green, blue, yellow, etc); and saturation is the purity of a color, the more saturated a color, the less mixture exists, indicates how much gray there is in the color (Montgomerie, 2006).
To describe the chromatic variation of the visual model (Vorobyev et al., 1998) we employed the formulas of Cassey et al. (2008) and implemented by Delhey et al. (2015), in R software (R Core Team, 2021). This approach reduces the measured spectrophotometric variables to a set of three (XYZ) chromatic coordinates that define their position in avian tetrahedral colorspace (Jones et al., 2022). The X-axis shows the relative stimulation of the S cone relative to the UVS cone; the Y-axis represents the relative stimulation of the M cone in relation to the UVS and S cones, and the Z-axis shows the relative stimulation of the L cone in relation to the UVS, S, and M cones (Delhey et al., 2015). The chromatic coordinates incorporate information of noise-to-signal ratios of each cone type, where euclidean distances among points reflect Just Noticeable Differences (JNDs) (Delhey et al., 2015). The JNDs were calculated assuming a Weber ratio of 0.1 for the photoreceptor sensitivity to long wavelength, and the relative proportion of Blue Tit cones, wavelength: UVS = 1, S = 1.92, M = 2.68 and L = 2.7 (Hart, 2001). To determine that two colors are different, the distance between them must be greater than the discrimination threshold, these values were expressed as chromatic contrast (ΔS) and the units for ΔS are JNDs (Vorobyev and Osorio, 1998) (Appendix A). Theoretically, it is considered that values greater than one JND are perceptible by birds under ideal conditions, since there are no variables that interfere with the signal and the model conditions are controlled (Olsson et al., 2015); however, for this study a value of two JND was established as discrimination threshold, and a more reliable conclusion is obtained and comparison with other studies is facilitated (Benítez-Saldívar and Massoni, 2018). We performed a principal component analysis (PCA) on the three (XYZ) chromatic coordinates to summarize chromatic variation for each patch and sex. Using a covariance matrix to maintain the JND units of the original data (Delhey et al., 2015). We calculated the chromatic contrast (ΔS) of the two extreme colors on the main axis of PC1xyz to assess the biological relevance of the variation range, or in other words, whether it is perceptible to the receiver (Delhey et al., 2015).
While intrasexual chromatic variation can be assessed through the analysis of spectral shapes or visual models (Stoddard and Prum, 2008), the ultimate selection of variables to be utilized often relies on the researcher’s judgment so, introducing subjectivity into the process (Montgomerie, 2006). To mitigate this bias, we opted to adopt the methodology proposed by Delhey et al. (2015). Given our dataset included plumage patches exhibiting continuous color variation, this method allowed us to condense the chromatic diversity into a single primary axis (PC1xyz) directly derived from the cone stimuli. This approach facilitated the scrutiny of the association between the three-dimensional principal axis (PC1xyz) and each of the colorimetric variables, streamlining the interpretation process.
We captured birds from two populations were located 15 km apart, and we conducted the experiments in a specially fitted Behavioral Room designated for behavioral studies at the OYCA. Unlike the first phase (intrasexual chromatic variation), to prevent colored rings interfering with behavior during the experiment, only individuals in captivity had a metal ring on their right tarsus (Metz and Weatherhead, 1991). At the time of release, we added the color band once again. Captive conditions are described in Appendix A.
For each mate choice or dominance test, duos were formed, in which individuals differed in crown and throat color by more than two ΔS. In order to build a behavioral model for duos, preliminary analyses of intrasexual chromatic variation were conducted, identifying patterns that established color differences between individuals. Each individual was then classified as either higher (O+) or lower (O–) in saturated orange based on their PC1xyz coordinates. In addition to having differences in the coloration of the crown and throat, the individuals must have had a similar body condition index, which was calculated with the residuals of the ordinary least squares of body mass and the morphometric measure most correlated with body mass (Jakob et al., 1996). This avoided bias due to individual differences in body condition. We transferred individuals to the experimentation area in a dark compartment to avoid stress. At the time of the test, we released and uncovered the individuals from the dark compartment simultaneously without the presence of the researcher in the visual field of the birds. No birds died during this study.
We used individuals several times, but we formed all possible combinations of single duos for the experiments without duplicating duos. In the event where an individual could be part of several duos, we randomly selected only three of these. The identity of the individuals was included as a random factor in the models, in order to avoid for pseudoreplication. Individuals from duos were used multiple times per experiment, while chooser birds were used only once to ensure the independence of the choice avoiding bias for the preference of the chooser (Johnson and Marzluff, 1990). We used the same conditions as in the mate choice experiments to form the duos for the dominance trials.
To assess mate preference, an aviary (1.5 m×1.2 m×2 m) was used according to the model of Murphy et al. (2014) divided into two small and one large compartments, visually isolated from each other, a duo (two individuals with contrasting coloration of the same sex) and the chooser of the opposite sex were located in the large compartment. Before the exposure with the duo, the chooser remained in a phase of habituation in the aviary for 30 min isolated from sounds of the nearby birds. The experiment started as soon as the chooser moved calmly after opening the two windows that had previously prevented it from observing the duo. The experiment lasted 30 min. To prevent bias due to site preference in the choosers, the experiment was divided into two stages of 30 min each, after the first stage the individuals of the duo changed positions (Rutstein et al., 2007). Furthermore, in the first stage of the experiment there was the same number of trials with O+ individuals on the left as on the right. It was considered that there was a choice if the chooser was located on the perch closest (12 cm) to the small compartments and observed the individual of the opposite sex. We only consider the active choice, which was evaluated as the time that chooser directed the head towards the opposite sex; if the chooser was not observing the individual, it was considered a non-active choice. Duo individuals were from the same population avoiding bias due to local song dialects while the chooser bird came from other population avoinding previous encounters. Among all the individuals tested, only one sporadically emitted single-note vocalizations.
Laterality bias was determined if the chooser bird spent the majority of time during both choosing stages on one side of the aviary. Additionally, a preference for an individual of the opposite sex was considered if the chooser bird spent more than 60% of the active choosing time with the same individual during each stage of the experiment. Experiments where laterality bias was found were discarded from subsequent analyses.
We conducted a confrontation trials to assess intrasexual dominance by placing two unfamiliar individuals of the same sex with contrasting coloration in a cage. These trials were conducted separately for males and females. The cage (90×90×70 cm) had two perches on either side of a central feeder with translucent walls for easy observation of the food (a mix of commercial seeds); however, the feeder allowed only one bird to access the food at a time (Pryke and Griffith, 2006). The birds were deprived of food for 12 h, starting the previous night, to stimulate competition for the available food (Pryke et al., 2002). During the confrontation trial, the individuals were placed in the cage simultaneously, with the researcher remaining out of their line of sight. We measured the total time each individual monopolized the food and the number of aggressive interactions over a period of 20 min. Aggressive interactions included one individual replacing another on the perch (supplant flight) or engaging in physical contact. These interactions were considered a good measure of dominance. Generally, no signs of dominance were observed at feeding time, as non-feeding individuals did not intervene or actively forage. Dominant individuals actively defended their positions on the perches, where most aggressive interactions occurred. An individual was considered dominant if it won more than 80% of the confrontations. Confrontation trials in which no aggressive interactions were observed during the 20 min and dominance could not be determined were discarded from the analysis.
We performed the Anderson–Darling Test to test the normality of the colorimetric variables. Depending on whether the data fulfilled the normality assumption or not, we used either Pearson or Spearman to determine if there was a correlation between colorimetric variables (brightness, hue, saturation, and carotenoid chroma) and PC1xyz.
To set up preference experiments for both sexes and to eliminate potential bias, we evaluated the first and second phases of all mate choice experiments, including both sexes. We ran a generalized linear mixed model (GLMM) with a binomial error distribution to assess whether the location of the chosen individual (left/right, explanatory variable) affected “preference or choice” (response variable) while considering the identity of the bird as a random factor. We used four GLMMs to assess the influence of color variation on mate choice. Preference (chosen and not chosen) as the response variable, with region color (O+ and O–) as predictors. The first model assessed male preference based on female crown color, the second evaluated female preference based on male crown color, the third explored female preference based on male throat color, and the fourth assessed male preference based on female throat color. For the examination of dominance, we conducted three additional GLMMs, utilizing dominance (dominant and subordinate) as the response variable, with region color (O+ and O–) as predictors. The first two models assessed the dominance status of male-male and female-female by considering crown colors as predictors and the third model assessed the throat color of females as predictor. All GLMMs used a binomial error distribution, and the identity of each bird in the duo was considered as a random factor. The glmmTMB function from the ‘glmmTMB’ package in R software was used to run the models. The performance of the models was evaluated by comparing the full models against a null model using the likelihood ratio test (LRT) and examining AIC/BIC values for model selection (Fox and Weisberg, 2011). Using the sequential Bonferroni correction for multiple tests, the critical p-value at the 5% significance level was adjusted to 0.0125 (0.05/4) to account for multiple comparisons in the mate choice and dominance experiments (Hochberg, 1988). Statistical analyses were conducted using R software (R Core Team, 2021).
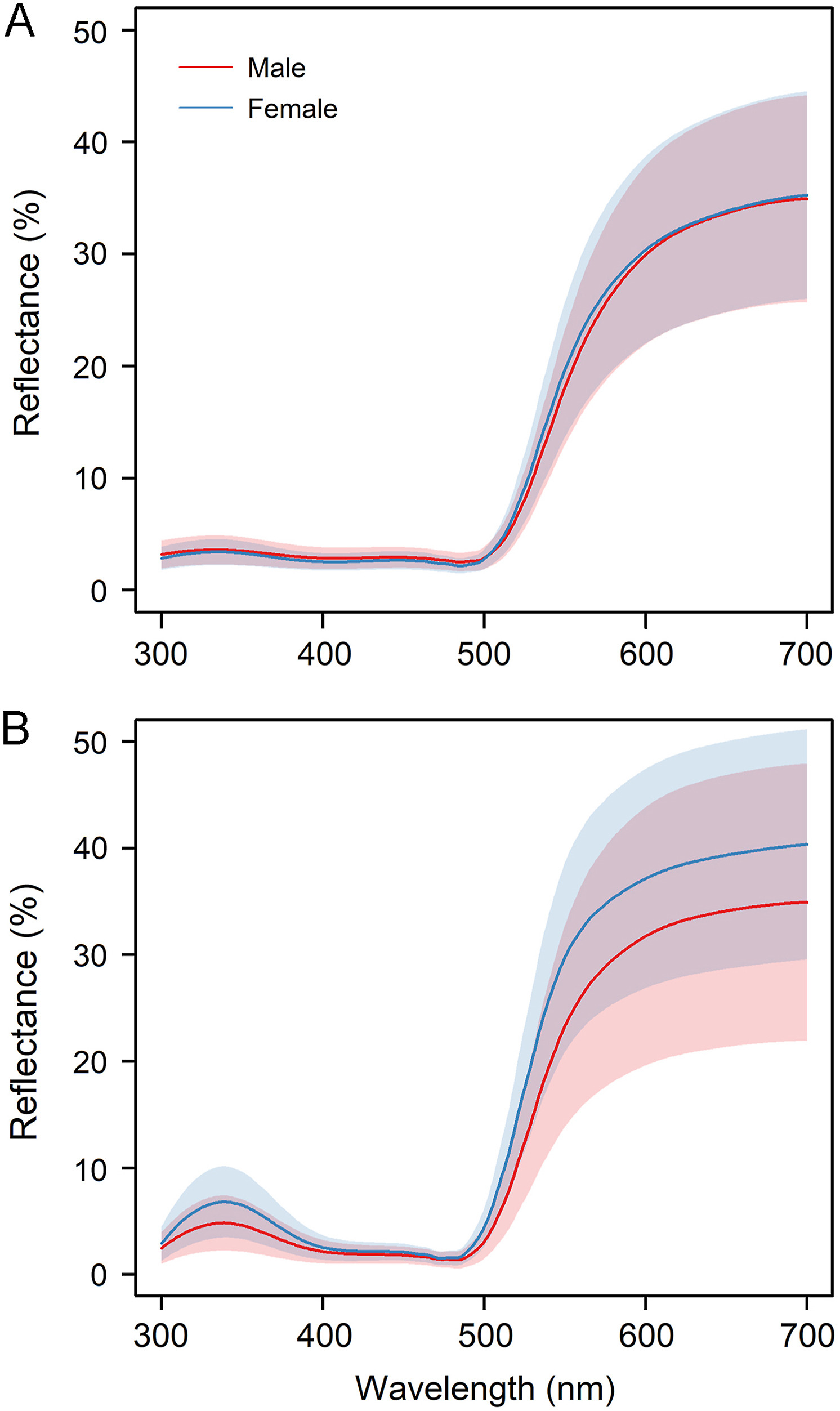
We captured a total of 68 females and 68 males. In the crown and throat, the reflectance was low in the short wavelength and high at long wavelengths (Fig. 1).
PC1xyz explained more than 69% of the variation in both patches (Table 1) presenting high loads in Y and Z axes and low load in X axis (Table 1, Figs. 2 and 3). This means that the color is mainly a consequence of the reflection and perception of medium and long wavelengths. All PCAxyz confirms the presence of one main axis of chromatic variation in the crown (females: ±1.21, range = 5.71 JNDs; males: ±1.31, range = 6.21 JNDs) and in the throat (females: ±1.74, range = 7.16 JNDs; males: ±2.66, range = 11.79 JNDs) that is perceptible by individuals having a range greater than two JND. The number of principal components was chosen based on Kaiser’s rule (Kaiser, 1961). In all axes, PC1xyz was the only one with eigenvalues greater than one (Table 1). There was no relationship between the patches color (PC1xyz) in both females (r = 0.1, t = 0.83, p = 0.4, N = 61) and males (r = 0.02, t = 0.2, p = 0.83, N = 61).
| Female crown | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 1.45 | 69.72 | 69.72 | -0.10 | 0.70 | 0.70 |
| 2 | 0.38 | 18.58 | 88.30 | -0.47 | -0.65 | 0.58 |
| 3 | 0.24 | 11.69 | 100 | -0.87 | 0.27 | -0.40 |
| Male crown | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 1.69 | 78.2 | 78.20 | -0.16 | 0.79 | 0.59 |
| 2 | 0.36 | 16.76 | 94.97 | -0.47 | -0.58 | 0.65 |
| 3 | 0.10 | 5.02 | 100 | -0.86 | 0.17 | -0.47 |
| Throat female | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 2.99 | 83.16 | 83.16 | -0.39 | 0.67 | 0.62 |
| 2 | 0.50 | 14.16 | 97.32 | -0.86 | -0.50 | -0.0007 |
| 3 | 0.09 | 2.67 | 100 | -0.31 | 0.54 | -0.77 |
| Throat male | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 6.88 | 89.75 | 89.75 | -0.11 | 0.73 | 0.66 |
| 2 | 0.61 | 7.98 | 97.73 | -0.86 | -0.40 | 0.29 |
| 3 | 0.17 | 2.22 | 100 | -0.48 | 0.54 | -0.68 |
When comparing the PC1xyz with colorimetric variables we found high a correlation in both patches to the colorimetric variables associated with saturation (saturation, carotenoid chroma, and r achieved) (Table 2). We assumed that the coordinates of PC1xyz indicate the saturation of the individuals, and the higher the values of chromatic coordinates were in the Y and Z axes, the more saturated was the color of the patch. We used these values to determine the most conspicuous individuals for the mate choice and dominance experiments.
| Female crown | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.35 | -0.55 | -0.11 | -2.93 | 0.006 | Pearson |
| Phi | 0.52 | 0.31 | 0.68 | 20006 | <0.001 | Spearman |
| r achieved | 0.91 | 0.85 | 0.94 | 3832 | <0.001 | Spearman |
| Luminance | -0.06 | -0.31 | 0.20 | 44214 | 0.63 | Spearman |
| Brightness | -0.15 | -0.38 | 0.10 | -1.16 | 0.25 | Pearson |
| Saturation | -0.77 | -0.86 | -0.65 | -9.55 | <0.001 | Pearson |
| Carotenoid chroma | 0.92 | 0.88 | 0.95 | 18.74 | <0.001 | Pearson |
| Hue | 0.35 | 0.11 | 0.55 | 2.88 | 0.007 | Pearson |
| Male crown | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.11 | -0.35 | 0.15 | 48508 | 0.39 | Spearman |
| Phi | 0.57 | 0.37 | 0.72 | 18904 | <0.001 | Spearman |
| r achieved | 0.95 | 0.92 | 0.97 | 23.88 | <0.001 | Pearson |
| Luminance | -0.11 | -0.34 | 0.14 | -0.85 | 0.47 | Pearson |
| Brightness | -0.15 | -0.38 | 0.10 | -1.17 | 0.33 | Pearson |
| Saturation | -0.96 | -0.98 | -0.93 | -26.70 | <0.001 | Pearson |
| Carotenoid chroma | 0.95 | 0.92 | 0.97 | 23.94 | <0.001 | Pearson |
| Hue | -0.02 | -0.27 | 0.23 | 44509 | 0.88 | Spearman |
| Female throat | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.64 | -0.77 | -0.46 | 59130 | <0.001 | Spearman |
| Phi | 0.65 | 0.47 | 0.77 | 6.46 | <0.001 | Pearson |
| r achieved | 0.97 | 0.96 | 0.98 | 32.73 | <0.001 | Pearson |
| Luminance | -0.51 | -0.68 | -0.30 | -4.53 | <0.001 | Pearson |
| Brightness | -0.54 | -0.70 | -0.33 | -4.90 | <0.001 | Pearson |
| Saturation | -0.93 | -0.96 | -0.89 | -19.28 | <0.001 | Pearson |
| Carotenoid chroma | 0.83 | 0.73 | 0.89 | 11.20 | <0.001 | Pearson |
| Hue | 0.64 | 0.45 | 0.77 | 13111.05 | <0.001 | Spearman |
| Male throat | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.33 | -0.53 | -0.09 | -2.77 | 0.01 | Pearson |
| Phi | 0.37 | 0.13 | 0.57 | 27538.82 | 0.005 | Spearman |
| r achieved | 0.48 | 0.26 | 0.66 | 22584.76 | <0.001 | Spearman |
| Luminance | -0.04 | -0.29 | 0.22 | 45309.02 | 0.77 | Spearman |
| Brightness | -0.20 | -0.43 | 0.06 | 52401.10 | 0.11 | Spearman |
| Saturation | -0.97 | -0.98 | -0.95 | 85977.48 | <0.001 | Spearman |
| Carotenoid chroma | 0.95 | 0.91 | 0.97 | 2398.53 | <0.001 | Spearman |
| Hue | 0.67 | 0.51 | 0.79 | 7.08 | <0.001 | Pearson |
| Confidence interval: 95%, significance level: 0.05. | ||||||
We captured and DNA-sexed a total of 45 individuals: 10 females and 10 males from Jamundí, and 10 females and 15 males from Cali. During the behavioral analysis, we used 28 of these individuals, including 14 females and 14 males, to form duos for the mate choice experiment based on color differences and similar body condition. We used the commissure measurement, which showed the highest correlation coefficient with body mass (r = 0.45, p = 0.001), to calculate the body condition index by using the residuals from the mass-commissure regression (Equation: Body mass = 5.927 + 2.15, Commissure, F = 11.54, p = 0.0014).
We conducted a total of 23 mate choice experiments, in 11 female choice of males experiments 5 (45.5%) presented bias due to direction or laterality, and 12 males choice of females experiments, 3 (25%) presented direction bias and 3 did not present a choice (25%), that is, the chooser did not present active choice. Individuals from duos were used 1.5 (±0.63) times/experiment. Since a bias with a marked preference for the right side was detected (GLMM, Wald χ2 test; χ21 = 5.05, p = 0.02, N = 80), eight experiments (34.78%) showing this right-side preference were discarded from the mate choice analysis.
No influence of crown color on mate choice was observed. In experiments where females chose males, six experiments (54.5%) showed mate choice, and no preference for crown color was identified. Three males with crown O+ and three with crown O- were chosen (GLMM, Z = 0, p = 1, N = 6). Similarly, no preference for crown color was found in experiments where males chose females. Six experiments (50%) displayed mate choice, with three females with crown O+ and three with crown O- being chosen (GLMM, Z = 0, p = 1, N = 6).
Females did not show a significant preference for males with a more saturated throat (5 O+ vs. 1 O-; GLMM, Z = -2.07, p = 0.03, N = 6, Table 3), nor did males exhibit a clear preference for saturation (3 O+ vs. 2 O-).
| Crown color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 22.64 | 24.09 | 16.64 | 0.0 | 1 | 1 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 22.64 | 24.09 | 16.64 | 0 | 1 | 1 |
| Throat color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 16.81 | 18.27 | 10.81 | 5.82 | 1 | 0.02 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 21.28 | 22.73 | 15.28 | 1.36 | 1 | 0.24 |
We used 11 females and 12 males in our confrontation trials, ensuring they had similar body conditions but differences in saturation. We conducted ten experiments among the males of both populations, in two there were no interactions between individuals (20%). The females faced each other 11 times and in five confrontations (45.45%) there were no interactions between the individuals. The individuals participated in the confrontations an average of 1.4 (±0.5) times. We observed aggressive interactions in 14 (66.6%) of the 21 confrontations, with a mean of 8.29 (±9.78) interactions per confrontation. There were a total of 174 interactions. The least frequent type involved physical contact, with only 11 interactions (6.32%) occurring in two experiments. The most frequent interaction was of one individual replacing another at the perch (supplant flight), accounting for 111 interactions (63.79%). The dominant individual won 94.42% (±0.004) of the aggressive interactions. Males had their first aggressive interaction at 5:21 min (±3:15), while females had theirs at 6:36 min (±3:25) after the start of the confrontation trial. In every case, the individual that won the first aggressive interaction turned out to be the dominant one. Overall, the dominant individual tended to move less and remained on its perch, whereas the other individual was more active, moving between perches and exploring the cage.
Males with O- crowns dominated those with O+ crowns; they won six confrontation trials (75%) of eight in which there were effective dominance interactions (GLMM, Z = 2.39, p = 0.01, N = 8; Table 4). Among females, those with O- crowns were dominant in five of six effective confrontations (83.33%) (GLMM, Z = 2.07, p = 0.03, N = 6; Table 4).
| Crown color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 24.65 | 26.20 | 20.65 | |||
| Model | 3 | 18.19 | 20.51 | 12.19 | 8.46 | 1 | 0.004 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.49 | 21.46 | 16.49 | |||
| Model | 3 | 16.81 | 18.27 | 10.81 | 5.68 | 1 | 0.02 |
| Throat color | |||||||
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.49 | 21.46 | 16.49 | |||
| Model | 3 | 21.24 | 22.69 | 15.24 | 1.26 | 1 | 0.26 |
When assessing whether throat color affected dominance in crown duos, O- and O+ males displayed an equal number of dominance responses (50%) in all six confrontation trials. The females present somewhat different results: with four (66.66%) experiments dominated by O- females and two (33.33%) by O+ (GLMM: Z = 0.85, p = 0.39, N = 6).
In order to evaluate the role of the throat and crown coloration in intraspecific interactions in Saffron Finches, a two-fold study was developed. In the first part, we developed the analysis of the chromatic variation in both plumage patches of the population; our results suggest that chromatic varation is perceptible among individuals, and that the Y and Z axes of the visual model (which estimates color perception based on the animal’s visual system and environmental conditions) are the ones that best explain the variation in the crown and throat. In the second phase, which consisted of behavioral experiments of dominance and mate choice, we found that the crown saturation was related to agonistic behaviors.
The greater weight in the Y and Z axes than in the X axis in the visual model was consistent with what one can expect for pigments such as carotenoids because these axes are associated with photoreceptors sensitive to medium and long wavelengths, respectively (Delhey et al., 2015). The JNDs PC1xyz’s range was higher than two in both plumage patches, and a strong correlation was found between PC1xyz and colorimetric variables associated with saturation. In carotenoid-based plumage saturation is a social signal in some passerines. For example, females prefer more saturated plumage in European Serins (Serinus serinus) (Leitão et al., 2014) and White-eyed Bulbuls (Pycnonotus xanthopygos) (Kabasakal et al., 2017). Furthermore, more saturated individuals are dominant among Common Waxbills (Estrilda astrild) (Beltrao et al., 2021) and Saffron Finches (Sicalis flaveola brasiliensis) in Brazil (Araújo-Silva et al., 2022). Results from the first phase of intra-sexual chromatic variation in Saffron Finches indicate noticeable differences for conspecifics in the main axis (primary direction of chromatic variation that explains the most significant portion of the color differences) in the crown and throat. This suggests that the color of both patches may play a role in communication, serving as social signals (Delhey and Peters, 2008). We found that the crown in males is significantly associated with dominance, while the throat patches in males appear to show a trend towards being linked to mate choice. Additionally, there are indications that the crown in females may influence dominance, though both results remain inconclusive.
The results of the mate choice experiment indicate that the color of the crown and throat does not influence females choice for males, contrary to what is known in passerines such as White-crowned Sparrows (Zonotrichia leucophrys) (Laubach et al., 2013) and Blue Tits (Henderson et al., 2013). In the case of Blue Tits, the blue color of the head exhibits a chromatic variability greater than four JNDs in both sexes, meaning that this color variation is perceptible to the birds (Delhey and Peters, 2008). It has been found in other passerines such as Green Finches (Carduelis chloris), Great Tits, and European Robins (Erithacus rubecula) that chromatic variability is greater in plumage patches associated with sexual selection or quality indicators (traits that convey honest information about an individual’s health, genetic fitness, or overall quality to potential rivals or mates) (Delhey and Peters, 2008).
Although female Saffron Finches did not exhibit a statistically significant preference for males with a more saturated throat, a marked trend was observed. The throat is exposed during social interactions such as courtship and territorial encounters between males during the dawn chorus (L. Cruz, unpublished data). We found that females prefer males with higher color saturation on the throat, which may reflect their overall condition and ability to access carotenoid-rich resources (Badyaev and Hill, 2000). Furthermore, given that this species is monogamous and males participate in chick care (Espinosa et al., 2017), the color could signal reproductive strategies and be linked to paternal care (Hill, 1991; Segura and Mahler, 2019). In passerine species, a preference for more colorful males with carotenoid-based plumage has been observed. For example, males with brighter overall plumage are favored by females in House Finches (Hill, 1993), while males with brighter head and throat coloration are preferred in Yellowhammers (Emberiza citrinella) (Sundberg, 1995). Additionally, females show a preference for males with bibs that exhibit higher values of brightness and hue in Yellowthroats (Geothlypis trichas) (Tarof et al., 2005).
Dominance experiments with Saffron Finches have shown that males with less saturated orange crown color tend to dominate over those with more saturated coloration. In females, although the result was not statistically significant, a similar trend was observed, with less saturated individuals displaying dominance over those with more saturated crown coloration. Given that the coloration is carotenoid-based, we expected individuals with more saturated coloration to exhibit greater aggressiveness (Badyaev and Hill, 2000). However, the results of this study differ from those observed in the Brazilian subspecies of Saffron Finch (S. f. brasiliensis), where more colorful individuals are dominant (Araújo-Silva et al., 2022). This finding contrasts with results in Red-Collared Widowbirds (Euplectes ardens) and Gouldian Finches, where redder males are more dominant (Pryke et al., 2002; Pryke and Griffith, 2006). However, researchers have also documented that, by intensifying the red color of the wing spots, in a wild population of males Red-Winged Blackbirds (Agelaius phoeniceus), color-modified individuals encounter greater difficulties in securing their territory, presenting more significant challenges in territorial competition and experiencing a higher frequency of losses in confrontations with non-color-modified individuals within the group (Yasukawa et al., 2009). This can be explained as a preventive action by neighboring males to reduce the likelihood of extra-pair breeding (Yasukawa et al., 2009). In a similar result, dull House Finches monopolized food in dominance experiments (McGraw and Hill, 2000). Therefore, less saturated individuals may be willing to risk more to acquire resources (McGraw and Hill, 2000).
In summary, the crown and throat show a degree of intraspecific variation that can be considered informative, as Saffron Finches are able to perceive color differences. The signal transmitted by the males’ crown influences intrasexual dominance in this species. The Saffron Finch is proposed as an ideal bird model for the study of the biology of color, due to the chromatic variability of its plumage patches, its wide distribution, and the differences in color between subspecies. This research provides insights into the behavioral ecology and plumage color of a tropical bird species, and is the third study to our knowledge that finds a negative association between coloration and dominance (McGraw and Hill, 2000; Yasukawa et al., 2009).
Camilo Ernesto Espinosa: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation, Conceptualization. James Montoya Lerma: Writing – review & editing, Methodology, Funding acquisition. Hector Fabio Rivera-Gutierrez: Writing – review & editing, Methodology, Funding acquisition. Lorena Cruz-Bernate: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
This study was evaluated by the Animal Ethics Committee of Universidad del Valle (Act No 008-2016) and had the permission as required by Colombian legislation (Resolution 149 of June 25, 2015). In addition, it is covered by the framework permit for the collection of specimens of wild species for non-commercial scientific research purposes from the National Environmental Licensing Authority-ANLA, which covers Universidad del Valle in its scientific research activities (Resolution 1070 of August 28, 2015).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2025.100241.
|
Andersson, M., 1994. Sexual Selection. Princeton University Press, Princeton.
|
|
Delhey, K., 2005. Sexual Selection and Blue Tit (Parus caeruleus) Crown Coloration. Doctoral Thesis. University of Munich, Germany.
|
|
Fox, J., Weisberg, S., 2011. An R Companion to Applied Regression. Sage Publications.
|
|
Gustincich, S., Manfioletti, G., Del Sal, G., Schneider, C., Carninci, P., 1989. A fast method for high-quality genomic DNA extraction from whole human blood. Biotechniques 11, 25.
|
|
Hilty, S.L., Brown, W.H., 2001. Guide to the Birds of Colombia. Universidad del Valle, Santiago de Cali, Valle del Cauca, Colombia.
|
|
Martin, G.R., Osorio, D., 2008. Vision in birds. In: Masland, R.H., Albright, T.D. (Eds.), The Senses, 1. Academic Press, London, pp. 25–52.
|
|
Montgomerie, R., 2006. Analyzing colors. In: Hill, G.E., McGraw, K.J. (Eds.), Bird Coloration, 1. Harvard University Press, Cambridge, pp. 90–147.
|
|
Rising, J., Jaramillo, A., Copete, J.L., Madge, S., Ryan, P., 2011. Family Emberizidae (buntings and new world sparrows). In: Del Hoyo, J., Elliot, A., Christie, A. (Eds.), Handbook of the Birds of the World. Volume 16: Tanagers to New World Blackbirds. Lynx Editions, Barcelona, Spain, pp. 428–876.
|
| Female crown | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 1.45 | 69.72 | 69.72 | -0.10 | 0.70 | 0.70 |
| 2 | 0.38 | 18.58 | 88.30 | -0.47 | -0.65 | 0.58 |
| 3 | 0.24 | 11.69 | 100 | -0.87 | 0.27 | -0.40 |
| Male crown | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 1.69 | 78.2 | 78.20 | -0.16 | 0.79 | 0.59 |
| 2 | 0.36 | 16.76 | 94.97 | -0.47 | -0.58 | 0.65 |
| 3 | 0.10 | 5.02 | 100 | -0.86 | 0.17 | -0.47 |
| Throat female | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 2.99 | 83.16 | 83.16 | -0.39 | 0.67 | 0.62 |
| 2 | 0.50 | 14.16 | 97.32 | -0.86 | -0.50 | -0.0007 |
| 3 | 0.09 | 2.67 | 100 | -0.31 | 0.54 | -0.77 |
| Throat male | ||||||
| Principal component | Eigenvalue | % variance | % cumulative variance | Vector weight | ||
| X | Y | Z | ||||
| 1 | 6.88 | 89.75 | 89.75 | -0.11 | 0.73 | 0.66 |
| 2 | 0.61 | 7.98 | 97.73 | -0.86 | -0.40 | 0.29 |
| 3 | 0.17 | 2.22 | 100 | -0.48 | 0.54 | -0.68 |
| Female crown | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.35 | -0.55 | -0.11 | -2.93 | 0.006 | Pearson |
| Phi | 0.52 | 0.31 | 0.68 | 20006 | <0.001 | Spearman |
| r achieved | 0.91 | 0.85 | 0.94 | 3832 | <0.001 | Spearman |
| Luminance | -0.06 | -0.31 | 0.20 | 44214 | 0.63 | Spearman |
| Brightness | -0.15 | -0.38 | 0.10 | -1.16 | 0.25 | Pearson |
| Saturation | -0.77 | -0.86 | -0.65 | -9.55 | <0.001 | Pearson |
| Carotenoid chroma | 0.92 | 0.88 | 0.95 | 18.74 | <0.001 | Pearson |
| Hue | 0.35 | 0.11 | 0.55 | 2.88 | 0.007 | Pearson |
| Male crown | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.11 | -0.35 | 0.15 | 48508 | 0.39 | Spearman |
| Phi | 0.57 | 0.37 | 0.72 | 18904 | <0.001 | Spearman |
| r achieved | 0.95 | 0.92 | 0.97 | 23.88 | <0.001 | Pearson |
| Luminance | -0.11 | -0.34 | 0.14 | -0.85 | 0.47 | Pearson |
| Brightness | -0.15 | -0.38 | 0.10 | -1.17 | 0.33 | Pearson |
| Saturation | -0.96 | -0.98 | -0.93 | -26.70 | <0.001 | Pearson |
| Carotenoid chroma | 0.95 | 0.92 | 0.97 | 23.94 | <0.001 | Pearson |
| Hue | -0.02 | -0.27 | 0.23 | 44509 | 0.88 | Spearman |
| Female throat | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.64 | -0.77 | -0.46 | 59130 | <0.001 | Spearman |
| Phi | 0.65 | 0.47 | 0.77 | 6.46 | <0.001 | Pearson |
| r achieved | 0.97 | 0.96 | 0.98 | 32.73 | <0.001 | Pearson |
| Luminance | -0.51 | -0.68 | -0.30 | -4.53 | <0.001 | Pearson |
| Brightness | -0.54 | -0.70 | -0.33 | -4.90 | <0.001 | Pearson |
| Saturation | -0.93 | -0.96 | -0.89 | -19.28 | <0.001 | Pearson |
| Carotenoid chroma | 0.83 | 0.73 | 0.89 | 11.20 | <0.001 | Pearson |
| Hue | 0.64 | 0.45 | 0.77 | 13111.05 | <0.001 | Spearman |
| Male throat | ||||||
| Variable | r/rho | CI low | CI high | t/S | p-value | Method |
| Theta | -0.33 | -0.53 | -0.09 | -2.77 | 0.01 | Pearson |
| Phi | 0.37 | 0.13 | 0.57 | 27538.82 | 0.005 | Spearman |
| r achieved | 0.48 | 0.26 | 0.66 | 22584.76 | <0.001 | Spearman |
| Luminance | -0.04 | -0.29 | 0.22 | 45309.02 | 0.77 | Spearman |
| Brightness | -0.20 | -0.43 | 0.06 | 52401.10 | 0.11 | Spearman |
| Saturation | -0.97 | -0.98 | -0.95 | 85977.48 | <0.001 | Spearman |
| Carotenoid chroma | 0.95 | 0.91 | 0.97 | 2398.53 | <0.001 | Spearman |
| Hue | 0.67 | 0.51 | 0.79 | 7.08 | <0.001 | Pearson |
| Confidence interval: 95%, significance level: 0.05. | ||||||
| Crown color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 22.64 | 24.09 | 16.64 | 0.0 | 1 | 1 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 22.64 | 24.09 | 16.64 | 0 | 1 | 1 |
| Throat color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 16.81 | 18.27 | 10.81 | 5.82 | 1 | 0.02 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.64 | 21.61 | 16.64 | |||
| Model | 3 | 21.28 | 22.73 | 15.28 | 1.36 | 1 | 0.24 |
| Crown color | |||||||
| Males | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 24.65 | 26.20 | 20.65 | |||
| Model | 3 | 18.19 | 20.51 | 12.19 | 8.46 | 1 | 0.004 |
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.49 | 21.46 | 16.49 | |||
| Model | 3 | 16.81 | 18.27 | 10.81 | 5.68 | 1 | 0.02 |
| Throat color | |||||||
| Females | df | AIC | BIC | Deviance | LRT | ||
| Chisq | Chi df | p-value | |||||
| Null model | 2 | 20.49 | 21.46 | 16.49 | |||
| Model | 3 | 21.24 | 22.69 | 15.24 | 1.26 | 1 | 0.26 |

