| Citation: | Sue-Jeong Jin, Hae-Ni Kim, Jun-Seo Go, Myeong-Chan Cha, Heesoo Lee, Seongho Yun, Jin-Won Lee. 2025: Comparative analysis of female bubbling calls: Within- and between-species variation among the four species of Cuculus cuckoos. Avian Research, 16(1): 100240. DOI: 10.1016/j.avrs.2025.100240 |
In brood-parasitic Cuculus cuckoos, male vocalizations are species-specific and easily distinguishable, whereas female calls are remarkably similar across species, making species identification challenging. In this study, we examined the structural characteristics and variability of female bubbling calls among four Cuculus species (Common Cuckoo C. canorus, Oriental Cuckoo C. optatus, Indian Cuckoo C. micropterus, and Lesser Cuckoo C. poliocephalus) breeding in South Korea. Comprehensive acoustic analyses of seven call parameters, using recordings from 2021 to 2023, were conducted to quantify the characteristics of their calls and compare within- and between-individual variability across species. Significant differences were found across all call parameters, with the Common Cuckoo producing the highest number of notes and the Oriental Cuckoo the lowest-frequency calls. Despite these differences, the overall structure of the calls remained acoustically similar, with overlapping characteristics across species. Furthermore, female Common Cuckoos exhibited greater within-individual variability in their calls, while the other species showed higher between-individual variability, which may further complicate species identification based vocalization alone. These findings highlight the complexities of female vocalizations in Cuculus cuckoos and suggest that ecological, social, and evolutionary factors may contribute to this vocal variability.
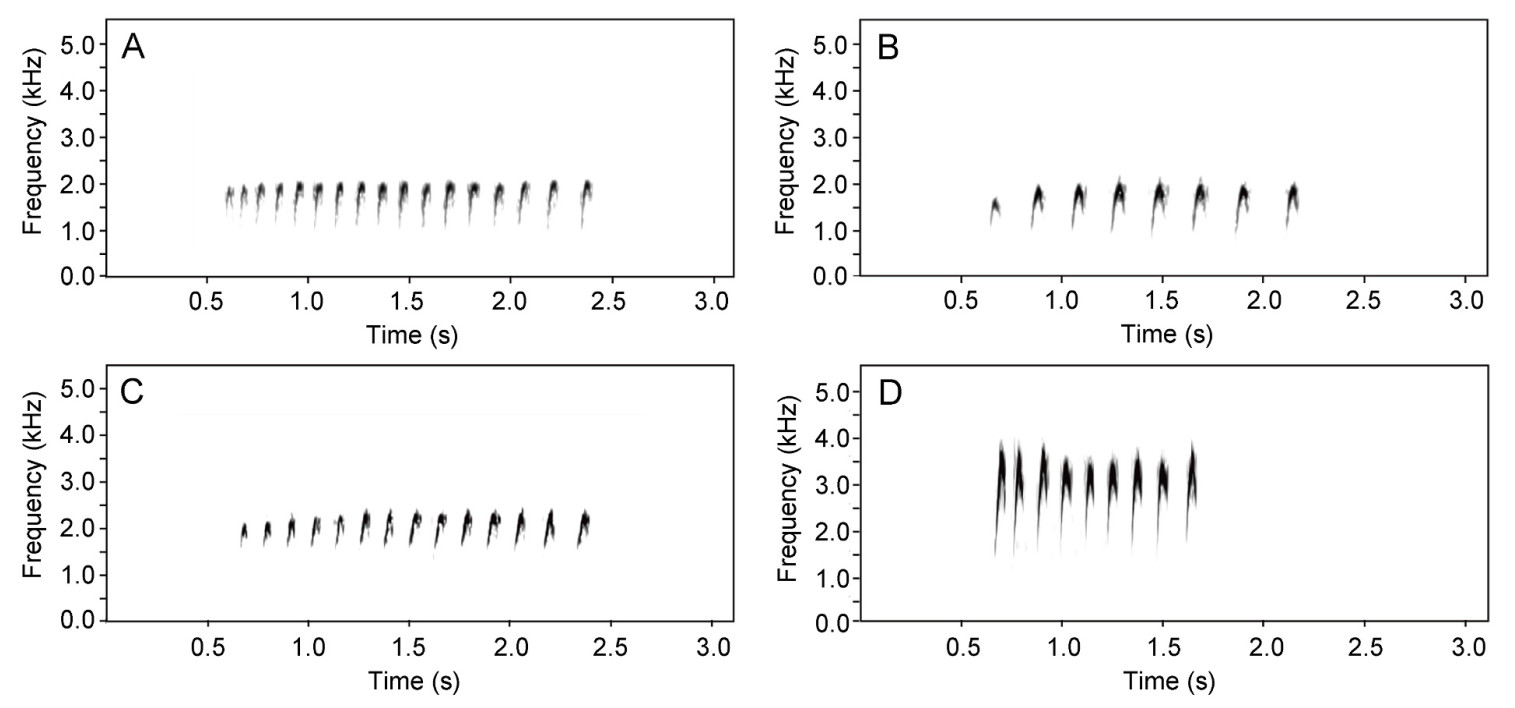
The four species of brood-parasitic Cuculus cuckoos (the Common Cuckoo C. canorus, Oriental Cuckoo C. optatus, Indian Cuckoo C. micropterus, and Lesser Cuckoo C. poliocephalus) arrive in Korea each spring (Lee, 2014). Upon arrival, they engage in intensive calling throughout the breeding season (del Hoyo et al., 1997). The male calls of these species, such as the iconic two-note ‘cu-coo’ of the Common Cuckoo, are elegantly simple yet highly distinctive, allowing for clear identification of each species. In contrast, the vocalizations of female cuckoos are less easily distinguishable. The female of all four Cuculus species produce calls that are remarkably similar, commonly referred to as bubbling calls (Chance, 1940; Payne and Sorensen, 2005; Erritzøe et al., 2012). These calls not only sound alike but also exhibit visually similar pattern when analyzed though sonograms (Kim et al., 2017a, b; Choi et al., 2022, Fig. 1). Despite extensive research into these vocalizations, however, the full extent of this similarity, both between and within species, remains largely unclear.
Both male and female Cuculus cuckoos use vocalizations for a variety of functions, including mate attraction and territorial defense (Lee et al., 2019; Moskát and Hauber, 2019, 2021; Xia et al., 2019; Yoo et al., 2020), but there are notable differences between their calls. In the Common Cuckoo, males are known to exhibit greater call variation between individuals than within individuals, enabling individual recognition (Jung et al., 2014; Li et al., 2017; Zsebők et al., 2017). However, Deng et al. (2019a) found that the consistency of male calls decreases as the breeding season progresses. Early in the season, calls remain stable early, but they become increasingly variable over time, reducing the accuracy of individual identification. This progressive decline in call stability suggests that male vocalizations may be more variable than previously thought, potentially complicating individual recognition. In contrast, female Common Cuckoos show lower within-individual consistency throughout the season compared to males, resulting in less predictable vocalization overall (Deng et al., 2019b). Although researches on female bubbling calls across species remain limited, existing studies have identified quantitative differences in call parameters, such as delta time and frequency, among the four Cuculus species in Korea (Kim et al., 2017a, b). These findings suggest that species-specific vocal traits may exist, providing a basis for understanding interspecific and intraspecific variability in female vocalizations.
However, the existing studies on female bubbling calls had several limitations. While some acoustic parameters have shown variation, they may not be sufficient on their own to reliably distinguish species. Moreover, the small sample size in previous research restricted the ability to comprehensively compare interspecies differences, and intraspecific variation was largely overlooked. Since these initial findings, no further quantitative analysis of bubbling calls across Cuculus species, nor has detailed research explored the underlying reasons for the apparent similarity in female bubbling calls across species. As a result, the degree of similarity and variation among these calls—despite sounding remarkably alike to human ears—remains poorly understood. These gaps highlight the need for more comprehensive studies to clarify the vocal characteristics of Cuculus species and their evolutionary significance.
In this study, we investigated the interspecific differences, underlying similarities, and individual consistency in the female bubbling calls of the four Cuculus species breeding in South Korea. Using a robust dataset of recordings, with the additional benefit of concurrent field recordings linked to individual identification, we conducted comprehensive cross-species comparisons. Through our analysis, we examined the unique characteristics of each species’ calls that arise from interspecies variation while also exploring why these calls appear acoustically similar to human listeners. Furthermore, by assessing the consistency of calls at the individual level across the four species, we identified specific parameters that can be used to differentiate individuals within a species. The insights gained from this study provide a detailed examination of the structure and individual variation in female bubbling calls, contributing to a deeper understanding of their evolutionary significance.
Fieldwork and recordings were conducted during the breeding season of Cuculus cuckoos from April to June in 2021–2023 across various region of South Korea. In South Korea, Oriental Cuckoo arrives first, typically in early to mid-April, followed by Indian Cuckoo in late April, and finally Common Cuckoo and Lesser Cuckoo in early to mid-May. While Common Cuckoo, Oriental Cuckoo and Indian Cuckoo are distributed nationwide, Lesser Cuckoo is primarily found in the southern regions (Lee et al., 2014). Recording was carried out nationwide, taking place daily from 4:00 to 12:00 and from 16:00 to 20:00. Most recording were captured using Zoom H1n Handy Recorder, which served as the primary recording device. Supplementary recordings were made with other devices, including the Zoom H4n Handy Recorder, cellphones (Note 9 (SM-N960N), iPhone X (A1901), and Galaxy S22+ (SM-S906N)). Fieldworks and recordings were not conducted on days with adverse weather conditions, such as strong wind or rain. Since female cuckoos vocalize infrequently and their calls are context-dependent, playback was used both to confirm their presence and to elicit vocal responses. The playback stimuli included calls from both male and female individuals of the same species, selected from different individuals to minimize potential bias. However, not all recorded calls were direct responses to playback, as some were naturally occurring vocalizations.
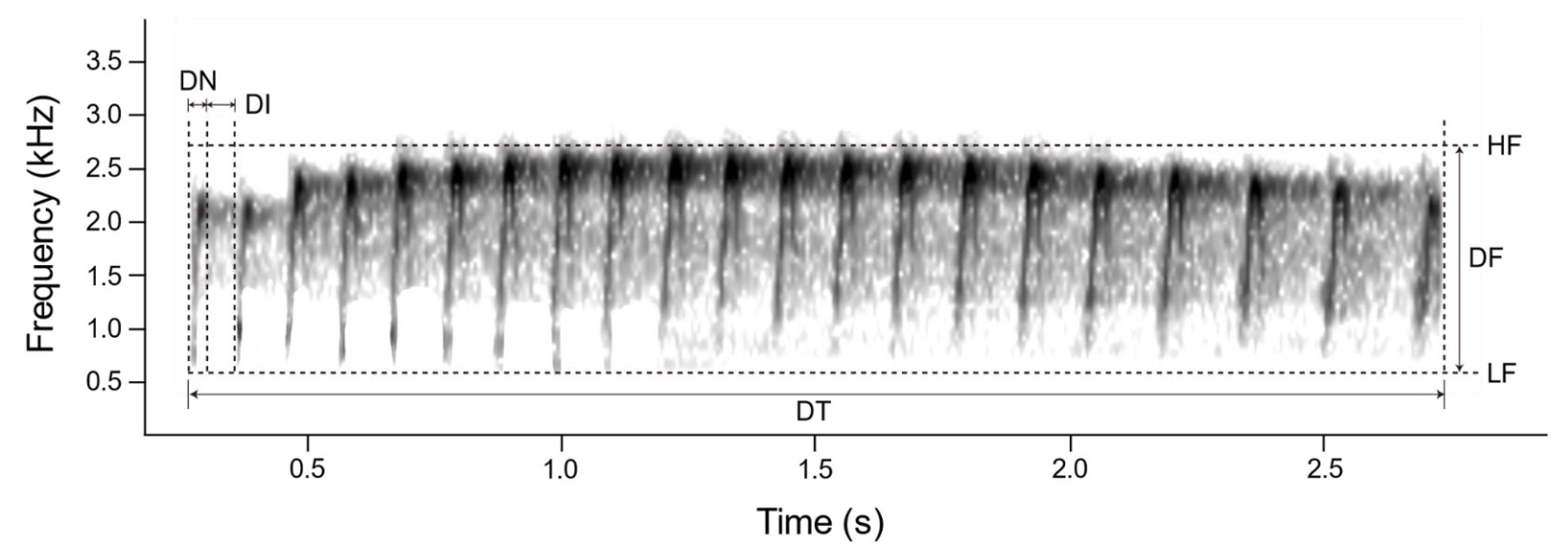
Acoustic data recorded by mobile phones were converted into.WAV files via Online Audio Converter (https://online-audio-converter.com/ko/). Using Audacity 3.2.4 (Audacity Team, Boston, MA), the sample rate of acoustic data was standardized to 44.1 kHz and 16 bit. In this study, a note refers to a single, continuous sound unit, while a syllable consists of one or more notes forming a distinct acoustic unit. Seven acoustic parameters were extracted per syllable using Raven Pro 1.6.5 (Lisa, 2023), including the number of notes (NN), delta time (DT), delta time of notes (DN), delta time of intervals (DI), highest frequency (HF), lowest frequency (LF), and delta frequency (DF) (Fig. 2). DN refers to the duration of individual notes, while DI represents the silent gap between consecutive notes. Since each syllable contains multiple notes, multiple DN and DI values were measured per syllable. For analysis, we calculated the average DN and DI values for each syllable and used these in statistical analyses. The further definition and extraction criteria for each parameter followed those outlined in Kim et al. (2017a). All acoustic analysis were performed by one person (Sue-Jeong Jin). The total number of female individuals analyzed was as follow: the 156 Common Cuckoos (187 files, 1417 syllables), 37 Oriental Cuckoos (41 files, 470 syllables), 17 Indian Cuckoos (21 files, 255 syllables), and 16 Lesser Cuckoos (24 files, 194 syllables).
We used the Kruskal–Wallis rank sum test to assess whether the call component values of female bubbling calls differed significantly among the four Cuculus species, as these values did not follow a normal distribution. To further investigate these differences, we performed a post hoc test on the Kruskal–Wallis rank sum test results using the ‘DescTolls’ package (Signorell, 2023). We calculated Spearman's rank correlation coefficient to assess the relationships between call components within each species. However, due to the limited sample size (n = 4), we assessed the relationship between body mass and the number of notes among the four Cuculus species by visually examining the trend rather than conducting statistical tests. The body mass data of the species were obtained from Table 1 of Go et al. (2021). For the analysis of variation in female bubbling calls, we included only individuals from which more than 10 syllables were recorded. This resulted in a dataset of 1012 syllables from the 55 Common Cuckoos, 390 syllables from 15 Oriental Cuckoos, 221 syllables from 7 Indian Cuckoos, and 165 syllables from 7 Lesser Cuckoos.
| Parameters | C. canorus (n = 156) | C. optatus (n = 37) | C. micropterus (n = 17) | C. poliocephalus (n = 16) | χ2 | p |
| Number of notes | 19 (17–22) | 8 (7–12) | 14 (11–17) | 8.5 (6–10) | 114.18 | <0.001 |
| Delta time (s) | 1.83 (1.66–2.01) | 1.58 (1.29–1.84) | 1.57 (1.35–1.9) | 0.97 (1.84–1.25) | 45.18 | <0.001 |
| Delta time of notes (s) | 0.03 (0.03–0.04) | 0.05 (0.04–0.06) | 0.05 (0.04–0.05) | 0.04 (0.03–0.05) | 77.44 | <0.001 |
| Delta time of intervals (s) | 0.07 (0.06–0.08) | 0.14 (0.11–0.16) | 0.08 (0.06–0.09) | 0.1 (0.09–0.11) | 102.57 | <0.001† |
| Highest frequency (kHz) | 2.29 (2.16–2.44) | 2.11 (1.99–2.27) | 2.44 (2.32–2.57) | 3.58 (3.35–3.66) | 60.31 | <0.001† |
| Lowest frequency (kHz) | 0.72 (0.62–0.81) | 0.57 (0.5–0.68) | 0.92 (0.8–1.02) | 1.29 (1.15–1.49) | 69.94 | <0.001† |
| Delta frequency (kHz) | 1.5 (1.42–1.72) | 1.49 (1.37–1.67) | 1.54 (1.45–1.6) | 2.17 (1.97–2.48) | 37.55 | <0.001 |
| A Kruskal–Wallis rank sum test was performed to assess whether the call parameter values differed significantly among the four species. A post hoc test was then conducted to identify specific differences between species. A dagger mark (†) indicates that all four species showed significant differences from each other based on the results of the post hoc analysis. | ||||||
The coefficient of variation of each call parameter was calculated using the bias-corrected formula CV = 100 × (1 + 1/(4 × n)) × SD/mean, where n is sample size and SD is standard deviation of sample. This correction, based on Sokal and Rohlf (1995), accounts for potential underestimation of variability in small sample sizes, providing a more accurate estimate of dispersion. For the within-individual coefficient of variation (CVi), we calculated the CV for each individual based on all the calls belonging to that individual, then averaged CVs across all individuals (Sokal and Rohlf, 1995). The between-individual CVb was obtained by first averaging the call parameters for each individual, followed by calculating the CV for these average values (Sokal and Rohlf, 1995). The potential individual coding (PIC) was measured by calculating the ratio of CVi/CVb (Robisson et al., 1993; Charrier et al., 2001). A PIC value greater than 1 indicates that the between individual variation exceeds the within individual variation (Robisson et al., 1993; Lengagne et al., 1997; Li et al., 2017).
Principal component analysis (PCA) was used to evaluate the differences between the four Cuculus species based on seven call parameters. PCA was computed using the ‘prcomp’ function, with calculations based on the correlation matrix to standardize the variables given their differing scales. All other settings followed the default configuration of the ‘prcomp’ function, including centering the data and retaining orthogonal axes without additional rotation. We retained the first three principal components (PCs), which together explain more than 80% of the total variation, following Kaiser's criterion (eigenvalue > 1) and examination of the scree plot (Crawley, 2012). To evaluate whether the PCs differed significantly among the four Cuculus species, we analyzed the component scores using the Kruskal–Wallis rank sum test. For pairwise species comparisons of PC values, we conducted a post hoc test using the package ‘dunn.test’ (Dinno, 2017). All statistical analyses were conducted using R version 4.3.0 (R Core team, 2023).
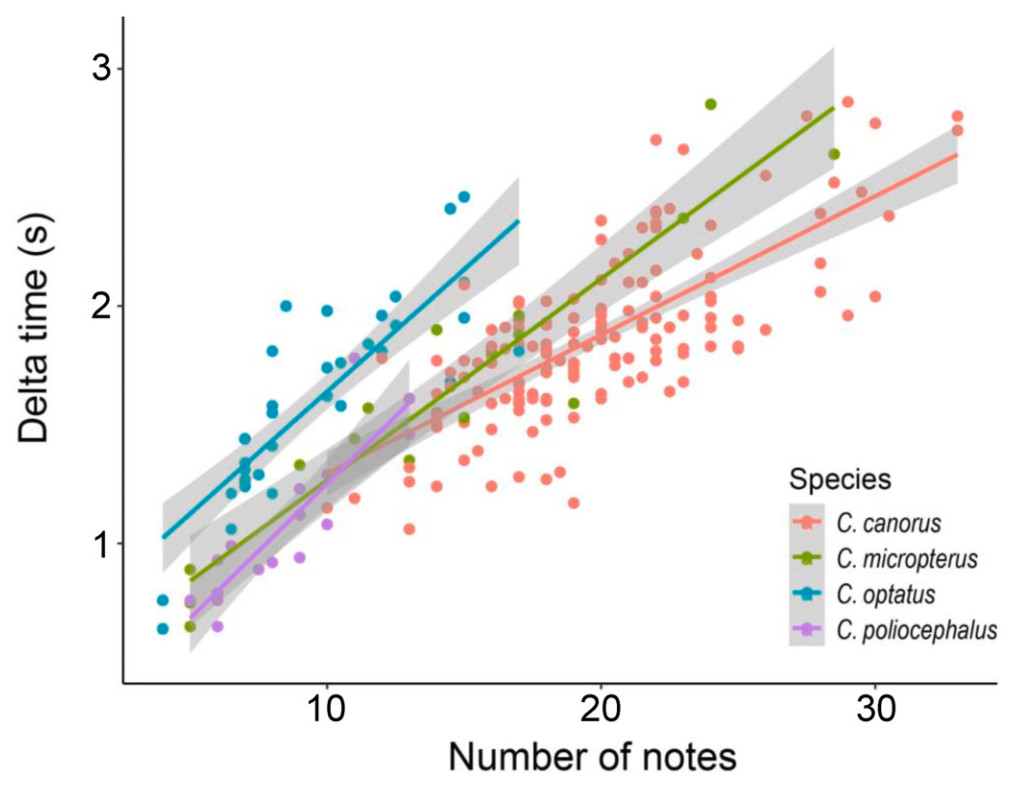
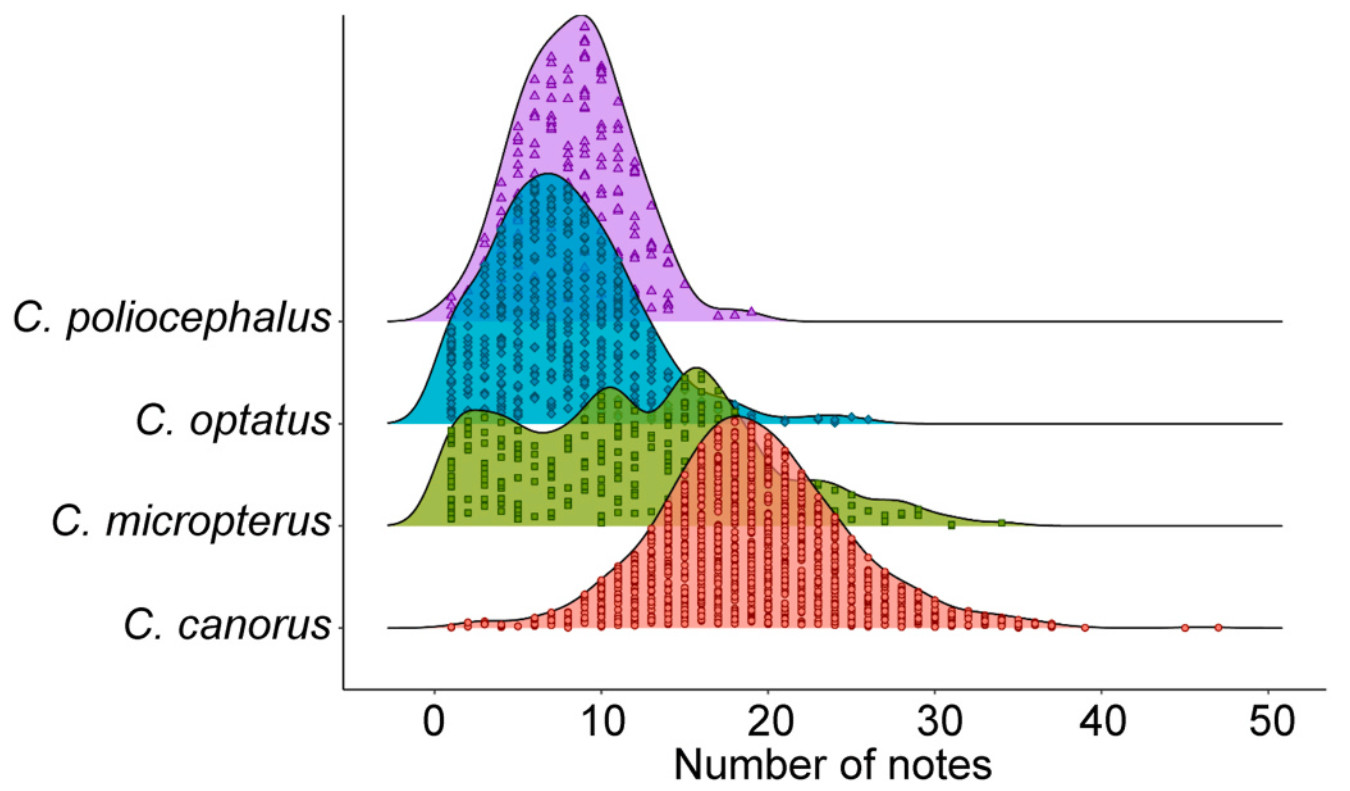
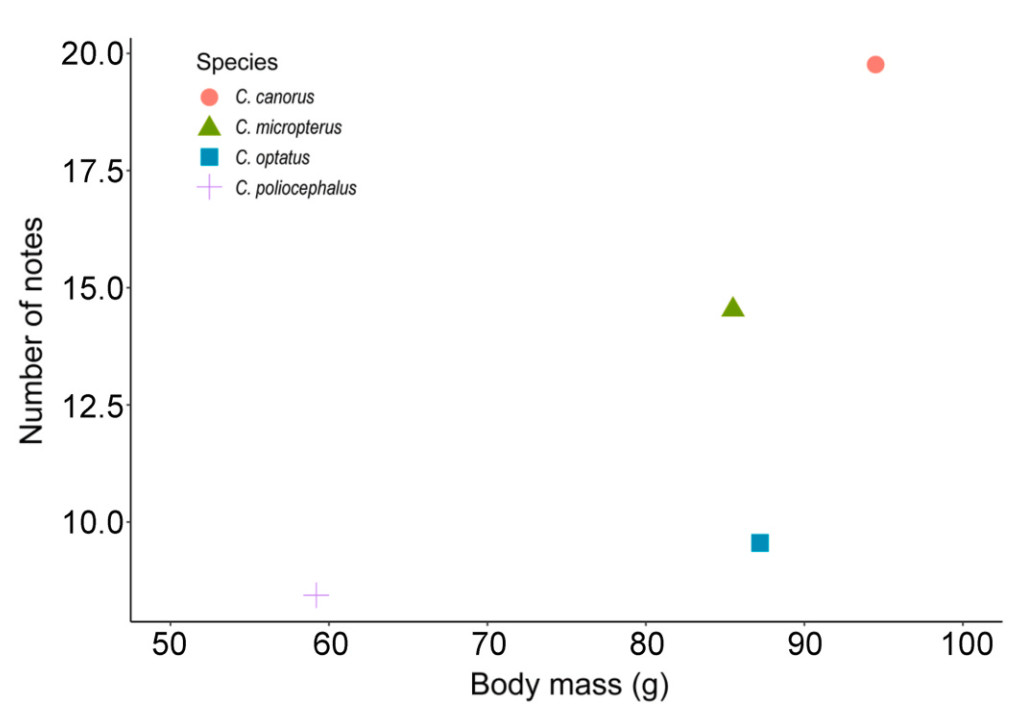
The female bubbling calls of the four Cuculus species exhibited structural similarities across species (Fig. 1). However, quantitative comparisons of seven call parameters revealed significant differences across all parameters (p < 0.001; Table 1). Notably, the delta time, high frequency and low frequency differed significantly among the species (Table 1). In particular, the Common Cuckoo showed distinct differences in all call parameters, except delta frequency, with the highest values in both the number of notes and delta time (Table 1). However, the correlation between the number of note and delta time was lowest in the Common Cuckoo (r = 0.69), followed by the Oriental Cuckoo (r = 0.87), Lesser Cuckoo (r = 0.91), and Indian Cuckoo (r = 0.92) (Fig. 3). The Common Cuckoo has the shortest delta time of notes and delta time of intervals among the four species, which may explain why it has a slightly shorter overall delta time compared to other species, despite producing more notes. Furthermore, the range in the number of notes produced by the Common Cuckoo was broader than that of the other three species, reflecting notable variability in its vocal output (Fig. 4), and this variation aligns with a positive relationship between body mass and the number of notes among the four Cuculus species (Fig. 5). However, due to the limited sample size (n = 4), statistical testing was not conducted, and this pattern should be interpreted with caution.
The Oriental Cuckoo exhibited the longest delta time of intervals and the lowest frequency values in all frequency parameters, indicating that it produces the slowest and lowest-pitched bubbling calls (Table 1). The Lesser Cuckoo, while producing a similar number of notes as the Oriental Cuckoo (p = 0.49), had the shortest delta time and the highest frequency values among the species (Table 1). Meanwhile, the Indian Cuckoo did not exhibit extreme values in any of parameters, but the highest frequency values were second only to the Lesser Cuckoo (Table 1). Therefore, the bubbling calls of the four Cuculus species exhibit distinct structural characteristics, each defined by species-specific call parameters.
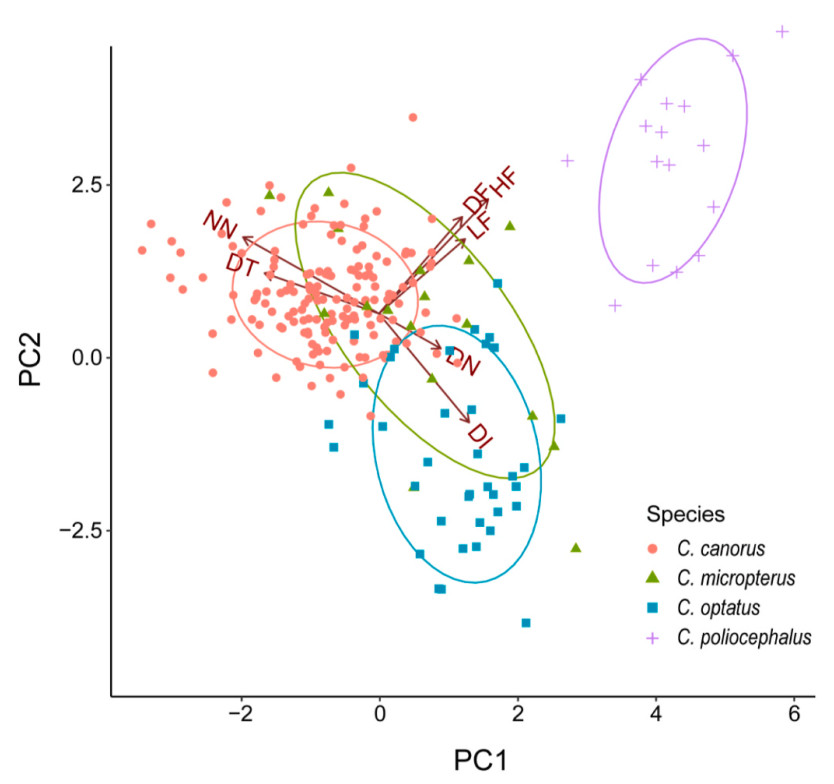
A principal component analysis (PCA) was performed to identify key parameters distinguishing the female bubbling calls among the four Cuculus species. Three principal components (PCs) were extracted, collectively accounting for ever 84% of the total variance, each with eigenvalues greater than 1 (Table 2). PC1, explaining 39% of the total variance, was negatively correlated with the number of notes and delta time. This means that species with fewer notes also had shorter delta time, and vice versa (Table 2; Fig. 6). PC2, accounting for 27% of the total variance, was negatively associated with the delta time of intervals and positively associated with high and delta frequencies. Higher PC2 values indicates faster calls with higher frequencies (Table 2; Fig. 6). PC3, which explain 18% the total variance, was positively correlated with the delta time of notes and negatively correlated with low frequency. This represents the relationship between note duration and frequency, where species with longer notes tend to produce lower-pitched call (Table 2; Fig. 6).
| Variables | PC1 (39%) | PC2 (27%) | PC3 (18%) |
| NN | −0.51 | 0.35 | 0.05 |
| DT | −0.43 | 0.18 | 0.40 |
| DN | 0.23 | −0.16 | 0.72 |
| DI | 0.34 | −0.49 | 0.07 |
| HF | 0.41 | 0.52 | 0.04 |
| LF | 0.33 | 0.34 | −0.4 |
| DF | 0.32 | 0.44 | 0.39 |
| NN, DT, DN, DI, HF, LF, DF represent Number of Notes, Delta Time, Delta Time of Notes, Delta Time of Intervals, Highest Frequency, Lowest Frequency and Delta Frequency, respectively. The percentages in parentheses show the amount of variation explained by each principal component (PC), and the numbers in bold represent the components loaded most highly for each parameter. Total explanation power 84%. | |||
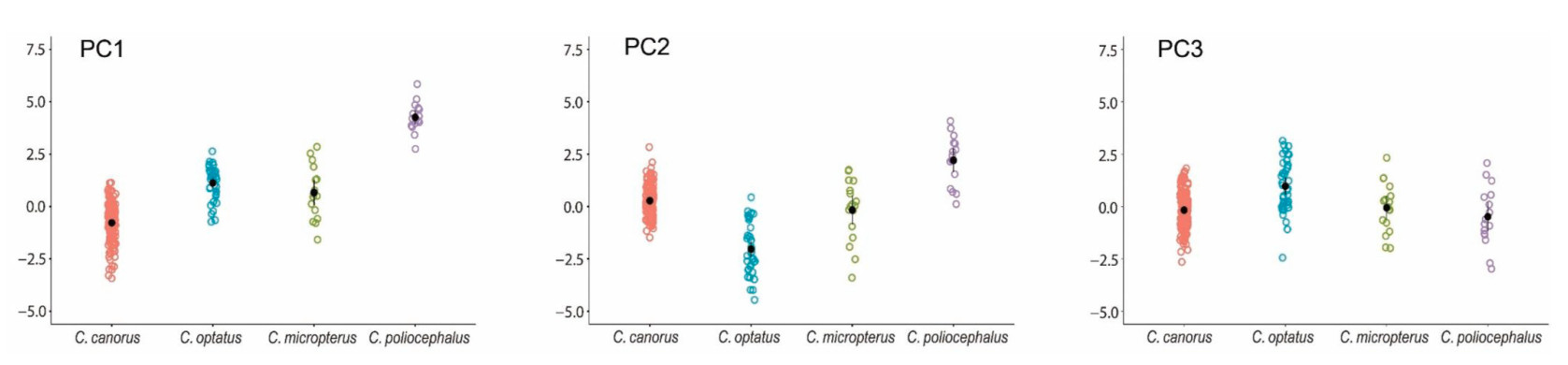
At the species level, all three principal components (PCs) showed significant differences among the four Cuculus species (p < 0.001). Post hoc test results revealed that PC1 showed significant differences between all species, except between the Oriental Cuckoo and Indian Cuckoo (p = 0.09; Fig. 7). The Lesser Cuckoo had the highest PC1 values, indicating that it produces the shortest and fastest bubbling calls. In contrast, the Common Cuckoo, with the lowest PC1, exhibited the opposite pattern, producing the longest call. For PC2, significant differences were found among species, except between the Common Cuckoo and Indian Cuckoo (p = 0.2; Fig. 7). The Lesser Cuckoo again had the highest values, while the Oriental Cuckoo showed the lowest values for PC2, indicating that the Lesser Cuckoo produces the highest-pitched calls with the shortest intervals, while the Oriental Cuckoo produces the lowest-pitched calls with the longest intervals. Regarding PC3, only the Oriental Cuckoo had significantly higher PC values compared to the other three species, and there were no significant differences in PC3 between the Common Cuckoo, Indian Cuckoo and Lesser Cuckoo (Common Cuckoo vs. Indian Cuckoo, p = 0.34; Common Cuckoo vs. Lesser Cuckoo, p = 0.18; Indian Cuckoo and Lesser Cuckoo, p = 0.16; Fig. 7). Therefore, the results showing that the three principal components differ significantly among the four Cuculus species, yet exhibit substantial overlap, suggest that key acoustic features contributing to species differentiation may not be perceptually salient to human listeners. This overlap in acoustic space may explain why bubbling calls are perceived as similar despite measurable differences in call parameters.
The call parameters exhibited varying degrees of within- and between-individual variation across the four Cuculus species (Table 3). In the Common Cuckoo, most call parameters show greater variation within individuals (CVi) than between individuals (CVb), with exception of the delta time of notes, where CVb was higher (PIC >1; Table 3). In contrast, in the other three species, three parameters in the Indian Cuckoo and Lesser Cuckoo, and four parameters in the Oriental Cuckoo, displayed greater CVb than CVi (PIC >1; Table 3). These results suggest that female Common Cuckoos exhibit a higher degree of call variability within individuals, indicating lower call consistency. Conversely, the other Cuculus species demonstrate greater call consistency within individuals and greater variability between individuals (Table 3).
| Species | Parameter | Mean (min–max) | CVb | CVi | PIC |
| C. canorus (n = 55) | NN | 18.8 (13–30.5) | 18.53 | 24.09 | 0.77 |
| DT | 1.81 (1.17–2.55) | 13.3 | 24.23 | 0.55 | |
| DN | 0.03 (0.02–0.05) | 20.18 | 18.53 | 1.09 | |
| DI | 0.07 (0.04–0.09) | 16.13 | 17.15 | 0.94 | |
| HF | 2.25 (1.95–2.67) | 7.15 | 8.49 | 0.84 | |
| LF | 0.74 (0.5–1.01) | 15.46 | 19.45 | 0.8 | |
| DF | 1.49 (1.23–1.95) | 9.6 | 13.02 | 0.74 | |
| C. optatus (n = 15) | NN | 7.4 (4–12) | 33.25 | 48.47 | 0.69 |
| DT | 1.38 (0.64–2) | 26.18 | 47.67 | 0.55 | |
| DN | 0.05 (0.03–0.06) | 19.13 | 16.7 | 1.15 | |
| DI | 0.16 (0.11–0.23) | 18.38 | 27.5 | 0.67 | |
| HF | 2.06 (1.8–2.43) | 164.7 | 11.71 | 14.06 | |
| LF | 0.63 (0.46–0.97) | 120 | 19.60 | 6.12 | |
| DF | 1.44 (1.11–1.87) | 180.4 | 15.4 | 11.72 | |
| C. micropterus (n = 7) | NN | 9.64 (5–16) | 37.98 | 62.01 | 0.61 |
| DT | 1.24 (0.65–1.81) | 33.02 | 58.14 | 0.57 | |
| DN | 0.04 (0.03–0.05) | 19.67 | 16.55 | 1.19 | |
| DI | 0.11 (0.08–0.19) | 41.95 | 46.94 | 0.89 | |
| HF | 2.39 (2.13–2.73) | 7.95 | 6.79 | 1.17 | |
| LF | 0.92 (0.72–1.1) | 14.48 | 15.14 | 0.96 | |
| DF | 1.50 (1.11–1.78) | 15.35 | 12.92 | 1.19 | |
| C. poliocephalus (n = 7) | NN | 9 (6.5–13) | 17.41 | 39.44 | 0.44 |
| DT | 1.12 (0.89–1.59) | 19.69 | 39.33 | 0.5 | |
| DN | 0.04 (0.03–0.05) | 22.29 | 16.51 | 1.35 | |
| DI | 0.1 (0.09–0.11) | 9.82 | 22.23 | 0.44 | |
| HF | 3.56 (2.92–4.43) | 10.67 | 9.16 | 1.17 | |
| LF | 1.33 (0.91–1.84) | 26.35 | 16.73 | 1.58 | |
| DF | 2.17 (1.64–2.52) | 13.9 | 14.87 | 0.94 | |
| Call parameters from individuals with recording containing more than 10 syllables were analyzed (C. canorus: 55 individuals, C. optatus: 15 individuals, C. micropterus and C. poliocephalus: 7 individuals each). The call parameters are defined as follows: NN (Number of Notes), DT (Delta Time), DN (Delta Time of Notes), DI (Delta Time of Intervals), HF (Highest Frequency), LF (Lowest Frequency), and DF (Delta Frequency). CVb represents between-individual variation, CVi represents within-individual variation, and PIC is the ratio of CVb to CVi. A PIC value greater than 1 indicates that between-individual variation exceeds within-individual variation. | |||||
This study explored the structural similarities and differences in the bubbling calls of the four Cuculus species, as well as within- and between-individual variations within each species. By quantifying and analyzing seven call parameters, our results revealed that all parameters are subtle but significantly different across species (all p < 0.001), enhancing previous research that identified six distinct parameters. Notably, while previous studies highlighted differences in delta time and frequency (Kim et al., 2017a, b), our results showed that the delta time of intervals, and highest frequency and lowest frequency, excluding delta frequency, of bubbling calls were also significantly different among the species. Consistent with previous results (Kim et al., 2017a, b), the number of notes remained the most variable parameter among the four species, with the Common Cuckoo producing the largest number of notes. Another notable finding in this regard is that the Common Cuckoo produced a boarder range of notes than the other three species, while using small numbers of notes less frequently. We suggest that this variability in vocal behavior aligns with the positive correlation observed between body mass and the number of notes among the four Cuculus cuckoos (Fig. 5). The larger body mass of female Common Cuckoos (94.5 ± 4.96, n = 12; Go et al., 2021) likely plays a crucial role in enabling the production of more notes, contributing to the distinct structure of their calls. These physical attributes may provide a physiological basis for the observed differences in call patterns, highlighting the interplay between morphology and vocal behavior in shaping species-specific vocal traits.
The sonogram analyzed in this study, consistent with previous studies (Kim et al., 2017a, b; Choi et al., 2022), revealed that while there are differences in call parameters between species, the overall call forms exhibit notable similarity. Principal component analysis (PCA) further supported this observation, indicating that the characteristics of bubbling calls overlap significantly among individuals of Cuculus species. In particular, the inter-specific overlap was most evident in PC3 (Explanatory power 18%), where the lower frequency tended to decrease as the delta time of the notes increased. Previous studies suggested that functional asymmetry between male and female calls may account for the large differentiation in male calls and greater similarity of female bubbling calls across species (Kim et al., 2017a, b). It was proposed that female cuckoos evolved less distinct vocalizations as they primarily respond to mate calls rather than attract mates (Kim et al., 2017a, b). In other words, male calls may have evolved to be distinctly different, increasing the likelihood that females can recognize conspecific males, while males may not have developed the ability to distinguish female calls. Therefore, the mechanism by which females distinguish male calls may be more critical than the reverse. However, recent studies have shown that female cuckoo calls serve multiple functions, including territorial defense and mate attraction, and in some concept, may overlap with the function of male vocalizations (e.g., Moskát and Hauber, 2019; Lee et al., 2019; Xia et al., 2019; Yoo et al., 2020; Moskát and Hauber, 2021). Consequently, the observed similarity in female bubbling calls cannot be solely attributed to functional asymmetry between the sexes.
More recent studies suggest that female bubbling calls may serve additional functions beyond conspecific communication. Some research has proposed that female calls may mimic raptor vocalizations, potentially deterring predators or manipulating host behavior (Welbergen and Davies, 2008, 2011; York and Davies, 2017). Other studies suggest that these calls might attract the host's attention, distracting or causing the host to leave the nest, thereby increasing the success of parasitism (Wang et al., 2022). These findings challenge the idea that the similarities in female calls across species are primarily associated with mate recognition and suggest that these vocalizations may have evolved under a combination of selective pressures, not only for conspecific communication but also for interaction with host species, potentially influencing parasitism and mimicry dynamic. Instead, the observed similarities in bubbling calls may still reflect shared ancestral traits, which have undergone limited changes across species (de Kort and ten Cate, 2004; Marler and Slabbekoorn, 2004). Subtle interspecific differences could result from physical constraints, such as body mass, or from selective pressures acting on vocal traits for species- or sex-specific functions. This perspective highlights the complexity of avian vocal evolution, suggesting that both shared ancestry and selective forces contribute to the observed patterns in bubbling call characteristics.
The individual consistencies of female bubbling calls of the four Cuculus species revealed distinct patterns of variability both within and between individuals across species. In the Oriental Cuckoo, Indian Cuckoo and Lesser Cuckoo, three or four call parameters exhibited greater consistency within individuals than between individuals, indicating that these species have greater between-individual variation with a strong individual call signature. In the Common Cuckoo, however, most call parameters showed lower within-individual consistency, suggesting that individual females have a flexible vocal repertoire. This variability contrasts with that of male Common Cuckoos, which exhibit relatively higher within-individual consistency across all vocal parameters (Jung et al., 2014; Deng et al., 2019b, but see Deng et al., 2019a). The lower call consistency observed either within or between individuals of females may also complicate species identification based solely on vocalizations. This is particularly evident in Common Cuckoo, where within-individual call variability is highest. In contrast, the higher call consistency within individuals of the other three species suggests that species recognition might be relatively easier in these species. Future studies should further investigate why the Common Cuckoo exhibits such elevated intra-individual variability. This could include exploring ecological and evolutionary factors, such as environmental influences, social interactions, or breeding strategies, that may shape these patterns of vocal consistency. Understanding these dynamics may provide insight into the adaptive significance of vocal flexibility in female Common Cuckoos.
In conclusion, this study provides a comprehensive analysis of the structural characteristics and variability of bubbling calls among the four Cuculus species. While significant statistical differences in call parameters were observed, the similarities and substantial variability among the calls highlight challenges in species identification. Addressing these challenges will require a more integrative approach that consider not only structural aspects but also overall acoustic similarity, including functional and perceptual dimension of the calls. Nevertheless, our findings clarify some of the complexities of female bubbling calls and provide a foundation for improving species identification. Future studies should investigate why such a marked difference exists between males and females in the Cuculus cuckoos, where male vocalizations are distinctly species-specific, while female calls are more similar across species. Understanding the ecological or evolutionary reasons behind this sexual dimorphism in vocalization could provide valuable insights into the evolution of avian vocalization.
Sue-Jeong Jin: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Hae-Ni Kim: Resources, Methodology, Investigation. Jun-Seo Go: Resources, Methodology, Investigation. Myeong-Chan Cha: Resources, Methodology, Investigation. Heesoo Lee: Resources, Methodology, Investigation. Seongho Yun: Resources, Methodology, Investigation. Jin-Won Lee: Writing – review & editing, Validation, Supervision, Resources, Methodology, Investigation, Funding acquisition, Conceptualization.
To prevent birds from experiencing confusion due to playback, no more playback was performed than necessary.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jin-Won Lee reports financial support was provided by National Research Foundation of Korea. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Hye-Kyoung Moon, Su-kyoung Youn, Hye-jeong Jeon and Ju-young Lee for their valuable help during the fieldwork and recording.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2025.100240.
|
Chance, E.P., 1940. The Truth about the Cuckoo. Country Life Ltd, London.
|
|
Crawley, M.J., 2012. The R Book, second ed. John Wiley & Sons, Chichester.
|
|
del Hoyo, J., Elliott, A., Sargatal, J., Cabot, J., 1997. Handbook of the Birds of the World, Vol. 4. Sandgrouse to Cuckoos. Lynx Edicions, Barcelona.
|
|
Erritzøe, J., Mann, C.F., Brammer, F., Fuller, R.A., 2012. Cuckoos of the World. A&C Black, London.
|
|
Kim, H., Lee, J.-W., Yoo, J.-C., 2017a. Characteristics of female calls of four Cuculus species breeding in Korea. Korean J. Ornithol. 24, 41–47.
|
|
Lee, J.-W., 2014. Searching for hosts of avian brood parasites breeding in Korea. Korean J. Ornithol. 21, 25–37.
|
|
Marler, P.R., Slabbekoorn, H., 2004. Nature’s Music, the Science of Birdsong. Academic Press, Cambridge.
|
|
Payne, R.B., Sorensen, M.D., 2005. The Cuckoos. Oxford University Press, UK.
|
|
Sokal, R.R., Rohlf, F.J., 1995. Biometry, third ed. Freeman, New York.
|
| Parameters | C. canorus (n = 156) | C. optatus (n = 37) | C. micropterus (n = 17) | C. poliocephalus (n = 16) | χ2 | p |
| Number of notes | 19 (17–22) | 8 (7–12) | 14 (11–17) | 8.5 (6–10) | 114.18 | <0.001 |
| Delta time (s) | 1.83 (1.66–2.01) | 1.58 (1.29–1.84) | 1.57 (1.35–1.9) | 0.97 (1.84–1.25) | 45.18 | <0.001 |
| Delta time of notes (s) | 0.03 (0.03–0.04) | 0.05 (0.04–0.06) | 0.05 (0.04–0.05) | 0.04 (0.03–0.05) | 77.44 | <0.001 |
| Delta time of intervals (s) | 0.07 (0.06–0.08) | 0.14 (0.11–0.16) | 0.08 (0.06–0.09) | 0.1 (0.09–0.11) | 102.57 | <0.001† |
| Highest frequency (kHz) | 2.29 (2.16–2.44) | 2.11 (1.99–2.27) | 2.44 (2.32–2.57) | 3.58 (3.35–3.66) | 60.31 | <0.001† |
| Lowest frequency (kHz) | 0.72 (0.62–0.81) | 0.57 (0.5–0.68) | 0.92 (0.8–1.02) | 1.29 (1.15–1.49) | 69.94 | <0.001† |
| Delta frequency (kHz) | 1.5 (1.42–1.72) | 1.49 (1.37–1.67) | 1.54 (1.45–1.6) | 2.17 (1.97–2.48) | 37.55 | <0.001 |
| A Kruskal–Wallis rank sum test was performed to assess whether the call parameter values differed significantly among the four species. A post hoc test was then conducted to identify specific differences between species. A dagger mark (†) indicates that all four species showed significant differences from each other based on the results of the post hoc analysis. | ||||||
| Variables | PC1 (39%) | PC2 (27%) | PC3 (18%) |
| NN | −0.51 | 0.35 | 0.05 |
| DT | −0.43 | 0.18 | 0.40 |
| DN | 0.23 | −0.16 | 0.72 |
| DI | 0.34 | −0.49 | 0.07 |
| HF | 0.41 | 0.52 | 0.04 |
| LF | 0.33 | 0.34 | −0.4 |
| DF | 0.32 | 0.44 | 0.39 |
| NN, DT, DN, DI, HF, LF, DF represent Number of Notes, Delta Time, Delta Time of Notes, Delta Time of Intervals, Highest Frequency, Lowest Frequency and Delta Frequency, respectively. The percentages in parentheses show the amount of variation explained by each principal component (PC), and the numbers in bold represent the components loaded most highly for each parameter. Total explanation power 84%. | |||
| Species | Parameter | Mean (min–max) | CVb | CVi | PIC |
| C. canorus (n = 55) | NN | 18.8 (13–30.5) | 18.53 | 24.09 | 0.77 |
| DT | 1.81 (1.17–2.55) | 13.3 | 24.23 | 0.55 | |
| DN | 0.03 (0.02–0.05) | 20.18 | 18.53 | 1.09 | |
| DI | 0.07 (0.04–0.09) | 16.13 | 17.15 | 0.94 | |
| HF | 2.25 (1.95–2.67) | 7.15 | 8.49 | 0.84 | |
| LF | 0.74 (0.5–1.01) | 15.46 | 19.45 | 0.8 | |
| DF | 1.49 (1.23–1.95) | 9.6 | 13.02 | 0.74 | |
| C. optatus (n = 15) | NN | 7.4 (4–12) | 33.25 | 48.47 | 0.69 |
| DT | 1.38 (0.64–2) | 26.18 | 47.67 | 0.55 | |
| DN | 0.05 (0.03–0.06) | 19.13 | 16.7 | 1.15 | |
| DI | 0.16 (0.11–0.23) | 18.38 | 27.5 | 0.67 | |
| HF | 2.06 (1.8–2.43) | 164.7 | 11.71 | 14.06 | |
| LF | 0.63 (0.46–0.97) | 120 | 19.60 | 6.12 | |
| DF | 1.44 (1.11–1.87) | 180.4 | 15.4 | 11.72 | |
| C. micropterus (n = 7) | NN | 9.64 (5–16) | 37.98 | 62.01 | 0.61 |
| DT | 1.24 (0.65–1.81) | 33.02 | 58.14 | 0.57 | |
| DN | 0.04 (0.03–0.05) | 19.67 | 16.55 | 1.19 | |
| DI | 0.11 (0.08–0.19) | 41.95 | 46.94 | 0.89 | |
| HF | 2.39 (2.13–2.73) | 7.95 | 6.79 | 1.17 | |
| LF | 0.92 (0.72–1.1) | 14.48 | 15.14 | 0.96 | |
| DF | 1.50 (1.11–1.78) | 15.35 | 12.92 | 1.19 | |
| C. poliocephalus (n = 7) | NN | 9 (6.5–13) | 17.41 | 39.44 | 0.44 |
| DT | 1.12 (0.89–1.59) | 19.69 | 39.33 | 0.5 | |
| DN | 0.04 (0.03–0.05) | 22.29 | 16.51 | 1.35 | |
| DI | 0.1 (0.09–0.11) | 9.82 | 22.23 | 0.44 | |
| HF | 3.56 (2.92–4.43) | 10.67 | 9.16 | 1.17 | |
| LF | 1.33 (0.91–1.84) | 26.35 | 16.73 | 1.58 | |
| DF | 2.17 (1.64–2.52) | 13.9 | 14.87 | 0.94 | |
| Call parameters from individuals with recording containing more than 10 syllables were analyzed (C. canorus: 55 individuals, C. optatus: 15 individuals, C. micropterus and C. poliocephalus: 7 individuals each). The call parameters are defined as follows: NN (Number of Notes), DT (Delta Time), DN (Delta Time of Notes), DI (Delta Time of Intervals), HF (Highest Frequency), LF (Lowest Frequency), and DF (Delta Frequency). CVb represents between-individual variation, CVi represents within-individual variation, and PIC is the ratio of CVb to CVi. A PIC value greater than 1 indicates that between-individual variation exceeds within-individual variation. | |||||