| Citation: | Johann H. van Niekerk. 2023: Bare-throated spurfowl (Pternistis spp.) males across Africa impress females with bright throat colours during courtship. Avian Research, 14(1): 100106. DOI: 10.1016/j.avrs.2023.100106 |
The role of bare body parts in sexual signalling in birds has received relatively little attention. I describe how the bare-throated spurfowl males saturate the colours of their throats to attract females. Of the 23 Afrotropical spurfowl species, the bare-throated subgroup includes Yellow-necked Spurfowl (Pternistis leucosceptus), Red-necked Spurfowl (P. afer), Grey-breasted Spurfowl (P. rufopictus) and Swainson's Spurfowl (P. swainsonii). The rest of the species include fully feathered throated spurfowls. Throat colour intensity of bare throats was scored using an extensive online digital photographic archive encompassing the four species across the year's seasons. Each throat (n = 836) was assigned to one of four colour-intensity categories to explore the relationship between colour intensities, breeding cycles, and environmental variation. Except for Swainson's Spurfowl male saturation of throat colours correlated with monthly rainfall, which peaks one or two months before egg laying. Swainson's Spurfowl peaks during egg laying. Yellow-necked Spurfowl has the largest bare throat. Bare-throated spurfowl males perform an elevated courtship display posture above the female to feature their throat colour. No such displays occur in feather-throated spurfowl. Males with low throat colour saturation harbour more ectoparasites on their bare throats than birds with saturated throats. Male Red-necked Spurfowls have significantly larger bare throats than females. The primary function of bare throats probably assists in thermoregulation, particularly in arid regions. The bare throat may have evolved a secondary role in mating. Yellow-necked, Red-necked, and Grey-breasted Spurfowls use their saturated throat colours as ornaments to court females during the breeding season. Unobtrusive female throat colours (unsaturated) may discourage male interlopers and predation during egg laying. Saturation appears to be carotenoid-food based. The different colours among the bare-throated species may serve as prezygotic mechanisms that inhibit cross-breeding and explain why females also have coloured throats.
The East Asian migratory flyway is recognised as the most species-rich but also the most poorly understood flyway globally, hosting approximately 477 species of land birds and a further 201 waterbirds (Newton, 2007; Kirby et al., 2008). In total 254 of those species are songbirds that undertake some latitudinal migration, of which 67% are long-distance migrants, flying thousands of kilometres to the wintering grounds in South and Southeast Asia (Yong et al., 2015). Little is known on the migration routes of these species, but novel tracking devices allow for an increasingly better understanding of their spatio-temporal distribution (Yong et al., 2021).
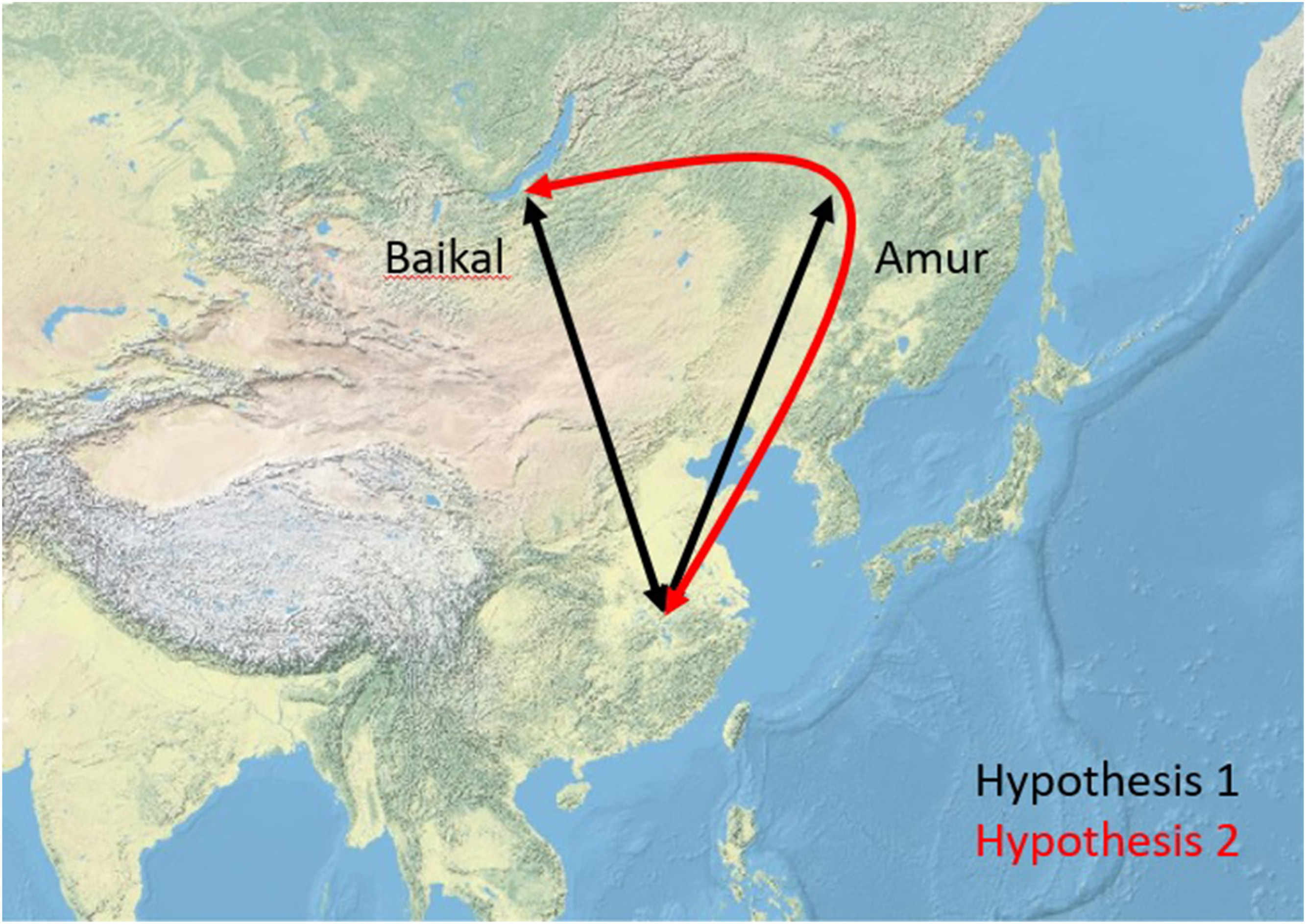
However, data are lacking for songbird populations breeding in Siberia, and it remains thus unknown whether species migrate along direct route through Mongolia and western China (hypothesis 1), or whether a detour through East Asia is taken (hypothesis 2) to their non-breeding destinations (Fig. 1). Even in the first half of the 20th century, there were ideas about the routes used by birds in northern Asia. Tugarinow (1931) summarised the knowledge to date and drew up eight possible routes. He suggested several migratory routes, but all of them avoided the high mountain ranges and were concentrated either on the East Asian coast or in Western Asia. A little later, Austin (1947), studying migration in Japan, concluded that thrushes and Bramblings (Fringilla montifringilla) came to winter on the island from as far north-central Russia, while some of the buntings reached Japan from north-eastern Russia and others from Sakhalin. None of the authors' assumptions could be substantiated. Austin (1949) was unable to prove the Russian origin of the ducks on the basis of a large number of data, and thus no evidence for a west-east migration direction was found. Hachisuka and Udagawa (1950), followed by Cheng (1963), highlighted the role of the East Asian coast in migration. Subsequently, more than 1 million birds were ringed in East Asia in the 1960s and 1970s as part of the MAPS programme, but the migration routes of songbirds, with a few exceptions, remained unknown (McClure, 1974). In the 1990s and 2000s, ringing data from several ringing stations in eastern Russia were used to locally study the songbird migration, but these did not provide data on the migration routes of the birds (Valchuk and Yuasa, 2002; Valchuk, 2003a, b; Valchuk et al., 2005; Lelyukhina and Valchuk, 2012; Maslovsky et al., 2014, 2018a, b; Lelyukhina et al., 2015; Maslovsky and Valchuk, 2015; Valchuk and Lelyukhina, 2015). Large numbers of migratory passerines ringed at bird ringing stations in Mongolia suggest that a direct route might be taken by many species (Davaasuren et al., 2020), and tracked Peregrine Falcons (Falco peregrinus) have also been shown to migrate along direct routes from Siberia to South Asia (Gu et al., 2021). But there is also some evidence for longitudinal migration through eastern Russia, which would be expected under hypothesis 2: at a stopover site in the Russian Far East, most warblers were captured during crosswinds (e.g. from the east) during spring migration (Bozó et al., 2018a, b), suggesting longitudinal migration (see Fig. 2).
Here, we compare spring and autumn migration phenologies of eight species of warblers trapped at stopover sites in the Russian Far East and at Lake Baikal in eastern Siberia, situated at similar latitudes but with a longitudinal difference of 1500 km areas. All of the studied species are regular migrants at both sites. In this study, we sought to answer the question whether migration phenology and biometrics of birds migrating through the two different geographical regions differ, thereby trying to infer that the birds fly over larger mountain ranges in the southern part of Asia (hypothesis 1) or are longitudinal migrants. If we could find evidence for hypothesis 1, we would expect that the migration timing does not differ between the two sites. If hypothesis 2 was supported by our data, we would expect that the studied species would 1) arrive earlier in spring in the East and 2) pass through earlier in autumn in the West. Furthermore, if different populations would be involved at the two sites (under hypothesis 1), we might expect morphological differences within the same species between the two sites, especially in those species which are known to occur in different subspecies at the two sites (del Hoyo and Elliott, 2006) with known differences in biometrical parameters (Svensson, 1992; Baker, 2010; Demongin, 2016). If we could provide evidence for hypothesis 2, the same populations might pass the two study sites, resulting in identical morphology of the birds captured at the two sites. However, as species and populations with longer migration routes are known to have longer wings (Winkler and Leisler, 1992; Marchetti et al., 1995; Tellería et al., 2001), we could also expect that under hypothesis 2, the share of longer-winged individuals is larger in the west than in the east, given that the individuals trapped in the west have to cover a significant detour which would prolong their route.
Birds were trapped at two study sites in the Asian part of Russia. The first study site is located at Muraviovka Park along the middle stream of the Amur River in the Russian Far East (hereafter: "Amur"), 60 km southeast of the city of Blagoveshchensk (49°55ʹ08.27″ N, 127°40ʹ19.93″ E). Birds were captured with standard mist-nets (Ecotone, Poland) and ringed within the Amur Bird Project (Heim and Smirenski, 2013, 2017) during spring (2013, 2015–2017) and autumn (2011–2015, 2017) migration. For details of the mist-netting effort and the habitats around the nets, see Bozó et al. (2018a, b) and Heim et al. (2018a, b).
The second study site is located in the buffer zone of Baikalsky State Nature Biosphere Reserve, which is situated on the SE coast of Lake Baikal, SW from the Mishikha River mouth on Pribaikalskaya flatland (hereafter: "Baikal"). Birds were captured with 20 Japanese and Chinese type mist-nets and ringed within the work of the Baikal Bird Ringing Station between 2014 and 2018.
We selected all Sylviid species that were trapped at both sites in sufficient numbers (n > 140 per site). Data analysis was based on 11,921 individuals of eight species (Arctic Warbler Phylloscopus borealis, Two-barred Warbler Ph. plumbeitarsus, Pallas's Leaf Warbler Ph. proregulus, Yellow-browed Warbler Ph. inornatus, Dusky Warbler Ph. fuscatus, Radde's Warbler Ph. schwarzii, Thick-billed Warbler Arundinax aedon, Pallas's Grasshopper-warbler Locustella certhiola) (Table 1).
| Spring | N | ASM | AEM | Median | χ2 | p | |||||||
| Amur | Baikal | Amur | Baikal | Amur | Baikal | Amur | Baikal | ||||||
| Thick-billed W. | 766 | 217 | 19 May | 29 May | 8 Jun | 12 Jun | 31 May | 2 Jun | 78.103 | < 0.0001 | |||
| Pallas's G-w. | 57 | 114 | 28 May | 3 Jun | 10 Jun | 18 Jun | 5 Jun | 12 Jun | ‒36.545 | < 0.0001 | |||
| Radde's W. | 94 | 246 | 12 May | 26 May | 5 Jun | 14 Jun | 25 May | 7 Jun | 80.31 | < 0.0001 | |||
| Dusky W. | 503 | 181 | 1 May | 23 May | 7 Jun | 13 Jun | 21 May | 2 Jun | ‒67.0 | < 0.0001 | |||
| Pallas's Leaf W. | 79 | 46 | 2 May | 18 May | 1 Jun | 15 Jun | 18 May | 27 May | 9.48 | < 0.002 | |||
| Yellow-browed W. | 2274 | 53 | 28 Apr | 13 May | 2 Jun | 3 Jun | 14 May | 23 May | 25.978 | < 0.0001 | |||
| Arctic W. | 236 | 102 | 19 May | 22 May | 7 Jun | 13 Jun | 28 May | 1 Jun | 22.755 | < 0.0001 | |||
| Two-barred W. | 65 | 108 | 23 May | 26 May | 7 Jun | 17 Jun | 31 May | 11 Jun | 90.577 | < 0.0001 | |||
| Autumn | |||||||||||||
| Thick-billed W. | 654 | 206 | 2 Aug | 9 Aug | 10 Sep | 27 Aug | 14 Aug | 18 Aug | 15.76 | < 0.0001 | |||
| Pallas's G-W. | 458 | 159 | 2 Aug | 15 Aug | 16 Sep | 20 Sep | 18 Aug | 29 Aug | 73.93 | < 0.0001 | |||
| Radde's W. | 218 | 275 | 10 Aug | 24 Aug | 24 Sep | 22 Sep | 11 Sep | 7 Sep | 47.447 | < 0.0001 | |||
| Dusky W. | 944 | 190 | 1 Aug | 4 Aug | 5 Oct | 24 Sep | 11 Sep | 28 Aug | 58.176 | < 0.0001 | |||
| Pallas's Leaf W. | 542 | 377 | 8 Sep | 26 Aug | 13 Oct | 9 Oct | 23 Sep | 17 Sep | 18.981 | < 0.0001 | |||
| Yellow-browed W. | 2113 | 168 | 29 Jul | 19 Aug | 6 Oct | 22 Sep | 7 Sep | 5 Sep | 0.805 | 0.369 | |||
| Arctic W. | 209 | 104 | 3 Aug | 5 Aug | 13 Sep | 1 Sep | 23 Aug | 23 Aug | 0.281 | 0.596 | |||
| Two-barred W. | 123 | 40 | 13 Aug | 11 Aug | 19 Sep | 15 Sep | 24 Aug | 29 Aug | 8.26 | 0.11 | |||
We included birds ringed during spring (25 April to 13 June at Amur, 10 May to 23 June at Baikal) and autumn migration (25 July to 17 October). Data for Muraviovka Park were collected during 2014–2017, and for Lake Baikal between 2014 and 2018. Wing length was measured by the 'maximum flattened chord method' (Svensson, 1992) to the nearest mm. To compare the wing length data between the two stations for each species, we used Mann–Whitney U-tests after testing the normality of data. This non-parametric test was used instead of t-test because of the data we used were not normally distributed. The migration was described by the following parameters: average earliest and latest capture dates, median dates. Average earliest and latest dates were obtained by averaging the first and last catches per season annually. For the analyses, we only used the data of first captures (no recaptures within the season). For the comparison of the median migration dates of each species between the two sites, we used Mood median.
Autumn migration finishes later in all species at Amur with the exception of the Pallas's Grasshopper-warbler. We found significant differences between the median dates of migration periods between the two study sites for five out of eight species (Thick-billed Warbler, Pallas's Grasshopper-warbler, Radde's Warbler, Dusky Warbler, Pallas's Leaf Warbler) (Table 1). All species start the migration later at Baikal with the exceptions of the Pallas's Leaf Warbler and the Two-barred Warbler. We found significant differences in wing length between the birds migrating through the two stations with the exception of the Yellow-browed Warbler and the Arctic Warbler (Table 2).
| Species | Season | N | Mean Wmax | SD | z | p | ΔWmax | |||||
| Amur | Baikal | Amur | Baikal | Amur | Baikal | |||||||
| Thick-billed Warbler | Spring | 757 | 217 | 78.5 | 82.1 | 1.88 | 2.27 | ‒17.70 | < 0.0001 | 3.6 | ||
| Autumn | 670 | 205 | 77.9 | 81.6 | 1.96 | 2.34 | ‒16.93 | < 0.0001 | 3.7 | |||
| Pallas's Grasshopper-warbler | Spring | 32 | 116 | 65 | 67.7 | 2.78 | 2.77 | ‒4.28 | < 0.0001 | 2.7 | ||
| Autumn | 400 | 153 | 61.7 | 64.7 | 2.07 | 2.47 | ‒12.24 | < 0.0001 | 3 | |||
| Radde's Warbler | Spring | 93 | 249 | 62.5 | 60.9 | 3.58 | 3.93 | ‒3.1 | 0.002 | ‒1.7 | ||
| Autumn | 285 | 255 | 61.5 | 60.5 | 3.78 | 3.81 | ‒3.09 | 0.002 | ‒1 | |||
| Dusky Warbler | Spring | 373 | 184 | 60.3 | 58.4 | 3.38 | 3.28 | ‒5.60 | < 0.0001 | ‒1.9 | ||
| Autumn | 1250 | 189 | 60.7 | 59.1 | 3.26 | 3.27 | ‒5.24 | < 0.0001 | ‒1.6 | |||
| Pallas's Leaf Warbler | Spring | 78 | 46 | 53 | 51.6 | 2.69 | 2.39 | ‒1.92 | 0.047 | ‒1.4 | ||
| Autumn | 532 | 380 | 53.6 | 51 | 2.74 | 2.39 | ‒12.71 | < 0.0001 | ‒2.6 | |||
| Yellow-browed Warbler | Spring | 2160 | 53 | 56.7 | 56.9 | 2.49 | 2.48 | ‒0.75 | 0.45 | 0.2 | ||
| Autumn | 2573 | 158 | 56.6 | 56 | 2.32 | 2.63 | ‒2.41 | 0.015 | ‒0.6 | |||
| Arctic Warbler | Spring | 234 | 101 | 67 | 66.3 | 2.56 | 3.66 | ‒0.82 | 0.41 | ‒0.7 | ||
| Autumn | 217 | 104 | 64.8 | 64.4 | 2.39 | 2.46 | ‒1.19 | 0.234 | ‒0.4 | |||
| Two-barred Warbler | Spring | 63 | 107 | 59.5 | 58.4 | 2.42 | 2.63 | ‒2.75 | 0.006 | ‒1.1 | ||
| Autumn | 137 | 35 | 58.2 | 56.4 | 2.48 | 2.21 | ‒1.95 | 0.047 | ‒1.7 | |||
In spring, all species start and finish the migration earlier at Amur than at Baikal. We found significant differences between the median dates of migration periods between the two study sites in all study species (Table 1). We found significant differences in wing length between the birds migrating through the two stations with the exception of the Arctic Warbler (Table 2).
We found clear differences in the migration phenology of warblers between the two study sites, with all species migrating significantly earlier in spring in the east. This may support our second hypothesis, suggesting large-scale longitudinal migration through eastern Russia as a result of a detour. Birds might benefit from circumventing the deserts and mountain ranges of Central Asia, and rather take a longer route, but with more suitable stopover habitats en route. Such migratory detours are well-known in other flyway systems (Chernetsov et al., 2008; Lindström et al., 2011; Vansteelant et al., 2017).
The observed time difference between the two study sites is considerable – in case of the Dusky Warbler, spring migration starts on average 22 days earlier in the east than in the west. This cannot only be explained by a longer migration distance, as the studied warbler species are capable of covering hundreds of kilometres in a single flight bout (Sander et al., 2020), and could therefore cover the distance between the two sites much faster.
Therefore, the differences in migration phenology may arise from the differences in climatic conditions in the two study regions. Spring starts later at Baikal than at Amur (World Climate Guide, 1991–2020), therefore birds must time their migration to pass here later due to the available food supply and the unfavourable weather conditions (strong winds, snow, etc.). Songbirds are known to track the vegetation phenology for resource availability en route (Thorup et al., 2017a, b), which has been shown for a songbird species migrating along the East Asian flyway as well (Heim et al., 2018a, b). The climate at the breeding destinations also affects vegetation growth, which may influence the start of nesting. Species primarily nesting in herbaceous vegetation (e.g. Thick-billed Warbler and Pallas's Grasshopper-warbler) (Bochenski and Kusnierczyk, 2003; del Hoyo and Elliott, 2006; Baker, 2010) might arrive as late as the vegetation is appropriate to build their nests. The Pallas's Grasshopper-warbler arrives latest in both areas, which is most likely related to its habitat requirements, as this species breeds in the densest herbaceous vegetation of all study species (Baker, 2010). Species nesting close to the ground, but not necessarily dependent on herbaceous vegetation (Dusky Warbler, Yellow-browed Warbler, Radde's Warbler and Arctic Warbler) (Forstmeier, 2002; del Hoyo and Elliott, 2006), might start their spring migration (and breeding) earlier in both places because of the available nesting places (roots of trees, fallen branches, bush etc.).
In autumn, we found that migration takes place later and that the migration period is longer in the east than in the west. All of the studied species have typically one brood per year, but some eastern populations might manage two broods per year (del Hoyo and Elliott, 2006). This would also cause eastern population to moult and migrate later in the year. Another possible reason for the elongated autumn migration period is that not only the local but more northern populations are also migrating through Muraviovka Park, while at Baikal we just trapped more local populations. Local populations at Muraviovka Park could start their migration earlier, while the more northerly populations arrive to the stopover site later. For European populations of Common Chiffchaff (Ph. collybita) the same pattern was found (Lövei, 1983). Furthermore, populations at Baikal might start the autumn migration later than the eastern populations, because they finish breeding later as a result of later spring arrival. The Pallas's Grasshopper-warbler was the only species in our study that finished the autumn migration on average later at Baikal than at Amur. This may be caused by the different timing of breeding in different geographical areas, or differences in the extent of post-breeding moult (Eilts et al., 2021). The populations migrating through Baikal may start and finish their breeding later resulting in a prolonged migration. The Pallas's Leaf Warbler and the Two-barred Warbler start their autumn migration later at Amur than at Baikal. This may be caused by the fact that these species breed in the taiga forest, which is close to our study site at Baikal but far away from the Amur. Therefore, it might take them more time to reach Amur.
Regarding the wing length, we found that Phylloscopus species have shorter or statistically not significantly longer wings in the west than in the east, while the opposite is true for the Thick-billed Warbler and the Pallas's Grasshopper-warbler. Overall, no significant differences were found. Therefore, our hypothesis 2 might be true for the Thick-billed Warbler and the Pallas's Grasshopper-warbler, but hypothesis 1 seems more likely for the Phylloscopus leaf warblers. Phylloscopus species migrating at Baikal might use another route to the wintering grounds, which might be shorter than the route used by the eastern birds, but it is also possible that at Muraviovka Park, more birds originated from more northerly breeding grounds, and thus had longer wings.
Regarding to the Thick-billed Warbler and the Pallas's Grashopper-warbler, different subspecies with different wing lengths are expected to occur at the two study sites. Thick-billed Warblers of the subspecies A. ae. aedon breed in SC Siberia and NW Mongolia – including Lake Baikal – while A. ae. rufescens occurs in E Siberia, NE Mongolia and NE China – including the Muraviovka Park (del Hoyo and Elliott, 2006; Gill et al., 2022). The nominate form has longer wings than A. ae. rufescens (Baker, 2010), which fits to our findings, as birds caught at Baikal had significantly longer wings than birds at Amur in both spring and autumn seasons. The Pallas's Grasshopper-warbler is a polytypic species with five subspecies. Following Kennerley and Pearson (2010), Pallas's Grasshopper-warblers breeding at Muraviovka Park belong most likely to nominate L. c. certhiola or L. c. minor, while L. c. rubescens could occur on migration. At Lake Baikal breeds the L. c. sparsimstriatus, while L. c. rubescens could occur on migration. The subspecies are very similar in their plumage (Svensson, 1992; Baker, 2010), and the biometric parameters also overlap (Baker, 2010). However, birds captured at Muraviovka Park have significantly shorter wings than birds at Baikal. The longer routes of western populations, possibly due to a detour as suggested under hypothesis 2, might explain the longer wings of Thick-billed Warblers and Pallas's Grasshopper-warblers at Baikal. In many songbirds, populations with longer migration routes have longer wings (Winkler and Leisler, 1992; Marchetti et al., 1995; Tellería et al., 2001).
In conclusion, we found significant differences in timing between the two sites, suggesting significant longitudinal migration, but a less clear pattern in the morphological differences. Our data underscore hypothesis 2, suggesting that landbirds from Siberia might opt for a detour through eastern Russia instead of a direct route. However, other factors shaping those differences cannot be excluded. Anecdotal evidence for our second hypothesis and a proof for a connection between the two sites comes from a single recovery of a bird ringed at Muraviovka Park, which was later recaptured at Baikal (Heim, 2016). However, this Red-flanked Bluetail (Tarsiger cyanurus) was ringed during spring migration in the east (21 April 2015), and was controlled during autumn migration in the west (8 October 2015). It might therefore be possible that this individual, and possibly many East Asian landbirds, follow a loop-migration pattern, with a detour through the east in spring, and a more direct route in autumn (Bozó et al., 2019). This migration strategy is most likely driven by the variation in food availability and/or prevailing winds en route between the spring and autumn seasons (Gauthreaux et al., 2006; Shaffer et al., 2006; Klaassen et al., 2011; Thorup et al., 2017a, b; Tøttrup et al., 2017). Loop migration could be detected by looking at the differences in wing lengths between spring and autumn at a given study site including adult birds (Jónás et al., 2018; Bozó et al., 2019). However, our data were not suitable to test this, so we cannot conclude whether loop migration is present in these species. Bozó et al. (2019) found for the six Siberian songbird species studied (including the Arctic Warbler), that this migration pattern is generally not specific to these species. The very late spring in north-east Asia might give migrants additional time for a longer spring route (Ktitorov et al., 2022). Tracking studies on Siberian landbird migrants may brig new facts to clarify the migration routes and patterns in this region.
LB, YA and WH collected field data. LB and WH performed data analyses and created the figures. LB wrote an early version of the manuscript. YA and WH revised and improved the manuscript. All authors have read and approved the final manuscript.
The authors confirm that all experiments were carried out under the current law for scientific bird ringing in Russia, and all necessary permissions were obtained.
The authors declare that they have no competing interests.
The authors want to thank the staff of Muraviovka Park and Baikal Bird Ringing Station Baikalsky State Nature Biosphere Reserve as well as the field teams of these the ringing stations stations for enabling these studies in Russia. We thank the editor and two anonymous reviewers for their constructive suggestions which greatly improved the paper.
|
Chiale, M.C., Rendón, M.A., Labaude, S., Deville, A.S., Garrido-Fernández, J., Pérez-Gálvez, A., et al., 2012. The color of greater flamingo feathers fades when no cosmetics are applied. Ecol. Evol. 11, 13773–13779.
|
|
Hall, B.P., 1963. The Francolins: A Study in Speciation. British Museum, London.
|
|
Hall, A., Sage, R.A., Madden, J.R., 2012. The effects of released pheasants on invertebrate populations in and around woodland release sites. Ecol. Evol. 11, 13559–13569.
|
|
Hernández, A., Martínez-Gómez, M., Beamonte-Barrientos, R., Montoya, B., 2012. Colourful traits in female birds relate to individual condition, reproductive performance and male-mate preferences: a meta-analytic approach. Biol. Lett. 17, 20210283.
|
|
Laitly, A., Callaghan, C.T., Delhey, K., Cornwell, W.K., 2012. Is color data from citizen science photographs reliable for biodiversity research? Ecol. Evol. 11, 4071–4083.
|
|
Little, R., 2016. Terrestrial Gamebirds and Snipes of Africa. Guineafowls, Francolins, Spurfowls, Quails, Sandgrouse and Snipes. Jacana, Johannesburg.
|
|
Liversage, R., 1978. The breeding seasons of African gamebirds. S. Afr. J. Wild. Res. Suppl. 1, 70–75.
|
|
Madge, S.M., McGowan, P., 2001. Pheasants, Partridges and Grouse. Christopher Helm, London.
|
|
Nwaerema, P., Diagi, B., Edokpa, D., Ajiere, S., 2019. Population variability and heat bias prediction of Africa from 2019 to 2049: an approach to sustainable continental heat management. World Sci. News 130, 265–285.
|
|
Pérez-Rodríguez, L., Martínez-Padilla, J., Mougeot, F., 2013. Carotenoid-based Ornaments as signals of health status in birds: evidences from two Galliform species, the Red-Legged Partridge (Alectoris rufa) and the Red Grouse (Lagopus lagopus scoticus). In: Yamaguchi, M. (Ed. ), Carotenoids: Food Sources, Production and Health Benefits. Nova Science Publishers, Hauppauge, pp. 173–198.
|
|
van Niekerk, J., 1983. Observations on courtship in Swainson's francolin. Bokmakierie 35, 90–92.
|
|
van Niekerk, J., 2001. Social and breeding behaviour of the crested francolin in the Rustenburg district, South Africa. S. Afr. J. Wildl. Res. 31, 35–42.
|
|
Withers, M.B., Hosking, D., 1996. Common Birds of East Africa. Harper Collins, London.
|
| 1. | Nazhong Zhang, Lizhi Zhou, Zhuqing Yang, et al. Effects of Food Changes on Intestinal Bacterial Diversity of Wintering Hooded Cranes (Grus monacha). Animals, 2021, 11(2): 433. DOI:10.3390/ani11020433 |
| 2. | Fernanda Bribiesca-Contreras, Ben Parslew, William I. Sellers. Functional morphology of the forelimb musculature reflects flight and foraging styles in aquatic birds. Journal of Ornithology, 2021, 162(3): 779. DOI:10.1007/s10336-021-01868-y |
| 3. | Guang Hu, Maxwell Wilson, Bing-Bing Zhou, et al. Spatiotemporal patterns and ecological consequences of a fragmented landscape created by damming. PeerJ, 2021, 9: e11416. DOI:10.7717/peerj.11416 |
| 4. | Federico A. Gianechini, Marcos D. Ercoli, Ignacio Díaz‐Martínez. Differential locomotor and predatory strategies of Gondwanan and derived Laurasian dromaeosaurids (Dinosauria, Theropoda, Paraves): Inferences from morphometric and comparative anatomical studies. Journal of Anatomy, 2020, 236(5): 772. DOI:10.1111/joa.13153 |
| 5. | Paweł Mackiewicz, Adam Dawid Urantówka, Aleksandra Kroczak, et al. Resolving Phylogenetic Relationships within Passeriformes Based on Mitochondrial Genes and Inferring the Evolution of Their Mitogenomes in Terms of Duplications. Genome Biology and Evolution, 2019, 11(10): 2824. DOI:10.1093/gbe/evz209 |
| 6. | Ying-Chi Chan, He-Bo Peng, Yong-Xiang Han, et al. 2019. DOI:10.1101/570556 |
| 7. | Chuang Zhou, Jiazheng Jin, Yinzhu Chen, et al. Two new complete mitochondrial genomes (Paradoxornis gularis and Niltava davidi) and their phylogenetic and taxonomic implications. Mitochondrial DNA Part B, 2019, 4(1): 820. DOI:10.1080/23802359.2019.1574682 |
| 8. | Houlang Duan, Shaoxia Xia, Xiyong Hou, et al. Conservation planning following reclamation of intertidal areas throughout the Yellow and Bohai Seas, China. Biodiversity and Conservation, 2019, 28(14): 3787. DOI:10.1007/s10531-019-01851-3 |
| 9. | Yuanqiu Dong, Xingjia Xiang, Guanghong Zhao, et al. Variations in gut bacterial communities of hooded crane (Grus monacha) over spatial-temporal scales. PeerJ, 2019, 7: e7045. DOI:10.7717/peerj.7045 |
| 10. | Wenjing Wan, Lizhi Zhou, Yunwei Song. Shifts in foraging behavior of wintering Hooded Cranes (Grus monacha) in three different habitats at Shengjin Lake, China. Avian Research, 2016, 7(1) DOI:10.1186/s40657-016-0047-0 |
| 11. | Jacqueline M. T. Nguyen, Simon Y. W. Ho. Mitochondrial rate variation among lineages of passerine birds. Journal of Avian Biology, 2016, 47(5): 690. DOI:10.1111/jav.00928 |
| 12. | Pierce Hutton, Kevin J. McGraw. Urban–Rural Differences in Eye, Bill, and Skull Allometry in House Finches (Haemorhous mexicanus). Integrative and Comparative Biology, 2016, 56(6): 1215. DOI:10.1093/icb/icw077 |
| 13. | Michal Šulc, Petr Procházka, Miroslav Capek, et al. Common cuckoo females are not choosy when removing an egg during parasitism. Behavioral Ecology, 2016. DOI:10.1093/beheco/arw085 |
| 14. | César González-Lagos, Javier Quesada. Avian Ecology in Latin American Cityscapes. DOI:10.1007/978-3-319-63475-3_6 |
| Spring | N | ASM | AEM | Median | χ2 | p | |||||||
| Amur | Baikal | Amur | Baikal | Amur | Baikal | Amur | Baikal | ||||||
| Thick-billed W. | 766 | 217 | 19 May | 29 May | 8 Jun | 12 Jun | 31 May | 2 Jun | 78.103 | < 0.0001 | |||
| Pallas's G-w. | 57 | 114 | 28 May | 3 Jun | 10 Jun | 18 Jun | 5 Jun | 12 Jun | ‒36.545 | < 0.0001 | |||
| Radde's W. | 94 | 246 | 12 May | 26 May | 5 Jun | 14 Jun | 25 May | 7 Jun | 80.31 | < 0.0001 | |||
| Dusky W. | 503 | 181 | 1 May | 23 May | 7 Jun | 13 Jun | 21 May | 2 Jun | ‒67.0 | < 0.0001 | |||
| Pallas's Leaf W. | 79 | 46 | 2 May | 18 May | 1 Jun | 15 Jun | 18 May | 27 May | 9.48 | < 0.002 | |||
| Yellow-browed W. | 2274 | 53 | 28 Apr | 13 May | 2 Jun | 3 Jun | 14 May | 23 May | 25.978 | < 0.0001 | |||
| Arctic W. | 236 | 102 | 19 May | 22 May | 7 Jun | 13 Jun | 28 May | 1 Jun | 22.755 | < 0.0001 | |||
| Two-barred W. | 65 | 108 | 23 May | 26 May | 7 Jun | 17 Jun | 31 May | 11 Jun | 90.577 | < 0.0001 | |||
| Autumn | |||||||||||||
| Thick-billed W. | 654 | 206 | 2 Aug | 9 Aug | 10 Sep | 27 Aug | 14 Aug | 18 Aug | 15.76 | < 0.0001 | |||
| Pallas's G-W. | 458 | 159 | 2 Aug | 15 Aug | 16 Sep | 20 Sep | 18 Aug | 29 Aug | 73.93 | < 0.0001 | |||
| Radde's W. | 218 | 275 | 10 Aug | 24 Aug | 24 Sep | 22 Sep | 11 Sep | 7 Sep | 47.447 | < 0.0001 | |||
| Dusky W. | 944 | 190 | 1 Aug | 4 Aug | 5 Oct | 24 Sep | 11 Sep | 28 Aug | 58.176 | < 0.0001 | |||
| Pallas's Leaf W. | 542 | 377 | 8 Sep | 26 Aug | 13 Oct | 9 Oct | 23 Sep | 17 Sep | 18.981 | < 0.0001 | |||
| Yellow-browed W. | 2113 | 168 | 29 Jul | 19 Aug | 6 Oct | 22 Sep | 7 Sep | 5 Sep | 0.805 | 0.369 | |||
| Arctic W. | 209 | 104 | 3 Aug | 5 Aug | 13 Sep | 1 Sep | 23 Aug | 23 Aug | 0.281 | 0.596 | |||
| Two-barred W. | 123 | 40 | 13 Aug | 11 Aug | 19 Sep | 15 Sep | 24 Aug | 29 Aug | 8.26 | 0.11 | |||
| Species | Season | N | Mean Wmax | SD | z | p | ΔWmax | |||||
| Amur | Baikal | Amur | Baikal | Amur | Baikal | |||||||
| Thick-billed Warbler | Spring | 757 | 217 | 78.5 | 82.1 | 1.88 | 2.27 | ‒17.70 | < 0.0001 | 3.6 | ||
| Autumn | 670 | 205 | 77.9 | 81.6 | 1.96 | 2.34 | ‒16.93 | < 0.0001 | 3.7 | |||
| Pallas's Grasshopper-warbler | Spring | 32 | 116 | 65 | 67.7 | 2.78 | 2.77 | ‒4.28 | < 0.0001 | 2.7 | ||
| Autumn | 400 | 153 | 61.7 | 64.7 | 2.07 | 2.47 | ‒12.24 | < 0.0001 | 3 | |||
| Radde's Warbler | Spring | 93 | 249 | 62.5 | 60.9 | 3.58 | 3.93 | ‒3.1 | 0.002 | ‒1.7 | ||
| Autumn | 285 | 255 | 61.5 | 60.5 | 3.78 | 3.81 | ‒3.09 | 0.002 | ‒1 | |||
| Dusky Warbler | Spring | 373 | 184 | 60.3 | 58.4 | 3.38 | 3.28 | ‒5.60 | < 0.0001 | ‒1.9 | ||
| Autumn | 1250 | 189 | 60.7 | 59.1 | 3.26 | 3.27 | ‒5.24 | < 0.0001 | ‒1.6 | |||
| Pallas's Leaf Warbler | Spring | 78 | 46 | 53 | 51.6 | 2.69 | 2.39 | ‒1.92 | 0.047 | ‒1.4 | ||
| Autumn | 532 | 380 | 53.6 | 51 | 2.74 | 2.39 | ‒12.71 | < 0.0001 | ‒2.6 | |||
| Yellow-browed Warbler | Spring | 2160 | 53 | 56.7 | 56.9 | 2.49 | 2.48 | ‒0.75 | 0.45 | 0.2 | ||
| Autumn | 2573 | 158 | 56.6 | 56 | 2.32 | 2.63 | ‒2.41 | 0.015 | ‒0.6 | |||
| Arctic Warbler | Spring | 234 | 101 | 67 | 66.3 | 2.56 | 3.66 | ‒0.82 | 0.41 | ‒0.7 | ||
| Autumn | 217 | 104 | 64.8 | 64.4 | 2.39 | 2.46 | ‒1.19 | 0.234 | ‒0.4 | |||
| Two-barred Warbler | Spring | 63 | 107 | 59.5 | 58.4 | 2.42 | 2.63 | ‒2.75 | 0.006 | ‒1.1 | ||
| Autumn | 137 | 35 | 58.2 | 56.4 | 2.48 | 2.21 | ‒1.95 | 0.047 | ‒1.7 | |||