| Citation: | Ying Ke, Tong Liu, Chenglong Han, Xue Yu, Jinmei Wang, Laixing Ding, Hongliang Pan, Xunqiang Mo, Xueqiang Lu. 2025: A review of eDNA technology in avian monitoring: Current status, challenges and future perspectives. Avian Research, 16(1): 100235. DOI: 10.1016/j.avrs.2025.100235 |
In recent years, environmental DNA (eDNA) has garnered significant attention as a novel tool in biodiversity monitoring, recognized for its efficiency, convenience, and non-invasiveness. Despite its extensive application in various ecological studies, such as conservation, invasion biology, biomonitoring and biodiversity survey assessment, its use in avian monitoring remains in its infancy. This review critically examines the potential and limitations of eDNA technology for avian monitoring, focusing on current advancements and ongoing challenges in this emerging field. Water and air are the primary environmental media for collecting avian eDNA, although other sources like spider webs and plant flowers have been explored as well. Notably, airborne eDNA has been reported to capture the highest diversity of avian species. While avian eDNA technology has shown promise for monitoring rare and endangered species and assessing avian diversity, significant challenges remain, particularly in sampling strategies, DNA extraction methodology, primer selection, and ascertain abundance. Additionally, we discussed the factors influencing the production, transportation, and degradation of avian eDNA in the environment. Finally, we suggested future research directions, including optimizing sampling strategies, developing avian-specific universal primers, expanding avian DNA barcode databases, enhancing eDNA detectability, and integrating environmental RNA (eRNA) and eDNA approaches.
Avian species, as an important component of ecosystem biodiversity, play a unique role in maintaining ecosystem function by contributing to plant and animal community stability through seed dispersal, pollination, and insect consumption (Anderson et al., 2011; Michel et al., 2020; Johnson, 2022; Luo et al., 2024). Traditional avian monitoring methods such as point sampling, line transect census, capture and marking and automated sound recording (Bibby et al., 2002), rely primarily on visual or auditory surveys (Nichols et al., 2009). However, these methods are often constrained by environmental conditions, particularly low visibility at dusk, dense vegetation, adverse weather like haze and ambient environmental noise. Furthermore, they require high-level expertise in species identification, and their outcomes can vary greatly depending on surveyors' skill and environmental conditions.
Environmental DNA (eDNA) technology offers a promising alternative to overcome the condition- and surveyor-dependent limitations of traditional monitoring. eDNA consists of DNA fragments shed by organisms into the environment through skin cells, feces, gametes, secretions, and other biological materials. Environmental samples carrying eDNA can be water, soil, sediment, feces, and even air (Ruppert et al., 2019). The technology involves extracting DNA from environmental samples, amplifying it by polymerase chain reaction (PCR), and performing sequencing analysis with high-throughput sequencing to identify target organisms. eDNA technology is versatile, enabling the monitoring of specific rare and endangered species (Neice and McRae, 2021) as well as overall biodiversity (Zhang et al., 2023). Compared to traditional methods, eDNA technology has advantages, including high efficiency, rapid processing, non-invasiveness, eco-friendliness, and standardized workflows, making it a promising tool for further development (Taberlet et al., 2012; Thomsen and Willerslev, 2015; Deiner et al., 2017).
Application of eDNA technology span a wide range of taxa, including fishes (Doi et al., 2017; Zhang et al., 2022), amphibians (Valentini et al., 2016; Goldberg et al., 2018), plants (Kodama et al., 2022), microorganisms (Laroche et al., 2018; Zhang et al., 2020), and birds (Ushio et al., 2018; Katayama et al., 2024). However, its effectiveness varies among species, due to factors such as target organism biology, spatiotemporal heterogeneity of eDNA distribution, and environmental conditions. Despite these challenges, eDNA for avian monitoring has gained increasing attention (Ushio et al., 2018; Schuetz et al., 2020; Neice and McRae, 2021).
An increasing number of eDNA studies have been conducted on monitoring avian diversity. Ushio et al. (2018) pioneered the use of modified fish/mammal universal primers to detect multiple avian species in both zoo and natural settings, demonstrating eDNA's potential for avian diversity assessments. Katayama et al. (2024) used eDNA metabarcoding to monitor avian species in paddy fields during breeding seasons, while Sohn and Song (2024) assessed seasonal variations in avian diversity in an urban ecological park. However, avian diversity monitoring is often incorporated within eDNA biodiversity monitoring targeting other taxonomic groups, such as vertebrates in urban areas (Zhang et al., 2023), wetlands (Saenz-Agudelo et al., 2022; Coleman et al., 2023), and freshwater and terrestrial ecosystems (Holm et al., 2023; Lynggaard et al., 2024).
Compared to traditional surveys, currently eDNA typically detects fewer avian species in overall diversity monitoring. For example, several avian species detected by eDNA technology vs. dozens of avian species in visual surveys (Coleman et al., 2023; Katayama et al., 2024). Compared to traditional camera traps, eDNA from soil and water detected significantly fewer bird species, while it identified certain species that were exclusively captured by eDNA (Holm et al., 2023; Tetzlaff et al., 2024). In contrast, airborne eDNA not only detected all bird species captured by cameras but also revealed a much greater diversity of species than camera traps alone (Polling et al., 2024). As airborne eDNA is considered low, the effectiveness and mechanisms of air eDNA technology should be further verified with more applications (Bohmann and Lynggaard, 2023; Lynggaard et al., 2024). Nonetheless, ascertaining abundance information using eDNA metabarcoding for whole avian communities remains a challenge.
For species-specific monitoring, although research on eDNA for rare and endangered avian species is still limited, existing studies have demonstrated its high efficiency. For example, Neice and McRae (2021) used eDNA to detect the globally endangered Black Rail (Laterallus jamaicensis), identifying it in 47% of eDNA from water and soil samples, compared to only 2% or two instances with traditional auditory and visual surveys. Day et al. (2019) developed an eDNA test for the endangered Gouldian Finch (Erythrura gouldiae), which detected the species in small water samples (20–200 mL) and demonstrated high specificity. Similarly, Guan et al. (2023) successfully applied eDNA to monitor Ridgway's Rail (Rallus obsoletus), while Schuetz et al. (2020) detected three European bird species (Platalea leucorodia, Recurvirostra avosetta, and Tringa tetanus) using the technology. These studies highlight eDNA's capability to detect elusive species at low population densities, which traditional methods often fail to achieve.
While eDNA technology shows significant potential for avian monitoring, this field is still in its early stages. This review aims to provide a comprehensive analysis of current state of eDNA applications in avian monitoring, identify key challenges, and outline future research directions to foster the effective integration of eDNA technology in avian studies.
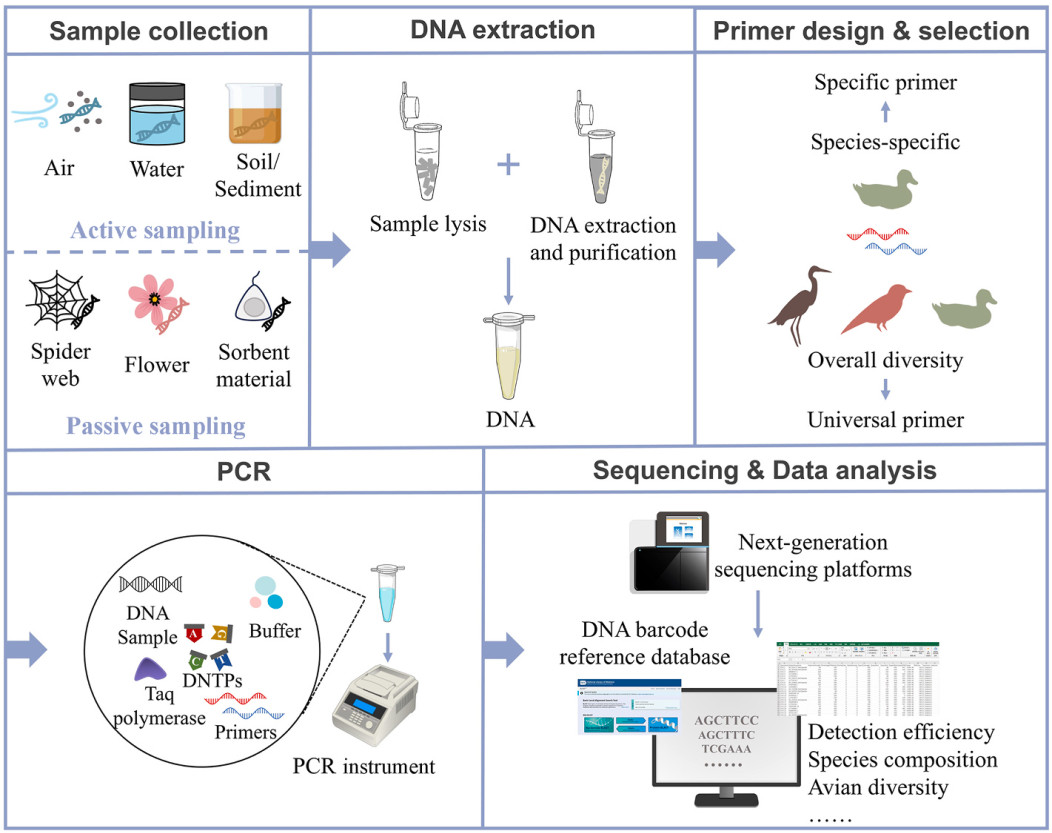
Avian species inhabit diverse ecosystems, including wetlands, forests, grasslands, and urban parks, resulting in varied eDNA sources such as water, sediment, soil, air, and even spider webs and flowers (Fig. 1, Table 1). Effective avian eDNA sampling strategies must consider these diverse origins.
| Monitoring type | Sample type | Sample amount (sample number) | Number of avian taxa detected | Mitochondrial genome region | Primer-Length | References |
| Species-specific (Laterallus jamaicensis) | Water; Soil | Water: 2000 mL (n = 29); Soil: 50 mL (n = 47) | 1 | COI | BLRA COI2 ~ 219 bp | Neice and McRae (2021) |
| Species-specific (Platalea leucorodia, Recurvirostra avosetta and Tringa totanus) | Water | 500 mL (n = 13) | 3 | COI | 3 species-specific primers ~ 160 bp; 188 bp; 199 bp | Schuetz et al. (2020) |
| Species-specific (Rallus obsoletus) | Water | 150–5000 mL (n = 23) | 2 | ND5 | RIRA-ND5-1 ~ 126 bp; RIRA-ND5-2 ~ 140 bp | Guan et al. (2023) |
| Species-specific (Erythrura gouldiae) | Water | 200 mL (n = 54) | 1 | – | FinchCR ~ 186 bp | Day et al. (2019) |
| Overall diversity | Air | Passive air sampler, 9 samples per two weeks (n = 198) | 4 | 16S | 16Smam ~ 140 bp | Johnson et al. (2023) |
| Overall diversity | Air | 8.8 m3/min for 30/60 min; 0.8 m3/min for 30/60 min/5 h; 0.8 m3/min for 30 h (n = 40) |
13 | 12S | 12SV05 ~ 97 bp | Lynggaard et al. (2022) |
| Overall diversity | Air | 1.1–3.5 m3/h for 12 h (n = 143) | 40 | 12S | 12SV05 ~ 97 bp | Lynggaard et al. (2024) |
| Overall diversity | Air | 0.01 m3/min for 24 h; 0.0165 m3/min for 24 h; 0.3 m3/min for 30 min (n = 64) |
113 | 16S; cytb | 16Smam ~ 140 bp; L15411F, H15546R–136 bp | Polling et al. (2024) |
| Overall diversity | Soil | 0.25 g (n = 86) | 8 | 12S | 12SV05 ~ 97 bp | Tetzlaff et al. (2024) |
| Overall diversity | Water | ~500 mL (n = 101) | 8 | 12S | MiBird-U ~ 171 bp | Katayama et al. (2024) |
| Overall diversity | Water | 500 mL (n = 120) | 13 | 12S | NA ~ 106 bp | Coleman et al. (2023) |
| Overall diversity | Water | 1000 mL (n = 18) | 15 | 12S | Tele02 ~ 167 bp | Macher et al. (2021) |
| Overall diversity | Water | 500–1000 mL (n = 65) | 20 | 12S | MiBird-U ~ 171 bp | Sohn and Song (2024) |
| Overall diversity | Water | 500 mL (n = 114) | 30 | 12S | Mamm01 ~ 59 bp; MiFish-U ~ 170 bp |
Holm et al. (2023) |
| Overall diversity | Water | 1000 mL (n = 147) | 31 | 12S | 12Sa-12Sh ~ 230 bp | Saenz-Agudelo et al. (2022) |
| Overall diversity | Water | 2000 mL (n = 69) | 32 | 12S | Tele02 ~ 167 bp | Mariani et al. (2021) |
| Overall diversity | Water | 1000 mL (n = 327) | 39 | 12S | Tele02 ~ 167 bp | Zhang et al. (2023) |
| Overall diversity | Water | 100–200 mL (zoo: n = 16; field: n = 3) | laboratory test: 22 zoo test: 16 field test: 5 |
12S | MiBird-U ~ 171 bp | Ushio et al. (2018) |
| Overall diversity | Spider webs | 1 web (n = 49) | 32 | 12S; 16S | 12SV05 ~ 97 bp; 16Smam1/2 ~ 130 bp |
Newton et al. (2024) |
| Overall diversity | Flowers | 5 flowers (n = 5) | 4 | 12S | MiBird-U ~ 171 bp | Jønsson et al. (2023) |
| Overall diversity | Flowers | 1 flower (n = 175) | 7 | 12S | BirdND2 ~ 229 bp | Newton et al. (2023) |
Water is a primary source of avian eDNA due to birds' frequent contact with aquatic environments. Detection probabilities vary across taxonomic groups and their lifestyles. Waterbirds, for example, exhibit higher eDNA detectability in water compared to forest birds (Zhang et al., 2023; Katayama et al., 2024). This is predictable, as their life histories are more water-dependent. Filtration is the standard method for capturing eDNA from water samples (Goldberg et al., 2016), typically involving volumes ranging from 100 mL to 5 L. Commonly used filters include mixed cellulose esters, glass fiber, cellulose nitrate filter and Sterivex filter, with pore sizes from 0.22 μm to 1.2 μm. Larger water volumes may improve detection sensitivity for low eDNA concentrations (Guan et al., 2023). Notably, lentic water bodies provide higher detection rates compared to lotic systems (Zhang et al., 2023), which serves as an important consideration for sampling.
In sediments and soils, eDNA distribution tends to be heterogeneous and localized due to deposition and limited diffusions, resulting in variability in species detection rates among samples (Guthrie et al., 2024). Therefore, comprehensive sampling strategies, such as multi-point mixing, can enhance detection rates, especially for species with low detectability or uneven distributions.
Airborne eDNA represents a significant advancement for avian monitoring, achieving the highest detection rates in current research. Collecting airborne eDNA requires specialized air samplers that can capture particles using media such as water, glass-fiber filters, adhesive tapes coated with petroleum jelly, or custom designs (Lynggaard et al., 2022, 2024; Polling et al., 2024). These samplers can be deployed on trees or in open spaces for continuous sampling. Detection probability increases with shorter bird-sampler distances, but adverse weather conditions must be mitigated to ensure effective autonomous sampling (Clare et al., 2022; Lynggaard et al., 2022, 2024).
Natural passive biofilters such as spider webs and flowers, offer unique experimental sampling opportunities. These materials require minimal preparation and effectively capture avian eDNA (Jønsson et al., 2023; Newton et al., 2023, 2024). Meanwhile, commercial sorbent materials like glass fiber filters can also serve as artificial passive samplers water eDNA (Chen et al., 2022).
Environmental factors such as temperature, ultraviolet light (UV), pH and salinity affect eDNA persistence and should be recorded during sampling to interpret results accurately (Harrison et al., 2019). Preservation is crucial due to the susceptibility of eDNA degradation. Samples should be transported on ice and processed promptly (e.g., filtering water samples), and stored at −20 ℃ or lower. On-site filtration, when feasible, offers superior preservation but requires an environment shielded from wind, dust, and sunlight. Filters should be temporarily frozen and stored until DNA extraction, avoiding repeated freeze-thaw cycles (Minamoto et al., 2020).
Given the generally extremely low concentration of avian eDNA, it is crucial to minimize contamination during DNA extraction. Equipment and materials must be sterilized with sodium hypochlorite serving as an effective disinfectant against DNA and PCR product contamination (Champlot et al., 2010). Commercial kits, such as the QIAGEN Blood and Tissue Kit, are commonly used for avian eDNA extraction. Practical adjustments, such as dissecting filter membranes and extending digestion time, can enhance DNA yield (Zhang et al., 2023; Lynggaard et al., 2024). To mitigate the influence of PCR inhibitors in environmental samples like humic acids, inhibitor removal kits (e.g., OneStep PCR Inhibitor Removal Kit) or sample dilution can be employed. Negative controls, including blank filtration and extraction controls are essential for detecting contamination. Extracted DNA should be stored at −20 ℃ or lower for subsequent analysis.
Species-specific detection relies on primers designed to amplify short DNA sequences unique to target species. Universal primers, by contrast, aim to detect multiple species within a single taxonomic group. Effective universal primers should balance broad detection with minimized amplification bias to avoid false negatives or false positives (Ficetola et al., 2010).
The mitochondrial 12S rRNA gene is widely utilized in avian eDNA studies, due to its ability to provide species-level identification and its short sequence length, which suits degraded eDNA samples (Di Finizio et al., 2007). For example, Ushio et al. (2018) developed the MiBird-U universal primer set specifically for avian species, and it has since been widely adopted (Jønsson et al., 2023; Katayama et al., 2024; Sohn and Song, 2024). Other primers corresponded to the 12S region such as 12SV05 (Lynggaard et al., 2022, 2024; Newton et al., 2024), Tele02 (Macher et al., 2021; Mariani et al., 2021; Zhang et al., 2023) also successfully detected avian species, despite being originally designed for other taxa.
Another common target is the COI region, which is widely used in species-specific avian monitoring (Schuetz et al., 2020; Neice and McRae, 2021). Interestingly, a study monitoring vertebrate diversity, researchers used the 16Smam (corresponded to the 16S region) primers targeting mammals and the L15411F and H15546R (corresponded to the cytb region) primers targeting rodents, successfully detected over a hundred avian species (Polling et al., 2024). Nonetheless, for specific avian eDNA monitoring, primers targeting particular avian groups or unique genes can enhance detection sensitivity and resolution.
Metabarcoding amplifies eDNA for next-generation sequencing by adding adapters to PCR products. Operational taxonomic unit (OTU) clustering and alignment with reference databases such as BOLD (the Barcode of Life Database System) or NCBI (National Center for Biotechnology Information), enable taxonomic classification.
Public databases provide accessible references but may lack quality control and coverage (Coleman et al., 2023). Custom-built databases allow researchers to verify sequence quality, improving identification accuracy (Iwasaki et al., 2013; Westfall et al., 2024). Expanding avian DNA barcodes in public and private repositories will further enhance eDNA-based monitoring.
Currently, avian eDNA technology is limited to detecting species composition and cannot precisely quantify their abundance in nature (Katayama et al., 2024; Polling et al., 2024). This challenge is not unique to avian studies and similarly affects eDNA for other species such as fishes (Tsuji et al., 2022), amphibians (Breton et al., 2022) and zooplanktons (Jo and Sasaki, 2024). Establishing a definitive relationship between species abundance to qPCR copy numbers, as well as the relative sequencing read counts, remains a subject of ongoing research.
Recent advancement in an airborne eDNA study has detected up to 113 avian species (Polling et al., 2024). However, most avian eDNA studies detect far fewer species, typically a few to several dozen (Table 1). Additionally, the number of species detected per eDNA sample is often limited, necessitating large sample sizes and increased costs. This discrepancy highlights a persistent issue of false negatives in eDNA-based avian diversity surveys. The detectability of avian eDNA is influenced by its production, transportation, and degradation in the environment (Fig. 2).
Avian eDNA is released into the environment via urine, feces, feathers, and saliva, aligning with bird physiological traits, including feather coverings and the stratum corneum on their skin (Zheng, 2012). Factors such as biomass, individual size, behavior, and metabolism significantly influence eDNA production (Pilliod et al., 2014; Jo et al., 2019; Maruyama et al., 2019).
Higher biomass (the total body weight of bird flock) correlates with increased detection frequency in eDNA from water and air (Lynggaard et al., 2022; Katayama et al., 2024). High biomass tends to be related to relatively large body sizes or individual counts, indicating increased DNA production and deposition, thus accompanied higher detection rate (Vanni and McIntyre, 2016; Yates et al., 2019). However, these relationships can be species-specific, reflecting variations in behavior and physiology (Harrison et al., 2019; Maruyama et al., 2019).
Enhancing our understanding of how avian eDNA production relates to these factors can refine sampling strategies and population assessments.
Once released, eDNA is transported through the environmental media, affecting its spatiotemporal detectability. Key factors influencing eDNA transport include medium type (e.g., water, air, soil), weather/climatic conditions, and human interference.
In aquatic environments, water flow (lentic or lotic), gradient, and substrate type significantly influence eDNA transport (Jerde et al., 2016; Jo and Yamanaka, 2022). For example, eDNA typically travels within 2 km in rivers (Jo and Yamanaka, 2022), whereas lentic water bodies allow greater accumulation (Zhang et al., 2023).
In the air, wind speed, direction, and rainfall can influence eDNA dispersal. Airborne eDNA spatial distribution is uneven and sparse, complicating detection. During bird migration, eDNA concentrations along routes increase, and weather conditions like rain can wash particulate matter out of the air, and particles may be tossed up and transported again when the landscape dries (Johnson et al., 2023).
In soil or sediments, eDNA is less mobile, offering higher spatial precision for detection (Zinger et al., 2019). However, the stochastic and localized distribution of eDNA in these media necessitates extensive sampling (Pawlowski et al., 2022). Human activities like dredging can significantly alter eDNA transportation in such environments.
eDNA transportation may dilute target eDNA concentration, reducing detectability. The detailed dynamics of eDNA release and dispersal still require further research and exploration. Understanding how eDNA transport and disperse under different environmental conditions is vital for accurately estimating avian species' spatiotemporal distributions using eDNA technology.
eDNA degradation in the environment is species-independent but accelerated by low avian eDNA abundance. It is influenced by biotic and abiotic factors, including microbial activity, nucleases, temperature, pH, and ultraviolet radiation.
Microbial activity and extracellular nucleases play pivotal roles in eDNA degradation (Harrison et al., 2019). Dissolved DNA serves as a nutrient source for microorganisms, which metabolize it through hydrolysis (Matsui et al., 2001; Salter, 2018). Seasonal variations in microbial nutrient responses affect eDNA persistence in marine environment (Salter, 2018). Extracellular nucleases directly degrade free DNA into smaller fragments, reducing its residence time in sediments to a few months under steady-state conditions (Corinaldesi et al., 2008). In soil, bacterial DNases are the primary cause of DNA degradation, as soil bacteria actively secrete nucleases (Levy-Booth et al., 2007).
High temperatures accelerated eDNA degradation, particularly in aquatic environments (Fu et al., 2012; Eichmiller et al., 2016; Jo et al., 2019; Kasai et al., 2020; Yu et al., 2022), while soil conditions may mitigate temperature impact, prolonging eDNA persistence (Guthrie et al., 2024).
pH significantly affects eDNA degradation (Seymour et al., 2018; Zhao et al., 2023), with neutral or slightly alkaline conditions favoring eDNA persistence (Lindahl, 1993). UV directly damages DNA (Lindahl, 1993; Nieto Moreno et al., 2023) or induces oxidative stress through reactive oxygen species (Leech et al., 2009; Zhang et al., 2020a, 2020b). UV effects are more pronounced in high altitudes or equatorial regions (Sun et al., 2024), though its relationship with eDNA degradation is nonlinear (Strickler et al., 2015). UV can also inhibit microbial degradation, enhancing eDNA persistence (Guthrie et al., 2024).
Nutrient status in water affects eDNA degradation, with degradation rates negatively correlated to dissolved organic carbon concentrations (Eichmiller et al., 2016). Humus can slow eDNA degradation by binding to free DNA, protecting it from enzymatic breakdown, despite potentially inhibiting PCR (Pietramellara et al., 2009).
Although understanding of eDNA degradation factors is currently limited, research consensus indicates that degradation occurs rapidly. These factors are essential for optimizing sampling protocols.
Building on eDNA technology, environmental RNA (eRNA) offers unique and complementary advantages. Unlike DNA, RNA originates only from actively transcribed genes, enabling distinctions between living and dead organisms (Veilleux et al., 2021). This property makes eRNA highly sensitive and yields a higher predictive value compared to eDNA, effectively reducing false positives and enhancing the monitoring of active, real-time biological processes (Li et al., 2024). Furthermore, eRNA demonstrates a stronger localized signal than eDNA, as its faster degradation rate ties positive detections more closely to specific temporal and spatial contexts, thereby improving spatiotemporal resolution (Yates et al., 2021; Veilleux et al., 2021).
eRNA offers a wealth of potential ecological information. Compared to fish, eRNA metabarcoding can detect a higher number of bird species than eDNA, contributing to its overall higher species numbers (Macher et al., 2024). Besides detecting species composition (Li et al., 2024; Macher et al., 2024; Zhang et al., 2024), it can also assess individual physiological states, the ecological health of populations, and community dynamics. This functional indicator reveals the status of populations or communities within ecosystems (Yates et al., 2021; Veilleux et al., 2021). For example, eRNA has the potential to detect different life-history stages, sexes, and even specific phenotypes within a species, surpassing the current taxonomic limitations of eDNA (Yates et al., 2021). Additionally, the analysis RNA expression can reveal organismal responses to environmental stressors, making it an effective tool for assessing chemical hazards, environmental pollution, or predicting unsuitable habitats (Veilleux et al., 2021; Greco et al., 2022; Hiki et al., 2023).
eRNA and eDNA data strongly complement each other in detecting taxonomic groups (Zhang et al., 2024). Integrating eRNA with eDNA enables a more profound understanding of how organism-level changes translate to biodiversity patterns. This integration allows for a more precise capture of dynamic ecological shifts, offering enriched information for avian monitoring. Nonetheless, the potential of eRNA metabarcoding as a routine monitoring method has yet to be fully realized. Significant gaps remain in optimizing sampling and analysis procedures to enhance the practically and reliability of eRNA applications in ecological studies. Further exploration and methodological improvement are essential to harness the full potential of eRNA.
Compared to traditional methods, eDNA technology detects fewer bird species and cannot quantify abundance in overall biodiversity monitoring due to its technical immaturity, with persistent issues of false negatives and false positives. However, traditional methods are time-consuming, labor-intensive, and highly susceptible to human influence. In contrast, eDNA technology, as an emerging survey approach, although still in its infancy, holds great potential for avian monitoring. To further advance the application of eDNA technology in avian monitoring, future research should prioritize (Fig. 3):
i) Optimizing sampling strategies: Improve avian eDNA capture by refining filter materials, pore sizes, and sampling protocols tailored to specific environments.
ii) Developing avian-specific universal primers: Minimize primer bias and improve detection accuracy by designing primers for specific taxonomic subgroups.
iii) Expanding avian DNA barcode databases: Establish comprehensive and high-quality reference libraries to enhance the reliability of eDNA analyses.
iv) Improving detection limits of trace eDNA: Develop methods to detect trace levels of avian eDNA amidst environmental noise, addressing biotic and abiotic degradation factors.
v) Achieving the efficiency of eDNA in quantify avian abundance: Break through technical barriers of eDNA in accurately quantify avian abundance.
vi) Integrating eDNA with eRNA: Select to conduct research on either eDNA or eRNA individually, or both simultaneously based on specific research requirements.
The potential of eDNA technology in monitoring rare and endangered avian species, as well as avian diversity, is clearly evident. Implementation of eDNA-based avian monitoring encompasses five key steps: sample collection, DNA extraction, PCR amplification, sequencing, and data analysis. However, heterogeneity across studies can occur at each of these steps, primarily due to variations in sample types (e.g., water, air, soil, sediment), sample volumes (e.g., 100 mL–5 L for water sample), filter types (e.g., mixed cellulose esters, glass fiber, cellulose nitrate filter and Sterivex filter), filter pore sizes (typically between 0.22 μm and 1.2 μm), genetic target selection (e.g., 12S, COI), and primer selection. These diverse and complex combinations hinder the establishment of standardized eDNA protocols. The unique biological characteristics of different avian species, the distinctive physicochemical properties of different environments, and spatiotemporal heterogeneity necessitate the ongoing refinement and adaptation of experimental methods to meet diverse avian research objectives.
In summary, while eDNA technology is not yet capable of fully replacing traditional survey methods, its potential for avian monitoring warrants further exploration. Recent breakthroughs in avian eDNA applications are poised to significantly advance avian monitoring systems. Furthermore, integrating eDNA with eRNA will provide additional promising ecological information. The selection of survey methods should balance the advantages and limitations of each approach in relation to specific research objectives. Currently, integration eDNA and eRNA technologies with traditional methods offers the most promise for achieving comprehensive and reliable outcomes.
Ying Ke: Writing – review & editing, Writing – original draft. Tong Liu: Writing – review & editing. Chenglong Han: Writing – review & editing. Xue Yu: Writing – review & editing. Jinmei Wang: Writing – review & editing. Laixing Ding: Writing – review & editing. Hongliang Pan: Writing – review & editing. Xunqiang Mo: Resources. Xueqiang Lu: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
| Monitoring type | Sample type | Sample amount (sample number) | Number of avian taxa detected | Mitochondrial genome region | Primer-Length | References |
| Species-specific (Laterallus jamaicensis) | Water; Soil | Water: 2000 mL (n = 29); Soil: 50 mL (n = 47) | 1 | COI | BLRA COI2 ~ 219 bp | Neice and McRae (2021) |
| Species-specific (Platalea leucorodia, Recurvirostra avosetta and Tringa totanus) | Water | 500 mL (n = 13) | 3 | COI | 3 species-specific primers ~ 160 bp; 188 bp; 199 bp | Schuetz et al. (2020) |
| Species-specific (Rallus obsoletus) | Water | 150–5000 mL (n = 23) | 2 | ND5 | RIRA-ND5-1 ~ 126 bp; RIRA-ND5-2 ~ 140 bp | Guan et al. (2023) |
| Species-specific (Erythrura gouldiae) | Water | 200 mL (n = 54) | 1 | – | FinchCR ~ 186 bp | Day et al. (2019) |
| Overall diversity | Air | Passive air sampler, 9 samples per two weeks (n = 198) | 4 | 16S | 16Smam ~ 140 bp | Johnson et al. (2023) |
| Overall diversity | Air | 8.8 m3/min for 30/60 min; 0.8 m3/min for 30/60 min/5 h; 0.8 m3/min for 30 h (n = 40) |
13 | 12S | 12SV05 ~ 97 bp | Lynggaard et al. (2022) |
| Overall diversity | Air | 1.1–3.5 m3/h for 12 h (n = 143) | 40 | 12S | 12SV05 ~ 97 bp | Lynggaard et al. (2024) |
| Overall diversity | Air | 0.01 m3/min for 24 h; 0.0165 m3/min for 24 h; 0.3 m3/min for 30 min (n = 64) |
113 | 16S; cytb | 16Smam ~ 140 bp; L15411F, H15546R–136 bp | Polling et al. (2024) |
| Overall diversity | Soil | 0.25 g (n = 86) | 8 | 12S | 12SV05 ~ 97 bp | Tetzlaff et al. (2024) |
| Overall diversity | Water | ~500 mL (n = 101) | 8 | 12S | MiBird-U ~ 171 bp | Katayama et al. (2024) |
| Overall diversity | Water | 500 mL (n = 120) | 13 | 12S | NA ~ 106 bp | Coleman et al. (2023) |
| Overall diversity | Water | 1000 mL (n = 18) | 15 | 12S | Tele02 ~ 167 bp | Macher et al. (2021) |
| Overall diversity | Water | 500–1000 mL (n = 65) | 20 | 12S | MiBird-U ~ 171 bp | Sohn and Song (2024) |
| Overall diversity | Water | 500 mL (n = 114) | 30 | 12S | Mamm01 ~ 59 bp; MiFish-U ~ 170 bp |
Holm et al. (2023) |
| Overall diversity | Water | 1000 mL (n = 147) | 31 | 12S | 12Sa-12Sh ~ 230 bp | Saenz-Agudelo et al. (2022) |
| Overall diversity | Water | 2000 mL (n = 69) | 32 | 12S | Tele02 ~ 167 bp | Mariani et al. (2021) |
| Overall diversity | Water | 1000 mL (n = 327) | 39 | 12S | Tele02 ~ 167 bp | Zhang et al. (2023) |
| Overall diversity | Water | 100–200 mL (zoo: n = 16; field: n = 3) | laboratory test: 22 zoo test: 16 field test: 5 |
12S | MiBird-U ~ 171 bp | Ushio et al. (2018) |
| Overall diversity | Spider webs | 1 web (n = 49) | 32 | 12S; 16S | 12SV05 ~ 97 bp; 16Smam1/2 ~ 130 bp |
Newton et al. (2024) |
| Overall diversity | Flowers | 5 flowers (n = 5) | 4 | 12S | MiBird-U ~ 171 bp | Jønsson et al. (2023) |
| Overall diversity | Flowers | 1 flower (n = 175) | 7 | 12S | BirdND2 ~ 229 bp | Newton et al. (2023) |