| Citation: | Na Zhu, Jing Shang, Shuping Zhang. 2023: Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds. Avian Research, 14(1): 100138. DOI: 10.1016/j.avrs.2023.100138 |
Night lighting has been shown to affect wild animals. To date, the effects of night lighting on the metabolic homeostasis of birds that spend short time in urban environments remain unclear. Using model bird species Zebra Finch (Taeniopygia guttata), we investigated the effects of short-term night lighting on liver transcriptome, blood glucose, triglyceride, and thyroxine (T4 and T3) levels in birds exposed to two different night lighting duration periods (three days and six days). After three days of night lighting exposure, the expression of genes involved in fat synthesis in the liver was upregulated while the expression of genes involved in fatty acid oxidation and triglyceride decomposition was downregulated. There was also a reduction in blood triglyceride, glucose, and T3 concentrations. However, after six days of night lighting, the expression of genes associated with fatty acid decomposition and hyperglycemia in the liver was upregulated, while the expression of genes involved in fat synthesis was downregulated. Simultaneously, blood glucose levels and T3 concentration increased. These findings indicate that short-term exposure to night lighting can disrupt the lipid and glucose metabolism of small passerine birds, and longer stopovers in urban area with intense night lighting may cause birds to consume more lipid energy.
The main objective of forest plantations is the production of timber and biomass. However, during the past two decades there has been a growing interest in society and among timber companies in improving the role of these industrial forests for the conservation of biodiversity (Spellerberg and Sawyer, 1996; Lindenmayer and Hobbs, 2004; Hayes et al., 2005; McFadden and Dirzo, 2018). An example of the latter is the “New Generation Plantations” project led by WWF which aims at exploring ways in which plantation management can enhance ecosystem integrity and forest biodiversity (Neves Silva, 2009).
It is now widely recognized that matrices of pine plantations, as opposed to solid monocultures, can offer suitable habitat to host a significant number of indigenous forest species (e.g., arthropods and birds in Chile (Estades and Escobar, 2005) and birds in Ireland (O’Callaghan et al., 2017)). Several factors influence the way in which forest plantations can contribute to the conservation of indigenous biodiversity. Local variables, such as plantation density, understory cover, etc., are known to have strong effects on the quality of plantations as habitat for wildlife (Hartley, 2002; Lindenmayer and Hobbs, 2004; Tomasevic and Estades, 2008; Spake et al., 2019).
Studies of the presence and abundance of forest bird species using exotic coniferous plantations indicate that, such mixed monocultures might represent an alternative and suitable habitat for sustaining populations (Estades and Temple, 1999; Vergara and Simonetti, 2004; Brockerhoff et al., 2008). The use of local density as a proxy for habitat quality relies on the assumption that the population fits an ideal free distribution. The ideal free distribution theory, proposed by Fretwell and Lucas (1969), predicts that individuals within a complex landscape of habitats of varying quality, distribute themselves in a way that results in equalized mean individual fitness among habitats if costs are negligible. The theory assumes that fitness (e.g., survival and/or fecundity) declines with increasing population density, and thus individuals seeking to maximize their own fitness by making optimal habitat choices, have evolved to assess, and take into account, population density when selecting habitat. This assumes also, however, the development of a co-evolved system in which individuals are able to assess habitat quality in relation to population density. In exotic plantation forests, such a perception cannot be assumed, and a variety of ecological factors might lead a bird to select a poorer-quality habitat than might be available to them: the so-called ecological trap (Johnson, 2007; Hollander et al., 2011; Hale and Swearer, 2016). Consequently, the density of animals in a habitat could, in some cases, be a misleading indicator of habitat quality (Van Horne, 1983). In such a scenario population density could offer a misleading index of habitat quality, so that assessing habitat quality would need to be complemented with other population or physiological parameters which might influence populations and fitness (Van Balen, 1973; Maron et al., 2012).
Within the austral temperate forest bird community, the White-crested Elaenia (Elaenia albiceps) is the only austral migrant (Ferrer et al., 2010) species to undertake long migrations between tropical and temperate forests in South American (Fjeldsa and Krabbe, 1990). It is an insectivorous-frugivore (Grigera, 1982), which, during the austral spring and summer, becomes the most abundant bird species in the temperate forests of South America (Ippi et al., 2009), where it arrives to reproduce, before returning to the tropics for the winter (Chesser, 1994; Fitzpatrick, 2004). The White-crested Elaenia plays an important functional role as the main seed disperser in southern forests ecosystems (Armesto et al., 1996), and its behavioral flexibility allows it to nest in industrial forest ecosystems, such as pine plantations, where it may also reach high population densities (Estades and Temple, 1999; Escobar et al., 2004). However, while these details make the White-crested Elaenia an attractive focal species for ecological study in this landscape system, the suitability of this novel habitat for this or other indigenous forest species has not been assessed, and the spatial distribution of White-crested Elaenia in these industrial forest ecosystems is not yet known.
As suggested above, bird abundance may not accurately indicate the suitability of a habitat. In this paper, we describe the status of White-crested Elaenia populations inhabiting native forests and exotic forest plantations in south-central Chile, and evaluate a number of population parameters for their ability to indicate habitat quality for birds. First, we assessed the relative abundance of the species in different components of these forested landscapes. We carried out bird surveys through the study area considering the possible effects of habitat borders and edges (Tubelis et al., 2007) as well as landscape features (McGarigal and McComb, 1995) on bird densities. Edge effects are known to increase bird density and diversity (Kroodsma, 1984). Second, in these forested landscapes we described White-crested Elaenia populations in terms of body condition, age structure and territory size. We assessed and compared habitat quality of native forest fragments or pine plantations by assessing the body condition of individuals trapped in the different habitats. We undertook this comparison on the assumption that the higher diversity of native forest fragments in terms of vegetation structure and composition, would be reflected in a greater diversity of trophic resources available for birds, which might then be reflected in better body condition of individuals maintaining territories in native fragments (Garnett, 1981; Ulfstrand et al., 1981; Riddington and Gosler, 1995). To test for a density dependent effect in the condition of individuals, we compare the mean density for the species between native forest and pine plantations. In this context, an inverse relationship with distance from streams covered with native vegetation has been described for the abundance of the bird and arthropod communities in forest landscapes of Central Chile (Estades and Temple, 1999; Fuentes et al., 2021). We suggest that a hierarchy in site quality, as modelled by Fretwell and Lucas (1969), might be reflected within pine plantation, through a sampling point’s distance to the native forest patch, and so we assess bird density in relation to this also.
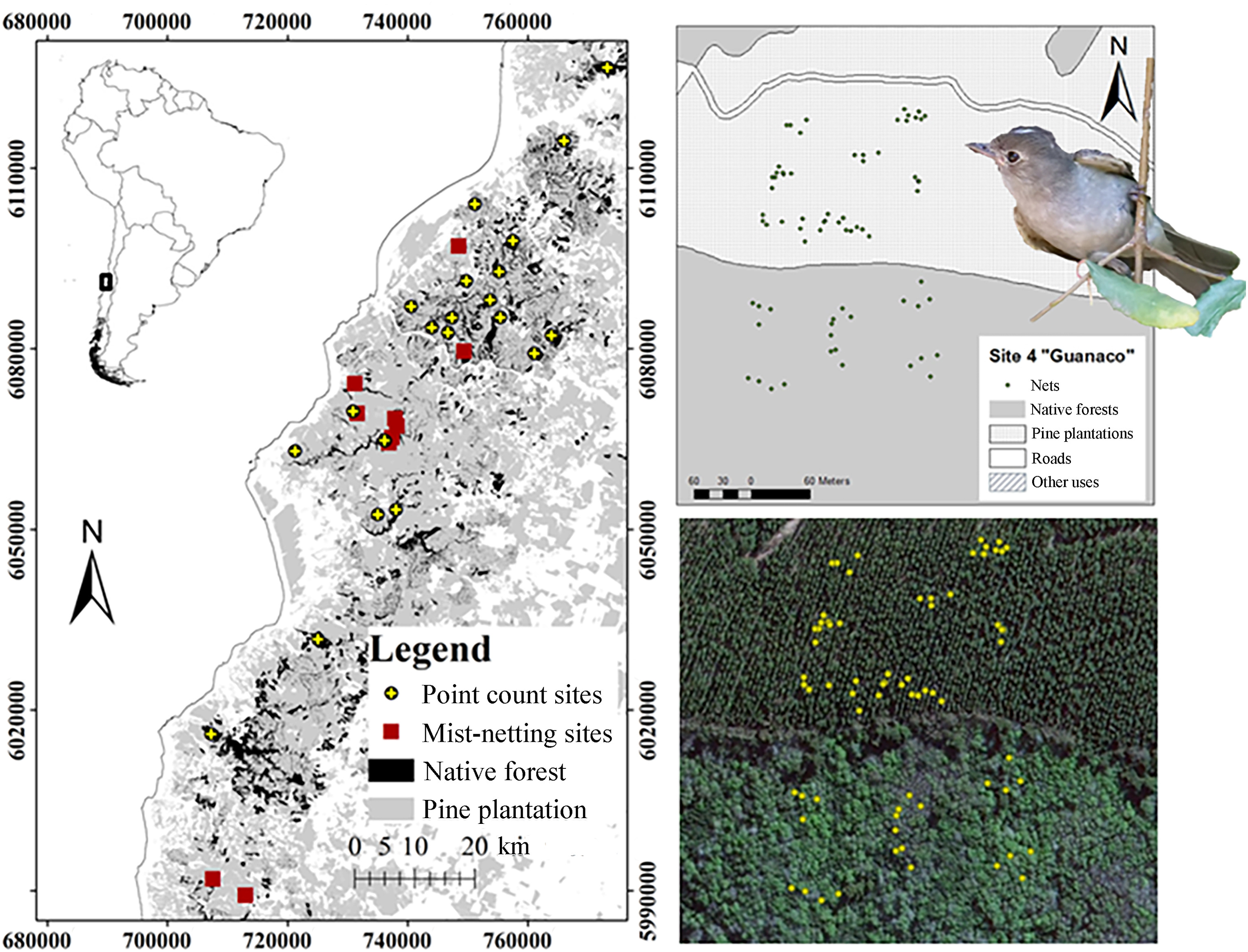
The study was carried out in the temperate forests of south-central Chile, in the “Del Maule” administrative region (Fig. 1). The climate in this region is temperate with warm and dry summers (Peel et al., 2007), a mean temperature of 13.9 ℃, and annual precipitation of 942 mm (Hajek and Di Castri, 1975). The study is focused on a 7500 km2 area of the “Roble-Hualo” forest type distributed over the coastal range, known as the Maulino forest. Ranging from the Itata river (36°24′ S, 72°44′ W) in the South to “Altos de Licanten” (34°58′ S, 72°02′ W) in the North. Geographically, the search for sites was constrained to locations on the western slope of the coastal range with an oceanic influence on the climate (Amigo and Ramírez, 1998). The natural vegetation corresponds to temperate deciduous forests dominated mainly by the tree Hualo (Losophonia glauca, Nothofagaceae), and having an understory that generally consists of shrubs or small trees with resistant evergreen leaves (Villagrán and Armesto, 2005). However, after decades of exploitation and substitution for forestry, the region is now dominated by a fast growing exotic tree culture, mainly Monterey Pine (Pinus radiata, D. Don). These planted forests now cover a large proportion of the region, leaving small scattered remnants of native forest.
In this study area we defined 20 landscape units, each of 4 km2, enough area to encompass the breeding home range of most forest-dwelling passerine species (McGarigal and McComb, 1995; Villard et al., 1999). Based on the Chilean Government Forest Service's updated Cartographic Forest Cover (CONAF-UACh, 2010), and QuickbirdTM imagery, the selection of landscape units aimed to represent the whole range of percentages of remaining native forest. In order to eliminate any disturbing effect made by young or clear-cut stands, the selection process considered continuous forest habitat as a main constraint, selecting only landscape units with at least 85% forest cover.
We used a stratified sampling design to survey each forest type proportionally in every landscape unit. To ensure independence of samples and to minimize edge effects, point counts were generally carried out at least 300 m from any other point and 25 m from a stand boundary for native forests plots, although in Pine plantation plots, the minimum distance was, by necessity, set at a minimum of 100 m from another patch type. In summary, distance constraints meant that all landscapes were sampled proportionally to their forest cover types, with c.12 points per landscape unit. This sampling effort was adequate to estimate species richness and the density of most Chilean forest bird species, as the number of species and individuals levelled off at 8 points sampled (Jiménez, 2000). Note that whilst all species were recorded in bird surveys, for the reasons outlined above, we report only for the White-crested Elaenia in this paper.
During the 2011–2012 breeding season, from October to December, we conducted bird counts on every landscape unit, using the variable circular-plot method with a 50-m maximum observation radius (Reynolds et al., 1980). Two 5-min counts were conducted at every visit, separated by a 5-min period. All counts were carried out during the first 5 h after dawn by a single trained observer (RT). To control for any season effect, between the beginning and the end of the breeding season, all plots were visited twice with visits separated by a month. No observations were conducted on rainy or misty days. All individuals seen or heard within the maximum radius were recorded, and their distance from the plot’s centre was estimated in 10 m increments to manage differences in detectability in each patch type (Buckland, 1987).We used the Distance 6.0 software (Thomas et al., 2010) to correct the data by detectability, fitting the data to a half-normal model curve (Buckland et al., 1993) considering the understory foliage volume as a covariate, following the methodology used by Estades and Temple (1999) in these forests.
Ten native forest fragments of at least 3 ha, surrounded by mature Monterey Pine plantations were selected to study their White-crested Elaenia populations. The selection of sites considered mature pine stands beside fragments of native Hualo forest.
During the 2012–2013 breeding season, birds were mist-netted in both habitats, the native forest fragments and within surrounding pine plantations. The fieldwork began in October and lasted until the middle of February. For this, 34 mist net sites of 6, 9 and 12 m were used, totalling 261 m in length. The nets in pine plantations were located at different distances from the border of the pine stand with the fragment of native vegetation (Fig. 1), recording their location using GPS for later estimation of their distance from to the edge of the fragment. In addition, each net was active for 9 h each day, except for during sporadic rain showers, representing a total effort of approximate 36,000 h m in native forest and 45,000 h m in pine plantations each year. In order to avoid a time effect on data, and to reduce the possibility of predation of birds in the nets, capture locations were moved every two days. Finally, sites were visited twice during the breeding season.
All captures were ringed and the following parameters were measured by a single observer: maximum wing length was measured using a 1 mm precision stopped rule; maximum tarsus length, depth and length of the bill to 0.1 mm were measured using a Vernier calliper (Ginn and Melville, 1983); and the weight of the individuals was recorded with a 0.1 g precision digital balance (American Weigh Scales, Georgia, USA). Sexing was based on reproductive traits such as cloacal protuberance and presence of brood patch when it was appropriated (Cueto et al., 2015). However, when no certainty existed regarding the sex of individuals, they were categorized as “not defined”. Finally, using the plumage molt and the colour of the palate as ageing criteria, we discriminated individuals considering three age categories; juveniles, younger adults and older adults (Thomson et al., 2016; see Gorosito et al., 2020 for an updated protocol).
White-crested Elaenias’ abundance in pine plantations and native fragments showed a normal but left skewed distribution. By applying a Mann-Whitney U test we assessed whether the mean abundance of White-crested Elaenia in pine plantations was different from that in native forest.
The residuals from a linear regression between weight and body size were used as a body condition index (BCI; Gosler et al., 1998). Body size was represented by the first axis derived from a principal component analysis of wing, tarsus and bill length was used as a measure of body size (Gosler et al., 1998), which captured 51.3% of the variance in these traits. We used a generalized linear mixed model in order to include and control any possible variability between sample sites, while it may cover a variety of models and distribution of response variables. We tested the following variables as fixed effects: time, sex, age, and habitat type (native or pine); in a linear mixed model explaining the body condition, using the estimated index as a dependent variable. Site was included as a random effect. To test for a possible effect of the distance from capture location to the border of the native fragment on the bird’s body condition, a subset of captures made in pine stands was fitted to a mixed linear model.
We compared the structure of populations inhabiting pine stands and native fragments by examining the proportion of young and older adults, and the proportion of female and male individuals captured during the study. Through a contingency table we assessed whether the pine stand populations of White-crested Elaenia differed in their age and sex structure from those of native forest populations.
Our large sample size allowed us to use a t-test to examine the mean recapture distance in each habitat type. The value was estimated as the average distance of all possible pairs of combinations among recapture locations for each individual. We use the dispersal distance as a proxy for territory size as mean dispersal distance is proportionally related to territory size (Bowman, 2003).
All statistical analyses were performed using R (R Development Core Team, 2012), with the packages lmerTest (Kuznetsova et al., 2013) for the mixed model analyses, and all of them considered a level of significance α = 0.05.
In total, 938 5-min counts spread throughout the surveyed landscapes were done. White-crested Elaenia was the most abundant species in forest landscapes during the breeding season. Population densities varied greatly between cover types. The density of White-crested Elaenia under native forest cover (5.50 ind./ha) was significantly greater than that in pine plantations (4.70 ind./ha) (W = 9458.5, P = 0.023). However, we found evidence for an edge effect, which suggests that were it not for the influence of native forest fragments nearby, the density of White-crested Elaenia would have been even lower. This is because twice as many birds were recorded in embedded fragment situations than further from the edge, and although not statistically significant (W = 26, P = 0.866), a greater density of individuals was seen in pine stands close to a native forest edge (8.75 ind./ha) than in the native fragment itself (7.28 ind./ha). Finally, no difference in capture rates was seen between pine stands (3.70 ind./1000 h m) and native fragments (2.87 ind./1000 h m) (W = 33, P = 0.612).
During the whole study period we ringed 547 individuals and had 183 recaptures. A difference in size among sexes in terms of wing length was found for the White-crested Elaenia, confirming that sexing through cloacal protuberance was accurate. Wing length for females (mean = 74.95, SD = 2.33, n = 164) and males (mean = 77.49, SD = 2.01, n = 104) were significantly different (t = −9.45, df = 242.07, P < 0.01).
As expected from detailed studies of other passerine birds the body condition of individuals increased over the day, most likely due to the increased in body fat and gut content (Gosler, 1996, Table 1), or in other words, individuals showed a substantial difference in body condition by comparing the status of individuals captured early in the morning and later in the day (Gosler, 2002).
| Species | BCI | |||
| Fixed effects | rcond2 | β | SE (β) | P |
| White-crested Elaenia | 0.13 | |||
| Intercept | −0.889 | 0.456 | 0.052 | |
| Time | 2.070 | 0.734 | 0.005 | |
| Habitat (Pine) | −0.426 | 0.188 | 0.024 | |
| Age (J) | −0.894 | 0.708 | 0.207 | |
| Age (ndf) | 0.372 | 0.987 | 0.706 | |
| Sex (m) | 0.507 | 0.176 | 0.004 | |
| Sex (ndf) | 0.637 | 0.282 | 0.024 | |
| Model explaining the body condition (BCI) of forest populations of White-crested Elaenia. Time: time of the day; Habitat: native forest or pine plantation. | ||||
Analyses show that environmental variables determine the BCI of the species. Hence, individuals captured in pine plantations (BCI = −0.17, CI = ± 0.18) were in poorer body condition than those inhabiting native forest (BCI = 0.45, CI = ± 0.33) (Table 1). Furthermore, the distance from native forest had a negative and significant effect on the body condition of White-crested Elaenia (β = −0.003, P = 0.024).
Through ageing White-crested Elaenia individuals as “young adults” and “adults”, a difference in the age structure for populations that inhabit the native forest fragments and pine plantations was found. In native forest, the White-crested Elaenia population consisted equally of adults and young adults (Fig. 2), but the population in pine plantations had a greater proportion of young adults over the other age class (χ2 = 5.62, df = 1, P = 0.017).
We were able to determine sexes via cloacal protuberance and/or brood patch to 198 individuals. We found no difference between habitats in the proportion of females and males captured (χ2 = 0.17, df = 1, P = 0.677).
The mean distance between capture locations, was larger in pine stands (mean = 81.3 m, SD = 53.4, n = 85) than in native fragments (mean = 66.1 m, SD = 47.7, n = 25), implying that birds in pine plantations either held larger territories than those in native forest, or that they were more likely to be transients. Given the timing in the breeding season, we suggest that the difference is most likely to indicate a difference in territory size. The difference in mean dispersal distance was statistically significant (t = −2.74, df = 43.24, P < 0.01).
White-crested Elaenia populations inhabiting existing forest landscapes in Chile reflect a patchy mosaic of habitat conditions. The estimated values of density of individuals do not necessarily represent the quality of the habitat. On the one hand, the arrangement of landscape elements, in terms of the number of native forest patches and their edge effects, influenced the observed bird densities, to the extent that it appears likely that birds are not able to assess the criteria necessary to distribute according to the Ideal Free Distribution model (see Fretwell and Lucas, 1969, and our introductory remarks). On the other hand, social dominance can segregate individuals to habitats of poorer quality, where they could increase the values of bird densities (Ulfstrand et al., 1981).
Estimating the abundance of a mobile passerine in two habitat types, scattered in a matrix or mosaic of patches, required us to consider the influence of neighbouring habitats. However, by including many landscapes and controlling the distance to habitat boundaries, we are confident in our assertion that the White-crested Elaenia is more abundant in native forest fragments than in exotic planted forests. Estimated densities for the species were twice as great, but the difference is reversed in the case of medium-sized native forest fragments surrounded by mature pine stands. This phenomenon was first presented by Estades and Temple (1999); habitat disturbance, such as habitat borders, and the not totally unsuitable vegetation surrounding native fragments for forest birds, create a condition where presence and abundance of most forest birds showed no relationship with fragment size, except for large species.
Pine plantations may well be a poor quality habitat for some forest bird species. For White-crested Elaenia, an austral migrant, birds holding territories in pine plantations had lower BCI than those in native forests. Furthermore, the body condition of White-crested Elaenia individuals inhabiting pine plantations worsened if their territories were located farther from native forests. This supports McIntyre and Hobbs (1999) suggestion that forested landscapes dominated by pine plantations are a gradient of alteration states.
The difference in body condition between individuals captured in these habitats does not correspond to a density dependent effect, as no differences were found in estimated densities of bird species in either habitat. Local densities seem to be a poor predictor of habitat quality, highlighting the importance for assessing other population parameters in order to have a thorough understanding of disturbance effects on populations. The influence of social dominance and prior occupancy is implicated in this study by the fact that White-crested Elaenia populations inhabiting pine plantations consisted of a greater proportion of younger adults, suggesting that young and less dominant individuals had been forced to find territories in sub-optimal coniferous plantations, as has been described for the Great Tit (Parus major) (Ulfstrand et al., 1981) and Pied Flycatcher (Ficedula hypoleuca) (Lundberg et al., 1981) in Sweden. This phenomenon has also been described in the neotropics. In the case of the American Redstart (Setophaga ruticilla), a neotropical migrant, older adult males tend to displace females and young males to poorer quality territories in wintering grounds (Marra et al., 1993). The process of settlement and gaining a breeding territory after migration remains undescribed for the White-crested Elaenias in these landscapes.
Habitat disturbance that could diminish habitat quality is expected to have cascade effects on various population parameters. According to Van Horne (1983), habitat quality has a direct effect on the survival rate of bird populations. Modern forestry may have detrimental effects on habitat quality that have not been assessed thoroughly yet. A previous study in this forest community has shown that body condition has a clear effect on the survival rate of some forest passerines inhabiting these forests (Thomson and Estades, 2012), with lower survival for individuals showing poor body condition. Presti et al. (2018) show that demography of White-crested Elaenia populations in Patagonian forests is easily affected by environmental changes, such as food limitation.
A concatenated reduction of the trophic niche breadth along with an increasing interspecific competition is seen for the species with this forest cover substitution (Thomson and Gosler, in review). Differences in food availability seem to be the key factor in explaining the latter and the lower BCI in individuals inhabiting the exotic coniferous plantations, which has a strong influence on reproduction and survival rates (Newton, 1998; Presti et al., 2018). Composition of upper trophic levels is entirely dependent on lower trophic levels, which is greater in native forest with a strong edge effect (Peralta et al., 2017).
These results reveal the impact that human-altered environments have on wildlife, in this case, modern forestry with fast growing exotic species in temperate South America. First, the occurrence and use of pine plantations by some species does not necessarily mean that there is preference for that habitat, but, as has been shown, they represent a less suitable habitat that could be used as an alternative for individuals of lower social rank. Resources in pine plantations seem to be inadequate in number and quality to sustain forest bird populations by themselves, as indicated strongly in our findings by the importance of natural forest patches for the birds and the strong influence of distance from native woodland on both the abundance and physiology of the birds. This corresponds with differences in the abundance and diversity of the arthropod community in these forests (Fierro et al., 2017; Ferrer et al., 2010). Hence, the contribution of small fragments to the conservation of wildlife in this ecosystem (Grez et al., 2005; Simonetti et al., 2012) could only be effective after restoring native landscape components directed to enhance the planted forest biodiversity.
Bird captures and ringing complied with all requirements and permissions from Chilean authorities: Ringing permit from the Servicio Agrícola y Ganadero (SAG), Gobierno de Chile # 5038.
AGG contributed to the study conception and design. RFT collected data, perfomed data analyses, and wrote the first draft of the manuscript. Both authors commented on previous versions of the manuscript, read and approved the final manuscript.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Our thanks to Professor Cristián F. Estades and all staff and volunteers of the Wildlife Ecology Lab (LEVS) from the Universidad de Chile who helped in the fieldwork. R.F. Thomson thanks to Comisión Nacional de Investigación Científica y Tecnológica CONICYT, Gobierno de Chile, for funding his graduate studies. Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT, Gobierno de Chile, supported this research through the proyect Id: 1120314.
| Species | BCI | |||
| Fixed effects | rcond2 | β | SE (β) | P |
| White-crested Elaenia | 0.13 | |||
| Intercept | −0.889 | 0.456 | 0.052 | |
| Time | 2.070 | 0.734 | 0.005 | |
| Habitat (Pine) | −0.426 | 0.188 | 0.024 | |
| Age (J) | −0.894 | 0.708 | 0.207 | |
| Age (ndf) | 0.372 | 0.987 | 0.706 | |
| Sex (m) | 0.507 | 0.176 | 0.004 | |
| Sex (ndf) | 0.637 | 0.282 | 0.024 | |
| Model explaining the body condition (BCI) of forest populations of White-crested Elaenia. Time: time of the day; Habitat: native forest or pine plantation. | ||||