| Citation: | Abel Souriau, Jorma Sorjonen, Adam Petrusek, Tereza Petrusková. 2025: Local song evolution after three decades in a complex songster, the Thrush Nightingale. Avian Research, 16(1): 100224. DOI: 10.1016/j.avrs.2025.100224 |
Birdsong is an important secondary sexual trait which may vary between but also within species. Intraspecific variation is generally studied either on the geographical or on the temporal scale; most of the studies exploring the variation of song over time, however, focused on species with rather simple songs. In this study, we explored the temporal changes in song of a complex songster, the Thrush Nightingale (Luscinia luscinia), recorded after 33 years (in 1986 and 2019) at the same locality in south-eastern Finland. Our analysis revealed a complete turnover of song types over the study period, with no song type shared between the two recording years. In contrast, 40% of the originally recorded syllable types were still found in the repertoires of recently recorded males. Their song type repertoires were significantly smaller but the songs themselves were on average longer compared to the 1986 recordings. Repertoires of both syllables and song types were more shared between males recorded in 1986 than between those from 2019. We discuss the processes that may have contributed to these temporal changes in song and call for more detailed studies of song evolution in wild populations.
Birdsong, i.e., learned vocalisation of oscine passerine birds, belongs to the most complex and diverse vocal signals in the animal kingdom and plays crucial roles in species-specific recognition, mate attraction and repelling of competitors (Todt and Naguib, 2000; Catchpole and Slater, 2014). It is a well-established and intensively studied example of animal social learning and cultural evolution (Lynch, 1996; Aplin, 2019) and, thanks to high diversity of songbirds and their ecologies, it exhibits outstanding inter-as well as intraspecific variation (e.g., Slater and Ince, 1979; Podos et al., 2004; Lachlan et al., 2018).
To achieve species-specific songs, young birds typically memorise songs heard from conspecifics shortly after hatching; subsequently, they practise and perform songs with high plasticity to finally reach their adult crystallised songs (Brainard and Doupe, 2002; Hultsch and Todt, 2004). Although this generalised process seems straightforward, song learning strategies in songbird species are very diverse—from precise imitation of tutors, through improvisation to invention (reviewed in Beecher and Brenowitz, 2005). As such, the accumulation of small variations in song characteristics commonly results in geographic variation of song structures or repertoires (Podos and Warren, 2007). Accordingly, spatial variation in song was documented in many different songbird species, regardless of the complexity of their song (Jenkins, 1977; Osiejuk et al., 2003; Roach and Phillmore, 2017; Barišić et al., 2018; Czocherová et al., 2022).
However, changes in songs within the same population may also occur over time. Studies comparing recordings obtained after a decade or more reported a whole range of different patterns, from remarkable stability (e.g., Ficken and Popp, 1995; Goodale and Podos, 2010; Ivanitskii et al., 2023) through partial turnovers (e.g., Chilton and Lein, 1996; Nelson et al., 2004; Luther and Baptista, 2010) to a complete lack of similarity between old and more recent recordings from the same area (e.g., Holland et al., 1996). Such changes of song at a single locality might have in return important consequences in the signalling functions. Multiple experimental studies documented that the changes in song could influence the behaviour of males (e.g., Thompson and Baker, 1993; Nelson and Soha, 2004; Szymkowiak and Kuczyński, 2017), females (e.g., Gentner and Hulse, 2000; Spencer et al., 2005; Caro et al., 2010), or both sexes (e.g., Catchpole et al., 1986; Balaban, 1988; Searcy et al., 1997; Anderson et al., 2007). From an evolutionary perspective, female preference for a local variant could, over time, constitute a behavioural barrier to gene flow via assortative mating and eventually favour a process of speciation (Derryberry, 2007).
Moreover, long-term studies of song variation focusing on a single population could provide a good background to explore the mechanisms behind song evolution, such as learning biases, selection on signal efficiency, or cultural drift (e.g., Williams and Lachlan, 2022). For example, a study on Chestnut-side Warblers (Dendroica pensylvanica) revealed a rapid change in one song type but almost none in another one, suggesting that multiple independent cultural traditions and probably multiple independent learning predispositions can evolve concurrently within a species (Byers et al., 2010). Similarly, the three parts of the song of Savannah Sparrows (Passerculus sandwichensis), likely serving different functions, apparently undergo changes at different rates and may be affected by different evolutionary mechanisms, both neutral and selective (Williams et al., 2013).
Most of the studies focusing on long-term temporal song evolution have so far examined species with rather simple songs (e.g., Gibbs, 1990; Grant and Grant, 1996; Hansen, 1999; Byers et al., 2010; Pipek et al., 2016). Recently, however, song repertoire evolution after almost four decades was documented in a population of the House Finch (Haemorhous mexicanus), a species with moderately complex song, showing that the song types had undergone a complete turnover while remaining structurally similar (Ju et al., 2019). Nonetheless, the opposite has been documented on a larger scale in Common Nightingales (Luscinia megarhynchos). In this species with very large song repertoires (over 190 song types per individual; Hultsch and Todt, 1981; Kipper et al., 2004), a remarkable stability of some of the most common song types was shown after four to nine decades (Jäckel et al., 2022).
In this study, we investigated the song evolution of the Thrush Nightingale (Luscinia luscinia), a sister species to the Common Nightingale. It also has a relatively large repertoire (though much smaller than the latter species) and complex syntax (Grießmann and Naguib, 2002; Ivanitskii et al., 2017), and shows a pronounced geographical song variation (Sorjonen, 1987; Souriau et al., 2019). We compared songs recorded at the same locality in Finland 33 years apart (in 1986 and 2019), focusing on both syllable and song type levels. We hypothesised that song and syllable type sharing would be overall lower between old and recent recordings than between recordings from the same year, and that syllables, as basic units of vocalisation, are more likely to persist over decades than song types but that at least some song types will be recorded in both years.
The Thrush Nightingale (Luscinia luscinia; Passeriformes: Muscicapidae) is a small migratory songbird breeding in the northern part of Eurasia and wintering in Southeast Africa (Cramp and Perrins, 1988; Collar, 2020). Males are highly philopatric (Sorjonen, 1987) and defend their territories by singing during both night and day (Sorjonen, 1977). As usual for small passerines, the males usually start breeding in the next year after hatching, and while the longest documented lifespans are nine to ten years (Cramp and Perrins, 1988; Glutz von Blotzheim, 1988), most individuals probably live much shorter. Natural song rate of territorial males usually ranges between six to eight songs per minute (Vokurková et al., 2013; Souriau et al., 2018, 2021), with individual songs separated by several-second-long pauses.
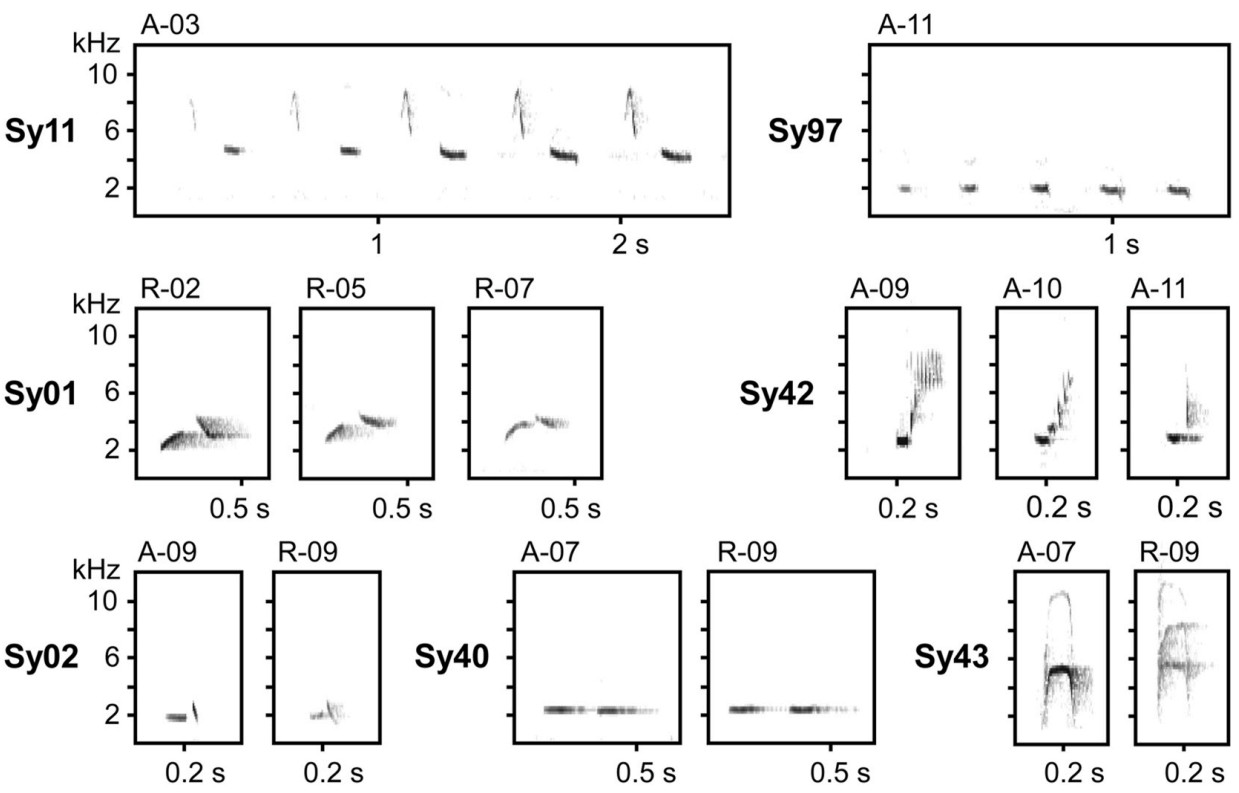
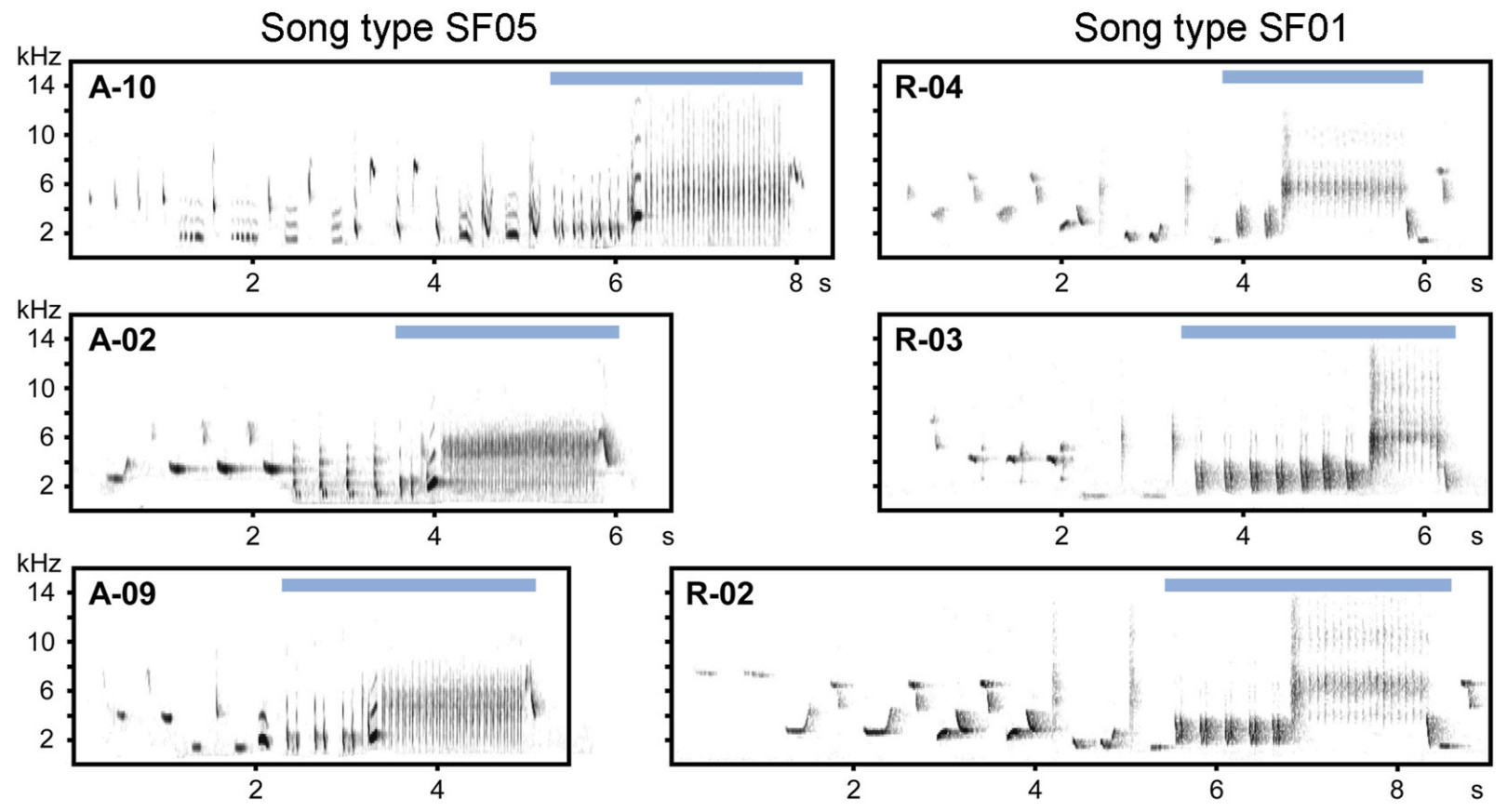
When characterising Thrush Nightingale song features in this study, we followed the general approach described in Souriau et al. (2019): a "syllable" is the smallest invariant distinct unit within a song consisting of one or several "elements" (i.e., continuous traces on the spectrogram) that are always repeated in the same sequential order. Individual songs of Thrush Nightingales, ca 5 s long, usually begin with a sequence of various initial syllables that is highly variable even among songs of the same individual, and end with a characteristic sequence of two consecutive phrases: loud "castanet" syllables followed by a deep "rattling" broadband trill (Fig. 1; Sorjonen, 1983; Souriau et al., 2019), often followed by a short terminal syllable. For the purposes of this study, we defined the "song type" as a set of songs that share a distinct conserved sequence of several syllables (or their repetitions) in the final part of the song (Fig. 1), usually covering both castanet and rattling phrases, i.e., structures that are typically used to characterise song types in this species (Grießmann and Naguib, 2002; Ivanitskii et al., 2017).
As a consequence of the song learning process, syllable as well as song type repertoires are shared by Thrush Nightingale males at the same location. For example, reported proportions of shared song types reached 30% on the Hiddensee island in the Baltic Sea (Grießmann and Naguib, 2002) and 47% in Moscow, Russia (Ivanitskii et al., 2017).
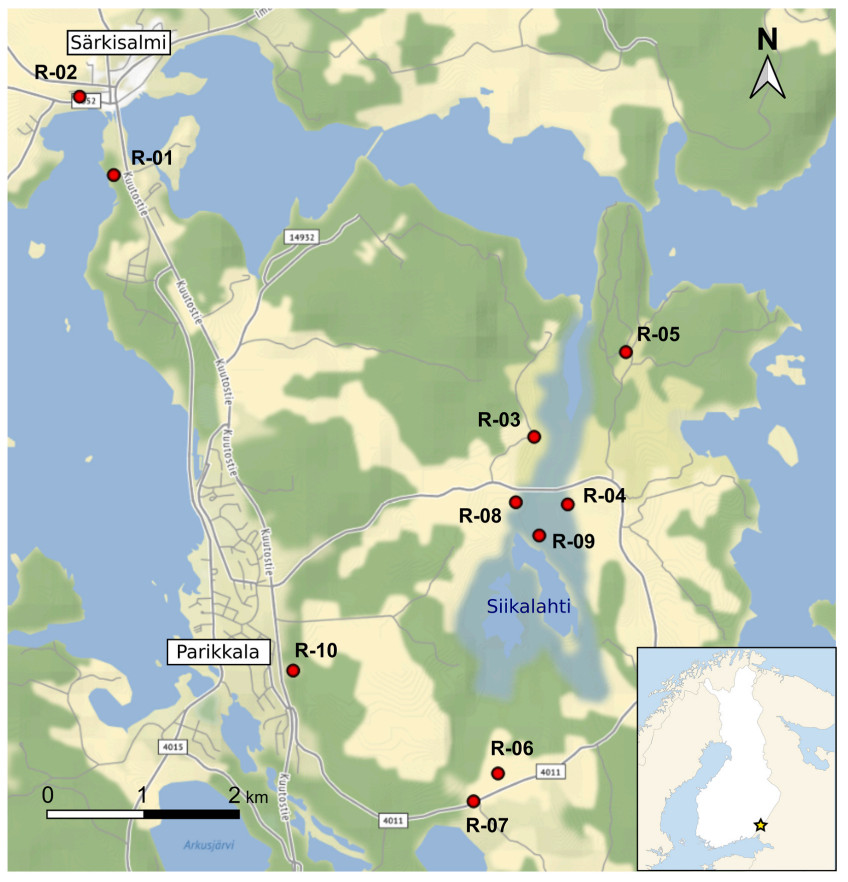
Song recordings were obtained in two breeding seasons, 1986 and 2019, in the region of Parikkala, south-eastern Finland, in an area surrounding the Siikalahti pond (radius of ca 6.5 km around 61.564° N, 29.560° E). The males were singing in semi-open habitats, from shrubs along roads and fields to denser vegetation and woodland edges, usually close to pond or lakes (Fig. 2). In this area, Thrush Nightingale population dynamics has been studied by J.S. in detail in the early 1980s, with proportion of returning individuals between years ranging from 9% to 40% (Sorjonen, 1987).
All males at the locality were recorded only once, in long uninterrupted recordings that lasted 11–32 min in 2019 and 15–25 min in 1986. Songs were recorded during short spring nights, between 20:20 and 05:45, from a distance of ca 10 m, close enough to maximise the recording signal to noise ratio but far enough not to disturb or interrupt the male’s singing activity. Analogue recordings in 1986 were obtained by J.S. using Uher 4400 Report IC and Uher M 517 microphones along with a JVC TL-E71 parabolic sound reflector, and were digitised at Luomus Helsinki Museum, Finland to 16bit PCM mono format with 96 kHz sampling frequency. For the purpose of our study, they were downsampled to 44.1 kHz. Recordings from 2019 were obtained by A.S. using an Olympus LS-5 handheld recorder with high sensitivity and low-cut filter settings, and saved in PCM format (16 bit, mono, 44.1 kHz).
The recording period lasted over one month in 1986 (mid-May to mid-June) and three days (May 30 to June 1) in 2019. As neighbouring males may converge in song use during the same breeding season (Sorjonen, 1987), the timing of the recording might have to some extent influenced the song type repertoires. However, we assume that the different duration of the recording periods should not have affected the qualitative differences (syllable and song type sharing patterns) between the two recording years substantially, especially considering the overall higher song type repertoire sizes in 1986 recordings, regardless of the recording date within the season (see Results, and Appendix Table S1).
Ten out of 17 males recorded in 2019 were selected for subsequent song analyses, based on the singing context (absence of direct territorial interaction with other males), and on the quality and duration of the recordings (low background noise such as wind, and at least 9 min of continuous singing of the focal male). Recordings of 11 males made in 1986 by J.S. were selected from sound archives based on the same criteria. Precise locations of those recordings were not available; however, they all originated from the same area as the recent ones. The selected recordings were cut to the duration of ca 10 min (keeping the last song in the sequence complete) for the subsequent processing. Details about the analysed recordings and key summary statistics are provided in Appendix Table S1.
Visualisation and analyses of spectrograms were performed with Avisoft-SASLab Pro 5.2.09 (Specht, 2014) with the following set of parameters: fast Fourier transform (FFT) length 512, frame size 100%, Hamming window, overlap 75%. For each individual, we analysed the truncated 10-min-long section of the recording, which yielded in total 1529 songs (1986: 818, 2019: 711) with a median value of 74 songs per recording (minimum 50, maximum 105).
All songs were analysed for syllable composition and assigned to song types by the same person (A.S.). Sonograms were checked visually and distinct elements or stable combinations of distinct elements were assigned to a particular syllable type. Certain elements, such as broadband "clicks", could have been classified in different categories, either as a separate syllable in the trill sequences, or as a part of more complex syllables in consistent association with other elements. We also considered syllable syntactic use within the song; syllables of a similar structure slightly differing in frequency characteristics were thus considered as belonging to the same syllable type if used in the same context in a particular song type (see Fig. 3).
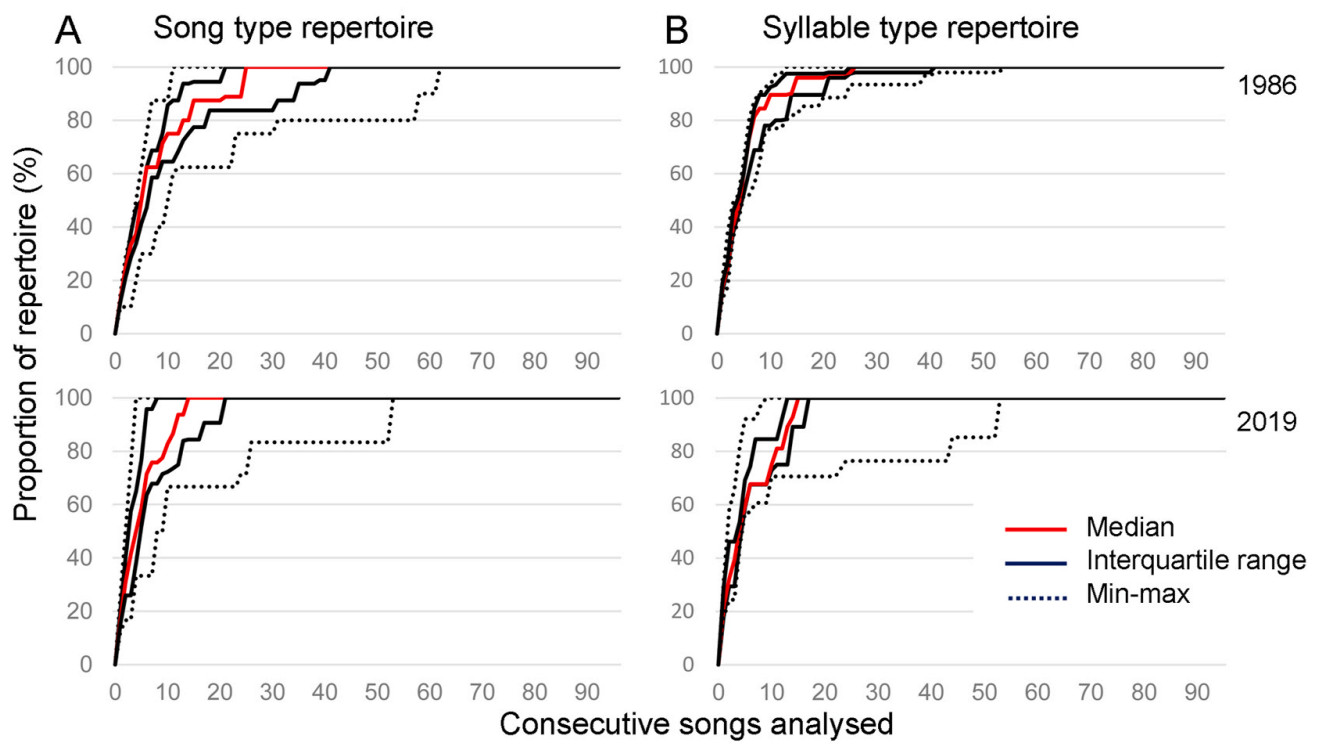
To assess how well the composition of individual repertoires was captured, we constructed accumulation curves in which the total number of detected song types was plotted against the number of processed songs within the analysed recording. We used the same approach to create accumulation curves for syllable types; however, to reduce the workload, this was only done for a randomly selected subset of males (five from 1986 and five from 2019).
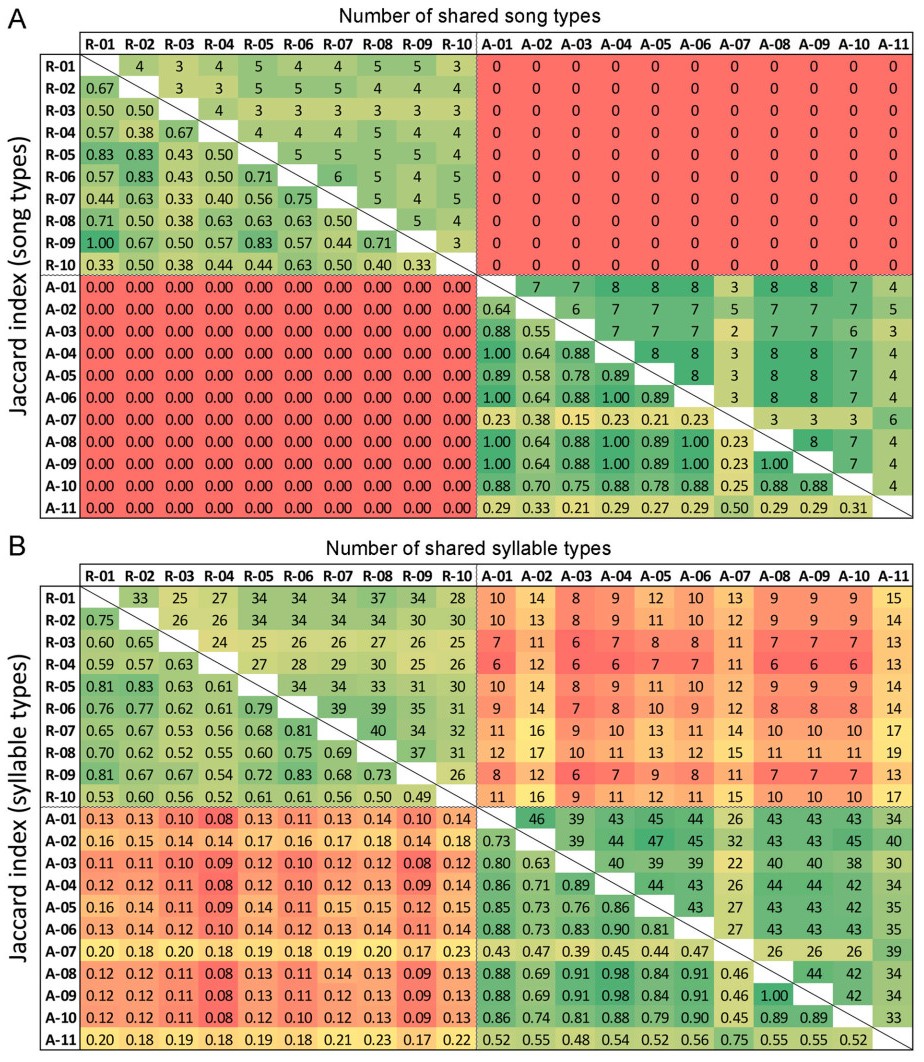
To estimate the syllable and song type repertoire sharing between individuals, we calculated the Jaccard similarity coefficient (as in Grießmann and Naguib, 2002), which ranges from 0 (no sharing) to 1 (all syllable or song types, respectively, shared between two individuals). Its complement, Jaccard distance, was used to visualise the song and syllable repertoire similarities between males and years in dendrograms, constructed by unweighted pair group method with arithmetic mean (UPGMA).
A two-sided non-parametric Mann-Whitney U test was used to evaluate the differences in syllable and song type repertoire sizes, and the extent of within-year repertoire sharing (based on mean value of Jaccard coefficient for each individual). A linear mixed-effect model (lmer function of the lmerTest R package; Kuznetsova et al., 2017), with the male identity as a random intercept, was used to test for differences in the song duration between the two years. The relationship between the geographic distances between individuals recorded in 2019 and their song and syllable repertoire dissimilarity (i.e., the Jaccard distance) was evaluated using a two-sided Mantel test (implemented in the ape package v. 5.0; Paradis and Schliep, 2019) permuted 10,000 times. All statistical analyses were performed with R software version 4.4.1 (R Core Team, 2024) along with the package ggplot2 (Wickham, 2016) for the visual exploration and figure plotting.
Data and song recordings underlying the present study are provided in the Zenodo repository (https://doi.org/10.5281/zenodo.14196640) or in the collections of the Luomus Helsinki Museum, Finland. The data in the online repository include all information available about the focal males (which are also provided in Appendix Table S1) and the analysed songs, catalogues of song types and syllables, and 10-min segments of recordings used in the study in a PCM format (sampling frequency 44.1 kHz, 16 bit, mono). Complete song recording from both 1986 and 2019 are available upon request from the Luomus Helsinki Museum.
The 1529 detected songs were classified into 31 song types (1986: 18, 2019: 13; none shared between the two years) composed of 122 syllable types (1986: 79, 2019: 73; 32 of those shared). The assessment of the relationship between the number of detected song and syllable types, respectively, and the number of analysed songs (Fig. 4) suggested that our sampling duration of 10 min was sufficient. For all analysed individuals, the accumulation curves reached a plateau well before the end of the given recording.
Repertoires of males recorded in 1986 comprised significantly more song types than those recorded in 2019 (median and range: 8 (7–10) vs. 6 (4–8); Mann-Whitney U test, n1 = 11, n2 = 10, W = 103, p < 0.001), and the same was found for syllable repertoires (median and range: 45 (39–61) vs. 38.5 (28–51); Mann-Whitney U test, W = 90, p = 0.014). Additionally, songs were significantly shorter in 1986 than in 2019 (mean ± SD: 5.0 ± 0.77 s vs. 6.1 ± 1.22 s; linear mixed model: difference = 1.09 s, SE = 0.44, t = 2.49, df = 18.9, p = 0.022; Appendix Fig. S1).
None of the song types recorded in 1986 was captured in recordings from 2019, though song type sharing within each studied year, expressed as pairwise Jaccard similarity values, was quite high (Fig. 5A and 6A; mean ± SD: J1986 = 0.65 ± 0.30, J2019 = 0.56 ± 0.16). Despite such song type turnover, the species’ characteristic song structure with typical ending was present in both years (Fig. 1), and over 40% of syllable types recorded in 1986 persisted until 2019 (Fig. 5B and 6B; J1986 = 0.72 ± 0.18, J2019 = 0.65 ± 10, J1986–2019 = 0.14 ± 0.03). The syllable sharing with any bird recorded in the same year was always higher than with any bird from the other recording year (Fig. 5B). The within-year sharing was more prominent in 1986 than in 2019 at both song type and syllable level (Figs. 5 and 6; Mann-Whitney U test, n1 = 11, n2 = 10, W = 25, p < 0.04 in both tests).
Despite the overall consistency in repertoires found within each recorded year, clusters of individuals with more similar vocalisation were also identified: this is well illustrated by the high similarity in both syllable and song types between males A-07 and A-11 recorded in 1986, which were distinct from the other 1986 males (Fig. 6). Although their song repertoires contained the three most common song types shared by the whole population (SF03, SF05 and SF13), these were the only two males not singing three other very common song types of that period (SF02, SF07 and SF26) while including in their repertoires three other song types unique to them (SF15, SF16, SF17; Appendix Fig. S2). However, increased similarity of song type repertoires was not related to the spatial distance between the recording locations in the recent population (Mantel test; p = 0.50, Z = 62,799). Indeed, males with most similar repertoires (e.g., R-01, R-09 and R-05, or R-02 and R-06) were not recorded at nearby territories (Fig. 2).
The songs of Thrush Nightingale males, recorded at the same locality in Finland, changed significantly over the course of 33 years. While almost half of the syllable types persisted, no song type was shared between the two years. Three decades represent a long time period for local song evolution in a small passerine species with short lifespan such as the Thrush Nightingale (Cramp and Perrins, 1988), and many new generations of birds replaced those recorded in 1986. Apart from temporal changes in repertoire composition, we also observed that individual song type as well as syllable repertoires were significantly smaller, and songs were longer in 2019 compared to 1986.
Despite a limited sample size of our study (11 and 10 males, respectively), the observed patterns were very consistent. As only two points in time were studied, it is not possible to differentiate between the alternative scenarios of the local song evolution, a slow successive modification of the existing song types on the one hand, or rapid "invasion" of new song types from a distant population on the other hand. These two scenarios, however, are not mutually exclusive, as both processes could have affected the local population’s repertoires.
The most noticeable change occurred at the song type level and was documented despite our inclusive approach, tolerating for high component variability and defining song types based on composition of the most stable song structures, usually by the combination of ending castanet and rattle sequences (Fig. 1; Sorjonen, 1987; Souriau et al., 2019). These results may seem contradictory with those from the study of Jäckel et al. (2022) on Common Nightingales, who showed that part of the most common song types from 1960 persisted till 2019. That study was much larger than ours in both geographic scale (14 countries) and size of the dataset (around 14,000 songs vs. our ca 1500). However, the specifics of Common Nightingale vocalisation may have played a key role in this contrast of the two congeneric species: despite the sharing of the song types among neighbours and some tendency to adjust repertoires to the local population (Hultsch and Todt, 1981; Kiefer et al., 2010), Common Nightingales show a remarkable lack of geographic song variation (Jäckel et al., 2022), and their repertoire sizes (over 190 song types per male; Hultsch and Todt, 1981) are substantially higher than in Thrush Nightingales. On the contrary, Thrush Nightingale males show a pronounced variation of song between locations (Sorjonen, 1987; Souriau et al., 2019) and despite substantial variation of their song, the reported song type repertoires are smaller: 4–10 (conservatively defined) song types recognised in our study, 16–30 in Grießmann and Naguib (2002), 7–23 in Ivanitskii et al. (2017). The persistence of a small fraction (1.2%) of the most common songs found in Common Nightingales (Jäckel et al., 2022), might then not be observable in the smaller repertoire of Thrush Nightingales.
In contrast to a complete turnover of song types, over 40% of syllable types of the 1986 syllable repertoire of Thrush Nightingales at our study locality were still present in 2019. From a syntactical point of view, the smaller song units—syllable types—should be easier to maintain than complex song types formed by their combinations. This suggestion is supported by a small-scale study comparing songs of seven territorial Thrush Nightingale males from an island population within a single breeding season, who generally shared 80% of their song elements but only 30% of their song types (Grießmann and Naguib, 2002).
While the variation of Thrush Nightingale song over time is well documented, consequences of such changes still need to be investigated. Both neutral processes, such as cultural drift and population fluctuations, and adaptive responses to the local environmental conditions or social contexts, may shape males’ repertoires. An interesting feature of Thrush Nightingale behaviour, highlighting its plasticity in vocalisation, is the tendency of the males to copy the songs of its closest related species, the Common Nightingale, in regions of their sympatry in a process known as "mixed singing" (Sorjonen, 1986; Vokurková et al., 2013). This prominent change in species’ song repertoire upon contact with its congener is considered adaptive in interspecific territorial interactions (Reif et al., 2015; Souriau et al., 2018). The capability to adapt to each season’s song type repertoire of competitors (Sorjonen, 1987; Grießmann and Naguib, 2002) is likely beneficial in intraspecific interactions of Thrush Nightingale males. Such behaviour, if persisting over a long period, together with occasional immigration of new individuals bringing new song variants, could thus eventually lead to a complete replacement of song types at the local population level.
A complete turnover of song types has also been observed in other songbird species with varying song complexity. For example, a population of the Hermit Warbler (Setophaga occidentalis) apparently changed its simple song over a decade only, with just a few remnant individuals singing the old song type (Janes and Ryker, 2013). Rapid repertoire change was also recently shown on a very large scale in the North American White-throated Sparrow (Zonotrichia albicollis), in which a characteristic dialect song ending spread from a local to a continental scale in just two decades (Otter et al., 2020). Temporal dynamics of song structures comparable to ours was also reported for the House Finch, a species of moderate song diversity, from a locality considered to be the species’ historical introduction site (Ju et al., 2019). After almost four decades, a complete turnover of song type repertoires occurred without any major change in song structures, and half of the syllable types still remained shared between the studied years. Such diversification of songs between individuals over time may have been caused by a rapid population expansion following a cultural founder effect (Ju et al., 2019).
The studied Thrush Nightingale population at our locality was well established and the potential influence of founder effects can be ruled out. We suggest that local population dynamics is the more likely reason for the observed changes in song. The Thrush Nightingale density in 2019 was apparently lower in comparison with 1986 (J.S., pers. observation). Lower density might result in less interactions between males, which therefore could result in longer songs, as observed in 2019, with a lower risk of interruption by counter-singing males. Similarly, interacting with fewer individuals might result in lower song type sharing and subsequently lead to smaller repertoires. Similar patterns were documented in a study on White-crowned Sparrows (Zonotrichia leucophrys oriantha) from populations of different size and territorial connectivity after 26 years (Harbison et al., 1999). In a large population, the song structure remained highly consistent, only accumulating small copying errors of syllables. In contrast, smaller populations from fragmented habitats showed an important loss of syllables and the introduction of new ones brought by individuals from nearby populations. The same was observed in a 40-year study on three endangered species of Hawaiian honeycreepers (Fringillidae: Drepanidinae) where a loss of song diversity and complexity followed substantial population declines (Paxton et al., 2019).
Such a dramatic event has also been reported from the Thrush Nightingale population at our study site at Siikalahti, Finland, when variation of song repertoires between 1980 and 1985 were compared (Sorjonen, 1987). The overall male’s tendency to converge in song repertoires was interrupted in 1984, likely due to a substantially higher rate of immigration compared to the other years, and more than half of the song types changed over a single year (Sorjonen, 1987). Similarly, the displacement of a Thrush Nightingale population due to unusual climatic conditions in the Moscow region, Russia, resulted in the invasion of "southern" song types into the northern population’s repertoire (Marova et al., 2015), and abrupt changes in song repertoires have also been described at the meta-population scale in the same region over a decade (Ivanitskii et al., 2022).
Species’ vocalisation may also adapt to changes of the surrounding environment, such as vegetation density (Tubaro et al., 1993; Derryberry, 2009) or anthropogenic noise levels (Slabbekoorn and Peet, 2003; Derryberry, 2009; Cardoso and Atwell, 2010; Luther and Baptista, 2010). Changes in response to increased anthropogenic noise have also been reported for the Thrush Nightingales (Ivanitskii et al., 2015). Although our study area has experienced woody plant encroachment over open and semi-open habitats over the recent years (J.S., pers. observations), the males themselves were found in similar habitats, preferred by the species, in both 1986 and 2019. Furthermore, if the change of vegetation structure drove repertoire composition of the studied Thrush Nightingale populations, we would expect that trills, the song structures particularly sensitive to acoustic degradation in close habitats (Sorjonen, 1983), would be particularly affected. However, the same syllables in both fast and slow trill types were used frequently in 1986 as well as in 2019. Therefore, we are convinced that adaptations to habitat changes were not responsible for the observed changes of Thrush Nightingale vocalisation between the study years.
To conclude, our analysis across a period of more than three decades revealed a substantial change in population repertoires, with a complete turnover of song types but only a partial change in syllable repertoires. The processes responsible for the observed song evolution, and whether the changes took place gradually or abruptly, cannot be assessed from this study, which was limited by sample size and two time points only. Interestingly, the song type repertoires of Thrush Nightingales turned to be less stable in our study area after three decades than was the case for its congener with substantially larger repertoire, the Common Nightingale, after almost seven decades (Jäckel et al., 2022). More studies on long term song variation in a broad number of songbird species, with different song complexity and learning mechanisms, are needed to better understand the processes and mechanisms affecting the cultural evolution of song and their outcomes.
Abel Souriau: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jorma Sorjonen: Writing – review & editing, Resources, Investigation, Conceptualization. Adam Petrusek: Writing – review & editing, Visualization, Validation, Methodology, Formal analysis, Conceptualization. Tereza Petrusková: Writing – review & editing, Writing – original draft, Supervision, Methodology, Formal analysis, Conceptualization.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We are grateful to Risto Väinölä and Jari Valkama, as well as the Finnish Museum of Natural History for making this collaborative project possible. We thank Reetta Vairimaa for bringing the light on the two sides of our ornithological work, and her help with connecting two generations of nightingale scientists over a short and prolific field season in the beautiful lake region of Eastern Finland. Pavel Linhart, Silke Kipper and anonymous referees provided useful insights during various phases of the study. As always, we extend our thanks to all the helpful colleagues and friends for their insightful comments and everyday support, and especially the Museum’s Bat Lab team for their warm hospitality.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.avrs.2025.100224.
|
Catchpole, C.K., Slater, P., 2014. Bird Song: Biological Themes and Variations, second ed. Cambridge University Press, New York.
|
|
Cramp, S., Perrins, C.M., 1988. Handbook of the Birds of Europe, the Middle East and North Africa: the Birds of the Western Palearctic. Volume Ⅴ: Tyrant Flycatchers to Thrushes. Oxford University Press, Oxford, New York.
|
|
Glutz von Blotzheim, U.N., 1988. Handbuch der Vögel Mitteleuropas. Band 11. In: Passeriformes (2. Teil). Aula-Verlag, Wiesbaden.
|
|
Hultsch, H., Todt, D., 2004. Learning to sing. In: Marler, P.R., Slabbekoorn, H. (Eds.), Nature’s Music: the Science of Birdsong. Elsevier Academic Press, London, pp. 80–106.
|
|
Lynch, A., 1996. The population memetics of birdsong. In: Kroodsma, D.E., Miller, E.H. (Eds.), Ecology and Evolution of Acoustic Communication in Birds. Cornell University Press, Ithaca, New York, pp. 181–197.
|
|
Osiejuk, T.S., Ratyńska, K., Cygan, J.P., Dale, S., 2003. Song structure and repertoire variation in ortolan bunting (Emberiza hortulana L.) from isolated Norwegian population. Ann. Zool. Fennici 40, 3–16.
|
|
Sorjonen, J., 1977. Seasonal and diel patterns in the song of the Thrush Nightingale Luscinia luscinia in SE Finland. Ornis Fenn. 54, 101–107.
|