| Citation: | Lili Sun, Hongyan Yang, Xiuyuan Lu, Ting Fu, Jia Guo, Sicheng Ren, Waner Liang, Qing Chen, Dongming Li, Theunis Piersma, Nicola Crockford, Yifei Jia, Guangchun Lei. 2025: Shallow water habitats provide high-quality foraging environments for the Spoon-billed Sandpiper at a critical staging site. Avian Research, 16(1): 100233. DOI: 10.1016/j.avrs.2025.100233 |
Abundant food supply is crucial for the survival of long-distance migratory birds. The continued population decline of the Spoon-billed Sandpiper (Calidris pygmeae), a critically endangered shorebird, is primarily attributed to habitat loss and degradation. However, significant gaps remain in research on their diet and foraging habitat selection, limiting effective conservation and restoration efforts. In this study, we investigated the composition of macrobenthic communities, analyzing habitat and prey selection at the main foraging area of SBS in Tiaozini, Jiangsu Province of eastern China—their most critical staging site during southward migration. Our findings revealed 25 species of macrobenthos in foraging areas, with mobile epibenthos comprising the largest group by biomass, accounting for 73%, and having higher density and biomass nearshore. Observations of foraging Spoon-billed Sandpiper individuals indicated that shallow water habitats were their preferred foraging environments, where mobile epibenthos, which thrive in these habitats after the tide recedes, made up 81% of their biomass intake. We propose that shallow water habitats in intertidal mudflats serve as essential refuges for mobile epibenthos after tidal retreat, thereby providing Spoon-billed Sandpipers with access to high-quality food resources. Habitat protection efforts should prioritize habitats harboring extensive microhabitats with shallow water, especially the nearshore area, and further research is needed to explore the mechanisms underlying the formation of these microhabitats, with the ultimate goal of restoring more high-quality habitats for the Spoon-billed Sandpiper.
The distribution of available (accessible and edible) food resources is an important factor affecting the habitat selection of birds. There are intertidal mudflats with diverse habitats (e.g., the Yellow Sea Ecoregion) providing the richest benthos communities in the world in the East Asian–Australasian Flyway (EAAF), which makes it the flyway with the most shorebirds among other flyways, supporting more than 60 species and millions of individuals of shorebirds, with the largest number of threatened migratory shorebirds worldwide (Barter, 2002; Piersma, 2007; Kirby et al., 2008; MacKinnon et al., 2012). Long-distance migratory shorebirds require large amounts of energy during migration, so the abundance of food resources at staging sites is critical for the survival of individuals, and sometimes even for the survival of populations (Battley et al., 2012; Piersma et al., 2016, 2017).
Unfortunately, the EAAF, especially the intertidal mudflats in the Yellow Sea, has been subjected to tremendous ecological pressures from humans in recent decades, for example more than 50% of the world's largest area of muddy intertidal mudflats have disappeared due to mudflat reclamation (Ma et al., 2014; Murray et al., 2015). The loss of the habitats with crucial food resources was considered to be one of the main reasons for the decline in populations of more than 80% of shorebird species in the EAAF, with species like Spoon-billed Sandpiper (Calidris pygmeae) and Great Knot (C. tenuirostris) declining at a particularly rapid rate (Zöckler et al., 2010; Iwamura et al., 2013; Conklin et al., 2014; Hua et al., 2015; Piersma et al., 2016; Green et al., 2021).
Although China has legislated to stop any approval of new coastal mudflat reclamation projects after 2018, China's coastal mudflats, especially the Yellow Sea ecoregion, are still facing disturbances such as invasion of Spartina alterniflora, industrial effluent discharges, and intensive aquaculture, which have resulted in degradation of the surviving mudflat habitats and a decrease in food resources for birds (Choi et al., 2020; Chang et al., 2021). Under these circumstances, understanding food availability, dietary preferences, and habitat selection at the remaining habitats is crucial for shorebird conservation and can provide scientific evidence for habitat restoration.
The Spoon-billed Sandpiper is a long-distance migratory shorebird endemic to the EAAF. The current global population is estimated to be around 773 individuals (95% CL = 569–978) (Green et al., 2021) and is still declining at a rate of 8% per year. It has been listed as Critically Endangered (CR) on the IUCN Red List since 2008 (IUCN International Union for Conservation of Nature, 2024). It has also been listed as a National First-class Protected Animal in China since 2021 and is treated as a key flagship and umbrella species for EAAF coastal wetlands. The Spoon-billed Sandpipers use various habitat types throughout their life cycle; thus, given their small population size, changes at any place could severely impact their survival, considering the loss and degradation of staging sites being key drivers of their decline (Zöckler et al., 2016; Kelly et al., 2017; Green et al., 2021). Therefore, the information of their habitat selection, diet and food distribution will be helpful for the species restoration.
Previous studies have described the foraging habitats and diet of the Spoon-billed Sandpiper. On breeding grounds Spoon-billed Sandpipers have been found to forage on dry (mossy) lands and in shallow water of freshwater ponds, tundra pools, and sandy lagoons, with the diet consisting of insects and their larvae, small amphipods, worms, and small snails (Dixon, 1918; Dementiev et al., 1951; Portenko, 1957, 1981; Burton, 1971; Kelly et al., 2017). On non-breeding grounds, Spoon-billed Sandpipers forage on intertidal mudflats and have been observed in soft muds, soft sandy muds without vegetation, dry surfaces, shallow muds and shallow waters (1–3 cm) (Jahn, 1942; Voronov, 1980; Piersma, 1986; Pedersen et al., 1998; Tong et al., 2012; Chowdhury et al., 2018; Aung et al., 2020). Spoon-billed Sandpipers have been noted to have a very clear preference for the rippled mudflats with a sand-mud mix compared to other habitats, especially sandy mudflats with a thin layer of mud on the surface (Bird et al., 2010; Kelly et al., 2017; Chowdhury et al., 2018; Aung et al., 2020). Spoon-billed Sandpipers mostly forage in shallow waters, small puddles or shallow muddy waters (Piersma, 1986; Bird et al., 2010; Zöckler et al., 2016; Kelly et al., 2017; Aung et al., 2020). Their diet includes fishes, shrimps, crabs, amphipods, polychaetes, and even flies (Voronov, 1980; Ali and Ripley, 1983; Sugathan, 1985; Cha and Young, 1990; Tong et al., 2012; Zöckler et al., 2016; Kelly et al., 2017; Lu et al., 2022). The above studies on its diet were all qualitative observations except for two studies where prey body length measurements or estimations and counts were made (Burton, 1971; Lu et al., 2022).
To date, within the entire distribution area of Spoon-billed Sandpipers, only three studies have quantitatively described food resources for this species. These studies were conducted at the migratory stopover sites in Tiaozini, Yangkou and Dongling in southern Jiangsu, China (Yang et al., 2020), the wintering sites in Mottama Bay, Myanmar (Aung et al., 2020) and Leizhou Bay, Zhanjiang, China (Lu et al., 2022). The former two studies sampled macrobenthos using a 15-cm diameter core sampler from locations where the foraging Spoon-billed Sandpipers were observed, but unfortunately, in the absence of definitive information on the local diet of Spoon-billed Sandpipers, potential instead of definitive food resources were speculated and described (Aung et al., 2020; Yang et al., 2020). The third study took a step forward in the exploration of available food resources for Spoon-billed Sandpipers based on their local diet, as well as using a novel frame sampler designed to minimize interference to mobile epibenthos (shrimps, crabs, fishes, etc.) at grid sampling stations (Lu et al., 2022).
To explain the habitat selection of a particular species, the diet selection criteria and food availability is the most critical information needed (Zwarts and Wanink, 1993; Piersma, 2012). With knowledge of the harvestable part of all prey, one can select the appropriate measure for food abundance, then map this harvestable prey and derive the relationship between food abundance and intake rates, which will help to predict the species distribution (Goss-Custard et al., 2006; Piersma, 2012). The Tiaozini Wetlands in Jiangsu Province of China are the most important migratory staging site for Spoon-billed Sandpipers use, with more than 50% of the world's population spending the entire moulting period there each autumn (Tong et al., 2012; Bai et al., 2015; Peng et al., 2017; Yang et al., 2020). This makes the site an ideal place to study the diet and food resources of Spoon-billed Sandpipers. Hence, we chose Tiaozini, the main foraging area of Spoon-billed Sandpipers, as our study area, and adopted quantitative methods to study the foraging ecology, aiming to provide a basis for better conservation of their foraging habitat. The study will aid us to better understand the foraging habitat selection, and food composition and distribution of Spoon-billed Sandpipers through video, photo and faecal analysis, as well as benthos sampling.
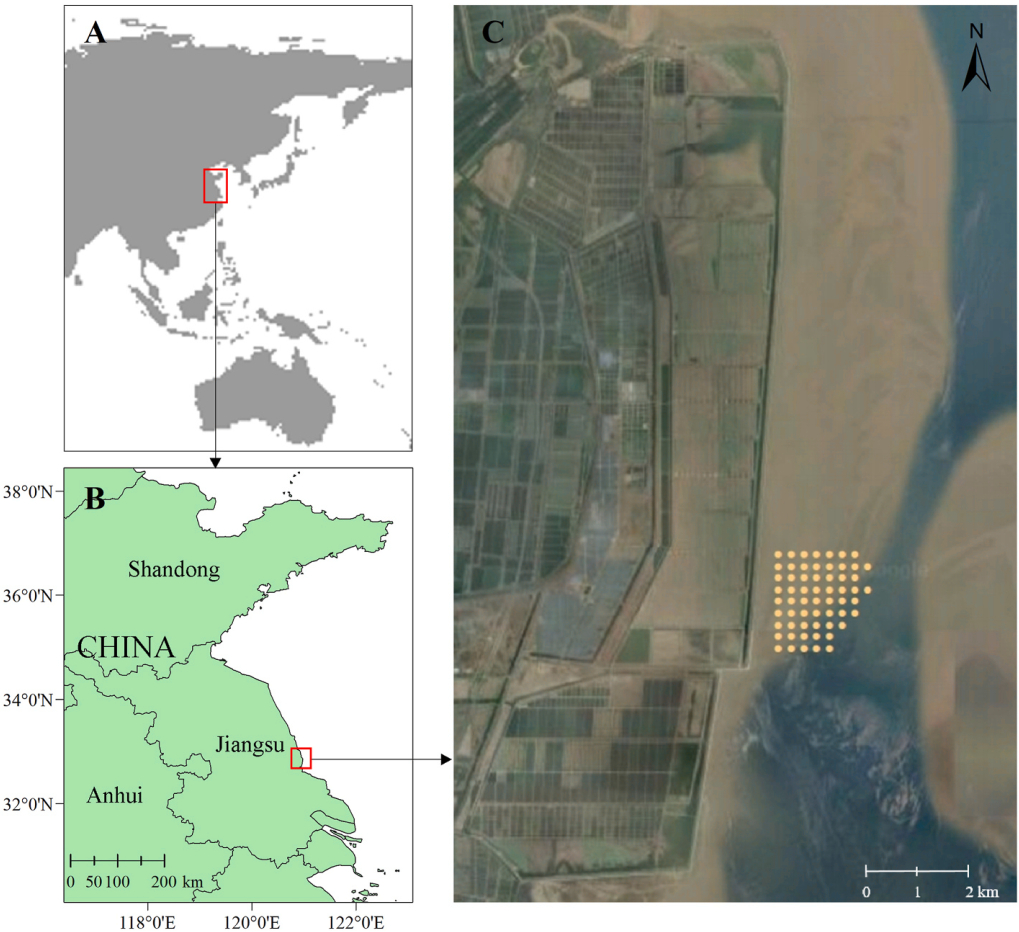
The study area (32°45′–52′ N, 120°58′–59′ E) is located at the Tiaozini Wetland of Dongtai, Yancheng, Jiangsu Province (Fig. 1). A reclamation project of 66,667 ha was planned from 2010 to 2014 in Tiaozini; however, due to the pressure of biodiversity conservation, only 20,000 ha was reclaimed finally. After stopping the reclamation, the Tiaozini Wetland was included in the list of China's Yellow (Bohai) Sea Migratory Bird Habitat (Phase I) World Heritage Site in 2019. This core area of the World Heritage Site includes the Dongtai Tiaozini Municipal Wetland Park, the Dongtai Tiaozini Wetland Mini Reserve, and the Dongtai Gaoni Muddy Flat Wetland Mini Reserve, with a total area of about 55,700 ha (CEZD Coastal Economic Zone of Dongtai City, 2022). On the seaward side of the seawall at Tiaozini Wetland is a coastal plain and shallow intertidal flats of the radial sand ridges (Zhang, 2013), and inside the seawall are reclamation zones made into artificial wetlands, such as fishponds and farmlands including rice paddies. The coastal mudflat is about 1–6 km wide with silty mud sediment, while the radial sand ridges are grey fine sand with a sand content of 80%. Tides are regular semidiurnal tides with a large mean tidal range of up to 3.9 m (Wang, 2017).
According to our long-term observation and the data from satellite-tagged individuals (Chang et al., 2021), in Tiaozini, feeding Spoon-billed Sandpipers concentrated in the south part of the mudflats; thus, we regarded this area as one of the main foraging areas for this species and referred to it as the core foraging area. But a major tidal channel on the mudflat shifted towards the seawall in the autumn and winter of 2019, causing significant changes in the mudflat landscape in the area. In 2020, it was found that the core foraging area slightly shifted to the north, and the density of foraging Spoon-billed Sandpipers in this area was much higher than that in other areas of Tiaozini Wetland during the low tide period, and therefore, we used this as the study area (Fig. 1). In our study area, the average peak number of Spoon-billed Sandpipers in each autumn was around 60 birds during our study (2020–2022) (Sun et al., in prep.).
Determining the food composition of birds is a prerequisite for studying their utilization and selection of foraging habitat (Piersma, 2012). Spoon-billed Sandpipers are small, sparsely distributed birds, and their prey is often small in size (Piersma, 1986; Bird et al., 2010; Kelly et al., 2017), making it difficult to study their diet. This study reconstructed the food composition of Spoon-billed Sandpipers simultaneously using two quantitative methods—predation images and faecal analysis—to try to compensate for their biases.
Due to the scarcity of Spoon-billed Sandpipers, in order to expand the sample size, we observed their foraging behavior during both the southward and the northward migrations. From autumn 2020 to autumn 2022, we tracced and observed Spoon-billed Sandpipers in the core foraging area during low tide when the waterline exited from the observation area, and took predation photos and foraging behavior videos. We also collected Spoon-billed Sandpiper predation photos taken between 2012 and 2019 on the mudflat within 3 km south of the study area, which was the core foraging area for Spoon-billed Sandpipers in Tiaozini during that time. We ended up with a sum of 845 foraging behavior videos recorded from August–October 2020, March–November 2021, and April–October 2022, totaling 376 min, and 66 predation photographs taken in autumns during the 2012–2022 period.
To quantify the time Spoon-billed Sandpipers spent foraging in different habitats, we classified Spoon-billed Sandpiper behavior into foraging-related (e.g., pecking, walking, running) and non-foraging (e.g., resting, preening, bathing) categories, and classified foraging habitats into five main categories: shallow water (a water area without mud surrounding and with a water depth lower than one leg length of Spoon-billed Sandpiper), large shallow pool (a pool with a depth lower than one bird leg length and a surface greater than two body length of the bird), dense small puddles (an area where more than three small puddles gather, with each puddle having a water surface less than two bird body length and a water depth lower than one bird leg length), edges of large deep pool (the edge area of a larger deep pool, which is a pool with a depth exceeding one bird leg length and a water surface greater than two bird body length), and wet mud (the surface of mudflat is wet but not covered with water) (Fig. 2). When analyzing foraging behavior videos, their foraging time in different habitats was calculated separately.
The prey in predation photos was identified at the species level as much as possible, and the prey size was estimated based on the ratio of its length to the bird's bill length (21–22 mm) (Burton, 1971; Wang et al., 2006). Then the biomass (ash-free dry mass) of each prey item was calculated based on the biomass of benthos samples with different species and body sizes obtained from the benthos sampling. Please note that there is a bias in the diet obtained by this method towards larger prey, as the photographers only capture moments when the prey is large enough to be visible through the lens.
To identify small preys of Spoon-billed Sandpipers, fecal analysis was adopted as a supplementary quantitative diet analysis. Throughout the study, when a foraging Spoon-billed Sandpiper on the mudflats was seen defecating after being traced and observed for a quiet long time by a very experienced investigator working on this bird species since 2014, if there were no same-sized shorebirds around and the faeces were not flooded, faecal samples were collected and placed in separate centrifuge tubes for freezing. However, Spoon-billed Sandpipers are very rare and they feed in shallow waters, so the intact faecal samples are extremely difficult to collect. Finally, only five faecal samples were collected during 3–31 September 2021, and four of them contained identifiable benthic structures. The exoskeletons of arthropods and fishbones are easily digested so they are hard to be found intact in faecal samples to identify the species. On the contrary, the bivalve hinges and polychaete jaws are robust, making them much more likely to remain relatively intact in the faecal sample, even if the prey item is small. Thus, much more prey items of bivalves and polychaetes can be identified in the faecal samples than in the predation photos. Although there were only four faecal samples, we can get more accurate information of the prey species and size (especially the small prey) based on the following progress.
The bivalve hinges and polychaete jaws in the thawed faecal samples were picked out, identified, measured and counted using a stereomicroscope (Phoenix Optical Technology, Shangrao, China). The numbers of prey items were calculated based on two hinges of the same size belonging to one bivalve and two or four jaws, depending on the species, of the same size belonging to one polychaete. The length of the prey animals was calculated based on an equation for the relationship between the size of identifiable structures and benthic body length for each species in Tiaozini Wetland (Table 1), which was constructed based on the available benthos data. In this way, we obtained the genre, number and length of benthos digested by Spoon-billed Sandpipers in faecal samples. The biomass (ash-free dry mass) was then calculated based on the biomass data associated with each size obtained from contemporaneous benthic samples, including Moerella iridescens (N = 50), Cyclina sinensis (N = 20), Mactra veneriformis (N = 169). Due to the lack of samples of the genus Ceratonereis, it was not possible to establish a formula for the relationship between the length of its jaws and body length, so the formula of Perinereis aibuhitensis, which belongs to the same subfamily (Nereidinae) as the genus Ceratonereis, was used to reconstruct its body length. Benthos data from other years in Tiaozini were used to calculate the biomass of polychaetes if data from the same period were lacking.
| Species | Relationship | R2 | N |
| Mactra veneriformis | L = 0.0062 × H2 + 0.0261 × H + 0.1059 | 0.96 | 30 |
| Moerella iridescens | L = 0.0664 × T + 0.0144 | 0.95 | 15 |
| Cyclina sinensis | L = 0.1322 × T + 0.0258 | 0.94 | 17 |
| L = 0.0753 × H + 0.0595 | 0.93 | ||
| Perinereis aibuhitensis | L = 85.917 × J2 – 81.983 × J + 59.358 | 0.98 | 5 |
| Glycera spp. | L = 131.09 × J2 – 46.687 × J + 15.703 | 0.99 | 6 |
| Note: T is the height of the bivalve hinge from the top of the shell, H is the height of the bivalve hinge, J is the length of the polychaete’s jaws, and L is the body length. | |||
Macrobenthic sampling was conducted from September 18–23, 2021 in Tiaozini. Based on the width of the intertidal zone, a grid sampling method was used to set up nine transects parallel to the latitude, and grid-like sampling stations 250 m apart were set up on each transect in a manner parallel to the longitude line. There was a total of 60 sampling stations covering the intertidal mudflats at low tide during the spring tide period in our study area (Fig. 1), and 56 of them were sampled when the mudflats were fully exposed. The benthos and sediment data used in this study were based on the samples from these 60 stations. But when there was no biomass of a specific size of a certain species from the study area, benthos samples from adjacent mudflats collected during the same period or in the same area but in other years were used. The distance of each sampling station to the seawall was measured using Google Earth.
As previous studies have revealed that Spoon-billed Sandpipers prefer to feed on benthos in the intertidal shallow pools (Pedersen et al., 1998; Bird et al., 2010; Zöckler et al., 2016; Kelly et al., 2017; Chowdhury et al., 2018; Aung et al., 2020), benthos were sampled in the upper substrate in shallow water habitats at each station. To effectively obtain data on the mobile arthropods and fishes on the mudflat surface, we adopted a sampling method that significantly reduced interference levels. At 10 m from each station, a 40 cm × 40 cm metal sampling frame was lifted into the air with a 4-m pole to a height of 2 m above the ground and slowly approached the station, where the frame was quickly snapped onto the mudflat surface and then the lower part of the frame was rapidly pressed into the sediment to prevent any escape of the mobile epibenthos inside the frame (Lu et al., 2022; Penning et al., 2022). A 30-cm D-shaped hand net was used to collect sediment from the upper 4 cm of the mudflat in the sampling frame and all liquids were filtered from within the frame. The filtered sediment left in the hand net was put into a 1-mm aperture metal sieve, and the sieved benthos were placed into labelled self-sealing bags and brought back to the laboratory to be stored in the refrigerator at −20 ℃.
Samples were thawed in the laboratory and identified to an identifiable taxonomic level using a stereomicroscope (Phoenix Optical Technology, Shangrao, China), and then the individuals were counted and their size was measured. A portion of the benthic sample was used to measure the biomass (ash-free dry mass, g) of different species of different sizes. The samples were dried at 60 ℃ for 3 days and weighed. After incineration at 550 ℃ for 5 h, the mass of the ashes was measured. Then the dry mass of the organic matter of the samples was calculated. Bivalves were deshelled before the above procedures. Based on the maximum lengths of Spoon-billed Sandpiper prey obtained from photos and faecal samples, we estimated the maximum lengths of different groups of benthos that are edible by Spoon-billed Sandpipers, and in this way, we were able to filter the food resources available to Spoon-billed Sandpipers from the benthos data.
During benthic sample collection, the proportion of shallow water area in the sampling frame at each station was estimated by taking photographs of the surface of the mudflat within the placed sampling frames. The percentage of water area within the sampling frames was 95 ± 5% (N = 60), and therefore the data from our study can be considered to be collected from the shallow water habitats of the mudflats. Photographs of the flat surface around the sampling frame were also taken during sampling at each station to estimate the percentage of water area within a 120-m radius of each station. The photographs taken when the mudflats were not fully exposed during the ebb tide were excluded from the estimation.
Sediment samples were collected at the 60 sample stations at the same time as the benthos sampling. Samples were taken at a depth of 4 cm from the surface of the mudflats using a 2.3-cm diameter sampling tube in areas of each sampling station that were not disturbed by human activity, and stored at −20 ℃. Mean sediment particle size (D50) and the distribution of various sizes were measured using a Mastersizer 2000 laser particle size analyser (Malvern Panalytical, Worcestershire, UK).
Spearman correlation analysis was used to analyze the relationship between the distribution of mobile epibenthos available to Spoon-billed Sandpipers and environmental factors (shallow water area, median grain size of sediment and distance to seawall). Statistical analysis was performed with IBM SPSS Statistics v.10.8 (SPSS Inc., Chicago, IL, USA) and plotted using the R software v. 4.1.3 (R Core Team, Vienna, Austria). Kriging interpolation is a method of weighting logarithmic data points based on spatial autocorrelation to provide estimates and prediction errors. The spatial correlation between interpolated points and known points was estimated by Variogram and weighted average was performed. Kriging was plotted using ArcGis software, version 10.8 (Esri, California, America).
The percentage of shallow water area on the mudflat surface around benthic sampling stations was 72 ± 17% (N = 56). The mean value of the median grain size of the sediment samples was 66.4 ± 14.6 μm (N = 60) with a median grain size ranging between 21.7 and 91.1 μm. The composition of the sediment of all samples was 60% very fine sand (grain size >63 μm and <125 μm) and 40% silt (grain size >4 μm and <63 μm). Thus, the sediments in the mudflats of Tiaozini contained mixed sand.
Spoon-billed Sandpiper foraging behavior video totaled 331 min, of which 329 min (99.3% of the total) were of them foraging in a variety of shallow water habitats (Fig. 2), and only 2 min (0.7% of the total) of them foraging on wet muds. Among shallow water habitats, they preferred larger areas of shallow water (Table 2).
| Empty Cell | Total foraging time | Shallow water | Large shallow pool | Dense small puddles | Edge of a large deep pool | Wet mud |
| Foraging time (min) | 331 | 114 | 111 | 76 | 28 | 2 |
| Percentage | 100% | 34.5% | 33.5% | 23% | 8.3% | 0.7% |
In total, we obtained 66 predation photos of Spoon-billed Sandpipers with identifiable prey (Fig. 3). Identified species include Parapenaeopsis tenella (N = 9), Exopalaemon modestu (N = 2), Macrophthalmus (Mareotis) japonicus (N = 7), Macrophthalmus dilatum (N = 6), Bullacta exarate (Philippi) (N = 7), Acanthogobius hasta (N = 1), Amoya caninus (N = 1), Solen dunkerianus (N = 1), and Cyclina sinensis (N = 1). Shrimps comprised the highest proportion of individuals and biomass among the observed prey (Table 3). Mobile epibenthos accounted for 79% of the total number and 81% of the total biomass. Based on the maximum lengths of different prey groups, we estimated the maximum length of these benthic groups that are edible by Spoon-billed Sandpipers (Table 3).
| Prey | Number (%) | Average length (size range; mm) | Edible size (mm) | Biomass (g; %) |
| Shrimp | 36 (55%) | 24 (13–39) | < 40 | 0.4002 (43.12%) |
| Crab | 12 (18%) | 9 (4–16) | <20 | 0.2138 (23.03%) |
| Mud snail | 7 (11%) | 9 (7–12) | <15 | 0.1612 (17.37%) |
| Polychaete | 5 (8%) | 35 (22–47) | <170a | 0.0053 (0.57%) |
| Fish | 4 (6%) | 29 (22–39) | <40 | 0.1333 (14.36%) |
| Bivalve | 2 (3%) | 11 (10–13) | <15 | 0.0144 (1.55%) |
| Total | 66 (100%) | 20 (4–47) | – | 0.9282 (100%) |
| Mobile epibenthos | 52 (79%) | 20 (4–39) | – | 0.7473 (80.51%) |
| a Determined by stereomicroscopic examination of faecal samples. Groups using italics were mobile epibenthos. | ||||
Two of the four faecal samples had all identifiable structures of bivalves, and the remaining two samples had all identifiable structures of polychaetes. Detailed results were as the following: in faeces 1, there were 3 Mactra veneriformis, 3 Cyclina sinensis, 1 Moerella iridescens; in faeces 2, there were 7 Mactra veneriformis and 1 Cyclina sinensis; in faeces 3, there were 2 Perinereis aibuhitensis, 2 Glycera spp. and 1 Ceratonereis sp.; in faeces 4, there were 7 Perinereis aibuhitensis. In sum, the four faecal samples comprised 56% bivalves and 44% polychaetes. Among them, Mactra veneriformis and Perinereis aibuhitensis were the most abundant, Perinereis aibuhitensis having the highest biomass (Table 4). The lengths of bivalves in the faecal samples were within the range of edible prey sizes estimated from photographs (Table 3). The length of polychaetes in the faecal samples ranged from 32 to 168 mm, with an average of 69 mm, which was greater than the edible size estimated from photos; therefore, the maximum length of polychaetes that is edible by Spoon-billed Sandpipers was set at 170 mm (Table 3).
| Species | Average length (size range; mm) | Number (%) | Biomass (g; %) |
| Mactra veneriformis | 3.9 (1–7) | 10 (37%) | 0.01243 (4.90%) |
| Cyclina sinensis | 2.8 (2–4) | 4 (15%) | 0.00067 (0.26%) |
| Moerella iridescens | 3.0 (3) | 1 (4%) | 0.00013 (0.05%) |
| Perinereis aibuhitensis | 79.0 (40–168) | 9 (33%) | 0.21355 (84.24%) |
| Glycera spp. | 38.5 (32–45) | 2 (7%) | 0.01551 (6.12%) |
| Ceratonereis sp. | 37 (37) | 1 (4%) | 0.01120 (4.42%) |
| Total | 32.5 (1–168) | 27 (100%) | 0.25349 (100%) |
| Bivalves | 3.5 (1–7) | 15 (56%) | 0.01323 (5.22%) |
| Polychaetes | 68.8 (32–168) | 12 (44%) | 0.24026 (94.78%) |
There were a total of 93 prey items obtained from images and faecal samples, with a size range of 1–168 mm. If 5 mm is used as the standard size for dividing prey, the biomass of small prey (1–5 mm) accounted for 1% (0.0085 g, N = 14) of the total biomass, while that of big prey (>5 mm) was as high as 99% (1.1731 g, N = 79). If 10 mm is used as the standard size, the biomass of small prey (1–10 mm) accounted for 19% (0.2248 g, N = 31) of the total biomass, and that of big prey (>10 mm) accounted for 81% (0.9568 g, N = 62). Thus, the big prey accounts for the majority of all prey in both quantity and biomass. We divided the photos into two groups for analysis: 2012–2019 and 2020–2022, based on the time of changes in the core foraging area. As the largest prey group, there was no significant difference in the proportion of shrimp to all food, both in number and biomass, between the two time periods (number: F = 1.47, p = 0.29; Biomass: F = 1.01, p = 0.37).
Using the maximum edible lengths of different benthic groups above estimated from diet analysis, the available food resources to Spoon-billed Sandpipers was filtered. There were a total of 25 benthos species available (accessible and edible) for Spoon-billed Sandpipers in Tiaozini Wetland, with 8 species of mobile epibenthos, including 7 arthropods species (1 species of hooded shrimp: Diastylis tricincta, 1 species of gammarid: Monoculodes koreanus, 3 species of shrimp: Exopalaemon modestus, Parapenaeopsis tenella, Ogyrides orientalis, 2 species of crab: Metaplax eleganu, Macrophthalmus (Mareotis) japonicus) and 1 species of fish (Acanthogobius hasta). The rest of the available benthos were six species of bivalves, Mud Snail (Bullacta exarate (Philippi)), nine species of Polychaeta and one species of Nemertea. The size of all 25 benthos species was edible for the Spoon-billed Sandpiper, except some big Mactra veneriformis.
The density of available food for Spoon-billed Sandpipers was 90.9 ind./m2 and biomass was 0.0596 g/m2, which accounted for 68% of density and 21% biomass of all benthos obtained from the samples, respectively (Table 5). The density of mobile epibenthos (small arthropod, shrimp, crab, and fish) accounted for 35.9% of the total density of the available food resources, while the biomass share was as high as 72.59%, of which shrimp had the highest biomass share of 38.37% (Table 5). The density of mobile epibenthos was 35.9 ind./m2 and the biomass was 0.0433 g/m2. Among them, two amphipod and decapod species (Monoculodes koreanus and Exopalaemon modestus) were the dominant species, accounting for 86% of the total mobile epibenthos numbers and 51% of the total mobile epibenthos biomass. Monoculodes koreanus had a density of 21 ind./m2, distributed in 73% of the stations, with biomass of 0.0035 g/m2, accounting for 8% of the total mobile epibenthos biomass, and Exopalaemon modestus had a density of 10 ind./m2, distributed in 33% of the stations, with biomass of 0.0186 g/m2, accounting for 43% of the total mobile epibenthos biomass.
| Group | No. of species | Average length (size range; mm) | Density (ind./m2) | Density proportion (%) | Biomass (g/m2) | Biomass proportion (%) |
| Small arthropod | 2 | 4.0 (2–7) | 21.4 | 23.51 | 0.0035 | 5.90 |
| Shrimp | 3 | 10.6 (6–17) | 12.8 | 14.10 | 0.0229 | 38.37 |
| Polychaete and Nemertea | 10 | 8.4 (1–26) | 4.0 | 4.36 | 0.0010 | 1.74 |
| Crab | 2 | 4.1 (1–9) | 1.4 | 1.49 | 0.0088 | 14.80 |
| Fish | 1 | 23.3 (8–35) | 0.3 | 0.34 | 0.0081 | 13.52 |
| Mollusc | 7 | 2.7 (1–15) | 51.0 | 56.20 | 0.0153 | 25.65 |
| Total | 25 | 5.4 (1–35) | 90.9 | 100 | 0.0596 | 100 |
| Mobile epibenthos | 8 | 7.6 (1–35) | 35.9 | 39.44 | 0.0433 | 72.59 |
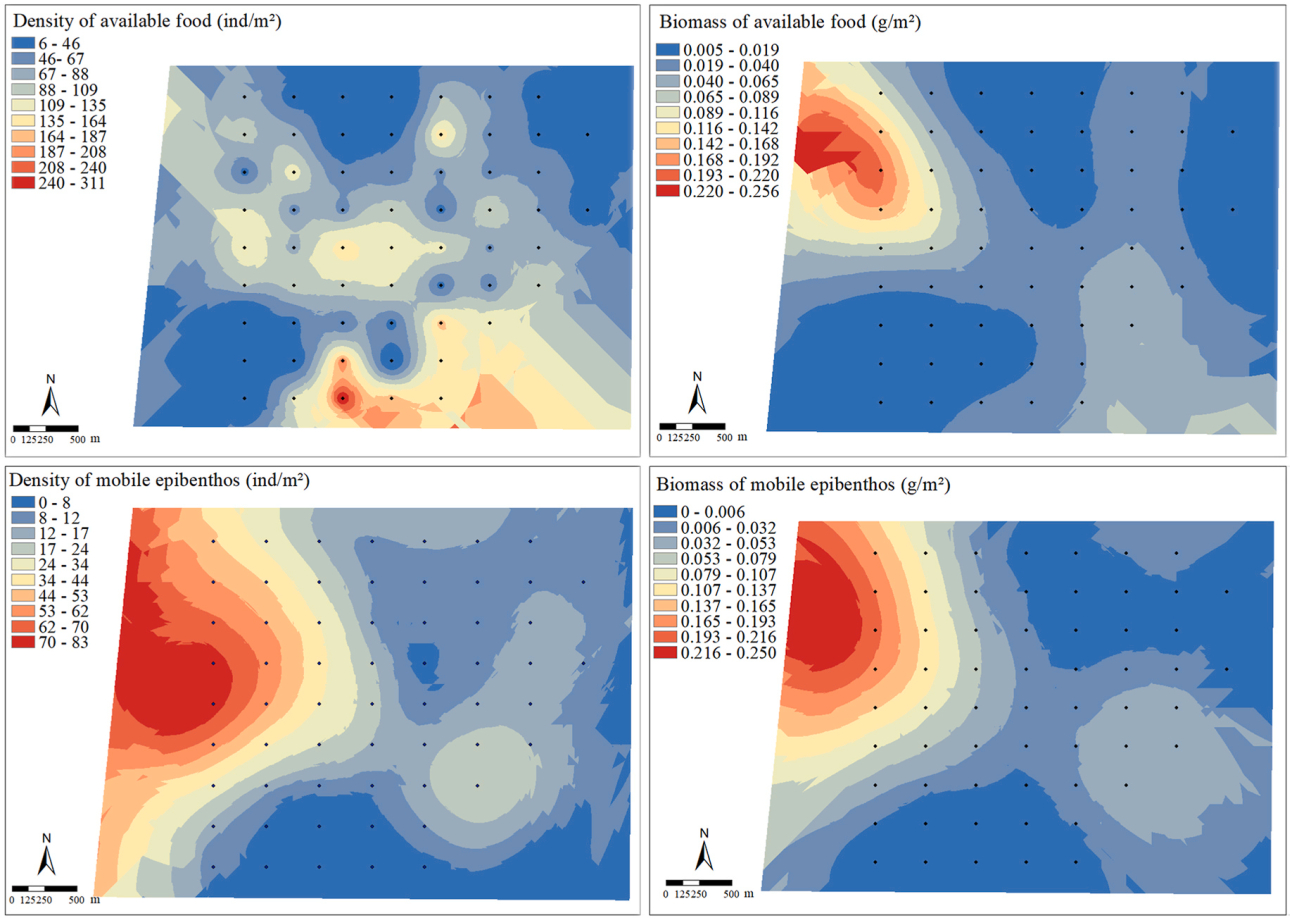
In terms of spatial distribution, the density of food available to Spoon-billed Sandpipers was higher in middle area of the mudflats while the biomass was higher in the nearshore area (Fig. 4). Both the density and biomass of mobile epibenthos were higher nearshore, with shrimps and crabs having higher densities nearshore, small arthropods having higher densities offshore, and all three taxa having higher biomass nearshore. Bivalves living inside the mud had a higher density in the middle area and a relatively uniform distribution of biomass, while polychaetes had a higher density and biomass in the northern part of the mudflat. The density and biomass of mud snails living on the mud surface were higher in the south. Spearman's correlation analysis showed that among the three environmental variables (distance to seawall, median grain size and water area), the distance to seawall played the most important role for the mobile epibenthos. The densities and biomass of shrimps (density: F = −0.433, p < 0.01; biomass: F = −0.260, p < 0.05) and crabs (density: F = −0.267, p < 0.05; biomass: F = −0.414, p < 0.01) were both significantly and negatively correlated with the distance to seawall, and the densities of small arthropods (F = 0.302, p < 0.05) were significantly and positively correlated with the distance to seawall.
Spoon-billed Sandpipers foraged in shallow water habitats on soft sediments with a thin layer of water on the sand-mud mixed mudflats in Tiaozini, never on the dry mud/sand, based on the foraging behavior videos, which was consistent with the findings of previous studies on their foraging in non-breeding areas (Jahn, 1942; Piersma, 1986; Pedersen et al., 1998; Bird et al., 2010; Zöckler et al., 2016; Kelly et al., 2017; Aung et al., 2020).
Previous dietary studies of the Spoon-billed Sandpiper on non-breeding grounds were mainly descriptions of prey species, which showed that their diet consists of shrimp, crab, amphipod, fish, polychaete, hard-shelled gastropod, and insect (Voronov, 1980; Ali and Ripley, 1983; Sugathan, 1985; Cha and Young, 1990; Tong et al., 2012; Zöckler et al., 2016; Kelly et al., 2017; Lu et al., 2022). Our study found a Spoon-billed Sandpiper diet composition that was largely consistent with the above findings, except for the absence of insect and hard-shelled gastropod, as well as the discovery that they also feed on bivalves and mud snail. From the predation photos in this study, mobile epibenthos (shrimp, crab, fish) accounted for 79% in number and 81% in biomass. Considering the bias of predation photos in favor of larger prey, the above results validate to some extent the speculation of previous studies that mobile epibenthos are the main food for the Spoon-billed Sandpiper at non-breeding sites (Aung et al., 2020; Yang et al., 2020; Lu et al., 2022). In addition, of the 27 prey items found in the four faecal samples based on identifiable benthic structures, bivalves comprised 56% of the total prey and polychaetes 44%, while polychaetes accounted for 95% of the total biomass of the total prey and bivalves only 5%, with an average of four bivalves and three polychaetes per faeces. Also, a certain amount of fragments of suspected crab shell or arthropod appendage was found in all four faecal samples, while a large amount of suspected crab shell fragments were present in the fifth faecal sample where no identifiable benthic structures were found.
Therefore, based on observed foraging habitat preference and diet composition of Spoon-billed Sandpipers, we consider the foraging strategy of this species to be specialized in feeding in mudflat pools on benthos living within the water of the pools (e.g., shrimp, gammaridea, and fish), or benthos exposed on the bottom surface of the pools (e.g., crab, polychaete, and bivalves, or even small- and moderate-sized mud snail), with the main energy source being provided by shrimps and probably polychaetes.
The benthic fauna sampling data obtained in this study showed that most of the benthos available to Spoon-billed Sandpipers in the shallow waters of the mudflats in the core foraging areas were within the size class observed to be edible by this bird. These potential prey were widely distributed over the mudflats (Fig. 4) at high density and biomass, especially the mobile epibenthos, which are the main observed prey of Spoon-billed Sandpipers. Among them, shrimps, which were observed to provide the main energy source for Spoon-billed Sandpipers, had quite high density and biomass in the shallow water habitats of the mudflats (Table 5), compared with the low density of shrimp in Tiaozini in northward migration (Lu et al., in prep.) and the density of shrimp in the most important wintering area in China (Lu et al., 2022). Therefore, the available food resources for Spoon-billed Sandpipers in the Tiaozini Wetland during their peak of southward migration were abundant. Analysis of foraging habitat selection by foraging behavioral videos revealed that Spoon-billed Sandpipers usually preferred larger patches of shallow waters. Meanwhile, distance to seawall was the most important factor influencing the distribution of mobile epibenthos. Thus, we concluded that nearshore shallow water had the highest availability of the preferred prey of Spoon-billed Sandpiper in Tiaozini in autumn, when they spend two months to moult (Yang et al., 2020), and hence also the priority area for Spoon-billed Sandpiper conservation during the peak of the southward migration at this staging site, which is consistent with findings of previous research on habitat use of other shorebird species in the Yellow Sea ecoregion (Mu and Wilcove, 2020).
The advantage of the predation photo analysis is that as long as the prey is large enough and the image is taken clearly enough, more prey species can be observed than faecal analysis. However, the disadvantage also lies in that the Spoon-billed Sandpipers seldom catch large prey; therefore, obtaining a suitable predation photo took a lot of time (Piersma, 1986; Bird et al., 2010; Aung et al., 2020; Lu et al., 2022). Furthermore, while all other groups of food resources obtained by predation photo analysis have biases toward larger prey (i.e., bivalves captured by Spoon-billed Sandpipers in photos were larger on average than those obtained from faecal analysis), the predation photo analysis may underestimate the size of polychaetes (i.e., polychaetes captured by Spoon-billed Sandpipers in photos were on average shorter than those from faecal analysis) because photos only showed a portion of a polychaetes' body while the rest was still in sediment. The structures in the faeces enable more accurate identification of prey to species level and the determination of their size through use of regression equations, including those of very small individuals, but the preference of Spoon-billed Sandpipers for foraging in water makes complete, unsoaked faecal samples very hard to come by, and also, for many groups of benthos it is difficult to retain any identifiable structure after digestion for identification and measurement. Fortunately, DNA macro-barcoding, a technique currently being improved, could be a supplement to predation photo and faecal analysis in studies that need to calculate the energy provided by food (Huang et al., 2022). In conclusion, we suggest that in order to describe the diet of the Spoon-billed Sandpiper as comprehensively and accurately as possible, and to quantify the diet composition, we need to apply image analysis (photos or videos) and faecal analysis, and supplement them with DNA barcoding of faecal samples.
The result of diet analysis of Spoon-billed Sandpipers in this study enabled this first attempt to quantitatively map the availability of prey of Spoon-billed Sandpipers on the intertidal mudflats. Due to mobile epibenthos, especially shrimps, being their main food source both at staging and wintering sites (Lu et al., 2022), efficient capture of mobile epibenthos becomes the key to evaluating the quality of foraging habitat for this species. The frame sampling method used in this study is good at reducing interference to mobile epibenthos and thus capturing them more efficiently. If combined with traditional core sampling methods of taking smaller but deeper (e.g., 20 cm deep) sediment cores, all types of macrobenthos including prey that moves horizontally (e.g., shrimp and fish) as well as vertically (e.g., polychaete and crab) could be captured (Kraan et al., 2009; Choi et al., 2014; Bom et al., 2018; Penning et al., 2022). In this way, the prey availability data for Spoon-billed Sandpipers could be collected more accurately, which in turn could be used to assess their habitat quality more comprehensively.
In recent years, the mudflat siltation after reclamation, the invasion of Spartina alterniflora, and the swing of tidal creeks have continued to bring about changes in the feeding habitats of the Spoon-billed Sandpiper at Tiaozini. Meanwhile, climate change induced sea level rise, rising sea temperature, and acidification may have long-term impacts on benthic communities of the mudflats (Iwamura et al., 2013; Hua et al., 2015; Peng et al., 2017). Therefore, in order to cope with these changes, it is necessary to launch long-term monitoring of the population, food resources and the factors affecting the food distribution of Spoon-billed Sandpipers and other shorebirds, to understand their requirements and even predict the changing trend of food resources, then design and test effective management measures to maintain and restore suitable habitat quality. Meanwhile, shallow water habitats may be the most preferred microhabitats for Spoon-billed Sandpipers. Areas with extensive distributions of such habitats should be prioritized for protection and investigation to identify additional habitats for Spoon-billed Sandpipers and safeguard potential ones. Research on the formation mechanisms of these microhabitats, considering factors such as hydrodynamics and bioturbation, will provide valuable scientific evidence for restoring habitats for Spoon-billed Sandpipers. Furthermore, it is requisite to carry out monitoring and assessment of food resources, and the conditions needed to provide them, in other key areas for Spoon-billed Sandpipers, to enable the provision of suitable conditions, through habitat management and restoration to ensure the recovery of their population and the conservation of the entire shorebird community dependent of the coastal wetlands of the EAAF.
Our study showed that Spoon-billed Sandpipers at their main autumn staging site forage mainly in areas with large shallow water patches on intertidal mudflats, and they prey on the vast majority of benthic fauna on the mudflats but mainly on the mobile epibenthos, which is related to their specialized spoon-shaped bill (Kelly et al., 2017). In terms of the spatial distribution of their main food resources, in areas where the distribution of available food resources for Spoon-billed Sandpipers is currently known (Tiaozini Wetland in Jiangsu in autumn, and Leizhou Wetlands in Guangdong in winter), priority should be given to protection of the nearshore habitats with a large number of shallow water areas, especially the intertidal mudflats with mixed sediment (Lu et al., 2022).
Also, while conducting a comprehensive survey of Spoon-billed Sandpiper populations along the coasts of southern China, it is urgent to carry out effective dietary studies and food resource surveys in as many known important habitats of the species as possible, in order to throw light on solving the mystery of the continuous decline in their population and provide important data to support the conservation of the species through appropriate habitat protection, management and restoration across the full range of this species in the EAAF.
Lili Sun: Writing – original draft, Investigation, Conceptualization. Hongyan Yang: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Xiuyuan Lu: Visualization, Investigation, Formal analysis, Data curation. Ting Fu: Visualization, Investigation, Formal analysis, Data curation. Jia Guo: Investigation, Formal analysis. Sicheng Ren: Investigation, Formal analysis. Waner Liang: Visualization, Formal analysis. Qing Chen: Investigation. Dongming Li: Investigation. Theunis Piersma: Writing – review & editing. Nicola Crockford: Writing – review & editing. Yifei Jia: Writing – review & editing, Writing – original draft, Project administration, Methodology, Funding acquisition, Conceptualization. Guangchun Lei: Writing – review & editing, Funding acquisition.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We acknowledge the strong support for this study from Dongtai Coastal Wetland Tourism and Resort Economic Zone Management Committee. We thank Yancheng Administration Center for Wetland and World Natural Heritage Protection and Yancheng National Nature Reserve for their support in our field research. We thank Huajin Sun for providing predation photos of Spoon-billed Sandpiper. We thank Lijun Wang and Hongbo Li for helping with the identification of benthos specimens. We thank all volunteers for their assistance for the fieldwork and labwork. We thank Ting Wu, Weixing Jiang, Taisheng Chen for helping to plot the Kernel Density analysis. The manuscript was improved with help from Li Wen and anonymous reviewers.
| Species | Relationship | R2 | N |
| Mactra veneriformis | L = 0.0062 × H2 + 0.0261 × H + 0.1059 | 0.96 | 30 |
| Moerella iridescens | L = 0.0664 × T + 0.0144 | 0.95 | 15 |
| Cyclina sinensis | L = 0.1322 × T + 0.0258 | 0.94 | 17 |
| L = 0.0753 × H + 0.0595 | 0.93 | ||
| Perinereis aibuhitensis | L = 85.917 × J2 – 81.983 × J + 59.358 | 0.98 | 5 |
| Glycera spp. | L = 131.09 × J2 – 46.687 × J + 15.703 | 0.99 | 6 |
| Note: T is the height of the bivalve hinge from the top of the shell, H is the height of the bivalve hinge, J is the length of the polychaete’s jaws, and L is the body length. | |||
| Empty Cell | Total foraging time | Shallow water | Large shallow pool | Dense small puddles | Edge of a large deep pool | Wet mud |
| Foraging time (min) | 331 | 114 | 111 | 76 | 28 | 2 |
| Percentage | 100% | 34.5% | 33.5% | 23% | 8.3% | 0.7% |
| Prey | Number (%) | Average length (size range; mm) | Edible size (mm) | Biomass (g; %) |
| Shrimp | 36 (55%) | 24 (13–39) | < 40 | 0.4002 (43.12%) |
| Crab | 12 (18%) | 9 (4–16) | <20 | 0.2138 (23.03%) |
| Mud snail | 7 (11%) | 9 (7–12) | <15 | 0.1612 (17.37%) |
| Polychaete | 5 (8%) | 35 (22–47) | <170a | 0.0053 (0.57%) |
| Fish | 4 (6%) | 29 (22–39) | <40 | 0.1333 (14.36%) |
| Bivalve | 2 (3%) | 11 (10–13) | <15 | 0.0144 (1.55%) |
| Total | 66 (100%) | 20 (4–47) | – | 0.9282 (100%) |
| Mobile epibenthos | 52 (79%) | 20 (4–39) | – | 0.7473 (80.51%) |
| a Determined by stereomicroscopic examination of faecal samples. Groups using italics were mobile epibenthos. | ||||
| Species | Average length (size range; mm) | Number (%) | Biomass (g; %) |
| Mactra veneriformis | 3.9 (1–7) | 10 (37%) | 0.01243 (4.90%) |
| Cyclina sinensis | 2.8 (2–4) | 4 (15%) | 0.00067 (0.26%) |
| Moerella iridescens | 3.0 (3) | 1 (4%) | 0.00013 (0.05%) |
| Perinereis aibuhitensis | 79.0 (40–168) | 9 (33%) | 0.21355 (84.24%) |
| Glycera spp. | 38.5 (32–45) | 2 (7%) | 0.01551 (6.12%) |
| Ceratonereis sp. | 37 (37) | 1 (4%) | 0.01120 (4.42%) |
| Total | 32.5 (1–168) | 27 (100%) | 0.25349 (100%) |
| Bivalves | 3.5 (1–7) | 15 (56%) | 0.01323 (5.22%) |
| Polychaetes | 68.8 (32–168) | 12 (44%) | 0.24026 (94.78%) |
| Group | No. of species | Average length (size range; mm) | Density (ind./m2) | Density proportion (%) | Biomass (g/m2) | Biomass proportion (%) |
| Small arthropod | 2 | 4.0 (2–7) | 21.4 | 23.51 | 0.0035 | 5.90 |
| Shrimp | 3 | 10.6 (6–17) | 12.8 | 14.10 | 0.0229 | 38.37 |
| Polychaete and Nemertea | 10 | 8.4 (1–26) | 4.0 | 4.36 | 0.0010 | 1.74 |
| Crab | 2 | 4.1 (1–9) | 1.4 | 1.49 | 0.0088 | 14.80 |
| Fish | 1 | 23.3 (8–35) | 0.3 | 0.34 | 0.0081 | 13.52 |
| Mollusc | 7 | 2.7 (1–15) | 51.0 | 56.20 | 0.0153 | 25.65 |
| Total | 25 | 5.4 (1–35) | 90.9 | 100 | 0.0596 | 100 |
| Mobile epibenthos | 8 | 7.6 (1–35) | 35.9 | 39.44 | 0.0433 | 72.59 |